 Found 1667 hits with Last Name = 'lamar' and Initial = 'j'
Found 1667 hits with Last Name = 'lamar' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cathepsin B
(Homo sapiens (Human)) | BDBM50137733
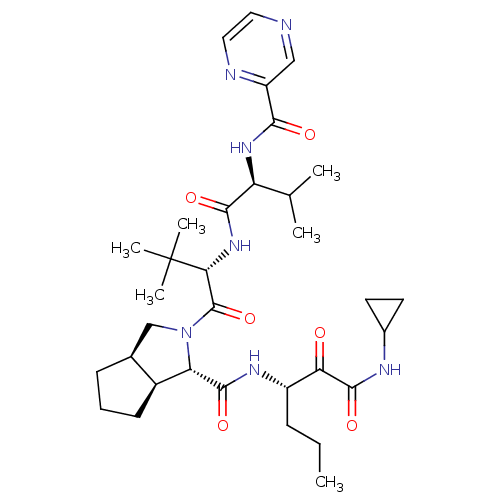
((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...)Show SMILES CCC[C@H](NC(=O)[C@@H]1[C@H]2CCC[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)(C)C)C(=O)C(=O)NC1CC1 Show InChI InChI=1S/C33H49N7O6/c1-7-9-22(26(41)31(45)36-20-12-13-20)37-30(44)25-21-11-8-10-19(21)17-40(25)32(46)27(33(4,5)6)39-29(43)24(18(2)3)38-28(42)23-16-34-14-15-35-23/h14-16,18-22,24-25,27H,7-13,17H2,1-6H3,(H,36,45)(H,37,44)(H,38,42)(H,39,43)/t19-,21-,22-,24-,25-,27+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against cathepsin B |
Bioorg Med Chem Lett 14: 257-61 (2003)
BindingDB Entry DOI: 10.7270/Q20P0ZDG |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM16250
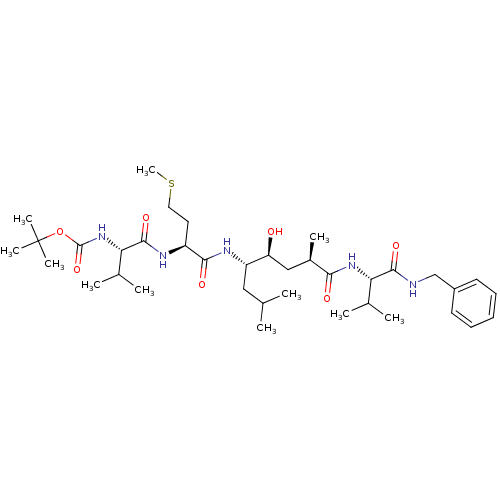
(CHEMBL290001 | N-(tert-butoxycarbonyl)-L-valyl-N-[...)Show SMILES CSCC[C@H](NC(=O)[C@@H](NC(=O)OC(C)(C)C)C(C)C)C(=O)N[C@@H](CC(C)C)[C@@H](O)C[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C37H63N5O7S/c1-22(2)19-28(29(43)20-25(7)32(44)41-30(23(3)4)34(46)38-21-26-15-13-12-14-16-26)40-33(45)27(17-18-50-11)39-35(47)31(24(5)6)42-36(48)49-37(8,9)10/h12-16,22-25,27-31,43H,17-21H2,1-11H3,(H,38,46)(H,39,47)(H,40,45)(H,41,44)(H,42,48)/t25-,27+,28+,29+,30+,31+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of Beta-secretase-1 in HEK293 (Human Embryonic Kidney) cell line. |
Bioorg Med Chem Lett 14: 239-43 (2003)
BindingDB Entry DOI: 10.7270/Q2MW2HP3 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50137736

((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...)Show SMILES CCC[C@H](NC(=O)[C@@H]1[C@H]2CCC[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)(C)C)C(=O)C(=O)NC(C)CC Show InChI InChI=1S/C34H53N7O6/c1-9-12-23(27(42)32(46)37-20(5)10-2)38-31(45)26-22-14-11-13-21(22)18-41(26)33(47)28(34(6,7)8)40-30(44)25(19(3)4)39-29(43)24-17-35-15-16-36-24/h15-17,19-23,25-26,28H,9-14,18H2,1-8H3,(H,37,46)(H,38,45)(H,39,43)(H,40,44)/t20?,21-,22-,23-,25-,26-,28+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against cathepsin B |
Bioorg Med Chem Lett 14: 257-61 (2003)
BindingDB Entry DOI: 10.7270/Q20P0ZDG |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50137720

((1S,3aR,6aS)-2-((S)-3-Methyl-2-{(S)-3-methyl-2-[(p...)Show SMILES CCCC(NC(=O)[C@@H]1[C@H]2CCC[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)C)C(=O)C(=O)NC1CC1 Show InChI InChI=1S/C32H47N7O6/c1-6-8-22(27(40)31(44)35-20-11-12-20)36-30(43)26-21-10-7-9-19(21)16-39(26)32(45)25(18(4)5)38-29(42)24(17(2)3)37-28(41)23-15-33-13-14-34-23/h13-15,17-22,24-26H,6-12,16H2,1-5H3,(H,35,44)(H,36,43)(H,37,41)(H,38,42)/t19-,21-,22?,24-,25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against cathepsin B |
Bioorg Med Chem Lett 14: 257-61 (2003)
BindingDB Entry DOI: 10.7270/Q20P0ZDG |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50137730
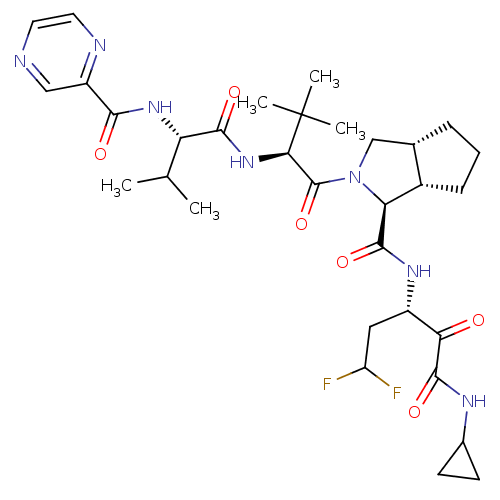
((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...)Show SMILES CC(C)[C@H](NC(=O)c1cnccn1)C(=O)N[C@H](C(=O)N1C[C@@H]2CCC[C@@H]2[C@H]1C(=O)N[C@@H](CC(F)F)C(=O)C(=O)NC1CC1)C(C)(C)C Show InChI InChI=1S/C32H45F2N7O6/c1-16(2)23(39-27(43)21-14-35-11-12-36-21)28(44)40-26(32(3,4)5)31(47)41-15-17-7-6-8-19(17)24(41)29(45)38-20(13-22(33)34)25(42)30(46)37-18-9-10-18/h11-12,14,16-20,22-24,26H,6-10,13,15H2,1-5H3,(H,37,46)(H,38,45)(H,39,43)(H,40,44)/t17-,19-,20-,23-,24-,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against cathepsin B |
Bioorg Med Chem Lett 14: 257-61 (2003)
BindingDB Entry DOI: 10.7270/Q20P0ZDG |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50150593

(3,4-Dihydro-1H-isoquinoline-2-carboxylic acid (S)-...)Show SMILES CCCC(NC(=O)[C@@H]1CC(CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)CCCCn1cnnn1)C(C)C)C(C)C)OC(=O)N1CCc2ccccc2C1)C(=O)C(=O)NC1CC1 Show InChI InChI=1S/C40H58N10O8/c1-6-11-30(35(52)38(55)42-28-15-16-28)43-36(53)31-20-29(58-40(57)48-19-17-26-12-7-8-13-27(26)21-48)22-50(31)39(56)34(25(4)5)45-37(54)33(24(2)3)44-32(51)14-9-10-18-49-23-41-46-47-49/h7-8,12-13,23-25,28-31,33-34H,6,9-11,14-22H2,1-5H3,(H,42,55)(H,43,53)(H,44,51)(H,45,54)/t29?,30?,31-,33-,34-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory potency against HCV NS3 protease |
Bioorg Med Chem Lett 14: 4333-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.078
BindingDB Entry DOI: 10.7270/Q2TX3DVP |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50137720

