

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50086681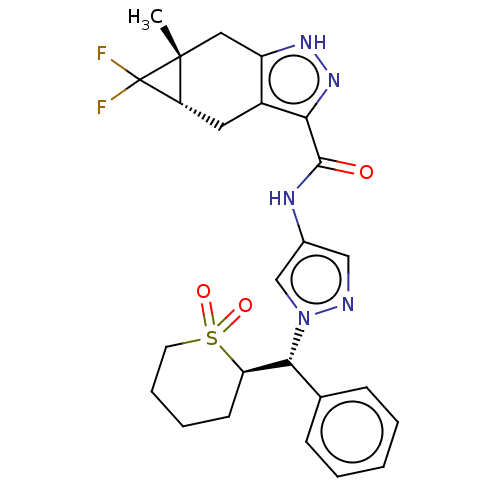 (CHEMBL3426309) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant full length human ITK using AcEFPIYDFLPAKKK-NH2 as substrate after 35 mins by Morrison plot analysis | J Med Chem 58: 3806-16 (2015) Article DOI: 10.1021/jm501998m BindingDB Entry DOI: 10.7270/Q28K7BTH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50015266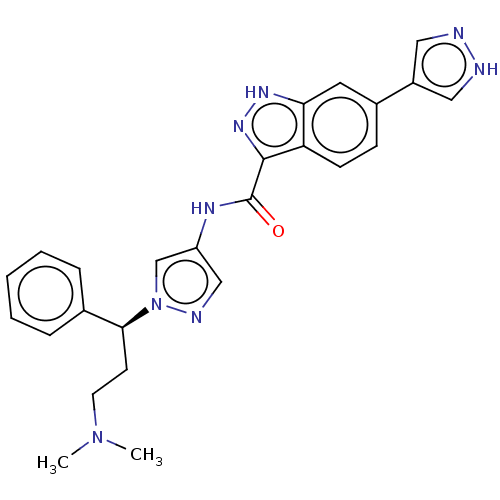 (CHEMBL3263053) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of GST-tagged full length ITK (unknown origin) using BLK peptide as substrate after 35 mins | J Med Chem 57: 5714-27 (2014) Article DOI: 10.1021/jm500550e BindingDB Entry DOI: 10.7270/Q2ZK5J7T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50086604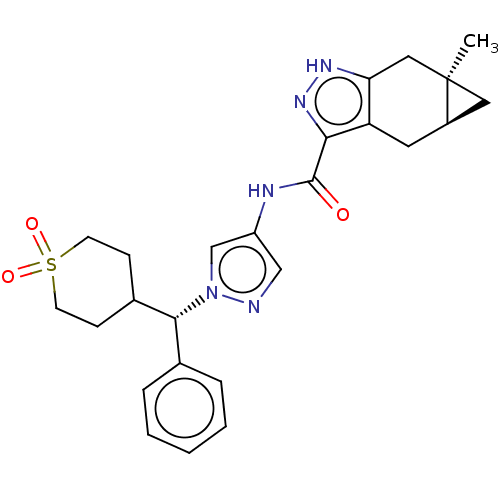 (CHEMBL3426308) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant full length human ITK using AcEFPIYDFLPAKKK-NH2 as substrate after 35 mins by Morrison plot analysis | J Med Chem 58: 3806-16 (2015) Article DOI: 10.1021/jm501998m BindingDB Entry DOI: 10.7270/Q28K7BTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50022940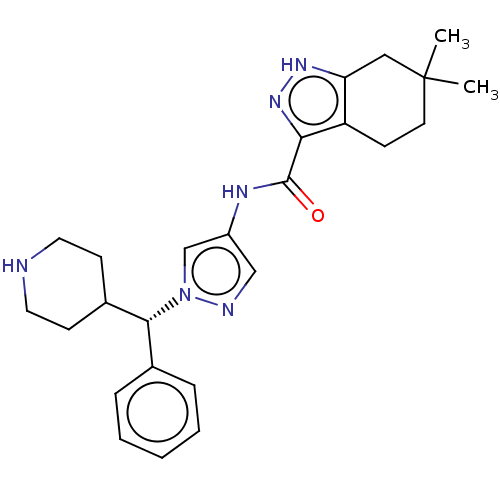 (CHEMBL3298373) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of GST-tagged full length ITK (unknown origin) using BLK peptide as substrate after 35 mins | J Med Chem 57: 5714-27 (2014) Article DOI: 10.1021/jm500550e BindingDB Entry DOI: 10.7270/Q2ZK5J7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50086659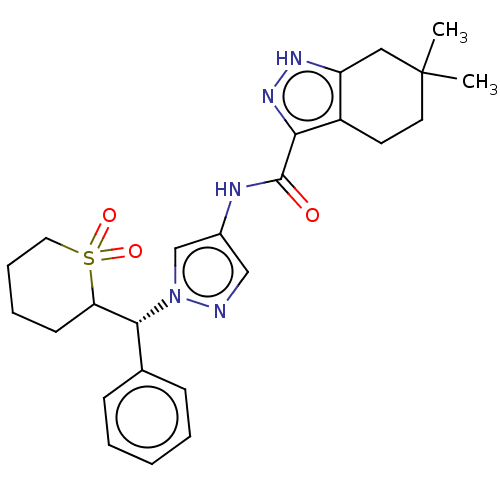 (CHEMBL3426303) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant full length human ITK using AcEFPIYDFLPAKKK-NH2 as substrate after 35 mins by Morrison plot analysis | J Med Chem 58: 3806-16 (2015) Article DOI: 10.1021/jm501998m BindingDB Entry DOI: 10.7270/Q28K7BTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50086605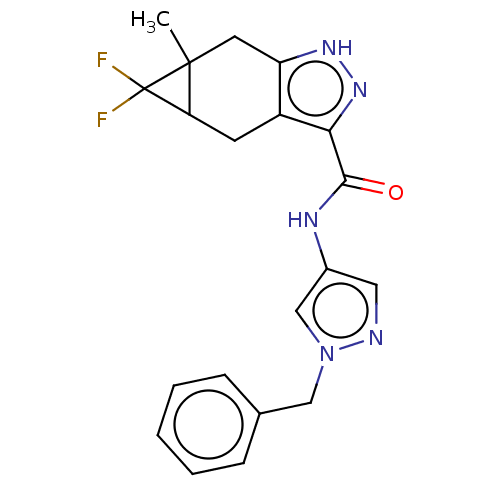 (CHEMBL3426307) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant full length human ITK using AcEFPIYDFLPAKKK-NH2 as substrate after 35 mins by Morrison plot analysis | J Med Chem 58: 3806-16 (2015) Article DOI: 10.1021/jm501998m BindingDB Entry DOI: 10.7270/Q28K7BTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50086671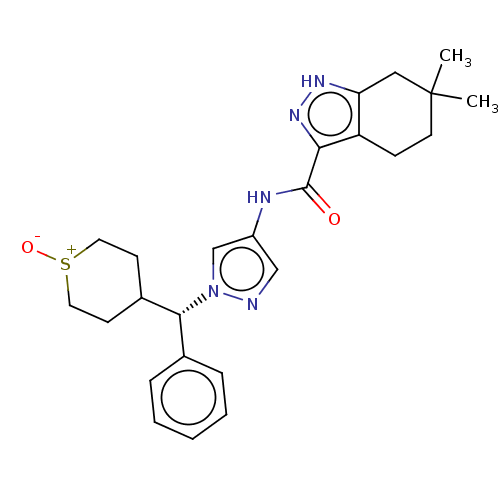 (CHEMBL3426305) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant full length human ITK using AcEFPIYDFLPAKKK-NH2 as substrate after 35 mins by Morrison plot analysis | J Med Chem 58: 3806-16 (2015) Article DOI: 10.1021/jm501998m BindingDB Entry DOI: 10.7270/Q28K7BTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50086671 (CHEMBL3426305) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant full length human ITK using AcEFPIYDFLPAKKK-NH2 as substrate after 35 mins by Morrison plot analysis | J Med Chem 58: 3806-16 (2015) Article DOI: 10.1021/jm501998m BindingDB Entry DOI: 10.7270/Q28K7BTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50086676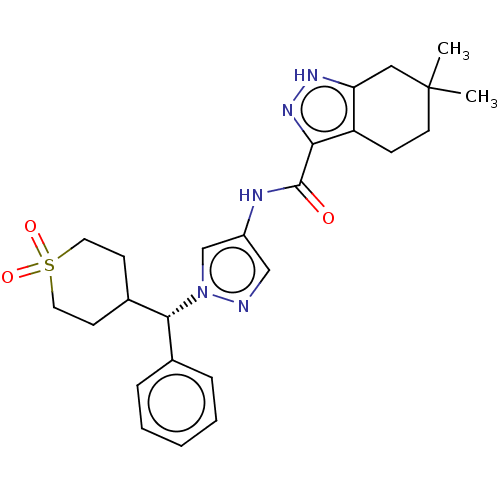 (CHEMBL3426304) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant full length human ITK using AcEFPIYDFLPAKKK-NH2 as substrate after 35 mins by Morrison plot analysis | J Med Chem 58: 3806-16 (2015) Article DOI: 10.1021/jm501998m BindingDB Entry DOI: 10.7270/Q28K7BTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50181362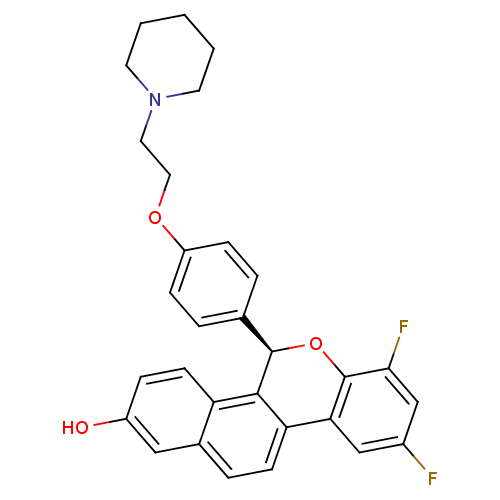 ((R)-(+)-7,9-difluoro-5-[4-(2-piperidin-1-ylethoxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]17beta-estradiol from ERalpha | J Med Chem 49: 843-6 (2006) Article DOI: 10.1021/jm0509795 BindingDB Entry DOI: 10.7270/Q20R9Q60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50086659 (CHEMBL3426303) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant full length human ITK using AcEFPIYDFLPAKKK-NH2 as substrate after 35 mins by Morrison plot analysis | J Med Chem 58: 3806-16 (2015) Article DOI: 10.1021/jm501998m BindingDB Entry DOI: 10.7270/Q28K7BTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50343775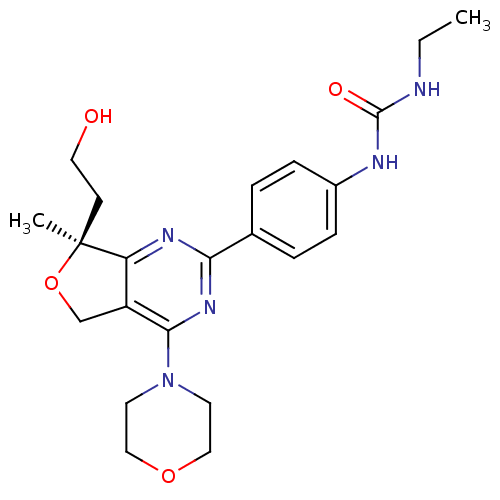 ((S)-1-ethyl-3-(4-(7-(2-hydroxyethyl)-7-methyl-4-mo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of human recombinant mTOR expressed in insect cells using 4E-BP1 substrate after 30 mins by fluorescence resonance energy transfer assay | J Med Chem 54: 3426-35 (2011) Article DOI: 10.1021/jm200215y BindingDB Entry DOI: 10.7270/Q29C6XRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM19441 (2-(4-hydroxyphenyl)-3-({4-[2-(piperidin-1-yl)ethox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for estrogen receptor alpha | Bioorg Med Chem Lett 13: 1907-10 (2003) BindingDB Entry DOI: 10.7270/Q2K35T16 