

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin D (Homo sapiens (Human)) | BDBM50076285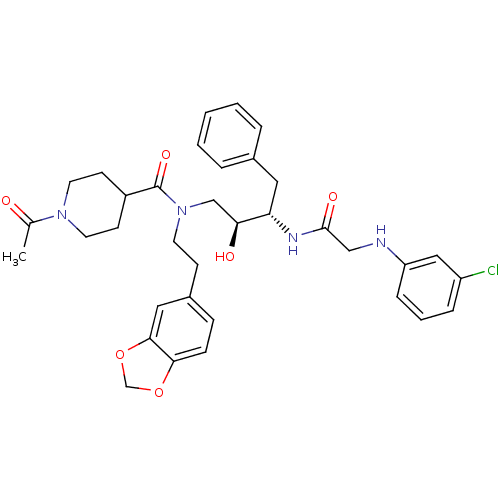 (1-Acetyl-piperidine-4-carboxylic acid (2-benzo[1,3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Compound was tested for the inhibitory activity against human liver cathepsin D | J Med Chem 42: 1428-40 (1999) Article DOI: 10.1021/jm980641t BindingDB Entry DOI: 10.7270/Q2MS3RZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50076294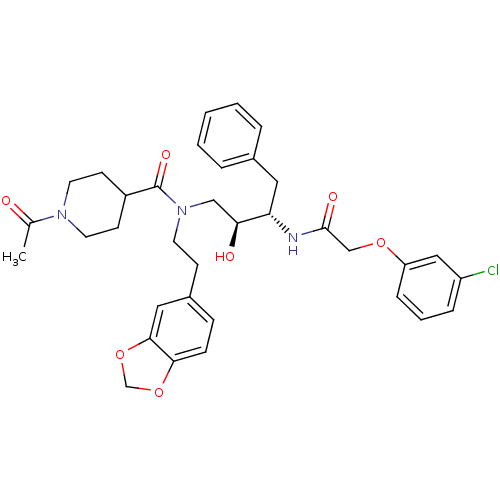 (1-Acetyl-piperidine-4-carboxylic acid (2-benzo[1,3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Compound was tested for the inhibitory activity against human liver cathepsin D | J Med Chem 42: 1428-40 (1999) Article DOI: 10.1021/jm980641t BindingDB Entry DOI: 10.7270/Q2MS3RZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50076292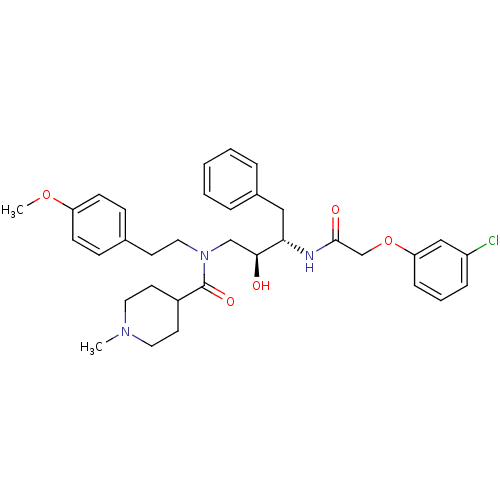 (1-Methyl-piperidine-4-carboxylic acid {(2S,3S)-3-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity against Plasmepsin 2 | J Med Chem 42: 1428-40 (1999) Article DOI: 10.1021/jm980641t BindingDB Entry DOI: 10.7270/Q2MS3RZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50076291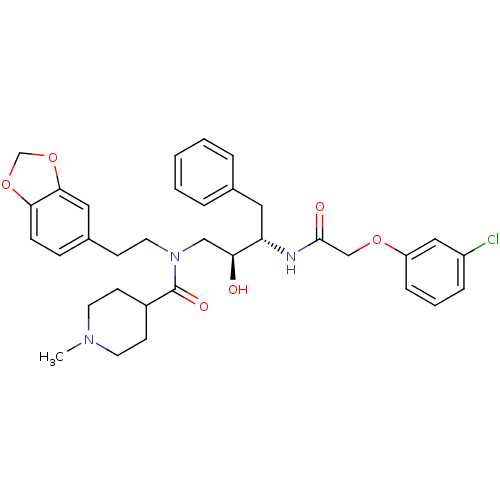 (1-Methyl-piperidine-4-carboxylic acid (2-benzo[1,3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity against Plasmepsin 2 | J Med Chem 42: 1428-40 (1999) Article DOI: 10.1021/jm980641t BindingDB Entry DOI: 10.7270/Q2MS3RZX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50076285 (1-Acetyl-piperidine-4-carboxylic acid (2-benzo[1,3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity against Plasmepsin 2 | J Med Chem 42: 1428-40 (1999) Article DOI: 10.1021/jm980641t BindingDB Entry DOI: 10.7270/Q2MS3RZX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50076294 (1-Acetyl-piperidine-4-carboxylic acid (2-benzo[1,3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity against Plasmepsin 2 | J Med Chem 42: 1428-40 (1999) Article DOI: 10.1021/jm980641t BindingDB Entry DOI: 10.7270/Q2MS3RZX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50076287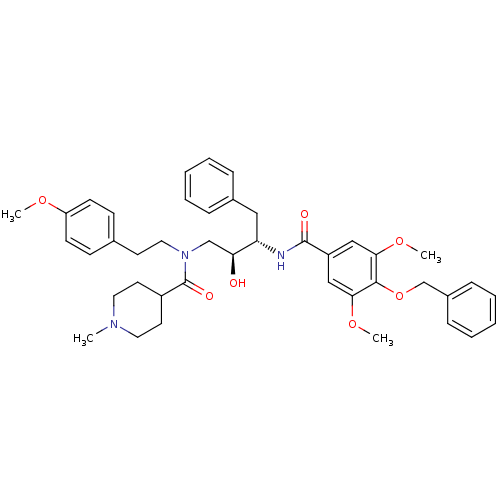 (1-Methyl-piperidine-4-carboxylic acid [(2S,3S)-3-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity against Plasmepsin 2 | J Med Chem 42: 1428-40 (1999) Article DOI: 10.1021/jm980641t BindingDB Entry DOI: 10.7270/Q2MS3RZX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50076293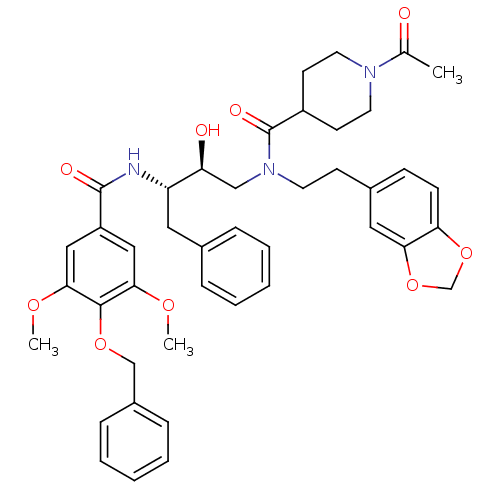 (1-Acetyl-piperidine-4-carboxylic acid (2-benzo[1,3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Compound was tested for the inhibitory activity against human liver cathepsin D | J Med Chem 42: 1428-40 (1999) Article DOI: 10.1021/jm980641t BindingDB Entry DOI: 10.7270/Q2MS3RZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM8019 (2-(3-chlorophenoxy)-N-[(2S,3S)-3-hydroxy-4-{N-[2-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity against Plasmepsin 2 | J Med Chem 42: 1428-40 (1999) Article DOI: 10.1021/jm980641t BindingDB Entry DOI: 10.7270/Q2MS3RZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50076293 (1-Acetyl-piperidine-4-carboxylic acid (2-benzo[1,3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity against Plasmepsin 2 | J Med Chem 42: 1428-40 (1999) Article DOI: 10.1021/jm980641t BindingDB Entry DOI: 10.7270/Q2MS3RZX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50076291 (1-Methyl-piperidine-4-carboxylic acid (2-benzo[1,3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Compound was tested for the inhibitory activity against human liver cathepsin D | J Med Chem 42: 1428-40 (1999) Article DOI: 10.1021/jm980641t BindingDB Entry DOI: 10.7270/Q2MS3RZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50076288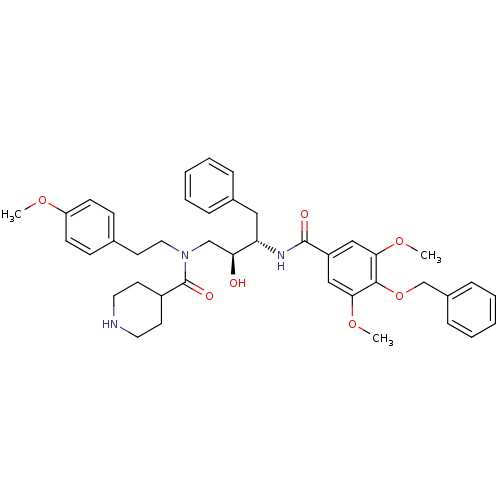 (CHEMBL284955 | Piperidine-4-carboxylic acid [(2S,3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity against Plasmepsin 2 | J Med Chem 42: 1428-40 (1999) Article DOI: 10.1021/jm980641t BindingDB Entry DOI: 10.7270/Q2MS3RZX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50076292 (1-Methyl-piperidine-4-carboxylic acid {(2S,3S)-3-[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Compound was tested for the inhibitory activity against human liver cathepsin D | J Med Chem 42: 1428-40 (1999) Article DOI: 10.1021/jm980641t BindingDB Entry DOI: 10.7270/Q2MS3RZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50076286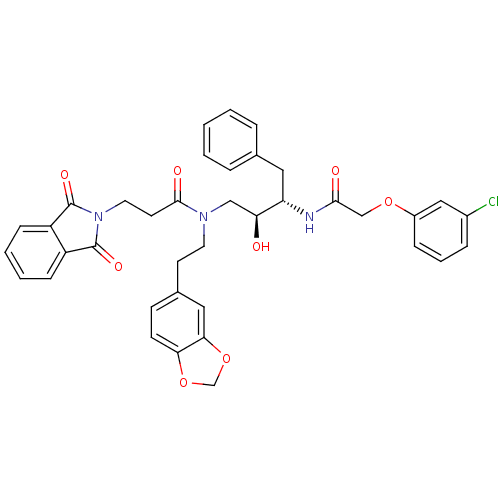 (CHEMBL34051 | N-(2-Benzo[1,3]dioxol-5-yl-ethyl)-N-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Compound was tested for the inhibitory activity against human liver cathepsin D | J Med Chem 42: 1428-40 (1999) Article DOI: 10.1021/jm980641t BindingDB Entry DOI: 10.7270/Q2MS3RZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50076287 (1-Methyl-piperidine-4-carboxylic acid [(2S,3S)-3-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Compound was tested for the inhibitory activity against human liver cathepsin D | J Med Chem 42: 1428-40 (1999) Article DOI: 10.1021/jm980641t BindingDB Entry DOI: 10.7270/Q2MS3RZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM8019 (2-(3-chlorophenoxy)-N-[(2S,3S)-3-hydroxy-4-{N-[2-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Compound was tested for the inhibitory activity against human liver cathepsin D | J Med Chem 42: 1428-40 (1999) Article DOI: 10.1021/jm980641t BindingDB Entry DOI: 10.7270/Q2MS3RZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50076288 (CHEMBL284955 | Piperidine-4-carboxylic acid [(2S,3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Compound was tested for the