((1S,3aR,6aS)-2-((S)-3-Methyl-2-{(S)-3-methyl-2-[(p...)Show SMILES CCCC(NC(=O)[C@@H]1[C@H]2CCC[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)C)C(=O)C(=O)NC1CC1 Show InChI InChI=1S/C32H47N7O6/c1-6-8-22(27(40)31(44)35-20-11-12-20)36-30(43)26-21-10-7-9-19(21)16-39(26)32(45)25(18(4)5)38-29(42)24(17(2)3)37-28(41)23-15-33-13-14-34-23/h13-15,17-22,24-26H,6-12,16H2,1-5H3,(H,35,44)(H,36,43)(H,37,41)(H,38,42)/t19-,21-,22?,24-,25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against cathepsin L |
Bioorg Med Chem Lett 14: 257-61 (2003)
BindingDB Entry DOI: 10.7270/Q20P0ZDG |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Human rhinovirus B) | BDBM50137724
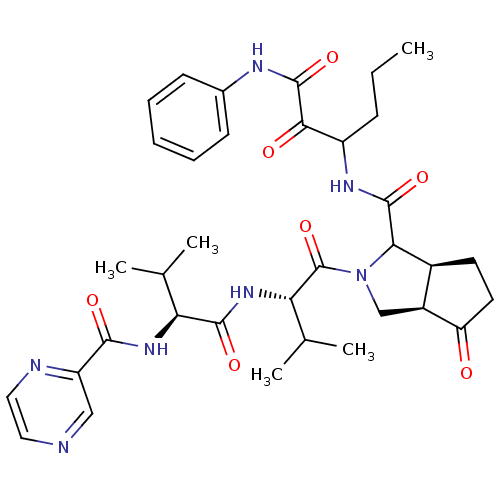
((3aR,5S)-2-((S)-3-Methyl-2-{(S)-3-methyl-2-[(pyraz...)Show SMILES CCCC(NC(=O)C1[C@H]2CCC(=O)[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)C)C(=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C35H45N7O7/c1-6-10-24(30(44)34(48)38-21-11-8-7-9-12-21)39-33(47)29-22-13-14-26(43)23(22)18-42(29)35(49)28(20(4)5)41-32(46)27(19(2)3)40-31(45)25-17-36-15-16-37-25/h7-9,11-12,15-17,19-20,22-24,27-29H,6,10,13-14,18H2,1-5H3,(H,38,48)(H,39,47)(H,40,45)(H,41,46)/t22-,23-,24?,27-,28-,29?/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Binding affinity towards Protease using PNA assay in rats |
Bioorg Med Chem Lett 14: 251-6 (2003)
BindingDB Entry DOI: 10.7270/Q24B30R9 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50152750
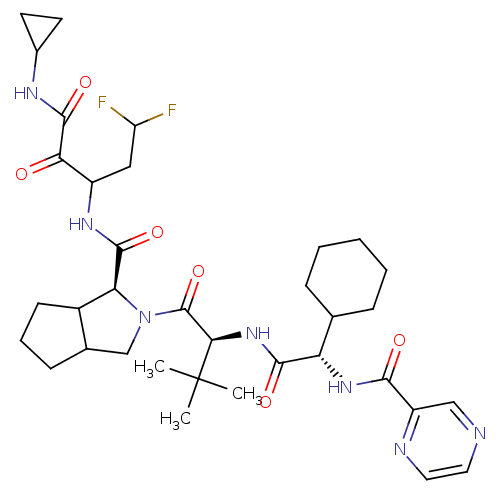
(2-((S)-2-{(S)-2-Cyclohexyl-2-[(pyrazine-2-carbonyl...)Show SMILES CC(C)(C)[C@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C1CCCCC1)C(=O)N1CC2CCCC2[C@H]1C(=O)NC(CC(F)F)C(=O)C(=O)NC1CC1 Show InChI InChI=1S/C35H49F2N7O6/c1-35(2,3)29(43-31(47)26(19-8-5-4-6-9-19)42-30(46)24-17-38-14-15-39-24)34(50)44-18-20-10-7-11-22(20)27(44)32(48)41-23(16-25(36)37)28(45)33(49)40-21-12-13-21/h14-15,17,19-23,25-27,29H,4-13,16,18H2,1-3H3,(H,40,49)(H,41,48)(H,42,46)(H,43,47)/t20?,22?,23?,26-,27-,29+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of HCV NS3 protease in the pNA based inhibition assay |
Bioorg Med Chem Lett 14: 5007-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.007
BindingDB Entry DOI: 10.7270/Q29P314N |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50150591
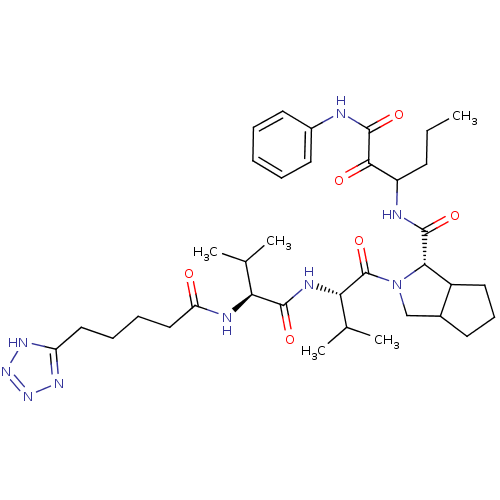
((S)-2-{(S)-3-Methyl-2-[(S)-3-methyl-2-(5-1H-tetraz...)Show SMILES CCCC(NC(=O)[C@@H]1C2CCCC2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)CCCCc1nnn[nH]1)C(C)C)C(C)C)C(=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C36H53N9O6/c1-6-13-26(32(47)35(50)37-24-15-8-7-9-16-24)38-34(49)31-25-17-12-14-23(25)20-45(31)36(51)30(22(4)5)40-33(48)29(21(2)3)39-28(46)19-11-10-18-27-41-43-44-42-27/h7-9,15-16,21-23,25-26,29-31H,6,10-14,17-20H2,1-5H3,(H,37,50)(H,38,49)(H,39,46)(H,40,48)(H,41,42,43,44)/t23?,25?,26?,29-,30-,31-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory potency against HCV NS3 protease |
Bioorg Med Chem Lett 14: 4333-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.078
BindingDB Entry DOI: 10.7270/Q2TX3DVP |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Human rhinovirus B) | BDBM50137747
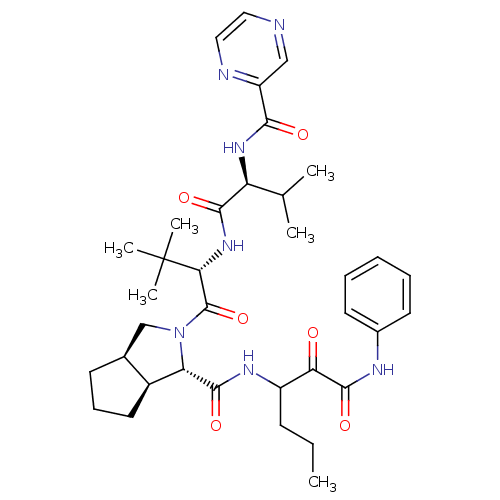
((1S,3aR,6aS)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2...)Show SMILES CCCC(NC(=O)[C@@H]1[C@H]2CCC[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)(C)C)C(=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C36H49N7O6/c1-7-12-25(29(44)34(48)39-23-14-9-8-10-15-23)40-33(47)28-24-16-11-13-22(24)20-43(28)35(49)30(36(4,5)6)42-32(46)27(21(2)3)41-31(45)26-19-37-17-18-38-26/h8-10,14-15,17-19,21-22,24-25,27-28,30H,7,11-13,16,20H2,1-6H3,(H,39,48)(H,40,47)(H,41,45)(H,42,46)/t22-,24-,25?,27-,28-,30+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Binding affinity towards Protease using PNA assay in rats |
Bioorg Med Chem Lett 14: 263-6 (2003)
BindingDB Entry DOI: 10.7270/Q2VX0FX5 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50137732

((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...)Show SMILES CCC[C@H](NC(=O)[C@@H]1[C@H]2CCC[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)(C)C)C(=O)C(=O)N[C@@H](C)c1ccccc1 Show InChI InChI=1S/C38H53N7O6/c1-8-13-27(31(46)36(50)41-23(4)24-14-10-9-11-15-24)42-35(49)30-26-17-12-16-25(26)21-45(30)37(51)32(38(5,6)7)44-34(48)29(22(2)3)43-33(47)28-20-39-18-19-40-28/h9-11,14-15,18-20,22-23,25-27,29-30,32H,8,12-13,16-17,21H2,1-7H3,(H,41,50)(H,42,49)(H,43,47)(H,44,48)/t23-,25-,26-,27-,29-,30-,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against cathepsin L |
Bioorg Med Chem Lett 14: 257-61 (2003)
BindingDB Entry DOI: 10.7270/Q20P0ZDG |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50137730