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50428131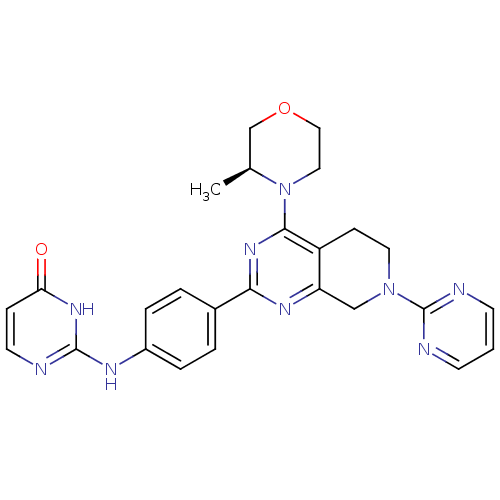 (CHEMBL2331687) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant mTOR (1360 to 2549)+GBL (unknown origin) using GFP-4E-BP1 as substrate after 30 mins by FRET assay | ACS Med Chem Lett 4: 103-7 (2013) Article DOI: 10.1021/ml3003132 BindingDB Entry DOI: 10.7270/Q28C9XK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50532834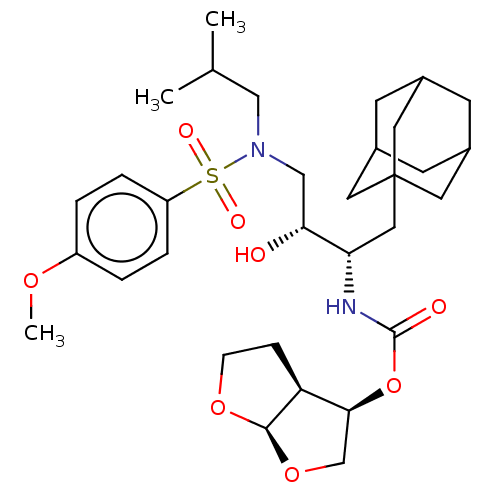 (CHEMBL4516606) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Abz-NleF*-6 as substrate preincubated for 5 to 10 mins followed by substrate addition measured after 1 hr by fluoro... | J Med Chem 59: 6826-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00639 BindingDB Entry DOI: 10.7270/Q2CJ8HZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50532834 (CHEMBL4516606) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Abz-NleF*-6 as substrate preincubated for 5 to 10 mins followed by substrate addition measured after 1 hr by fluoro... | J Med Chem 59: 6826-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00639 BindingDB Entry DOI: 10.7270/Q2CJ8HZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50181362 ((R)-(+)-7,9-difluoro-5-[4-(2-piperidin-1-ylethoxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]17beta-estradiol from ERbeta | J Med Chem 49: 843-6 (2006) Article DOI: 10.1021/jm0509795 BindingDB Entry DOI: 10.7270/Q20R9Q60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM20608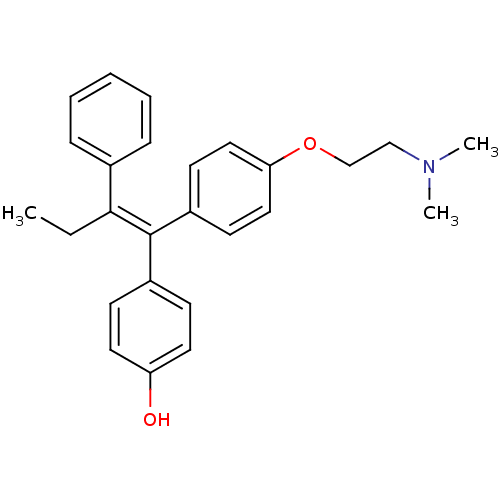 (4-Hydroxytamoxifen | 4-Hydroxytamoxifen (9) | 4-[(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for estrogen receptor alpha | Bioorg Med Chem Lett 13: 1907-10 (2003) BindingDB Entry DOI: 10.7270/Q2K35T16 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50443182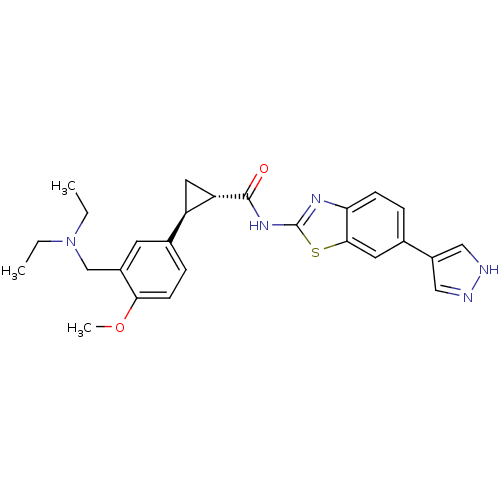 (CHEMBL3086538) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd Curated by ChEMBL | Assay Description Inhibition of full length GST-tagged ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis | Bioorg Med Chem Lett 23: 6331-5 (2013) Article DOI: 10.1016/j.bmcl.2013.09.069 BindingDB Entry DOI: 10.7270/Q2GX4D16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50086680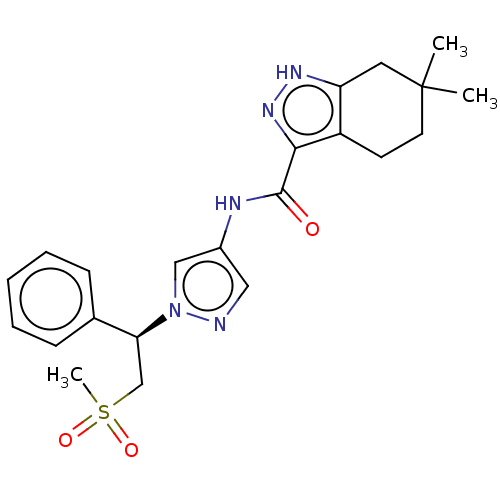 (CHEMBL3426306) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant full length human ITK using AcEFPIYDFLPAKKK-NH2 as substrate after 35 mins by Morrison plot analysis | J Med Chem 58: 3806-16 (2015) Article DOI: 10.1021/jm501998m BindingDB Entry DOI: 10.7270/Q28K7BTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM20608 (4-Hydroxytamoxifen | 4-Hydroxytamoxifen (9) | 4-[(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for estrogen receptor beta | Bioorg Med Chem Lett 13: 1907-10 (2003) BindingDB Entry DOI: 10.7270/Q2K35T16 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50532840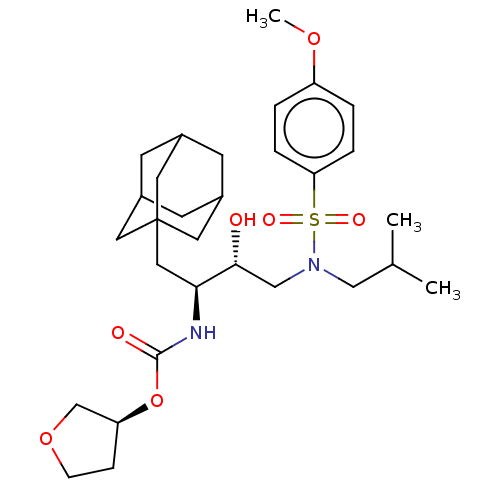 (CHEMBL4464672) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Abz-NleF*-6 as substrate preincubated for 5 to 10 mins followed by substrate addition measured after 1 hr by fluoro... | J Med Chem 59: 6826-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00639 BindingDB Entry DOI: 10.7270/Q2CJ8HZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50532840 (CHEMBL4464672) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Abz-NleF*-6 as substrate preincubated for 5 to 10 mins followed by substrate addition measured after 1 hr by fluoro... | J Med Chem 59: 6826-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00639 BindingDB Entry DOI: 10.7270/Q2CJ8HZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50128396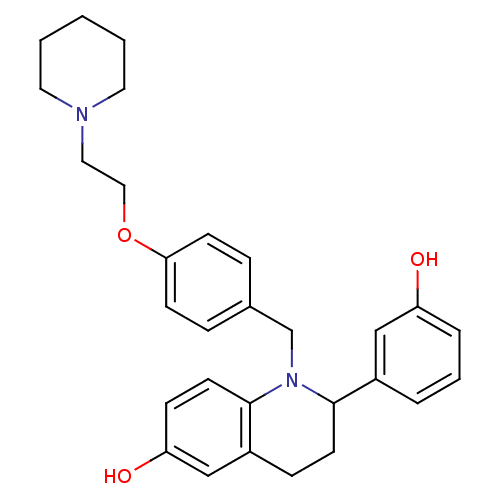 (2-(3-Hydroxy-phenyl)-1-[4-(2-piperidin-1-yl-ethoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for estrogen receptor alpha | Bioorg Med Chem Lett 13: 1907-10 (2003) BindingDB Entry DOI: 10.7270/Q2K35T16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50443181 (CHEMBL3086535) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd Curated by ChEMBL | Assay Description Inhibition of full length GST-tagged ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis | Bioorg Med Chem Lett 23: 6331-5 (2013) Article DOI: 10.1016/j.bmcl.2013.09.069 BindingDB Entry DOI: 10.7270/Q2GX4D16 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50128392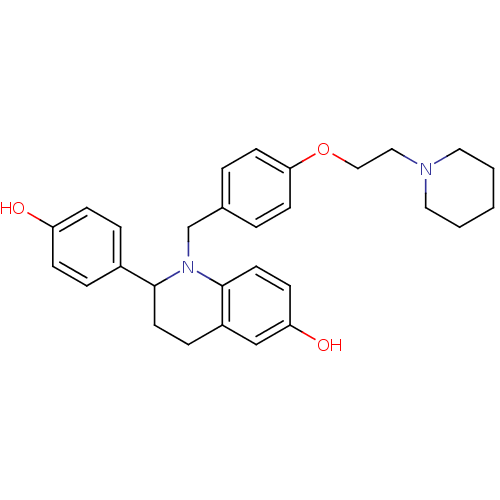 (2-(4-Hydroxy-phenyl)-1-[4-(2-piperidin-1-yl-ethoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for estrogen receptor alpha | Bioorg Med Chem Lett 13: 1907-10 (2003) BindingDB Entry DOI: 10.7270/Q2K35T16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50343776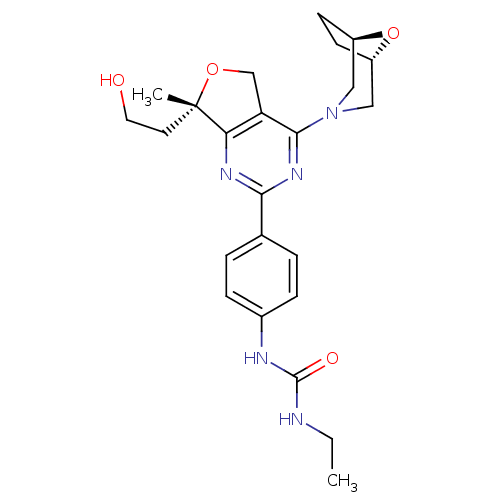 (1-(4-((S)-4-((1R,5S)-8-oxa-3-azabicyclo[3.2.1]octa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of human recombinant mTOR expressed in insect cells using 4E-BP1 substrate after 30 mins by fluorescence resonance energy transfer assay | J Med Chem 54: 3426-35 (2011) Article DOI: 10.1021/jm200215y BindingDB Entry DOI: 10.7270/Q29C6XRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50343774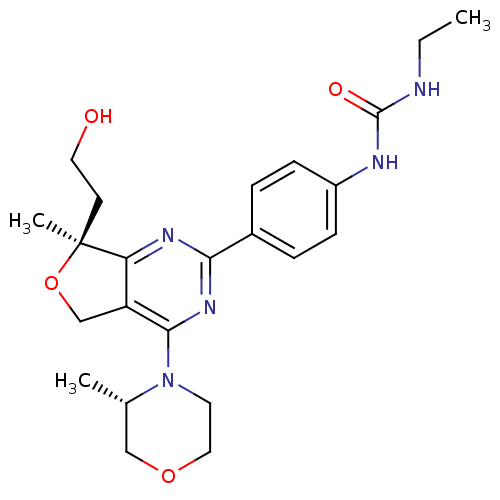 (1-Ethyl-3-(4-((S)-7-(2-hydroxyethyl)-7-methyl-4-((...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of human recombinant mTOR expressed in insect cells using 4E-BP1 substrate after 30 mins by fluorescence resonance energy transfer assay | J Med Chem 54: 3426-35 (2011) Article DOI: 10.1021/jm200215y BindingDB Entry DOI: 10.7270/Q29C6XRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50443180 (CHEMBL3086536) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd Curated by ChEMBL | Assay Description Inhibition of full length GST-tagged ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis | Bioorg Med Chem Lett 23: 6331-5 (2013) Article DOI: 10.1016/j.bmcl.2013.09.069 BindingDB Entry DOI: 10.7270/Q2GX4D16 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50022934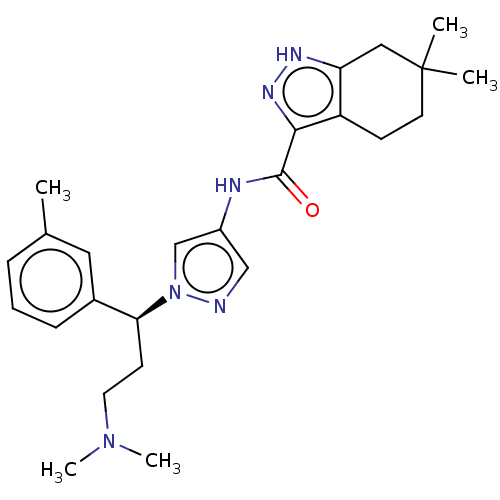 (CHEMBL3298375) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of GST-tagged full length ITK (unknown origin) using BLK peptide as substrate after 35 mins | J Med Chem 57: 5714-27 (2014) Article DOI: 10.1021/jm500550e BindingDB Entry DOI: 10.7270/Q2ZK5J7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50022932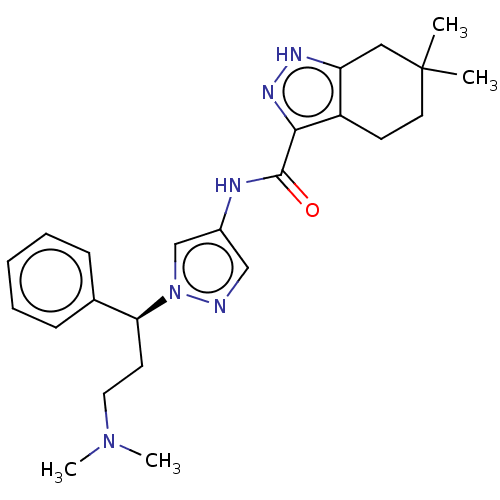 (CHEMBL3298371) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of GST-tagged full length ITK (unknown origin) using BLK peptide as substrate after 35 mins | J Med Chem 57: 5714-27 (2014) Article DOI: 10.1021/jm500550e BindingDB Entry DOI: 10.7270/Q2ZK5J7T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50532833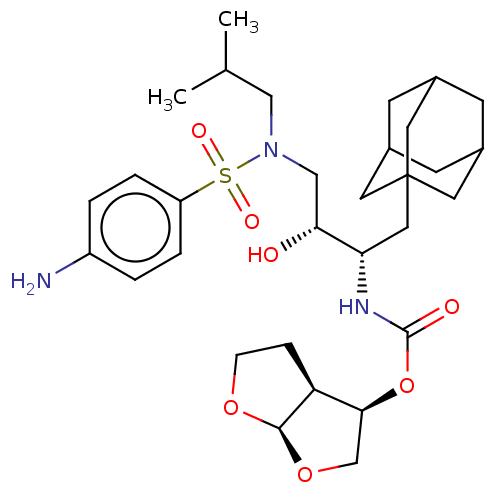 (CHEMBL4581883) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Abz-NleF*-6 as substrate preincubated for 5 to 10 mins followed by substrate addition measured after 1 hr by fluoro... | J Med Chem 59: 6826-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00639 BindingDB Entry DOI: 10.7270/Q2CJ8HZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50532833 (CHEMBL4581883) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Abz-NleF*-6 as substrate preincubated for 5 to 10 mins followed by substrate addition measured after 1 hr by fluoro... | J Med Chem 59: 6826-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00639 BindingDB Entry DOI: 10.7270/Q2CJ8HZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50343769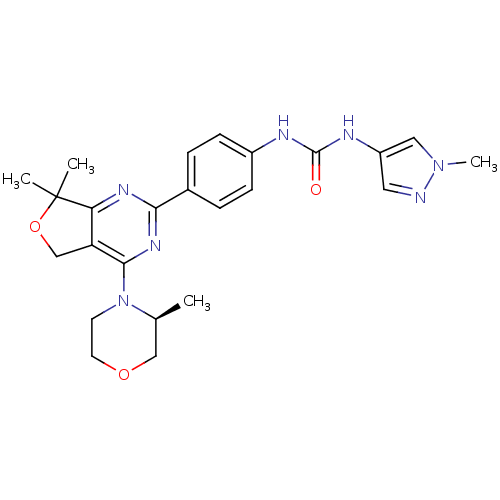 ((S)-1-(4-(7,7-Dimethyl-4-(3-methylmorpholino)-5,7-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of human recombinant mTOR expressed in insect cells using 4E-BP1 substrate after 30 mins by fluorescence resonance energy transfer assay | J Med Chem 54: 3426-35 (2011) Article DOI: 10.1021/jm200215y BindingDB Entry DOI: 10.7270/Q29C6XRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50022927 (CHEMBL3298370) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of GST-tagged full length ITK (unknown origin) using BLK peptide as substrate after 35 mins | J Med Chem 57: 5714-27 (2014) Article DOI: 10.1021/jm500550e BindingDB Entry DOI: 10.7270/Q2ZK5J7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50086669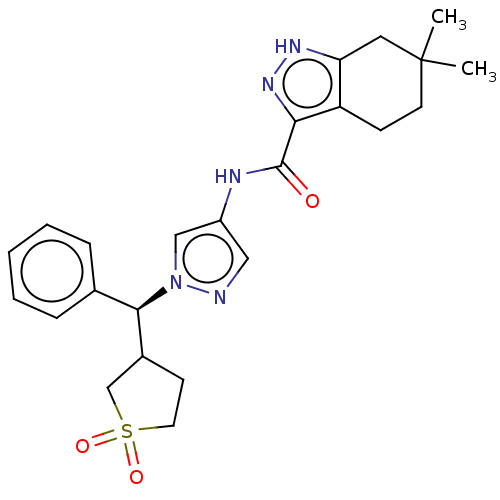 (CHEMBL3426301) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant full length human ITK using AcEFPIYDFLPAKKK-NH2 as substrate after 35 mins by Morrison plot analysis | J Med Chem 58: 3806-16 (2015) Article DOI: 10.1021/jm501998m BindingDB Entry DOI: 10.7270/Q28K7BTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50128402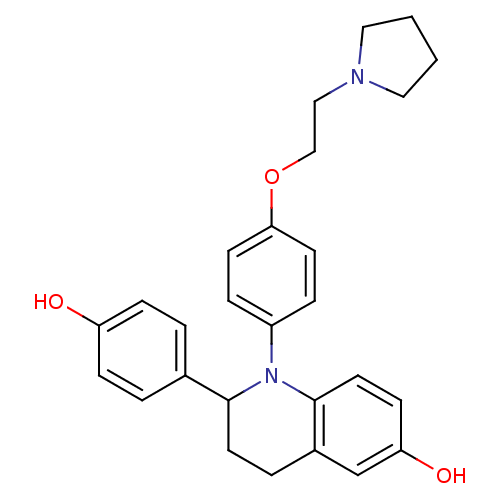 (2-(4-Hydroxy-phenyl)-1-[4-(2-pyrrolidin-1-yl-ethox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for estrogen receptor alpha | Bioorg Med Chem Lett 13: 1907-10 (2003) BindingDB Entry DOI: 10.7270/Q2K35T16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50086669 (CHEMBL3426301) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant full length human ITK using AcEFPIYDFLPAKKK-NH2 as substrate after 35 mins by Morrison plot analysis | J Med Chem 58: 3806-16 (2015) Article DOI: 10.1021/jm501998m BindingDB Entry DOI: 10.7270/Q28K7BTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50129719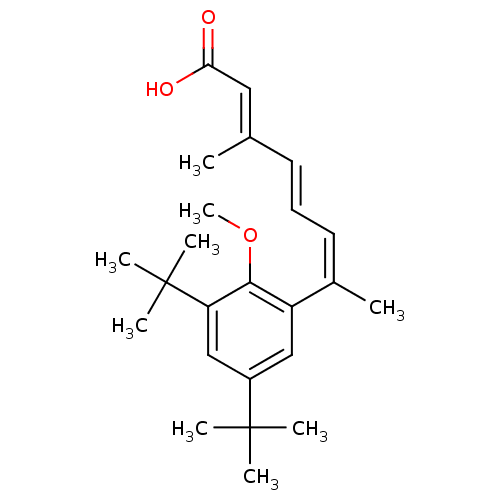 (7-(3,5-Di-tert-butyl-2-methoxy-phenyl)-3-methyl-oc...