inhibitory activity against human liver cathepsin D | J Med Chem 42: 1428-40 (1999) Article DOI: 10.1021/jm980641t BindingDB Entry DOI: 10.7270/Q2MS3RZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50076289 (CHEMBL32997 | N-((1S,2S)-3-{(2-Benzo[1,3]dioxol-5-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | MMDB Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity against Plasmepsin 2 | J Med Chem 42: 1428-40 (1999) Article DOI: 10.1021/jm980641t BindingDB Entry DOI: 10.7270/Q2MS3RZX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50076286 (CHEMBL34051 | N-(2-Benzo[1,3]dioxol-5-yl-ethyl)-N-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity against Plasmepsin 2 | J Med Chem 42: 1428-40 (1999) Article DOI: 10.1021/jm980641t BindingDB Entry DOI: 10.7270/Q2MS3RZX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50076290 (CHEMBL284151 | N-(2-Benzo[1,3]dioxol-5-yl-ethyl)-N...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity against Plasmepsin 2 | J Med Chem 42: 1428-40 (1999) Article DOI: 10.1021/jm980641t BindingDB Entry DOI: 10.7270/Q2MS3RZX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Anthranilate phosphoribosyltransferase (Mycobacterium tuberculosis) | BDBM93073 (ACS No 172) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description The initial screen was performed in a 96-well plate using the fluoresence-based assay previously described. An enzyme-coupled absorbance-based assay... | Biochemistry 52: 1776-1787 (2013) Article DOI: 10.1021/bi301387m BindingDB Entry DOI: 10.7270/Q2B56HBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate phosphoribosyltransferase (Mycobacterium tuberculosis) | BDBM93074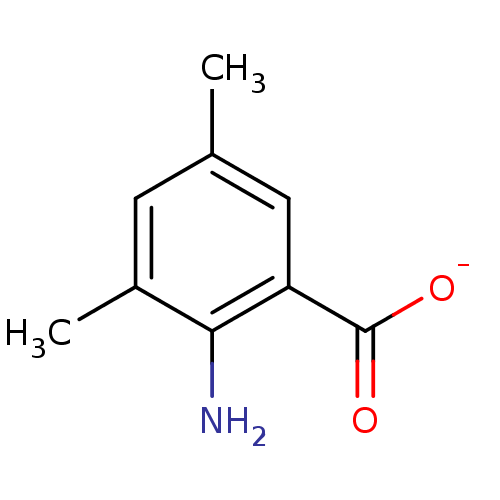 (ACS No 142) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description The initial screen was performed in a 96-well plate using the fluoresence-based assay previously described. An enzyme-coupled absorbance-based assay... | Biochemistry 52: 1776-1787 (2013) Article DOI: 10.1021/bi301387m BindingDB Entry DOI: 10.7270/Q2B56HBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate phosphoribosyltransferase (Mycobacterium tuberculosis) | BDBM93075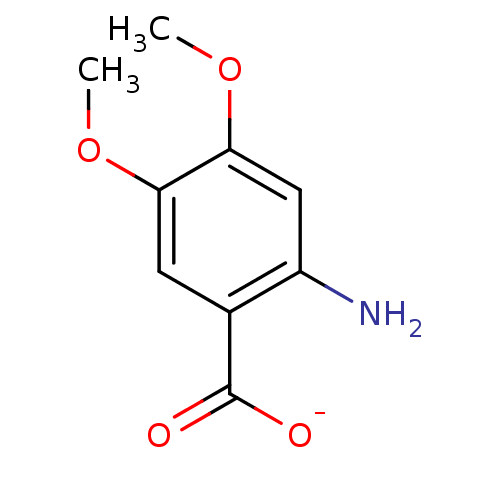 (ACS No 145) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description The initial screen was performed in a 96-well plate using the fluoresence-based assay previously described. An enzyme-coupled absorbance-based assay... | Biochemistry 52: 1776-1787 (2013) Article DOI: 10.1021/bi301387m BindingDB Entry DOI: 10.7270/Q2B56HBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate phosphoribosyltransferase (Mycobacterium tuberculosis) | BDBM93076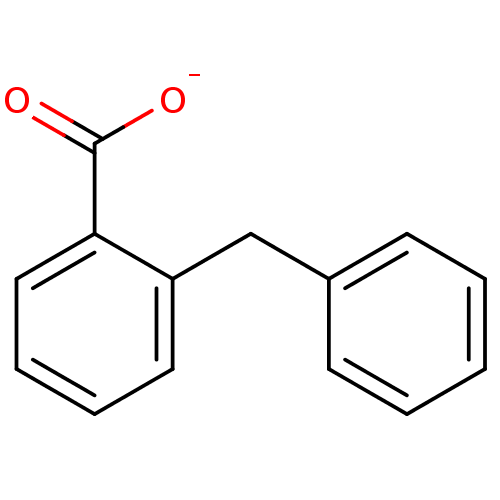 (ACS No 174) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description The initial screen was performed