

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16318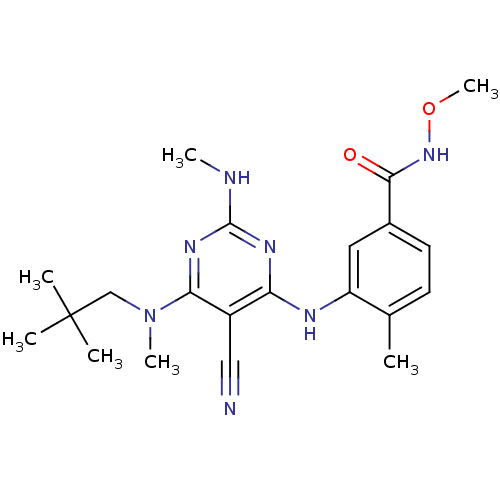 (3-({5-cyano-6-[(2,2-dimethylpropyl)(methyl)amino]-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0470 | -58.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16319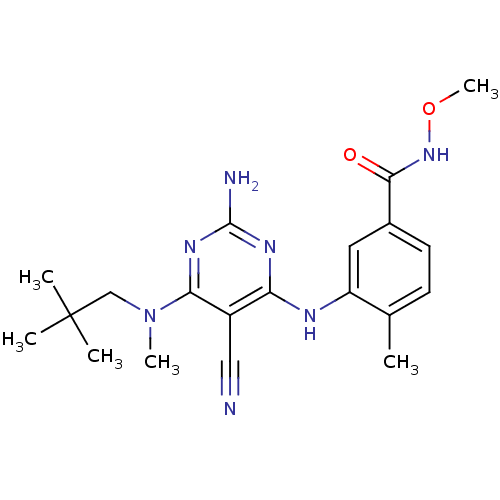 (3-({2-amino-5-cyano-6-[(2,2-dimethylpropyl)(methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | -58.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16317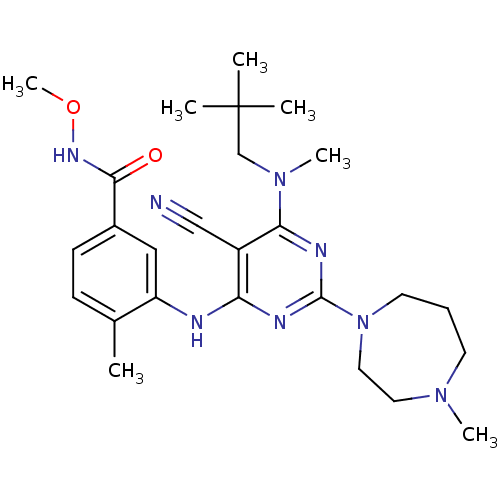 (3-({5-cyano-6-[(2,2-dimethylpropyl)(methyl)amino]-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0570 | -57.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16329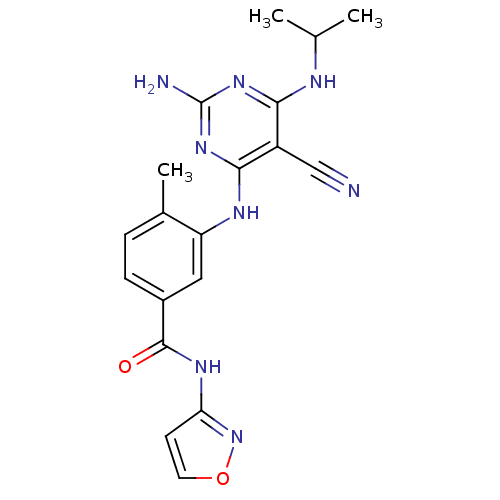 (3-({2-amino-5-cyano-6-[(1-methylethyl)amino]pyrimi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.0570 | -57.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16320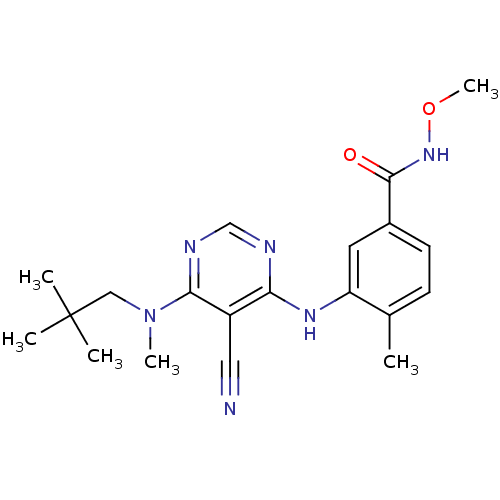 (3-({5-cyano-6-[(2,2-dimethylpropyl)(methyl)amino]p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.150 | -55.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16330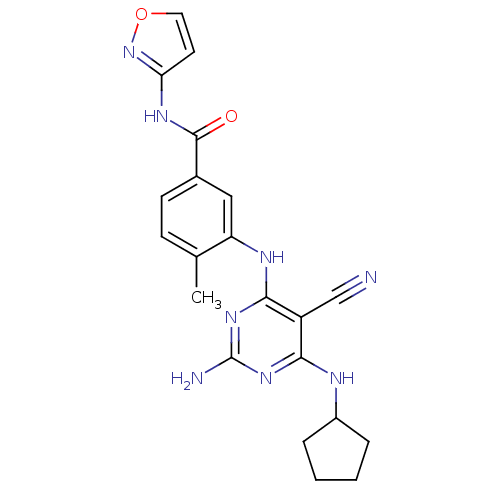 (3-{[2-amino-5-cyano-6-(cyclopentylamino)pyrimidin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.160 | -55.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-1 (Homo sapiens (Human)) | BDBM50538006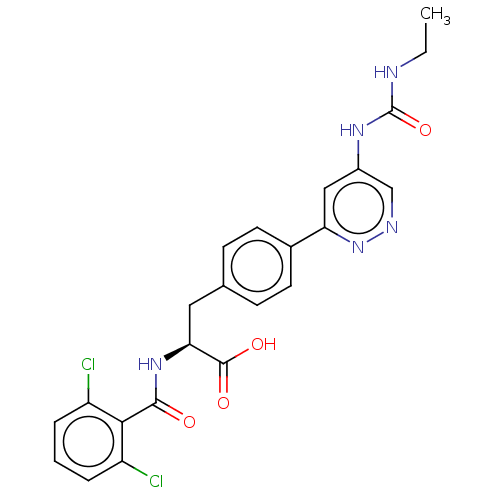 (CHEMBL4649232) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.166 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pliant Therapeutics Curated by ChEMBL | Assay Description