

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM31225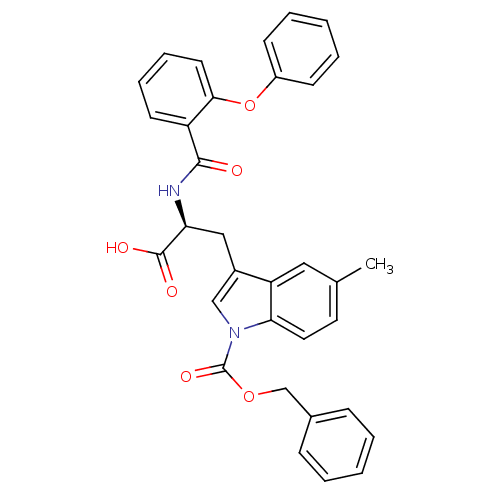 (2-phenoxybenzoyltryptophan derivative, R5C3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 100 | -39.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 23 |
Schering-Plough Research Institute | Assay Description Ki values were determined from a competition experiment in which serial dilutions of inhibitor were added to compete against a fixed concentration (1... | Anal Biochem 331: 138-46 (2004) Article DOI: 10.1016/j.ab.2004.03.009 BindingDB Entry DOI: 10.7270/Q2VX0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM31224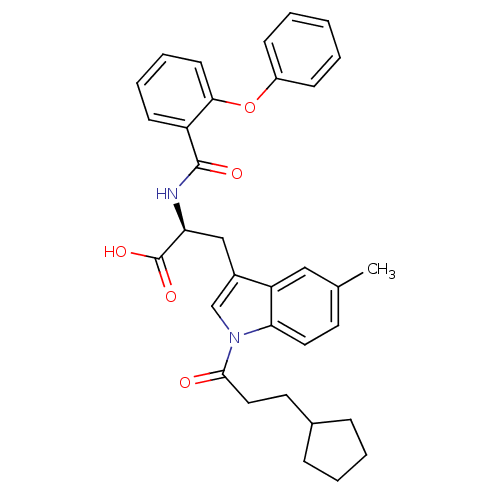 (2-phenoxybenzoyltryptophan derivative, R5C2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 200 | -38.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 23 |
Schering-Plough Research Institute | Assay Description Ki values were determined from a competition experiment in which serial dilutions of inhibitor were added to compete against a fixed concentration (1... | Anal Biochem 331: 138-46 (2004) Article DOI: 10.1016/j.ab.2004.03.009 BindingDB Entry DOI: 10.7270/Q2VX0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM31216 (2-phenoxybenzoyltryptophan derivative, R3C2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 400 | -36.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 23 |
Schering-Plough Research Institute | Assay Description Ki values were determined from a competition experiment in which serial dilutions of inhibitor were added to compete against a fixed concentration (1... | Anal Biochem 331: 138-46 (2004) Article DOI: 10.1016/j.ab.2004.03.009 BindingDB Entry DOI: 10.7270/Q2VX0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM31209 (2-phenoxybenzoyltryptophan derivative, R1C3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 500 | -35.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 23 |
Schering-Plough Research Institute | Assay Description Ki values were determined from a competition experiment in which serial dilutions of inhibitor were added to compete against a fixed concentration (1... | Anal Biochem 331: 138-46 (2004) Article DOI: 10.1016/j.ab.2004.03.009 BindingDB Entry DOI: 10.7270/Q2VX0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM31226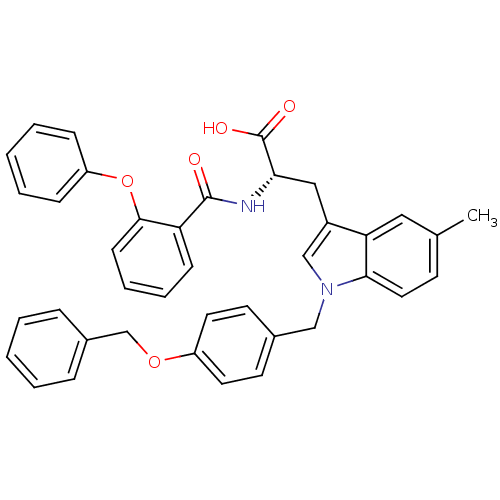 (2-phenoxybenzoyltryptophan derivative, R5C4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 600 | -35.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 23 |