in a 96-well plate using the fluoresence-based assay previously described. An enzyme-coupled absorbance-based assay... | Biochemistry 52: 1776-1787 (2013) Article DOI: 10.1021/bi301387m BindingDB Entry DOI: 10.7270/Q2B56HBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate phosphoribosyltransferase (Mycobacterium tuberculosis) | BDBM93077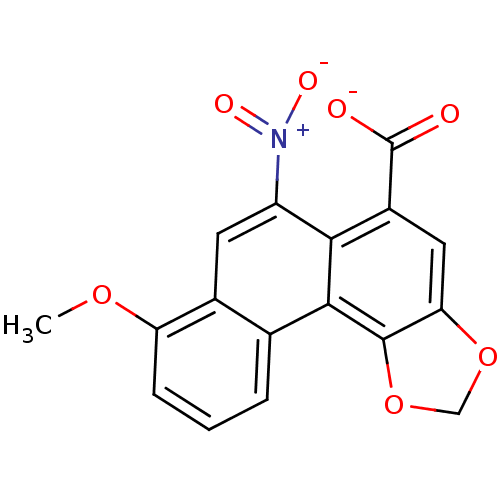 (ACS No 179) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description The initial screen was performed in a 96-well plate using the fluoresence-based assay previously described. An enzyme-coupled absorbance-based assay... | Biochemistry 52: 1776-1787 (2013) Article DOI: 10.1021/bi301387m BindingDB Entry DOI: 10.7270/Q2B56HBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate phosphoribosyltransferase (Mycobacterium tuberculosis) | BDBM93078 (ACS No 126) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description The initial screen was performed in a 96-well plate using the fluoresence-based assay previously described. An enzyme-coupled absorbance-based assay... | Biochemistry 52: 1776-1787 (2013) Article DOI: 10.1021/bi301387m BindingDB Entry DOI: 10.7270/Q2B56HBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate phosphoribosyltransferase (Mycobacterium tuberculosis) | BDBM93079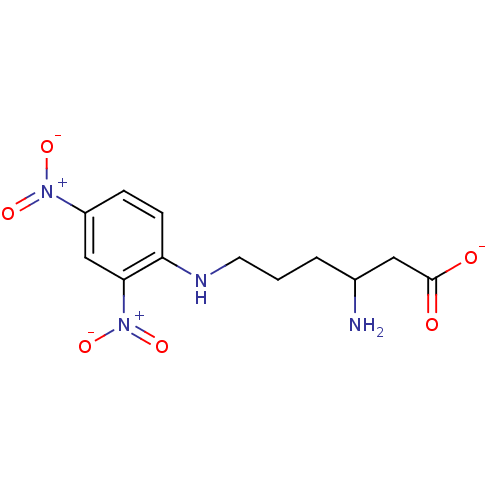 (ACS No 165) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 7.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description The initial screen was performed in a 96-well plate using the fluoresence-based assay previously described. An enzyme-coupled absorbance-based assay... | Biochemistry 52: 1776-1787 (2013) Article DOI: 10.1021/bi301387m BindingDB Entry DOI: 10.7270/Q2B56HBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate phosphoribosyltransferase (Mycobacterium tuberculosis) | BDBM85513 (ACS No 10 | CAS_54-21-7 | NSC_5900 | Sodium salicy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1.19E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description The initial screen was performed in a 96-well plate using the fluoresence-based assay previously described. An enzyme-coupled absorbance-based assay... | Biochemistry 52: 1776-1787 (2013) Article DOI: 10.1021/bi301387m BindingDB Entry DOI: 10.7270/Q2B56HBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial PAS domain-containing protein 1 (Homo sapiens (Human)) | BDBM50105306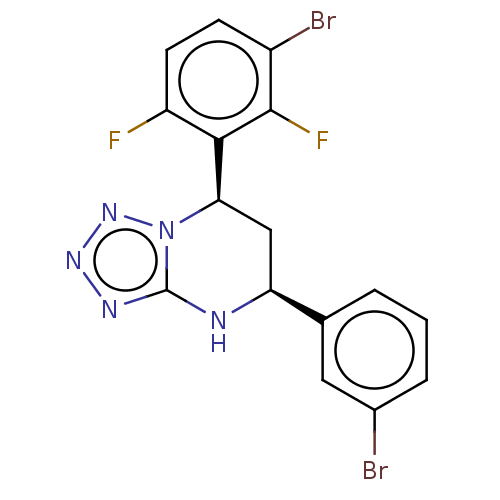 (CHEMBL3597782) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Southwestern Medical Center Curated by ChEMBL | Assay Description Binding affinity to wild type GST-tagged HIF-2alpha PAS-B (240 to 350) (unknown origin) expressed in Escherichia coli assessed as inhibition of inter... | J Med Chem 58: 5930-41 (2015) Article DOI: 10.1021/acs.jmedchem.5b00529 BindingDB