Displacement of [3H] C8 from human integrin alphavbeta1 incubated for 6 hrs by competition binding assay | J Med Chem 63: 5675-5696 (2020) Article DOI: 10.1021/acs.jmedchem.9b01869 BindingDB Entry DOI: 10.7270/Q2DR302Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50376226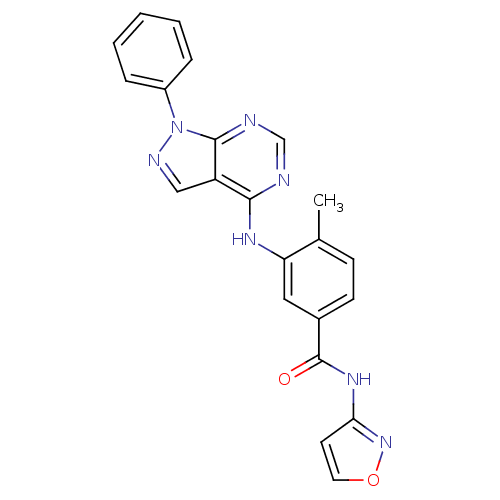 (CHEMBL259922) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant p38alpha-mediated myelin basic protein phosphorylation | Bioorg Med Chem Lett 18: 2652-7 (2008) Article DOI: 10.1016/j.bmcl.2008.03.019 BindingDB Entry DOI: 10.7270/Q2C53MR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50376226 (CHEMBL259922) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant p38alpha-mediated myelin basic protein phosphorylation | Bioorg Med Chem Lett 18: 2652-7 (2008) Article DOI: 10.1016/j.bmcl.2008.03.019 BindingDB Entry DOI: 10.7270/Q2C53MR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity of compound was determined against to human cannabinoid receptor 2 in chinese hamster ovary cells | J Med Chem 46: 2110-6 (2003) Article DOI: 10.1021/jm020329q BindingDB Entry DOI: 10.7270/Q2J67HPG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16325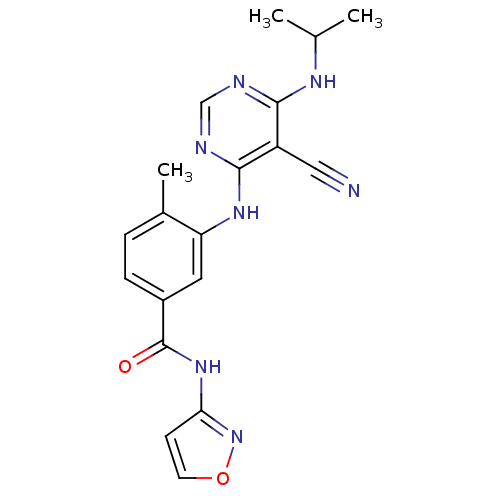 (3-({5-cyano-6-[(1-methylethyl)amino]pyrimidin-4-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.410 | -53.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50376456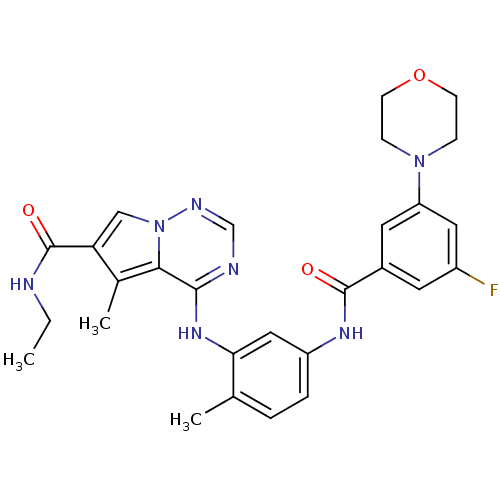 (CHEMBL262592) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of p38alpha | Bioorg Med Chem Lett 18: 2739-44 (2008) Article DOI: 10.1016/j.bmcl.2008.02.067 BindingDB Entry DOI: 10.7270/Q29887X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16324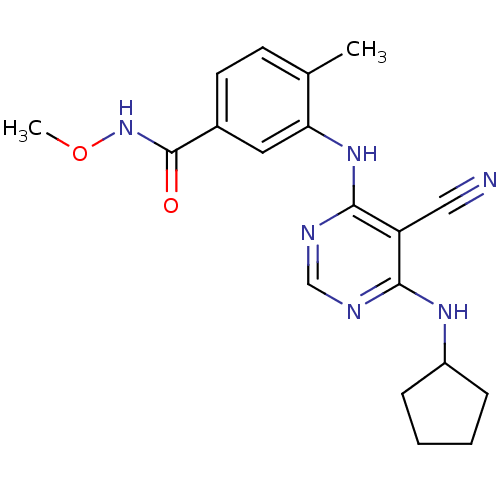 (3-{[5-cyano-6-(cyclopentylamino)pyrimidin-4-yl]ami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.420 | -53.