Schering-Plough Research Institute | Assay Description Ki values were determined from a competition experiment in which serial dilutions of inhibitor were added to compete against a fixed concentration (1... | Anal Biochem 331: 138-46 (2004) Article DOI: 10.1016/j.ab.2004.03.009 BindingDB Entry DOI: 10.7270/Q2VX0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM31210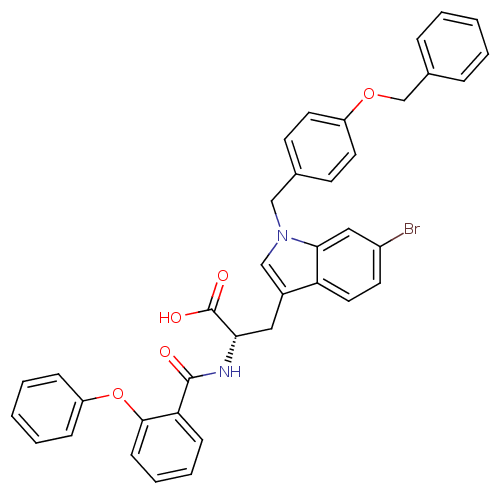 (2-phenoxybenzoyltryptophan derivative, R1C4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 600 | -35.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 23 |
Schering-Plough Research Institute | Assay Description Ki values were determined from a competition experiment in which serial dilutions of inhibitor were added to compete against a fixed concentration (1... | Anal Biochem 331: 138-46 (2004) Article DOI: 10.1016/j.ab.2004.03.009 BindingDB Entry DOI: 10.7270/Q2VX0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM31213 (2-phenoxybenzoyltryptophan derivative, R2C3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 600 | -35.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 23 |
Schering-Plough Research Institute | Assay Description Ki values were determined from a competition experiment in which serial dilutions of inhibitor were added to compete against a fixed concentration (1... | Anal Biochem 331: 138-46 (2004) Article DOI: 10.1016/j.ab.2004.03.009 BindingDB Entry DOI: 10.7270/Q2VX0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM31220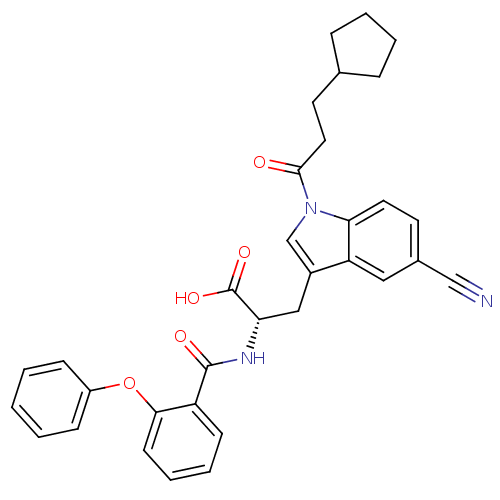 (2-phenoxybenzoyltryptophan derivative, R4C2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 600 | -35.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 23 |
Schering-Plough Research Institute | Assay Description Ki values were determined from a competition experiment in which serial dilutions of inhibitor were added to compete against a fixed concentration (1... | Anal Biochem 331: 138-46 (2004) Article DOI: 10.1016/j.ab.2004.03.009 BindingDB Entry DOI: 10.7270/Q2VX0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM31221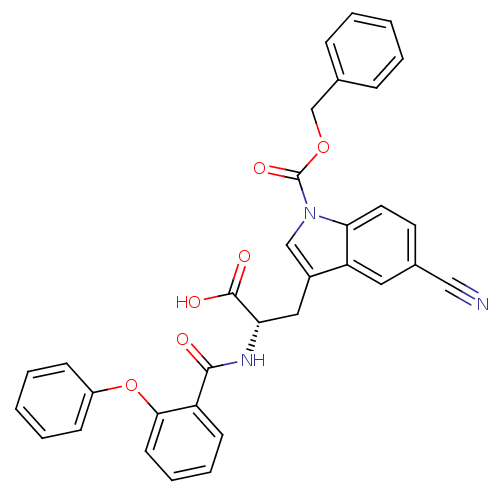 (2-phenoxybenzoyltryptophan derivative, R4C3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 600 | -35.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 23 |