Entry DOI: 10.7270/Q2G162K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial PAS domain-containing protein 1 (Homo sapiens (Human)) | BDBM50105310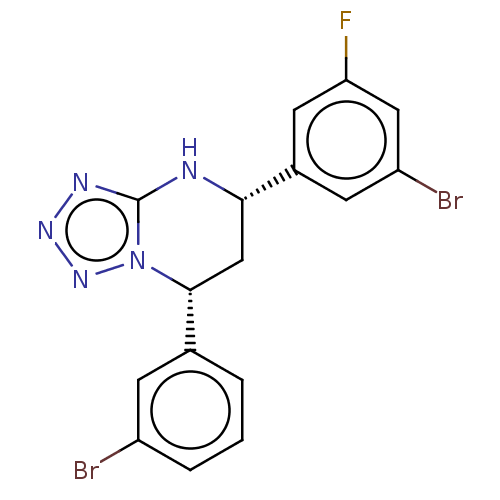 (CHEMBL3597786) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Southwestern Medical Center Curated by ChEMBL | Assay Description Binding affinity to wild type GST-tagged HIF-2alpha PAS-B (240 to 350) (unknown origin) expressed in Escherichia coli assessed as inhibition of inter... | J Med Chem 58: 5930-41 (2015) Article DOI: 10.1021/acs.jmedchem.5b00529 BindingDB Entry DOI: 10.7270/Q2G162K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial PAS domain-containing protein 1 (Homo sapiens (Human)) | BDBM50105280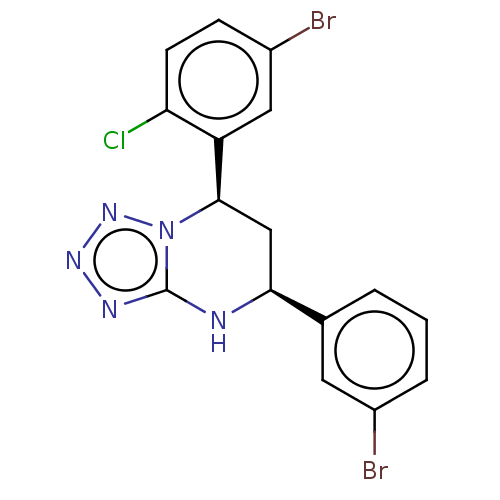 (CHEMBL3597780) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Southwestern Medical Center Curated by ChEMBL | Assay Description Binding affinity to wild type GST-tagged HIF-2alpha PAS-B (240 to 350) (unknown origin) expressed in Escherichia coli assessed as inhibition of inter... | J Med Chem 58: 5930-41 (2015) Article DOI: 10.1021/acs.jmedchem.5b00529 BindingDB Entry DOI: 10.7270/Q2G162K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial PAS domain-containing protein 1 (Homo sapiens (Human)) | BDBM50105308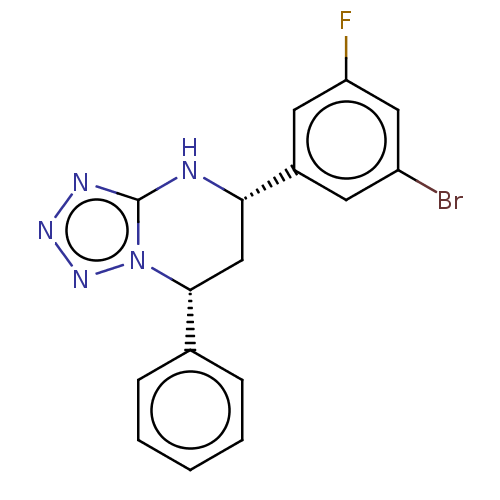 (CHEMBL3597784) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Southwestern Medical Center Curated by ChEMBL | Assay Description Binding affinity to wild type GST-tagged HIF-2alpha PAS-B (240 to 350) (unknown origin) expressed in Escherichia coli assessed as inhibition of inter... | J Med Chem 58: 5930-41 (2015) Article DOI: 10.1021/acs.jmedchem.5b00529 BindingDB Entry DOI: 10.7270/Q2G162K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial PAS domain-containing protein 1 (Homo sapiens (Human)) | BDBM50105278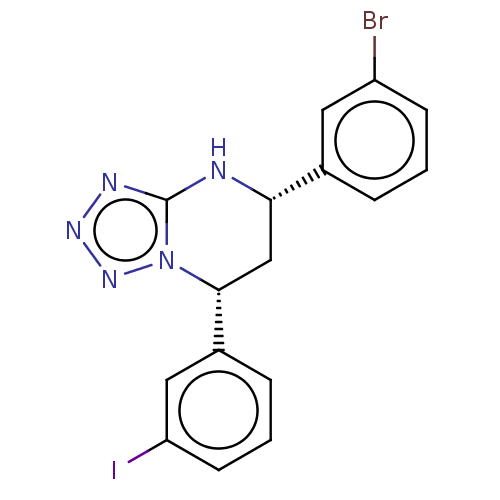 (CHEMBL3597778) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 108 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Southwestern Medical Center Curated by ChEMBL | Assay Description Binding affinity to wild type GST-tagged HIF-2alpha PAS-B (240 to 350) (unknown origin) expressed in Escherichia coli assessed as inhibition of inter... | J Med Chem 58: 5930-41 (2015) Article DOI: 10.1021/acs.jmedchem.5b00529 BindingDB Entry DOI: 10.7270/Q2G162K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial PAS domain-containing protein 1 (Homo sapiens (Human)) | BDBM50105276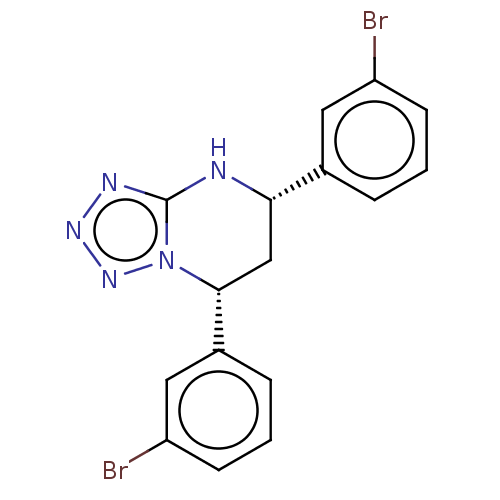 (CHEMBL3597698) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Southwestern Medical Center Curated by ChEMBL | Assay Description Binding affinity to wild type GST-tagged HIF-2alpha PAS-B (240 to 350) (unknown origin) expressed in Escherichia coli assessed as inhibition of inter... | J Med Chem 58: 