((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...)Show SMILES CC(C)[C@H](NC(=O)c1cnccn1)C(=O)N[C@H](C(=O)N1C[C@@H]2CCC[C@@H]2[C@H]1C(=O)N[C@@H](CC(F)F)C(=O)C(=O)NC1CC1)C(C)(C)C Show InChI InChI=1S/C32H45F2N7O6/c1-16(2)23(39-27(43)21-14-35-11-12-36-21)28(44)40-26(32(3,4)5)31(47)41-15-17-7-6-8-19(17)24(41)29(45)38-20(13-22(33)34)25(42)30(46)37-18-9-10-18/h11-12,14,16-20,22-24,26H,6-10,13,15H2,1-5H3,(H,37,46)(H,38,45)(H,39,43)(H,40,44)/t17-,19-,20-,23-,24-,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against cathepsin L |
Bioorg Med Chem Lett 14: 257-61 (2003)
BindingDB Entry DOI: 10.7270/Q20P0ZDG |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183974
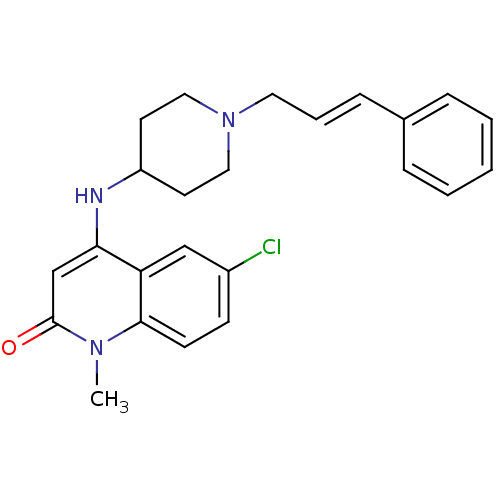
((E)-6-chloro-4-(1-cinnamylpiperidin-4-ylamino)-1-m...)Show SMILES Cn1c2ccc(Cl)cc2c(NC2CCN(C\C=C\c3ccccc3)CC2)cc1=O Show InChI InChI=1S/C24H26ClN3O/c1-27-23-10-9-19(25)16-21(23)22(17-24(27)29)26-20-11-14-28(15-12-20)13-5-8-18-6-3-2-4-7-18/h2-10,16-17,20,26H,11-15H2,1H3/b8-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50150599
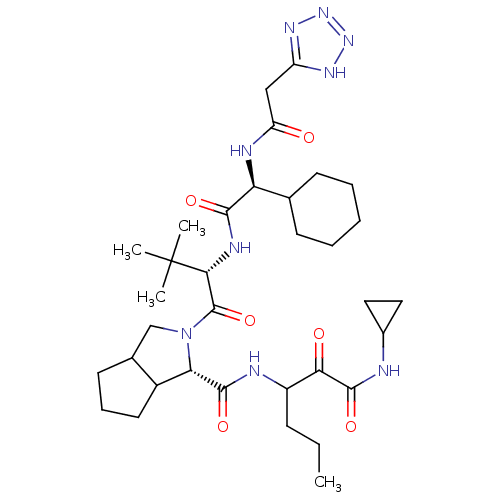
(2-{(S)-2-[(S)-2-Cyclohexyl-2-(2-2H-tetrazol-5-yl-a...)Show SMILES CCCC(NC(=O)[C@@H]1C2CCCC2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)Cc1nnn[nH]1)C1CCCCC1)C(C)(C)C)C(=O)C(=O)NC1CC1 Show InChI InChI=1S/C34H53N9O6/c1-5-10-23(28(45)32(48)35-21-15-16-21)36-31(47)27-22-14-9-13-20(22)18-43(27)33(49)29(34(2,3)4)38-30(46)26(19-11-7-6-8-12-19)37-25(44)17-24-39-41-42-40-24/h19-23,26-27,29H,5-18H2,1-4H3,(H,35,48)(H,36,47)(H,37,44)(H,38,46)(H,39,40,41,42)/t20?,22?,23?,26-,27-,29+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory potency against HCV NS3 protease |
Bioorg Med Chem Lett 14: 4333-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.078
BindingDB Entry DOI: 10.7270/Q2TX3DVP |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50150595
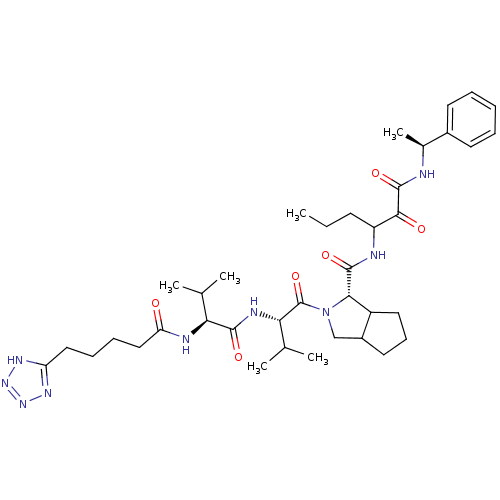
((S)-2-{(S)-3-Methyl-2-[(S)-3-methyl-2-(5-1H-tetraz...)Show SMILES CCCC(NC(=O)[C@@H]1C2CCCC2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)CCCCc1nnn[nH]1)C(C)C)C(C)C)C(=O)C(=O)N[C@@H](C)c1ccccc1 Show InChI InChI=1S/C38H57N9O6/c1-7-14-28(34(49)37(52)39-24(6)25-15-9-8-10-16-25)40-36(51)33-27-18-13-17-26(27)21-47(33)38(53)32(23(4)5)42-35(50)31(22(2)3)41-30(48)20-12-11-19-29-43-45-46-44-29/h8-10,15-16,22-24,26-28,31-33H,7,11-14,17-21H2,1-6H3,(H,39,52)(H,40,51)(H,41,48)(H,42,50)(H,43,44,45,46)/t24-,26?,27?,28?,31-,32-,33-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory potency against HCV NS3 protease |
Bioorg Med Chem Lett 14: 4333-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.078
BindingDB Entry DOI: 10.7270/Q2TX3DVP |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50137732

((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...)Show SMILES CCC[C@H](NC(=O)[C@@H]1[C@H]2CCC[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)(C)C)C(=O)C(=O)N[C@@H](C)c1ccccc1 Show InChI InChI=1S/C38H53N7O6/c1-8-13-27(31(46)36(50)41-23(4)24-14-10-9-11-15-24)42-35(49)30-26-17-12-16-25(26)21-45(30)37(51)32(38(5,6)7)44-34(48)29(22(2)3)43-33(47)28-20-39-18-19-40-28/h9-11,14-15,18-20,22-23,25-27,29-30,32H,8,12-13,16-17,21H2,1-7H3,(H,41,50)(H,42,49)(H,43,47)(H,44,48)/t23-,25-,26-,27-,29-,30-,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against cathepsin B |
Bioorg Med Chem Lett 14: 257-61 (2003)
BindingDB Entry DOI: 10.7270/Q20P0ZDG |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50137736

((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...)Show SMILES CCC[C@H](NC(=O)[C@@H]1[C@H]2CCC[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)(C)C)C(=O)C(=O)NC(C)CC Show InChI InChI=1S/C34H53N7O6/c1-9-12-23(27(42)32(46)37-20(5)10-2)38-31(45)26-22-14-11-13-21(22)18-41(26)33(47)28(34(6,7)8)40-30(44)25(19(3)4)39-29(43)24-17-35-15-16-36-24/h15-17,19-23,25-26,28H,9-14,18H2,1-8H3,(H,37,46)(H,38,45)(H,39,43)(H,40,44)/t20?,21-,22-,23-,25-,26-,28+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against cathepsin L |
Bioorg Med Chem Lett 14: 257-61 (2003)
BindingDB Entry DOI: 10.7270/Q20P0ZDG |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50137732

((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...)Show SMILES CCC[C@H](NC(=O)[C@@H]1[C@H]2CCC[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)(C)C)C(=O)C(=O)N[C@@H](C)c1ccccc1 Show InChI InChI=1S/C38H53N7O6/c1-8-13-27(31(46)36(50)41-23(4)24-14-10-9-11-15-24)42-35(49)30-26-17-12-16-25(26)21-45(30)37(51)32(38(5,6)7)44-34(48)29(22(2)3)43-33(47)28-20-39-18-19-40-28/h9-11,14-15,18-20,22-23,25-27,29-30,32H,8,12-13,16-17,21H2,1-7H3,(H,41,50)(H,42,49)(H,43,47)(H,44,48)/t23-,25-,26-,27-,29-,30-,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Chymotrypsin |
Bioorg Med Chem Lett 14: 257-61 (2003)
BindingDB Entry DOI: 10.7270/Q20P0ZDG |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50150606

(3,4-Dihydro-1H-isoquinoline-2-carboxylic acid (S)-...)Show SMILES CCCC(NC(=O)[C@@H]1CC(CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)CCCCC(O)=O)C(C)C)C(C)C)OC(=O)N1CCc2ccccc2C1)C(=O)C(=O)NC1CC1 Show InChI InChI=1S/C40H58N6O10/c1-6-11-29(35(50)38(53)41-27-16-17-27)42-36(51)30-20-28(56-40(55)45-19-18-25-12-7-8-13-26(25)21-45)22-46(30)39(54)34(24(4)5)44-37(52)33(23(2)3)43-31(47)14-9-10-15-32(48)49/h7-8,12-13,23-24,27-30,33-34H,6,9-11,14-22H2,1-5H3,(H,41,53)(H,42,51)(H,43,47)(H,44,52)(H,48,49)/t28?,29?,30-,33-,34-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory potency against HCV NS3 protease |
Bioorg Med Chem Lett 14: 4333-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.078
BindingDB Entry DOI: 10.7270/Q2TX3DVP |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Human rhinovirus B) | BDBM50137721