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50376433 (CHEMBL258895) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of p38alpha | Bioorg Med Chem Lett 18: 2739-44 (2008) Article DOI: 10.1016/j.bmcl.2008.02.067 BindingDB Entry DOI: 10.7270/Q29887X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50376434 (CHEMBL408150) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of p38alpha | Bioorg Med Chem Lett 18: 2739-44 (2008) Article DOI: 10.1016/j.bmcl.2008.02.067 BindingDB Entry DOI: 10.7270/Q29887X6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50376435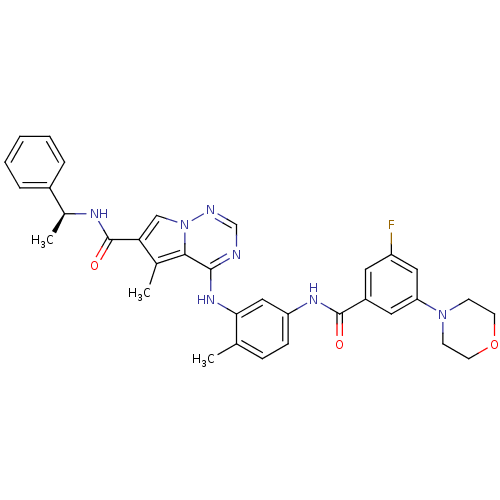 (CHEMBL261845) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of p38alpha | Bioorg Med Chem Lett 18: 2739-44 (2008) Article DOI: 10.1016/j.bmcl.2008.02.067 BindingDB Entry DOI: 10.7270/Q29887X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16323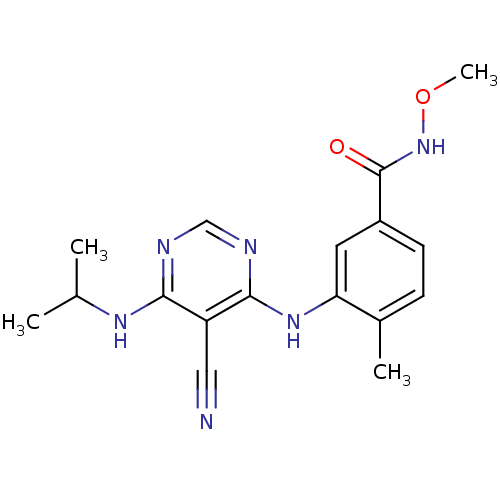 (3-({5-cyano-6-[(1-methylethyl)amino]pyrimidin-4-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.610 | -52.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16322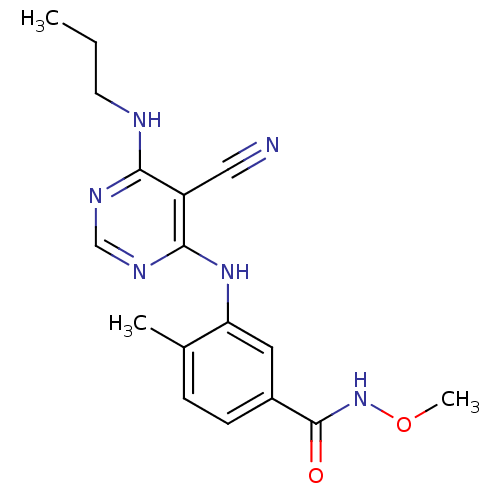 (3-{[5-cyano-6-(propylamino)pyrimidin-4-yl]amino}-N...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.970 | -50.