5930-41 (2015) Article DOI: 10.1021/acs.jmedchem.5b00529 BindingDB Entry DOI: 10.7270/Q2G162K1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Endothelial PAS domain-containing protein 1 (Homo sapiens (Human)) | BDBM50105274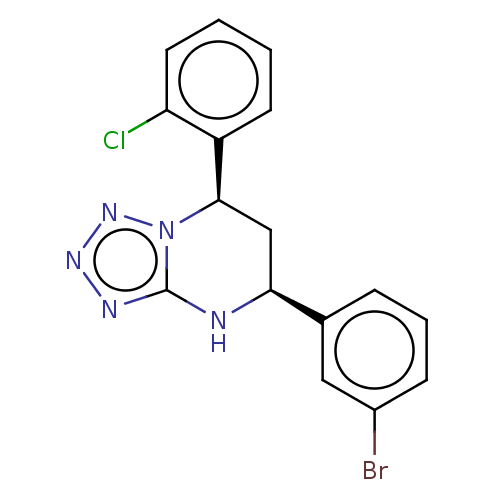 (CHEMBL3597696) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 195 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Southwestern Medical Center Curated by ChEMBL | Assay Description Binding affinity to wild type GST-tagged HIF-2alpha PAS-B (240 to 350) (unknown origin) expressed in Escherichia coli assessed as inhibition of inter... | J Med Chem 58: 5930-41 (2015) Article DOI: 10.1021/acs.jmedchem.5b00529 BindingDB Entry DOI: 10.7270/Q2G162K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial PAS domain-containing protein 1 (Homo sapiens (Human)) | BDBM50105268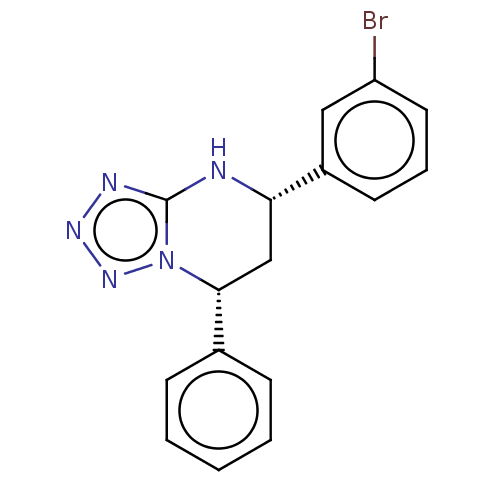 (CHEMBL3597690) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 248 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Southwestern Medical Center Curated by ChEMBL | Assay Description Binding affinity to wild type GST-tagged HIF-2alpha PAS-B (240 to 350) (unknown origin) expressed in Escherichia coli assessed as inhibition of inter... | J Med Chem 58: 5930-41 (2015) Article DOI: 10.1021/acs.jmedchem.5b00529 BindingDB Entry DOI: 10.7270/Q2G162K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial PAS domain-containing protein 1 (Homo sapiens (Human)) | BDBM50105272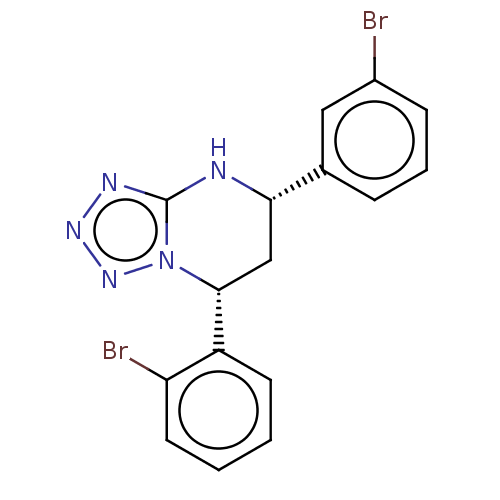 (CHEMBL3597694) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 314 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Southwestern Medical Center Curated by ChEMBL | Assay Description Binding affinity to wild type GST-tagged HIF-2alpha PAS-B (240 to 350) (unknown origin) expressed in Escherichia coli assessed as inhibition of inter... | J Med Chem 58: 5930-41 (2015) Article DOI: 10.1021/acs.jmedchem.5b00529 BindingDB Entry DOI: 10.7270/Q2G162K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial PAS domain-containing protein 1 (Homo sapiens (Human)) | BDBM50105310 (CHEMBL3597786) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Southwestern Medical Center Curated by ChEMBL | Assay Description Inhibition of HIF-2alpha in human Hep3B cells assessed as downregulation of EPO mRNA expression by RT-PCR analysis | J Med Chem 58: 5930-41 (2015) Article DOI: 10.1021/acs.jmedchem.5b00529 BindingDB Entry DOI: 10.7270/Q2G162K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial PAS domain-containing protein 1 (Homo sapiens (Human)) | BDBM50105270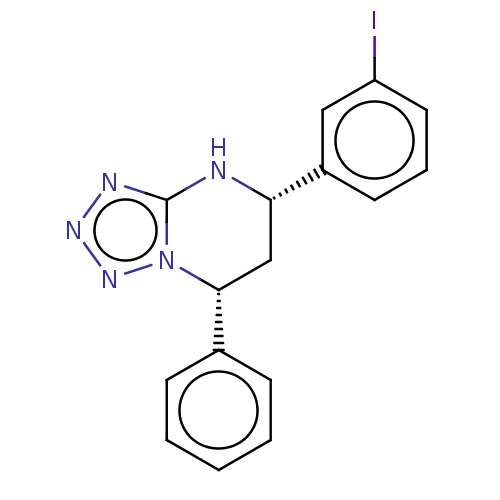 (CHEMBL3597692) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Southwestern Medical Center Curated by ChEMBL | Assay Description Binding affinity to wild type GST-tagged HIF-2alpha PAS-B (240 to 350) (unknown origin) expressed in Escherichia coli assessed as inhibition of inter... | J Med Chem 58: 5930-41 (2015) Article DOI: 10.1021/acs.jmedchem.5b00529 BindingDB Entry DOI: 10.7270/Q2G162K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50105308 (CHEMBL3597784) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Southwestern Medical Center Curated by ChEMBL | Assay Description Binding affinity to wild type GST-tagged HIF-2alpha PAS-B S304M mutant (240 to 350) (unknown origin) expressed in Escherichia coli assessed as inhibi... | J Med Chem 58: 5930-41 (2015) Article DOI: 10.1021/acs.jmedchem.5b00529 BindingDB Entry DOI: 10.7270/Q2G162K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50105274 (CHEMBL3597696) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Southwestern Medical Center Curated by ChEMBL | Assay Description Binding affinity to wild type GST-tagged HIF-2alpha PAS-B S304M mutant (240 to 350) (unknown origin) expressed in Escherichia coli assessed as inhibi... | J Med Chem 58: 5930-41 (2015) Article DOI: 10.1021/acs.jmedchem.5b00529 BindingDB Entry DOI: 10.7270/Q2G162K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial PAS domain-containing protein 1 (Homo sapiens (Human)) | BDBM50105307 (CHEMBL3597783) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Southwestern Medical Center Curated by ChEMBL | Assay Description Binding affinity to wild type GST-tagged HIF-2alpha PAS-B (240 to 350) (unknown origin) expressed in Escherichia coli assessed as inhibition of inter... | J Med Chem 58: 5930-41 (2015) Article DOI: 10.1021/acs.jmedchem.5b00529 BindingDB Entry DOI: 10.7270/Q2G162K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50105280 (CHEMBL3597780) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Southwestern Medical Center Curated by ChEMBL | Assay Description Binding affinity to wild type GST-tagged HIF-2alpha PAS-B S304M mutant (240 to 350) (unknown origin) expressed in Escherichia coli assessed as inhibi... | J Med Chem 58: 5930-41 (2015) Article DOI: 10.1021/acs.jmedchem.5b00529 BindingDB Entry DOI: 10.7270/Q2G162K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial PAS domain-containing protein 1 (Homo sapiens (Human)) | BDBM50105273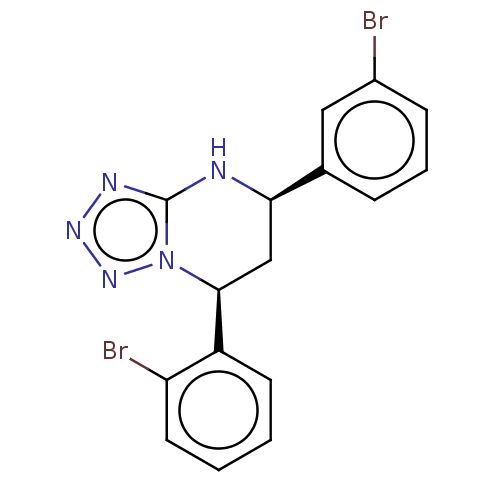 (CHEMBL3597695) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Southwestern Medical Center Curated by ChEMBL | Assay Description Binding affinity to wild type GST-tagged HIF-2alpha PAS-B (240 to 350) (unknown origin) expressed in Escherichia coli assessed as inhibition of inter... | J Med Chem 58: 5930-41 (2015) Article DOI: 10.1021/acs.jmedchem.5b00529 BindingDB Entry DOI: 10.7270/Q2G162K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50105279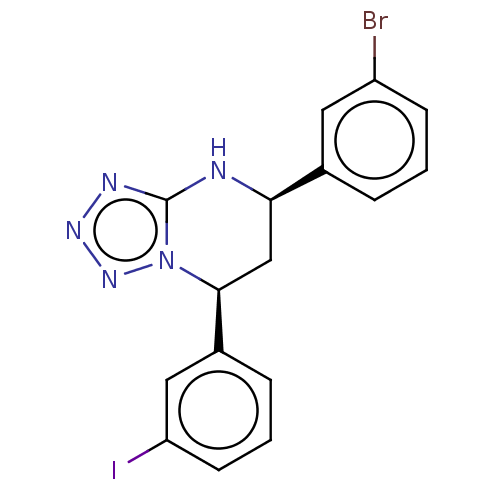 (CHEMBL3597779) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Southwestern