(2-(3-{[(1S,5S,6R)-2-((S)-3-Methyl-2-{(S)-3-methyl-...)Show SMILES CCCC(NC(=O)[C@@H]1[C@H]2CCC[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)C)C(=O)C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C38H51N7O8/c1-6-11-26(32(46)36(50)42-27(38(52)53)18-23-12-8-7-9-13-23)41-35(49)31-25-15-10-14-24(25)20-45(31)37(51)30(22(4)5)44-34(48)29(21(2)3)43-33(47)28-19-39-16-17-40-28/h7-9,12-13,16-17,19,21-22,24-27,29-31H,6,10-11,14-15,18,20H2,1-5H3,(H,41,49)(H,42,50)(H,43,47)(H,44,48)(H,52,53)/t24-,25-,26?,27-,29-,30-,31-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Binding affinity towards Protease using PNA assay in rats |
Bioorg Med Chem Lett 14: 251-6 (2003)
BindingDB Entry DOI: 10.7270/Q24B30R9 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50152754
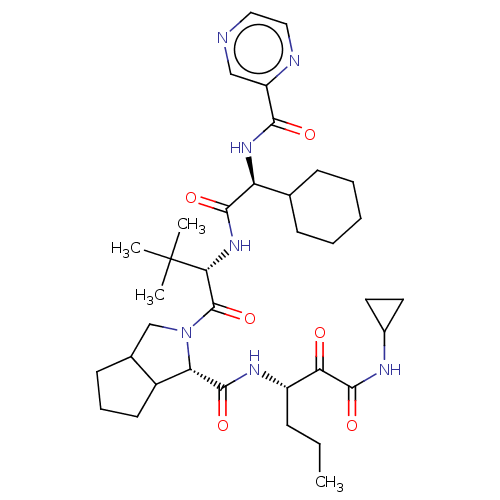
(2-((S)-2-{(S)-2-Cyclohexyl-2-[(pyrazine-2-carbonyl...)Show SMILES CCC[C@H](NC(=O)[C@@H]1C2CCCC2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C1CCCCC1)C(C)(C)C)C(=O)C(=O)NC1CC1 Show InChI InChI=1S/C36H53N7O6/c1-5-10-25(29(44)34(48)39-23-15-16-23)40-33(47)28-24-14-9-13-22(24)20-43(28)35(49)30(36(2,3)4)42-32(46)27(21-11-7-6-8-12-21)41-31(45)26-19-37-17-18-38-26/h17-19,21-25,27-28,30H,5-16,20H2,1-4H3,(H,39,48)(H,40,47)(H,41,45)(H,42,46)/t22?,24?,25?,27-,28-,30+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of HCV NS3 protease in the pNA based inhibition assay |
Bioorg Med Chem Lett 14: 5007-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.007
BindingDB Entry DOI: 10.7270/Q29P314N |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50150602
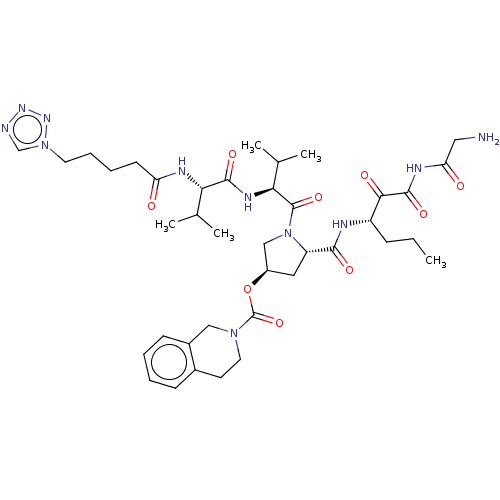
(3,4-Dihydro-1H-isoquinoline-2-carboxylic acid (S)-...)Show SMILES CCC[C@H](NC(=O)[C@@H]1C[C@H](CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)CCCCn1cnnn1)C(C)C)C(C)C)OC(=O)N1CCc2ccccc2C1)C(=O)C(=O)NC(=O)CN Show InChI InChI=1S/C39H57N11O9/c1-6-11-28(34(53)37(56)44-31(52)19-40)42-35(54)29-18-27(59-39(58)48-17-15-25-12-7-8-13-26(25)20-48)21-50(29)38(57)33(24(4)5)45-36(55)32(23(2)3)43-30(51)14-9-10-16-49-22-41-46-47-49/h7-8,12-13,22-24,27-29,32-33H,6,9-11,14-21,40H2,1-5H3,(H,42,54)(H,43,51)(H,45,55)(H,44,52,56)/t27?,28?,29-,32-,33-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory potency against HCV NS3 protease |
Bioorg Med Chem Lett 14: 4333-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.078
BindingDB Entry DOI: 10.7270/Q2TX3DVP |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50152753

((S)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[(pyrazi...)Show SMILES CCC[C@H](NC(=O)[C@@H]1C2CCCC2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)[C@@H](C)CC)C(C)(C)C)C(=O)C(=O)NC1CC1 Show InChI InChI=1S/C34H51N7O6/c1-7-10-23(27(42)32(46)37-21-13-14-21)38-31(45)26-22-12-9-11-20(22)18-41(26)33(47)28(34(4,5)6)40-30(44)25(19(3)8-2)39-29(43)24-17-35-15-16-36-24/h15-17,19-23,25-26,28H,7-14,18H2,1-6H3,(H,37,46)(H,38,45)(H,39,43)(H,40,44)/t19?,20?,22?,23?,25-,26-,28+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of HCV NS3 protease in the pNA based inhibition assay |
Bioorg Med Chem Lett 14: 5007-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.007
BindingDB Entry DOI: 10.7270/Q29P314N |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50152748
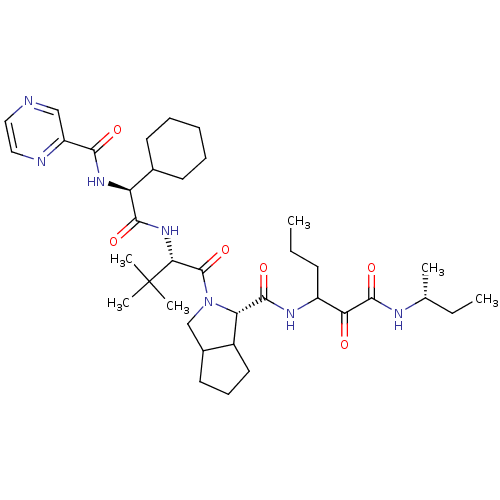
((S)-2-((S)-2-{(S)-2-Cyclohexyl-2-[(pyrazine-2-carb...)Show SMILES CCCC(NC(=O)[C@@H]1C2CCCC2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C1CCCCC1)C(C)(C)C)C(=O)C(=O)N[C@H](C)CC Show InChI InChI=1S/C37H57N7O6/c1-7-13-26(30(45)35(49)40-22(3)8-2)41-34(48)29-25-17-12-16-24(25)21-44(29)36(50)31(37(4,5)6)43-33(47)28(23-14-10-9-11-15-23)42-32(46)27-20-38-18-19-39-27/h18-20,22-26,28-29,31H,7-17,21H2,1-6H3,(H,40,49)(H,41,48)(H,42,46)(H,43,47)/t22-,24?,25?,26?,28+,29+,31-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of HCV NS3 protease in the pNA based inhibition assay |
Bioorg Med Chem Lett 14: 5007-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.007
BindingDB Entry DOI: 10.7270/Q29P314N |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50137733