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50376432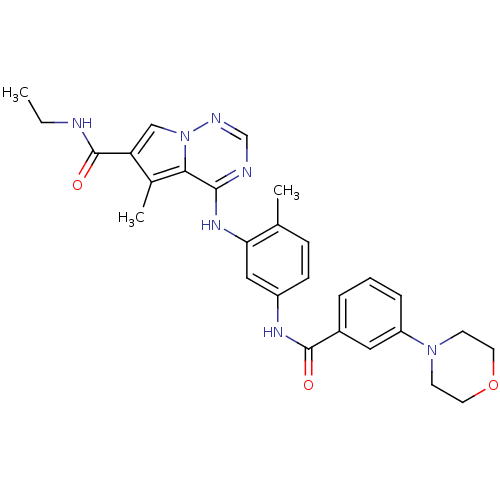 (CHEMBL258748) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of p38alpha | Bioorg Med Chem Lett 18: 2739-44 (2008) Article DOI: 10.1016/j.bmcl.2008.02.067 BindingDB Entry DOI: 10.7270/Q29887X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50192752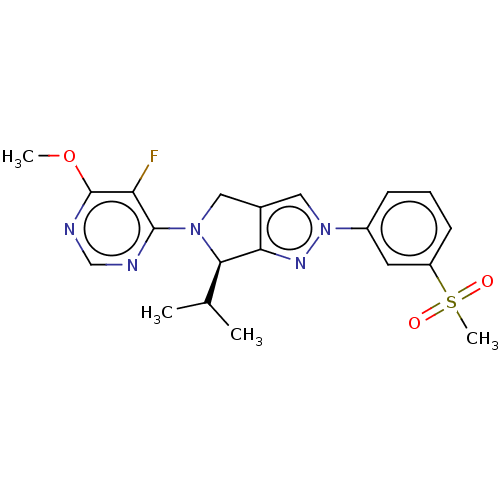 (CHEMBL3905741) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin) | Bioorg Med Chem Lett 26: 5044-5050 (2016) Article DOI: 10.1016/j.bmcl.2016.08.089 BindingDB Entry DOI: 10.7270/Q28P62GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM304444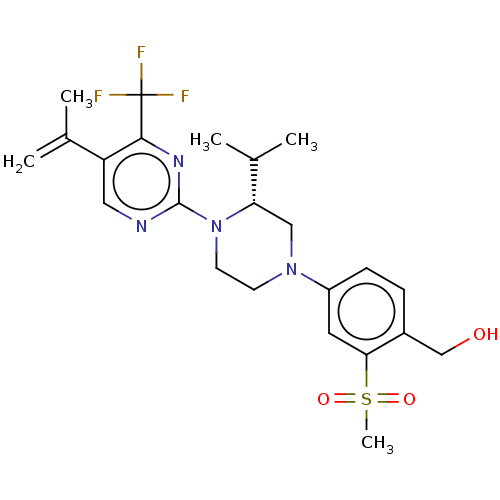 ((R)-(4-(3-isopropyl-4-(5-(prop-1-en-2-yl)-4-(trifl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan... | US Patent US10144715 (2018) BindingDB Entry DOI: 10.7270/Q2PV6NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM304526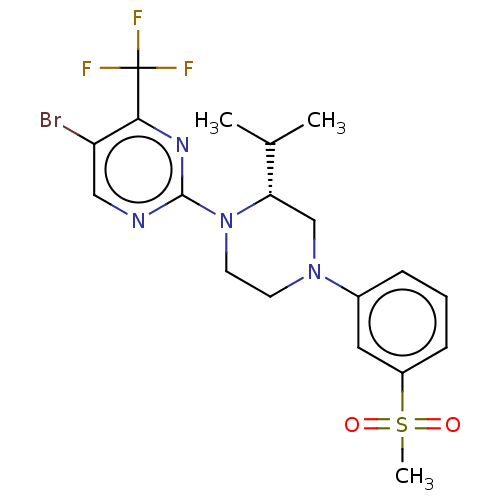 ((R)-5-cyclopropyl-2-(4-(4-fluoro-3-(methylsulfonyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan... | US Patent US10144715 (2018) BindingDB Entry DOI: 10.7270/Q2PV6NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50177012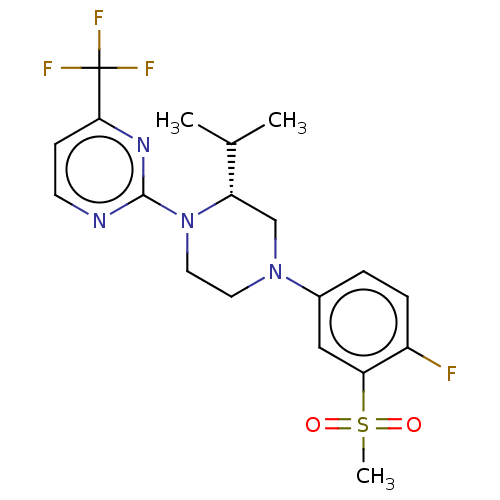 (CHEMBL3814153 | US10144715, Compound 7-32) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM304505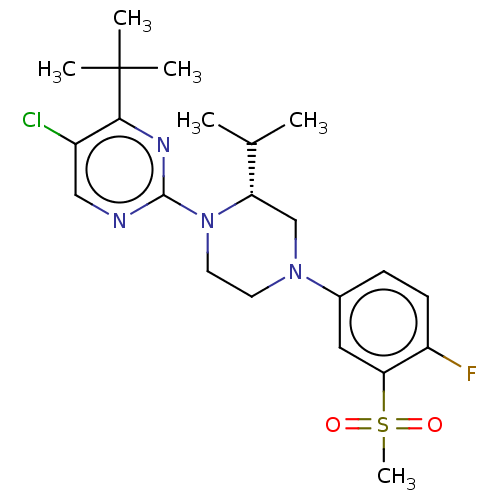 ((R)-5-chloro-2-(4-(4-fluoro-3-(methylsulfonyl)phen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan... | US Patent US10144715 (2018) BindingDB Entry DOI: 10.7270/Q2PV6NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM304452 ((R)-2-(4-(4-fluoro-3-(methylsulfonyl)phenyl)-2-iso...