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Binding affinity against Retinoic acid receptor RXR-alpha was determined in vitro by using [3H]-9-cis-RA as radioligand | J Med Chem 46: 2683-96 (2003) Article DOI: 10.1021/jm020340q BindingDB Entry DOI: 10.7270/Q27H1HZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50439517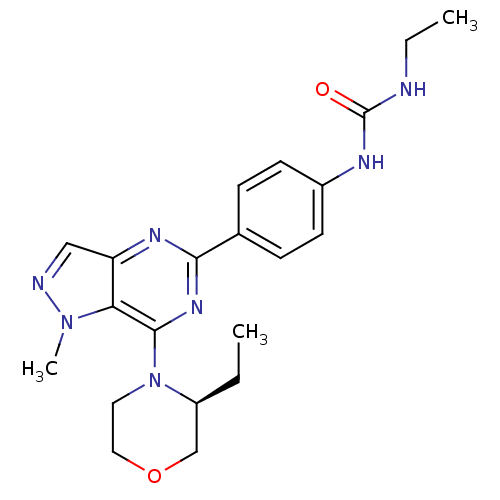 (CHEMBL2418349) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of human recombinant mTOR (1360-2549) expressed in insect cells assessed as phosphorylation of recombinant (GFP)-4-EBP1 after 30 mins by L... | Bioorg Med Chem Lett 23: 5097-104 (2013) Article DOI: 10.1016/j.bmcl.2013.07.027 BindingDB Entry DOI: 10.7270/Q2QN687D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50343779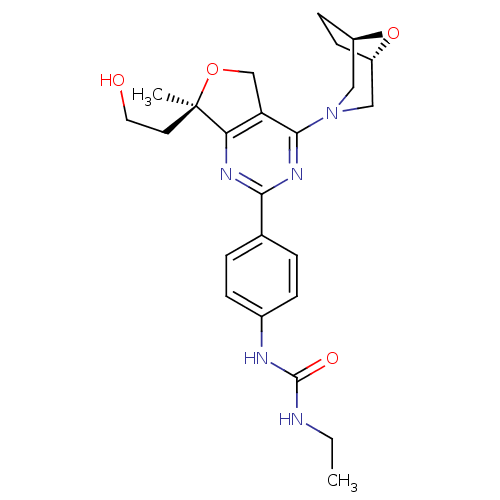 (1-(4-((R)-4-((1R,5S)-8-oxa-3-azabicyclo[3.2.1]octa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of human recombinant mTOR expressed in insect cells using 4E-BP1 substrate after 30 mins by fluorescence resonance energy transfer assay | J Med Chem 54: 3426-35 (2011) Article DOI: 10.1021/jm200215y BindingDB Entry DOI: 10.7270/Q29C6XRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50343772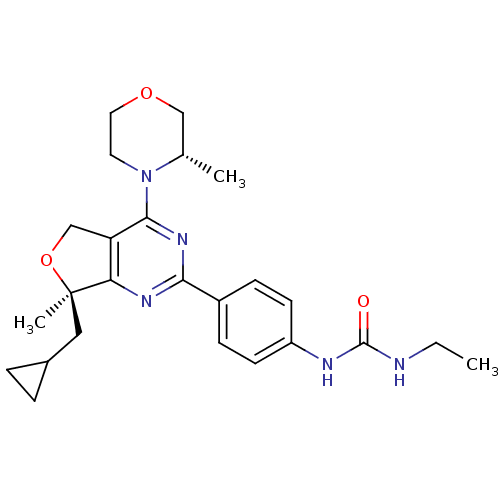 (1-(4-((R)-7-(cyclopropylmethyl)-7-methyl-4-((S)-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of human recombinant mTOR expressed in insect cells using 4E-BP1 substrate after 30 mins by fluorescence resonance energy transfer assay | J Med Chem 54: 3426-35 (2011) Article DOI: 10.1021/jm200215y BindingDB Entry DOI: 10.7270/Q29C6XRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50129723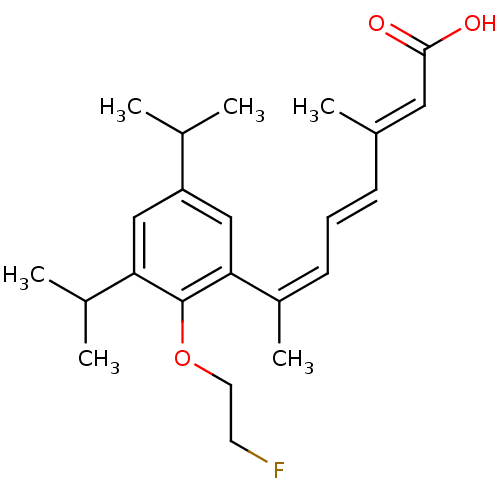 (7-[2-(2-Fluoro-ethoxy)-3,5-diisopropyl-phenyl]-3-m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Binding affinity against Retinoic acid receptor RXR-alpha was determined in vitro by using [3H]-9-cis-RA as radioligand | J Med Chem 46: 2683-96 (2003) Article DOI: 10.1021/jm020340q BindingDB Entry DOI: 10.7270/Q27H1HZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50532841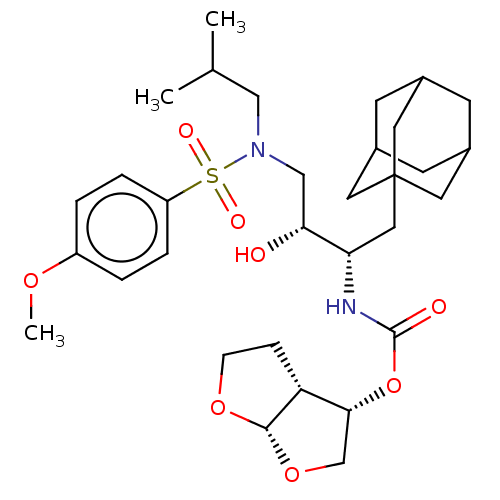 (CHEMBL4592535) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Abz-NleF*-6 as substrate preincubated for 5 to 10 mins followed by substrate addition measured after 1 hr by fluoro... | J Med Chem 59: 6826-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00639 BindingDB Entry DOI: 