((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...)Show SMILES CCC[C@H](NC(=O)[C@@H]1[C@H]2CCC[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)(C)C)C(=O)C(=O)NC1CC1 Show InChI InChI=1S/C33H49N7O6/c1-7-9-22(26(41)31(45)36-20-12-13-20)37-30(44)25-21-11-8-10-19(21)17-40(25)32(46)27(33(4,5)6)39-29(43)24(18(2)3)38-28(42)23-16-34-14-15-35-23/h14-16,18-22,24-25,27H,7-13,17H2,1-6H3,(H,36,45)(H,37,44)(H,38,42)(H,39,43)/t19-,21-,22-,24-,25-,27+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of HCV NS3 protease in the pNA based inhibition assay |
Bioorg Med Chem Lett 14: 5007-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.007
BindingDB Entry DOI: 10.7270/Q29P314N |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50150604
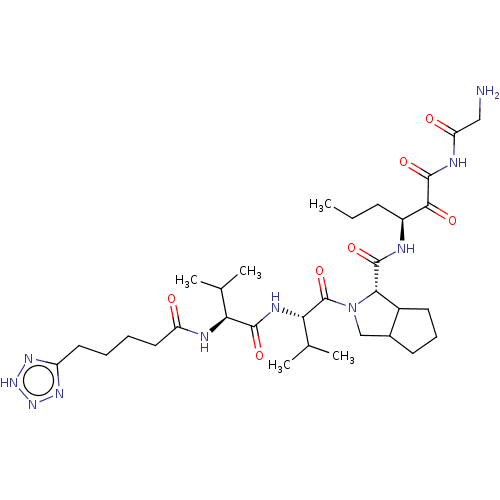
(2-{(S)-3-Methyl-2-[(S)-3-methyl-2-(5-1H-tetrazol-5...)Show SMILES CCC[C@H](NC(=O)[C@@H]1C2CCCC2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)CCCCc1nn[nH]n1)C(C)C)C(C)C)C(=O)C(=O)NC(=O)CN Show InChI InChI=1S/C32H52N10O7/c1-6-10-21(28(45)31(48)36-24(44)15-33)34-30(47)27-20-12-9-11-19(20)16-42(27)32(49)26(18(4)5)37-29(46)25(17(2)3)35-23(43)14-8-7-13-22-38-40-41-39-22/h17-21,25-27H,6-16,33H2,1-5H3,(H,34,47)(H,35,43)(H,37,46)(H,36,44,48)(H,38,39,40,41)/t19?,20?,21?,25-,26-,27-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory potency against HCV NS3 protease |
Bioorg Med Chem Lett 14: 4333-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.078
BindingDB Entry DOI: 10.7270/Q2TX3DVP |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Human rhinovirus B) | BDBM50137713
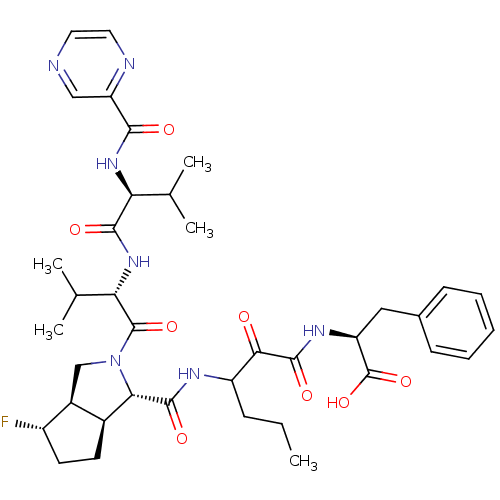
((S)-2-((S)-3-{[(1S,5S,6R)-4-Fluoro-2-((S)-3-methyl...)Show SMILES CCCC(NC(=O)[C@@H]1[C@H]2CC[C@H](F)[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)C)C(=O)C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C38H50FN7O8/c1-6-10-26(32(47)36(51)43-27(38(53)54)17-22-11-8-7-9-12-22)42-35(50)31-23-13-14-25(39)24(23)19-46(31)37(52)30(21(4)5)45-34(49)29(20(2)3)44-33(48)28-18-40-15-16-41-28/h7-9,11-12,15-16,18,20-21,23-27,29-31H,6,10,13-14,17,19H2,1-5H3,(H,42,50)(H,43,51)(H,44,48)(H,45,49)(H,53,54)/t23-,24-,25-,26?,27-,29-,30-,31-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Binding affinity towards Protease using PNA assay in rats |
Bioorg Med Chem Lett 14: 251-6 (2003)
BindingDB Entry DOI: 10.7270/Q24B30R9 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Human rhinovirus B) | BDBM50137733

((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...)Show SMILES CCC[C@H](NC(=O)[C@@H]1[C@H]2CCC[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)(C)C)C(=O)C(=O)NC1CC1 Show InChI InChI=1S/C33H49N7O6/c1-7-9-22(26(41)31(45)36-20-12-13-20)37-30(44)25-21-11-8-10-19(21)17-40(25)32(46)27(33(4,5)6)39-29(43)24(18(2)3)38-28(42)23-16-34-14-15-35-23/h14-16,18-22,24-25,27H,7-13,17H2,1-6H3,(H,36,45)(H,37,44)(H,38,42)(H,39,43)/t19-,21-,22-,24-,25-,27+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards Protease using PNA assay in rats |
Bioorg Med Chem Lett 14: 257-61 (2003)
BindingDB Entry DOI: 10.7270/Q20P0ZDG |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Human rhinovirus B) | BDBM50137739
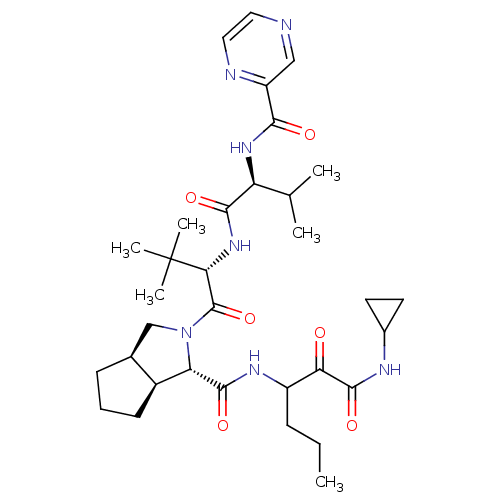
((1S,3aR,6aS)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2...)Show SMILES CCCC(NC(=O)[C@@H]1[C@H]2CCC[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)(C)C)C(=O)C(=O)NC1CC1 Show InChI InChI=1S/C33H49N7O6/c1-7-9-22(26(41)31(45)36-20-12-13-20)37-30(44)25-21-11-8-10-19(21)17-40(25)32(46)27(33(4,5)6)39-29(43)24(18(2)3)38-28(42)23-16-34-14-15-35-23/h14-16,18-22,24-25,27H,7-13,17H2,1-6H3,(H,36,45)(H,37,44)(H,38,42)(H,39,43)/t19-,21-,22?,24-,25-,27+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Binding affinity towards Protease using PNA assay in rats |
Bioorg Med Chem Lett 14: 263-6 (2003)
BindingDB Entry DOI: 10.7270/Q2VX0FX5 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183965
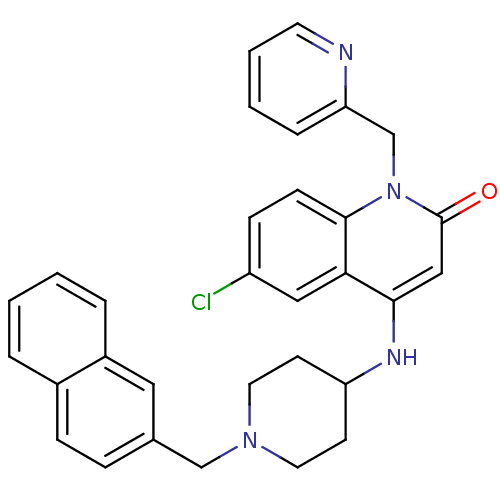
(6-chloro-4-(1-(naphthalen-2-ylmethyl)piperidin-4-y...)Show SMILES Clc1ccc2n(Cc3ccccn3)c(=O)cc(NC3CCN(Cc4ccc5ccccc5c4)CC3)c2c1 Show InChI InChI=1S/C31H29ClN4O/c32-25-10-11-30-28(18-25)29(19-31(37)36(30)21-27-7-3-4-14-33-27)34-26-12-15-35(16-13-26)20-22-8-9-23-5-1-2-6-24(23)17-22/h1-11,14,17-19,26,34H,12-13,15-16,20-21H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Human rhinovirus B) | BDBM50137718
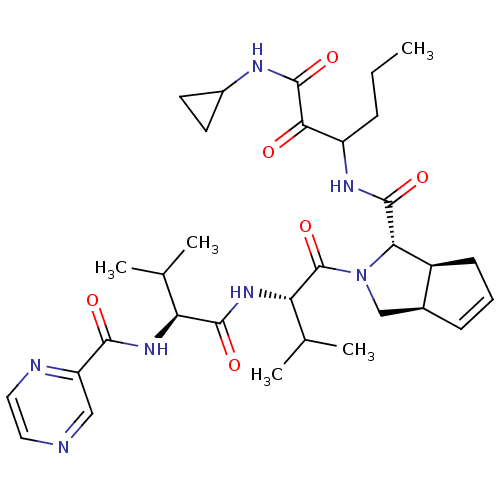
((1S,5S,6R)-2-((S)-3-Methyl-2-{(S)-3-methyl-2-[(pyr...)Show SMILES CCCC(NC(=O)[C@@H]1[C@H]2CC=C[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)C)C(=O)C(=O)NC1CC1 |c:10| Show InChI InChI=1S/C32H45N7O6/c1-6-8-22(27(40)31(44)35-20-11-12-20)36-30(43)26-21-10-7-9-19(21)16-39(26)32(45)25(18(4)5)38-29(42)24(17(2)3)37-28(41)23-15-33-13-14-34-23/h7,9,13-15,17-22,24-26H,6,8,10-12,16H2,1-5H3,(H,35,44)(H,36,43)(H,37,41)(H,38,42)/t19-,21-,22?,24-,25-,26-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Binding affinity towards Protease using PNA assay in rats |
Bioorg Med Chem Lett 14: 251-6 (2003)
BindingDB Entry DOI: 10.7270/Q24B30R9 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50152751