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan... | US Patent US10144715 (2018) BindingDB Entry DOI: 10.7270/Q2PV6NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50177012 (CHEMBL3814153 | US10144715, Compound 7-32) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan... | US Patent US10144715 (2018) BindingDB Entry DOI: 10.7270/Q2PV6NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50272598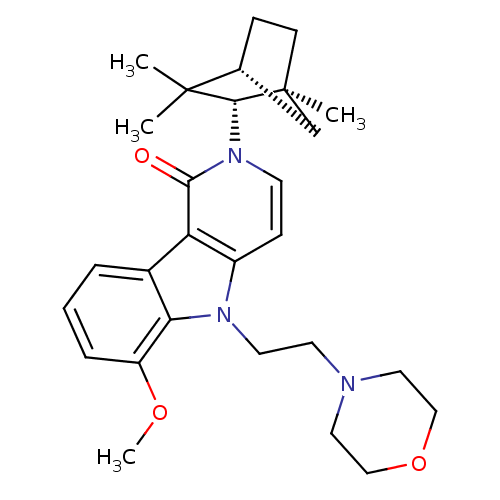 (6-Methoxy-5-(2-morpholin-4-yl-ethyl)-2-(1,3,3-trim...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity against human cannabinoid receptor 2 in chinese hamster ovary cells using WIN-55212-2 mesylate[57-3H] | J Med Chem 46: 2110-6 (2003) Article DOI: 10.1021/jm020329q BindingDB Entry DOI: 10.7270/Q2J67HPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50177016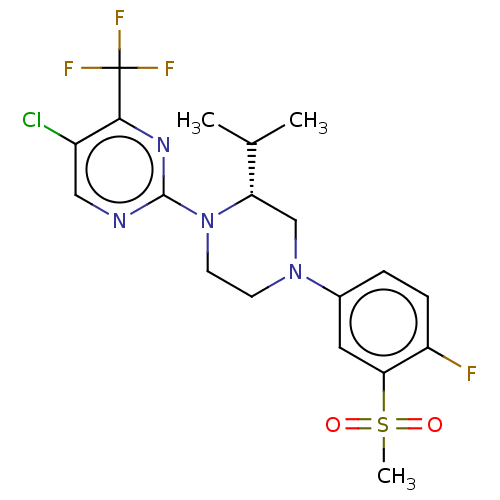 (CHEMBL3814501) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50177010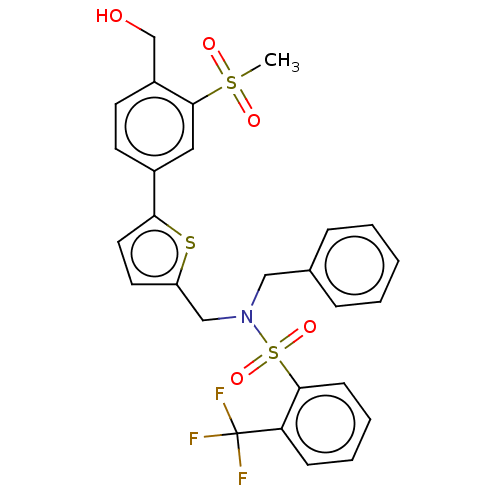 (CHEMBL3814006) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16331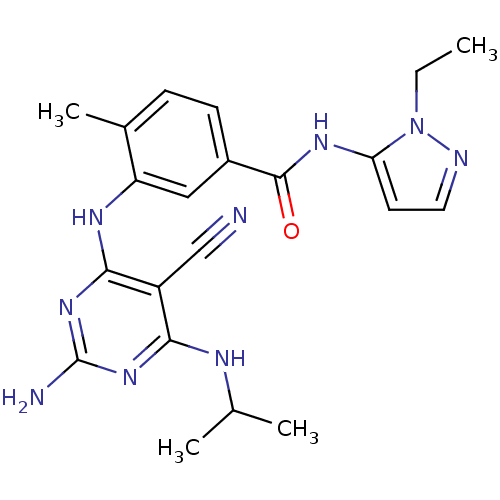 (3-({2-amino-5-cyano-6-[(1-methylethyl)amino]pyrimi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.5 | -49.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16326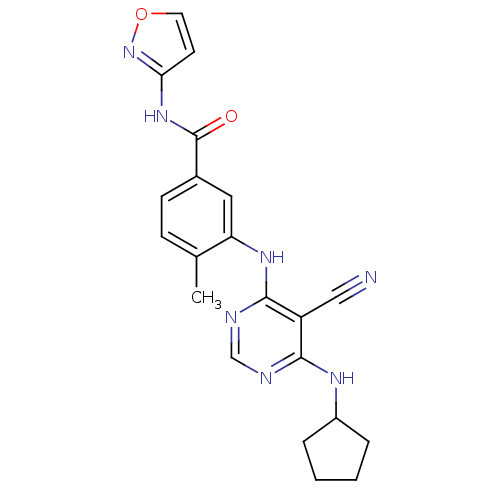 (3-{[5-cyano-6-(cyclopentylamino)pyrimidin-4-yl]ami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.60 | -49.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16332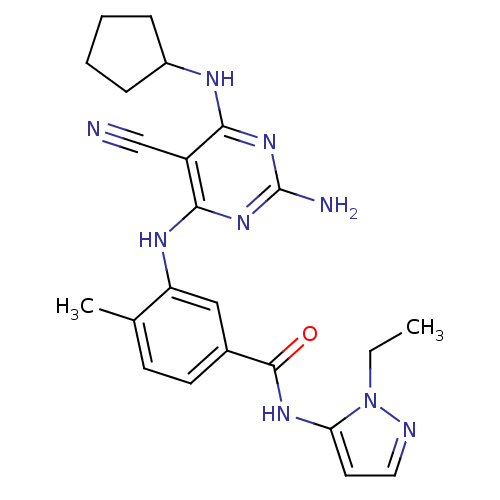 (3-{[2-amino-5-cyano-6-(cyclopentylamino)pyrimidin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.90 | -49.