10.7270/Q2CJ8HZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50532841 (CHEMBL4592535) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Abz-NleF*-6 as substrate preincubated for 5 to 10 mins followed by substrate addition measured after 1 hr by fluoro... | J Med Chem 59: 6826-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00639 BindingDB Entry DOI: 10.7270/Q2CJ8HZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50129717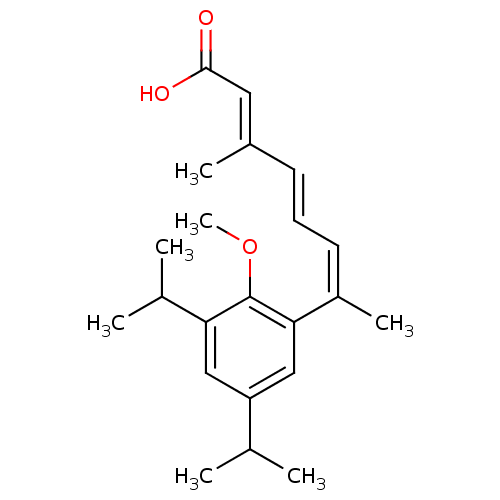 (7-(3,5-Diisopropyl-2-methoxy-phenyl)-3-methyl-octa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Binding affinity against Retinoic acid receptor RXR-alpha was determined in vitro by using [3H]-9-cis-RA as radioligand | J Med Chem 46: 2683-96 (2003) Article DOI: 10.1021/jm020340q BindingDB Entry DOI: 10.7270/Q27H1HZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50532838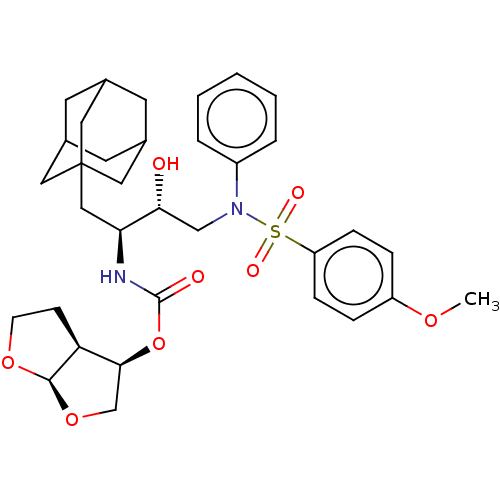 (CHEMBL4456683) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Abz-NleF*-6 as substrate preincubated for 5 to 10 mins followed by substrate addition measured after 1 hr by fluoro... | J Med Chem 59: 6826-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00639 BindingDB Entry DOI: 10.7270/Q2CJ8HZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50532838 (CHEMBL4456683) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Abz-NleF*-6 as substrate preincubated for 5 to 10 mins followed by substrate addition measured after 1 hr by fluoro... | J Med Chem 59: 6826-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00639 BindingDB Entry DOI: 10.7270/Q2CJ8HZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50128394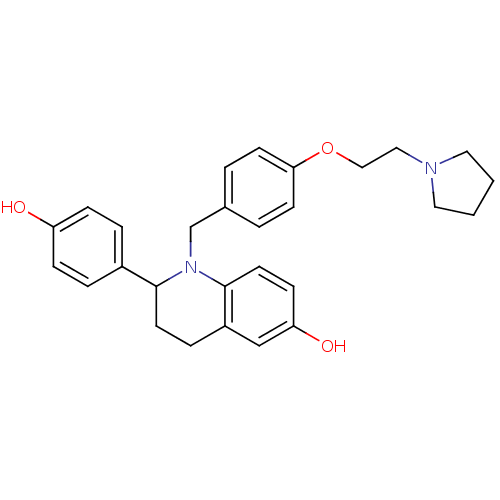 (2-(4-Hydroxy-phenyl)-1-[4-(2-pyrrolidin-1-yl-ethox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for estrogen receptor alpha | Bioorg Med Chem Lett 13: 1907-10 (2003) BindingDB Entry DOI: 10.7270/Q2K35T16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50015271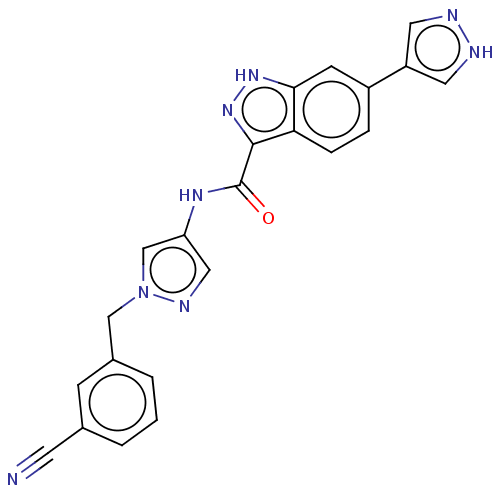 (CHEMBL3263036) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of GST-tagged full length ITK (unknown origin) using BLK peptide as substrate after 35 mins | J Med Chem 57: 5714-27 (2014) Article DOI: 10.1021/jm500550e BindingDB Entry DOI: 10.7270/Q2ZK5J7T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 1931 total ) | Next | Last >> |