(2-((S)-2-{(S)-2-Cyclohexyl-2-[((R)-pyrazine-2-carb...)Show SMILES CCCC(NC(=O)[C@@H]1C2CCCC2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C1CCCCC1)C(C)(C)C)C(=O)C(=O)N[C@H](C)c1ccccc1 Show InChI InChI=1S/C41H57N7O6/c1-6-14-30(34(49)39(53)44-25(2)26-15-9-7-10-16-26)45-38(52)33-29-20-13-19-28(29)24-48(33)40(54)35(41(3,4)5)47-37(51)32(27-17-11-8-12-18-27)46-36(50)31-23-42-21-22-43-31/h7,9-10,15-16,21-23,25,27-30,32-33,35H,6,8,11-14,17-20,24H2,1-5H3,(H,44,53)(H,45,52)(H,46,50)(H,47,51)/t25-,28?,29?,30?,32+,33+,35-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of HCV NS3 protease in the pNA based inhibition assay |
Bioorg Med Chem Lett 14: 5007-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.007
BindingDB Entry DOI: 10.7270/Q29P314N |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50150605
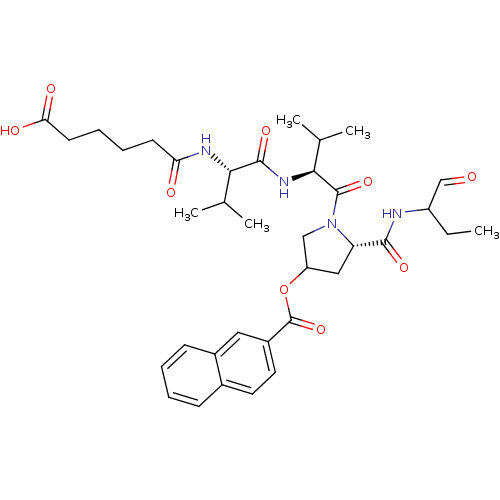
(CHEMBL360805 | Naphthalene-2-carboxylic acid (S)-1...)Show SMILES CCC(NC(=O)[C@@H]1CC(CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)CCCCC(O)=O)C(C)C)C(C)C)OC(=O)c1ccc2ccccc2c1)C=O Show InChI InChI=1S/C36H48N4O9/c1-6-26(20-41)37-33(45)28-18-27(49-36(48)25-16-15-23-11-7-8-12-24(23)17-25)19-40(28)35(47)32(22(4)5)39-34(46)31(21(2)3)38-29(42)13-9-10-14-30(43)44/h7-8,11-12,15-17,20-22,26-28,31-32H,6,9-10,13-14,18-19H2,1-5H3,(H,37,45)(H,38,42)(H,39,46)(H,43,44)/t26?,27?,28-,31-,32-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory potency against HCV NS3 protease |
Bioorg Med Chem Lett 14: 4333-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.078
BindingDB Entry DOI: 10.7270/Q2TX3DVP |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183969
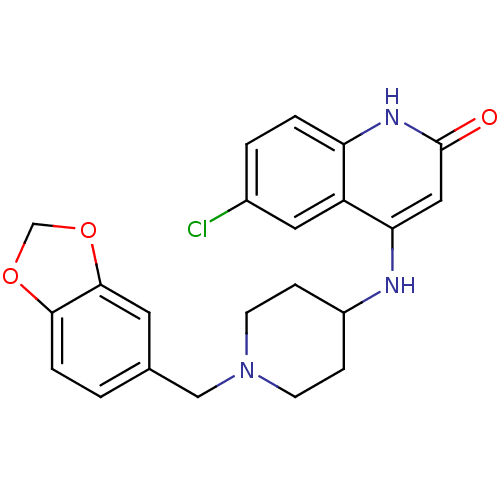
(4-(1-(benzo[d][1,3]dioxol-5-ylmethyl)piperidin-4-y...)Show SMILES Clc1ccc2[nH]c(=O)cc(NC3CCN(Cc4ccc5OCOc5c4)CC3)c2c1 Show InChI InChI=1S/C22H22ClN3O3/c23-15-2-3-18-17(10-15)19(11-22(27)25-18)24-16-5-7-26(8-6-16)12-14-1-4-20-21(9-14)29-13-28-20/h1-4,9-11,16H,5-8,12-13H2,(H2,24,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50150598
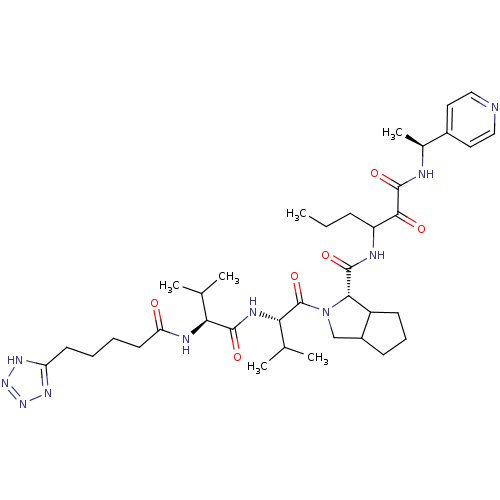
((S)-2-{(S)-3-Methyl-2-[(S)-3-methyl-2-(5-1H-tetraz...)Show SMILES CCCC(NC(=O)[C@@H]1C2CCCC2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)CCCCc1nnn[nH]1)C(C)C)C(C)C)C(=O)C(=O)N[C@@H](C)c1ccncc1 Show InChI InChI=1S/C37H56N10O6/c1-7-11-27(33(49)36(52)39-23(6)24-16-18-38-19-17-24)40-35(51)32-26-13-10-12-25(26)20-47(32)37(53)31(22(4)5)42-34(50)30(21(2)3)41-29(48)15-9-8-14-28-43-45-46-44-28/h16-19,21-23,25-27,30-32H,7-15,20H2,1-6H3,(H,39,52)(H,40,51)(H,41,48)(H,42,50)(H,43,44,45,46)/t23-,25?,26?,27?,30-,31-,32-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory potency against HCV NS3 protease |
Bioorg Med Chem Lett 14: 4333-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.078
BindingDB Entry DOI: 10.7270/Q2TX3DVP |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183966
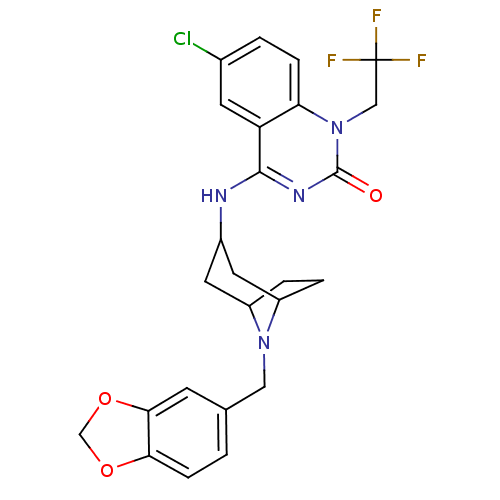
(4-(8-(benzo[d][1,3]dioxol-5-ylmethyl)-8-aza-bicycl...)Show SMILES FC(F)(F)Cn1c2ccc(Cl)cc2c(NC2CC3CCC(C2)N3Cc2ccc3OCOc3c2)nc1=O |TLB:14:15:22:18.19| Show InChI InChI=1S/C25H24ClF3N4O3/c26-15-2-5-20-19(8-15)23(31-24(34)33(20)12-25(27,28)29)30-16-9-17-3-4-18(10-16)32(17)11-14-1-6-21-22(7-14)36-13-35-21/h1-2,5-8,16-18H,3-4,9-13H2,(H,30,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Human rhinovirus B) | BDBM50137720