Medical Center Curated by ChEMBL | Assay Description Binding affinity to wild type GST-tagged HIF-2alpha PAS-B S304M mutant (240 to 350) (unknown origin) expressed in Escherichia coli assessed as inhibi... | J Med Chem 58: 5930-41 (2015) Article DOI: 10.1021/acs.jmedchem.5b00529 BindingDB Entry DOI: 10.7270/Q2G162K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50105304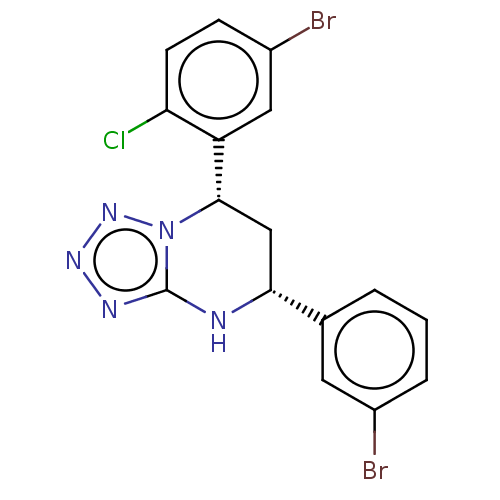 (CHEMBL3597781) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Southwestern Medical Center Curated by ChEMBL | Assay Description Binding affinity to wild type GST-tagged HIF-2alpha PAS-B S304M mutant (240 to 350) (unknown origin) expressed in Escherichia coli assessed as inhibi... | J Med Chem 58: 5930-41 (2015) Article DOI: 10.1021/acs.jmedchem.5b00529 BindingDB Entry DOI: 10.7270/Q2G162K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50105278 (CHEMBL3597778) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Southwestern Medical Center Curated by ChEMBL | Assay Description Binding affinity to wild type GST-tagged HIF-2alpha PAS-B S304M mutant (240 to 350) (unknown origin) expressed in Escherichia coli assessed as inhibi... | J Med Chem 58: 5930-41 (2015) Article DOI: 10.1021/acs.jmedchem.5b00529 BindingDB Entry DOI: 10.7270/Q2G162K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50105277 (CHEMBL3597699) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Southwestern Medical Center Curated by ChEMBL | Assay Description Binding affinity to wild type GST-tagged HIF-2alpha PAS-B S304M mutant (240 to 350) (unknown origin) expressed in Escherichia coli assessed as inhibi... | J Med Chem 58: 5930-41 (2015) Article DOI: 10.1021/acs.jmedchem.5b00529 BindingDB Entry DOI: 10.7270/Q2G162K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50105276 (CHEMBL3597698) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Southwestern Medical Center Curated by ChEMBL | Assay Description Binding affinity to wild type GST-tagged HIF-2alpha PAS-B S304M mutant (240 to 350) (unknown origin) expressed in Escherichia coli assessed as inhibi... | J Med Chem 58: 5930-41 (2015) Article DOI: 10.1021/acs.jmedchem.5b00529 BindingDB Entry DOI: 10.7270/Q2G162K1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50105275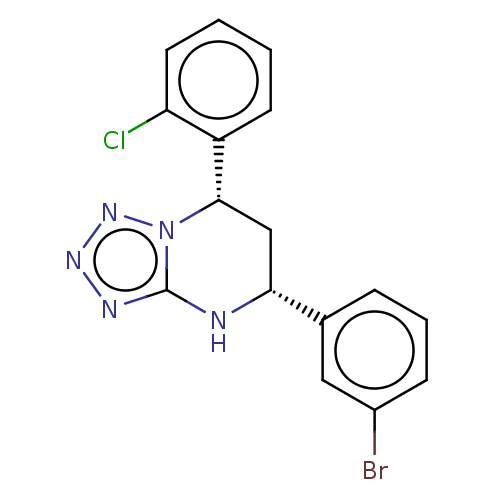 (CHEMBL3597697) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Southwestern Medical Center Curated by ChEMBL | Assay Description Binding affinity to wild type GST-tagged HIF-2alpha PAS-B S304M mutant (240 to 350) (unknown origin) expressed in Escherichia coli assessed as inhibi... | J Med Chem 58: 5930-41 (2015) Article DOI: 10.1021/acs.jmedchem.5b00529 BindingDB Entry DOI: 10.7270/Q2G162K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 115 total ) | Next | Last >> |