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity of compound was determined against to human cannabinoid receptor 1 in chinese hamster ovary cells | J Med Chem 46: 2110-6 (2003) Article DOI: 10.1021/jm020329q BindingDB Entry DOI: 10.7270/Q2J67HPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM304419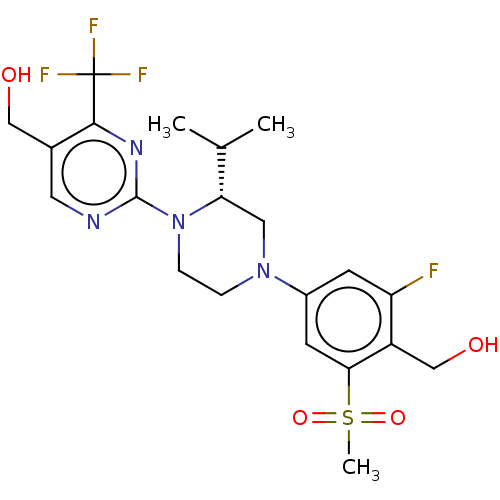 ((R)-(2-fluoro-4-(4-(5-(hydroxymethyl)-4-(trifluoro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan... | US Patent US10144715 (2018) BindingDB Entry DOI: 10.7270/Q2PV6NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50128092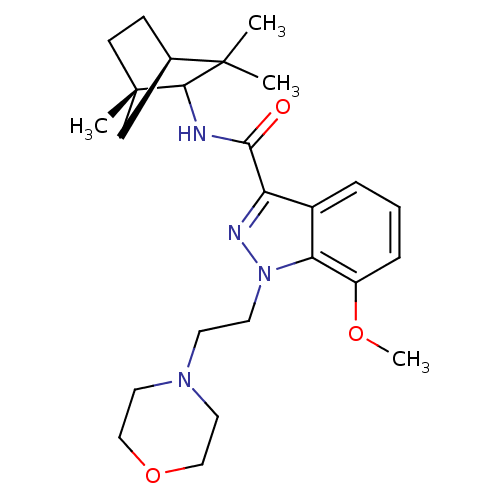 (7-Methoxy-1-(2-morpholin-4-yl-ethyl)-1H-indazole-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity against human cannabinoid receptor 2 in chinese hamster ovary cells using WIN-55212-2 mesylate[57-3H] | J Med Chem 46: 2110-6 (2003) Article DOI: 10.1021/jm020329q BindingDB Entry DOI: 10.7270/Q2J67HPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM168079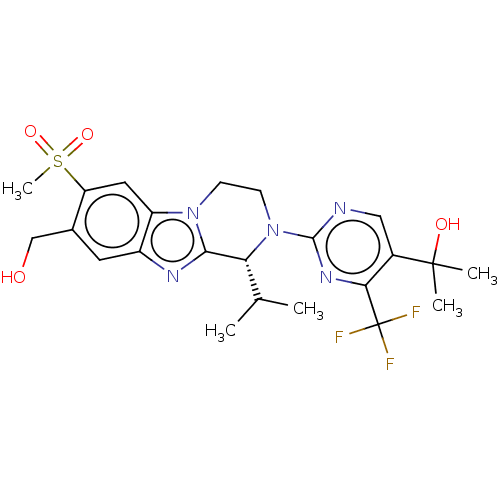 (US9073931, E27a | US9073931, E7a) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2 | -49.7 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan... | US Patent US9073931 (2015) BindingDB Entry DOI: 10.7270/Q26W98VC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM304616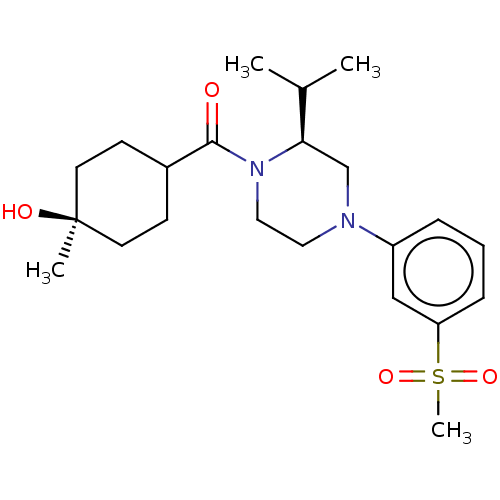 ((S)-2-isopropyl-4-(3-(methylsulfonyl)phenyl)-1-(((...