((1S,3aR,6aS)-2-((S)-3-Methyl-2-{(S)-3-methyl-2-[(p...)Show SMILES CCCC(NC(=O)[C@@H]1[C@H]2CCC[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)C)C(=O)C(=O)NC1CC1 Show InChI InChI=1S/C32H47N7O6/c1-6-8-22(27(40)31(44)35-20-11-12-20)36-30(43)26-21-10-7-9-19(21)16-39(26)32(45)25(18(4)5)38-29(42)24(17(2)3)37-28(41)23-15-33-13-14-34-23/h13-15,17-22,24-26H,6-12,16H2,1-5H3,(H,35,44)(H,36,43)(H,37,41)(H,38,42)/t19-,21-,22?,24-,25-,26-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards Protease using PNA assay in rats |
Bioorg Med Chem Lett 14: 257-61 (2003)
BindingDB Entry DOI: 10.7270/Q20P0ZDG |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Human rhinovirus B) | BDBM50137738
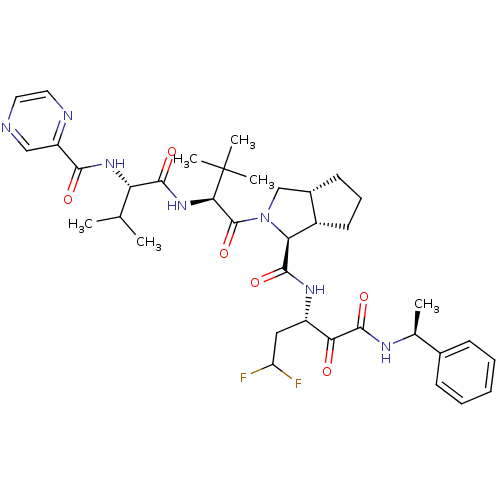
((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...)Show SMILES CC(C)[C@H](NC(=O)c1cnccn1)C(=O)N[C@H](C(=O)N1C[C@@H]2CCC[C@@H]2[C@H]1C(=O)N[C@@H](CC(F)F)C(=O)C(=O)N[C@@H](C)c1ccccc1)C(C)(C)C Show InChI InChI=1S/C37H49F2N7O6/c1-20(2)28(44-32(48)26-18-40-15-16-41-26)33(49)45-31(37(4,5)6)36(52)46-19-23-13-10-14-24(23)29(46)34(50)43-25(17-27(38)39)30(47)35(51)42-21(3)22-11-8-7-9-12-22/h7-9,11-12,15-16,18,20-21,23-25,27-29,31H,10,13-14,17,19H2,1-6H3,(H,42,51)(H,43,50)(H,44,48)(H,45,49)/t21-,23-,24-,25-,28-,29-,31+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards Protease using PNA assay in rats |
Bioorg Med Chem Lett 14: 257-61 (2003)
BindingDB Entry DOI: 10.7270/Q20P0ZDG |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Human rhinovirus B) | BDBM50137720

((1S,3aR,6aS)-2-((S)-3-Methyl-2-{(S)-3-methyl-2-[(p...)Show SMILES CCCC(NC(=O)[C@@H]1[C@H]2CCC[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)C)C(=O)C(=O)NC1CC1 Show InChI InChI=1S/C32H47N7O6/c1-6-8-22(27(40)31(44)35-20-11-12-20)36-30(43)26-21-10-7-9-19(21)16-39(26)32(45)25(18(4)5)38-29(42)24(17(2)3)37-28(41)23-15-33-13-14-34-23/h13-15,17-22,24-26H,6-12,16H2,1-5H3,(H,35,44)(H,36,43)(H,37,41)(H,38,42)/t19-,21-,22?,24-,25-,26-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 123 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Cytotoxic activity in rat liver Huh-7 cells |
Bioorg Med Chem Lett 14: 263-6 (2003)
BindingDB Entry DOI: 10.7270/Q2VX0FX5 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50150601
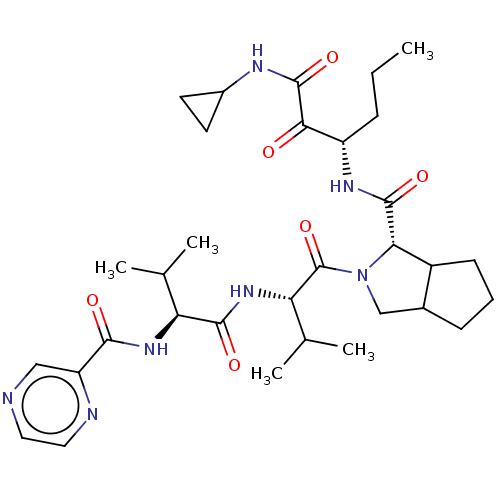
(2-((S)-3-Methyl-2-{(S)-3-methyl-2-[(pyrazine-2-car...)Show SMILES CCC[C@H](NC(=O)[C@@H]1C2CCCC2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)C)C(=O)C(=O)NC1CC1 Show InChI InChI=1S/C32H47N7O6/c1-6-8-22(27(40)31(44)35-20-11-12-20)36-30(43)26-21-10-7-9-19(21)16-39(26)32(45)25(18(4)5)38-29(42)24(17(2)3)37-28(41)23-15-33-13-14-34-23/h13-15,17-22,24-26H,6-12,16H2,1-5H3,(H,35,44)(H,36,43)(H,37,41)(H,38,42)/t19?,21?,22?,24-,25-,26-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 123 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory potency against HCV NS3 protease |
Bioorg Med Chem Lett 14: 4333-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.078
BindingDB Entry DOI: 10.7270/Q2TX3DVP |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Human rhinovirus B) | BDBM50137720

((1S,3aR,6aS)-2-((S)-3-Methyl-2-{(S)-3-methyl-2-[(p...)Show SMILES CCCC(NC(=O)[C@@H]1[C@H]2CCC[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)C)C(=O)C(=O)NC1CC1 Show InChI InChI=1S/C32H47N7O6/c1-6-8-22(27(40)31(44)35-20-11-12-20)36-30(43)26-21-10-7-9-19(21)16-39(26)32(45)25(18(4)5)38-29(42)24(17(2)3)37-28(41)23-15-33-13-14-34-23/h13-15,17-22,24-26H,6-12,16H2,1-5H3,(H,35,44)(H,36,43)(H,37,41)(H,38,42)/t19-,21-,22?,24-,25-,26-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 123 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Binding affinity towards Protease using PNA assay in rats |
Bioorg Med Chem Lett 14: 251-6 (2003)
BindingDB Entry DOI: 10.7270/Q24B30R9 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183960
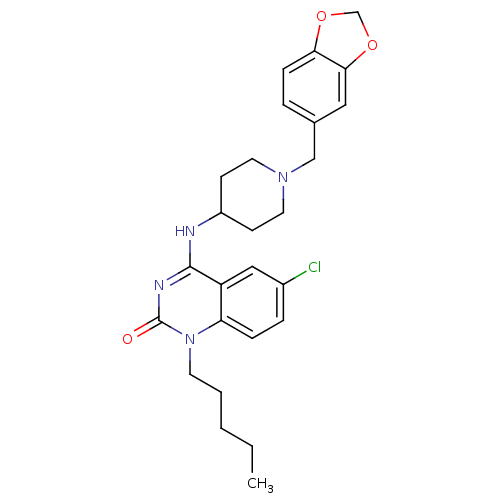
(4-(1-(benzo[d][1,3]dioxol-5-ylmethyl)piperidin-4-y...)Show SMILES CCCCCn1c2ccc(Cl)cc2c(NC2CCN(Cc3ccc4OCOc4c3)CC2)nc1=O Show InChI InChI=1S/C26H31ClN4O3/c1-2-3-4-11-31-22-7-6-19(27)15-21(22)25(29-26(31)32)28-20-9-12-30(13-10-20)16-18-5-8-23-24(14-18)34-17-33-23/h5-8,14-15,20H,2-4,9-13,16-17H2,1H3,(H,28,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 135 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183971
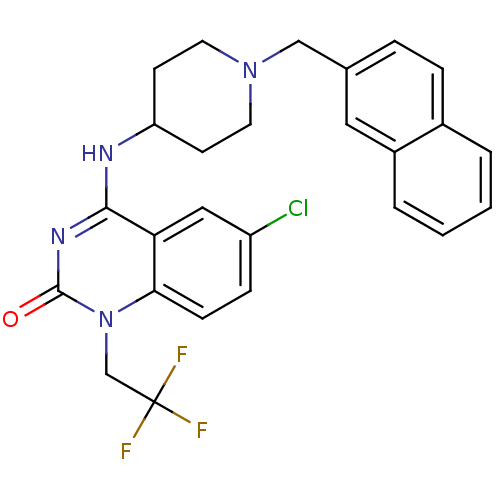
(6-chloro-4-(1-(naphthalen-2-ylmethyl)piperidin-4-y...)Show SMILES FC(F)(F)Cn1c2ccc(Cl)cc2c(NC2CCN(Cc3ccc4ccccc4c3)CC2)nc1=O Show InChI InChI=1S/C26H24ClF3N4O/c27-20-7-8-23-22(14-20)24(32-25(35)34(23)16-26(28,29)30)31-21-9-11-33(12-10-21)15-17-5-6-18-3-1-2-4-19(18)13-17/h1-8,13-14,21H,9-12,15-16H2,(H,31,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183972
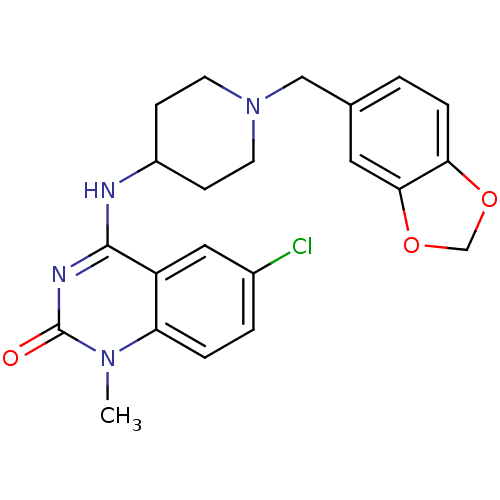
(4-(1-(benzo[d][1,3]dioxol-5-ylmethyl)piperidin-4-y...)Show SMILES Cn1c2ccc(Cl)cc2c(NC2CCN(Cc3ccc4OCOc4c3)CC2)nc1=O Show InChI InChI=1S/C22H23ClN4O3/c1-26-18-4-3-15(23)11-17(18)21(25-22(26)28)24-16-6-8-27(9-7-16)12-14-2-5-19-20(10-14)30-13-29-19/h2-5,10-11,16H,6-9,12-13H2,1H3,(H,24,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50152755
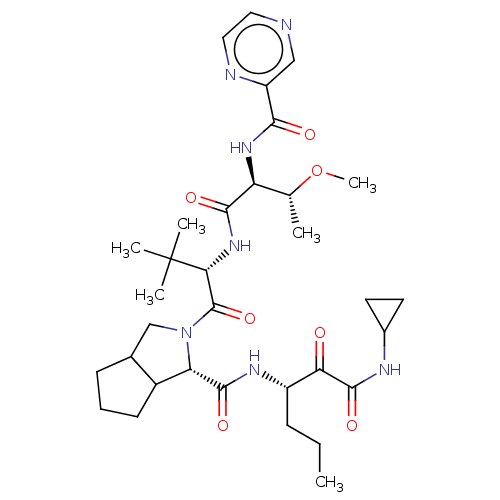
(2-((S)-2-{(S)-3-Methoxy-2-[(pyrazine-2-carbonyl)-a...)Show SMILES CCC[C@H](NC(=O)[C@@H]1C2CCCC2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)[C@@H](C)OC)C(C)(C)C)C(=O)C(=O)NC1CC1 Show InChI InChI=1S/C33H49N7O7/c1-7-9-22(26(41)31(45)36-20-12-13-20)37-30(44)25-21-11-8-10-19(21)17-40(25)32(46)27(33(3,4)5)39-29(43)24(18(2)47-6)38-28(42)23-16-34-14-15-35-23/h14-16,18-22,24-25,27H,7-13,17H2,1-6H3,(H,36,45)(H,37,44)(H,38,42)(H,39,43)/t18?,19?,21?,22?,24-,25-,27+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of HCV NS3 protease in the pNA based inhibition assay |
Bioorg Med Chem Lett 14: 5007-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.007
BindingDB Entry DOI: 10.7270/Q29P314N |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50137733

((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...)Show SMILES CCC[C@H](NC(=O)[C@@H]1[C@H]2CCC[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)(C)C)C(=O)C(=O)NC1CC1 Show InChI InChI=1S/C33H49N7O6/c1-7-9-22(26(41)31(45)36-20-12-13-20)37-30(44)25-21-11-8-10-19(21)17-40(25)32(46)27(33(4,5)6)39-29(43)24(18(2)3)38-28(42)23-16-34-14-15-35-23/h14-16,18-22,24-25,27H,7-13,17H2,1-6H3,(H,36,45)(H,37,44)(H,38,42)(H,39,43)/t19-,21-,22-,24-,25-,27+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 154 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against cathepsin L |
Bioorg Med Chem Lett 14: 257-61 (2003)
BindingDB Entry DOI: 10.7270/Q20P0ZDG |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50152752
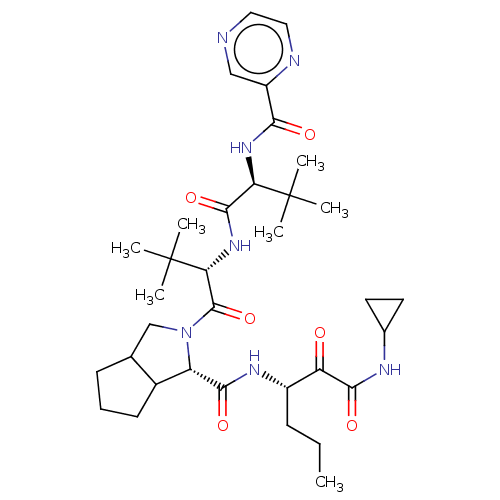
((S)-2-((S)-2-{(S)-3,3-Dimethyl-2-[(pyrazine-2-carb...)Show SMILES CCC[C@H](NC(=O)[C@@H]1C2CCCC2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)(C)C)C(C)(C)C)C(=O)C(=O)NC1CC1 Show InChI InChI=1S/C34H51N7O6/c1-8-10-22(25(42)30(45)37-20-13-14-20)38-29(44)24-21-12-9-11-19(21)18-41(24)32(47)27(34(5,6)7)40-31(46)26(33(2,3)4)39-28(43)23-17-35-15-16-36-23/h15-17,19-22,24,26-27H,8-14,18H2,1-7H3,(H,37,45)(H,38,44)(H,39,43)(H,40,46)/t19?,21?,22?,24-,26+,27+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of HCV NS3 protease in the pNA based inhibition assay |
Bioorg Med Chem Lett 14: 5007-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.007
BindingDB Entry DOI: 10.7270/Q29P314N |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183968
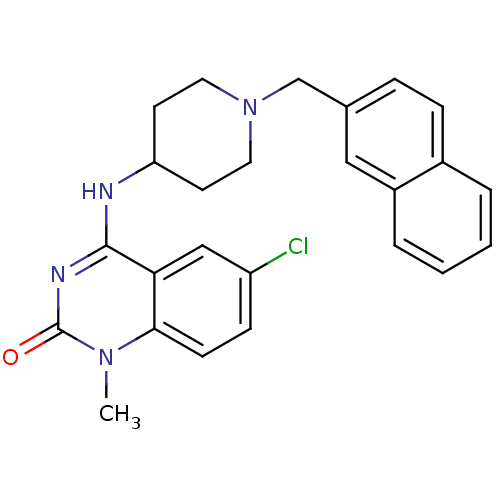
(6-chloro-1-methyl-4-(1-(naphthalen-2-ylmethyl)pipe...)Show SMILES Cn1c2ccc(Cl)cc2c(NC2CCN(Cc3ccc4ccccc4c3)CC2)nc1=O Show InChI InChI=1S/C25H25ClN4O/c1-29-23-9-8-20(26)15-22(23)24(28-25(29)31)27-21-10-12-30(13-11-21)16-17-6-7-18-4-2-3-5-19(18)14-17/h2-9,14-15,21H,10-13,16H2,1H3,(H,27,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50150594
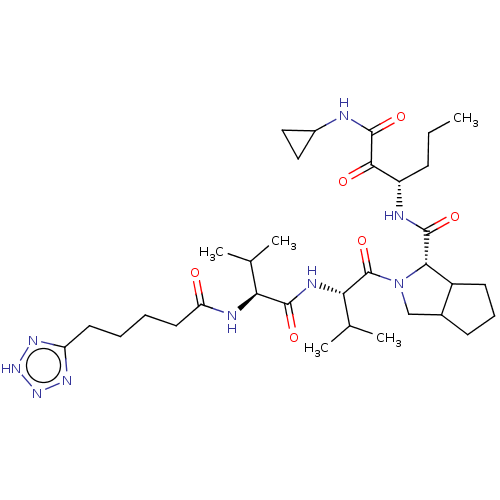
((S)-2-{(S)-3-Methyl-2-[(S)-3-methyl-2-(5-1H-tetraz...)Show SMILES CCC[C@H](NC(=O)[C@@H]1C2CCCC2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)CCCCc1nn[nH]n1)C(C)C)C(C)C)C(=O)C(=O)NC1CC1 Show InChI InChI=1S/C33H53N9O6/c1-6-10-23(29(44)32(47)34-21-15-16-21)35-31(46)28-22-12-9-11-20(22)17-42(28)33(48)27(19(4)5)37-30(45)26(18(2)3)36-25(43)14-8-7-13-24-38-40-41-39-24/h18-23,26-28H,6-17H2,1-5H3,(H,34,47)(H,35,46)(H,36,43)(H,37,45)(H,38,39,40,41)/t20?,22?,23?,26-,27-,28-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory potency against HCV NS3 protease |
Bioorg Med Chem Lett 14: 4333-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.078
BindingDB Entry DOI: 10.7270/Q2TX3DVP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data