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan... | US Patent US10144715 (2018) BindingDB Entry DOI: 10.7270/Q2PV6NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM304525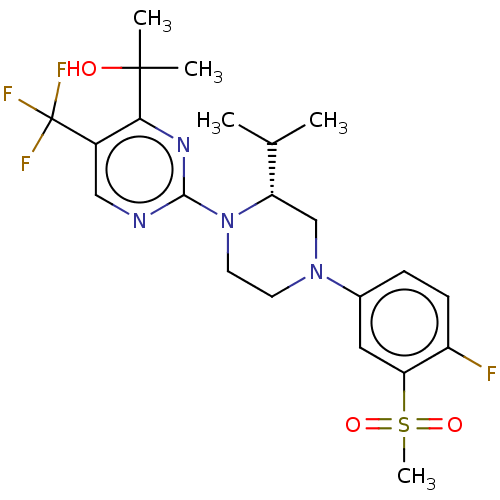 ((R)-2-(2-(4-(4-fluoro-3-(methylsulfonyl)phenyl)-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan... | US Patent US10144715 (2018) BindingDB Entry DOI: 10.7270/Q2PV6NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM304681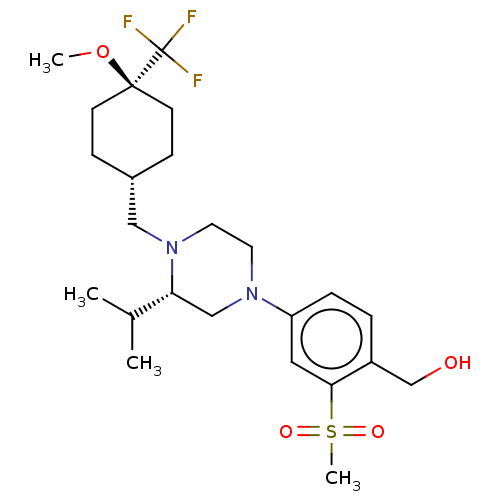 ((4-((S)-3-isopropyl-4-(((1r,4S)-4-methoxy-4-(trifl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan... | US Patent US10144715 (2018) BindingDB Entry DOI: 10.7270/Q2PV6NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM304523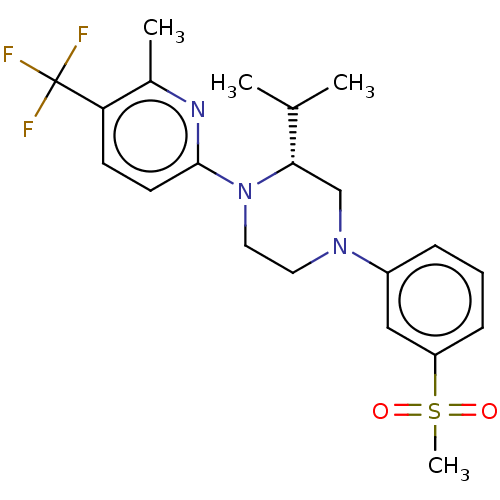 ((R)-2-isopropyl-1-(6-methyl-5-(trifluoromethyl)pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan... | US Patent US10144715 (2018) BindingDB Entry DOI: 10.7270/Q2PV6NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50177016 (CHEMBL3814501) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from LXRalpha ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM304682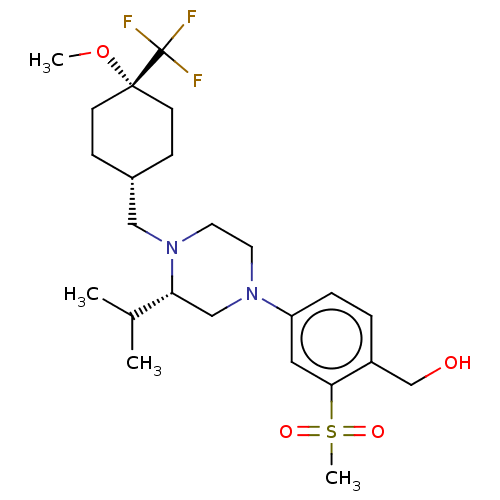 ((4-((S)-3-isopropyl-4-(((1s,4R)-4-methoxy-4-(trifl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan... | US Patent US10144715 (2018) BindingDB Entry DOI: 10.7270/Q2PV6NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50177015 (CHEMBL3814206 | US10144715, Compound 19-1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan... | US Patent US10144715 (2018) BindingDB Entry DOI: 10.7270/Q2PV6NFD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50177015 (CHEMBL3814206 | US10144715, Compound 19-1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM304505 ((R)-5-chloro-2-(4-(4-fluoro-3-(methylsulfonyl)phen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan... | US Patent US10144715 (2018) BindingDB Entry DOI: 10.7270/Q2PV6NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM304512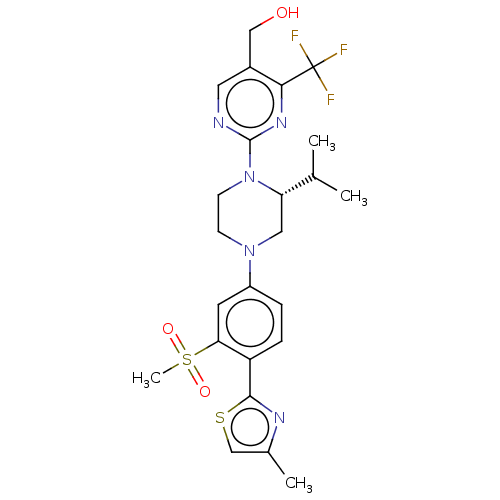 ((R)-2-(2-(4-(4-(hydroxymethyl)-3-(methylsulfonyl)p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan... | US Patent US10144715 (2018) BindingDB Entry DOI: 10.7270/Q2PV6NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM304514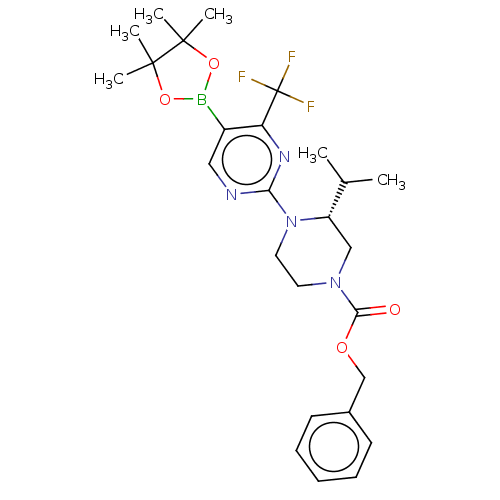 ((R)-2-(4-(4-fluoro-3-(methylsulfonyl)phenyl)-2-iso...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan... | US Patent US10144715 (2018) BindingDB Entry DOI: 10.7270/Q2PV6NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM304522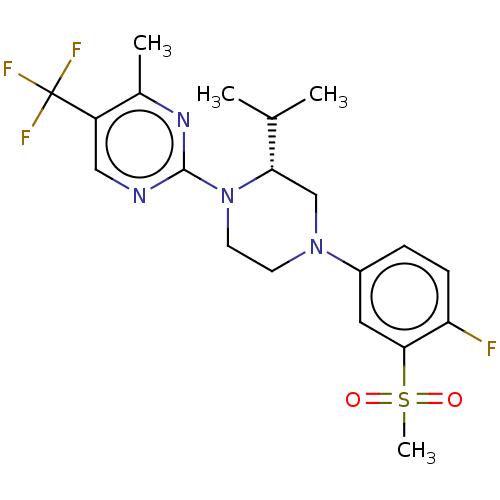 ((R)-2-(4-(4-fluoro-3-(methylsulfonyl)phenyl)-2-iso...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan... | US Patent US10144715 (2018) BindingDB Entry DOI: 10.7270/Q2PV6NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM304524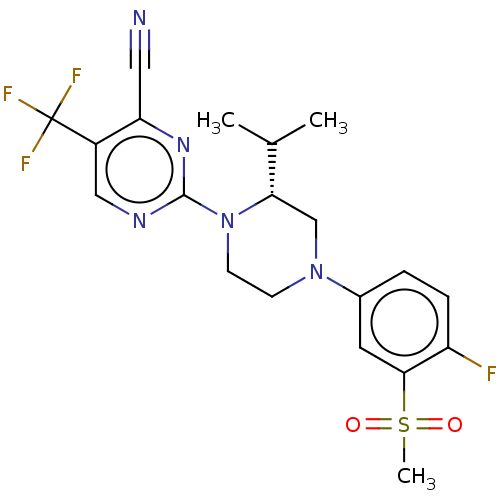 (1-(2-((R)-4-(4-fluoro-3-(methylsulfonyl)phenyl)-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan... | US Patent US10144715 (2018) BindingDB Entry DOI: 10.7270/Q2PV6NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM304431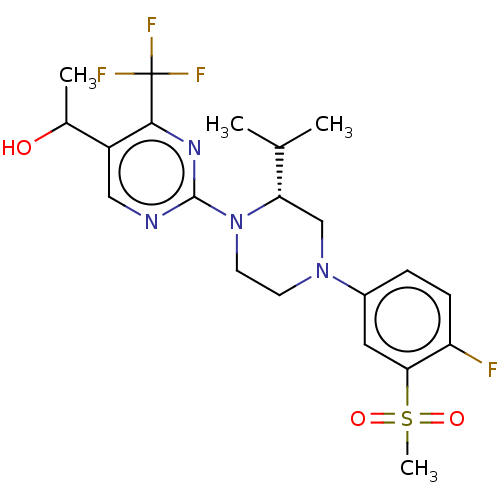 (1-(2-((R)-4-(4-fluoro-3-(methylsulfonyl)phenyl)-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan... | US Patent US10144715 (2018) BindingDB Entry DOI: 10.7270/Q2PV6NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 7452 total ) | Next | Last >> |