Schering-Plough Research Institute | Assay Description Ki values were determined from a competition experiment in which serial dilutions of inhibitor were added to compete against a fixed concentration (1... | Anal Biochem 331: 138-46 (2004) Article DOI: 10.1016/j.ab.2004.03.009 BindingDB Entry DOI: 10.7270/Q2VX0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM31217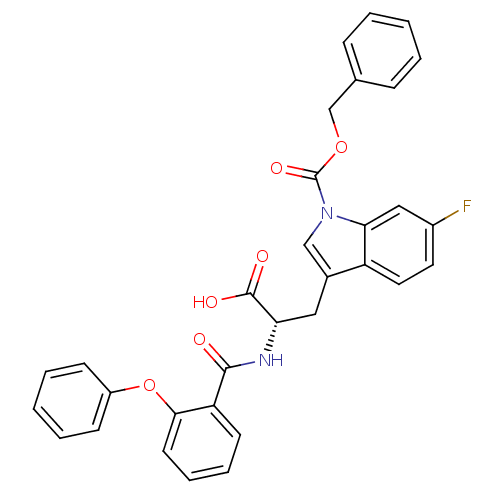 (2-phenoxybenzoyltryptophan derivative, R3C3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 800 | -34.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 23 |
Schering-Plough Research Institute | Assay Description Ki values were determined from a competition experiment in which serial dilutions of inhibitor were added to compete against a fixed concentration (1... | Anal Biochem 331: 138-46 (2004) Article DOI: 10.1016/j.ab.2004.03.009 BindingDB Entry DOI: 10.7270/Q2VX0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM31212 (2-phenoxybenzoyltryptophan derivative, R2C2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 800 | -34.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 23 |
Schering-Plough Research Institute | Assay Description Ki values were determined from a competition experiment in which serial dilutions of inhibitor were added to compete against a fixed concentration (1... | Anal Biochem 331: 138-46 (2004) Article DOI: 10.1016/j.ab.2004.03.009 BindingDB Entry DOI: 10.7270/Q2VX0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM31208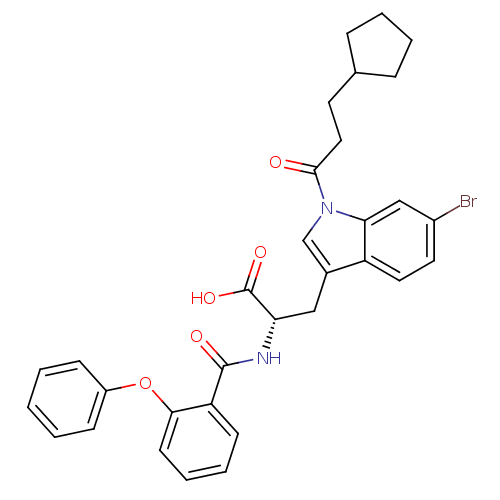 (2-phenoxybenzoyltryptophan derivative, R1C2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 800 | -34.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 23 |
Schering-Plough Research Institute | Assay Description Ki values were determined from a competition experiment in which serial dilutions of inhibitor were added to compete against a fixed concentration (1... | Anal Biochem 331: 138-46 (2004) Article DOI: 10.1016/j.ab.2004.03.009 BindingDB Entry DOI: 10.7270/Q2VX0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM31222 (2-phenoxybenzoyltryptophan derivative, R4C4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | -34.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 23 |
Schering-Plough Research Institute | Assay Description Ki values were determined from a competition experiment in which serial dilutions of inhibitor were added to compete against a fixed concentration (1... | Anal Biochem 331: 138-46 (2004) Article DOI: 10.1016/j.ab.2004.03.009 BindingDB Entry DOI: 10.7270/Q2VX0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM31214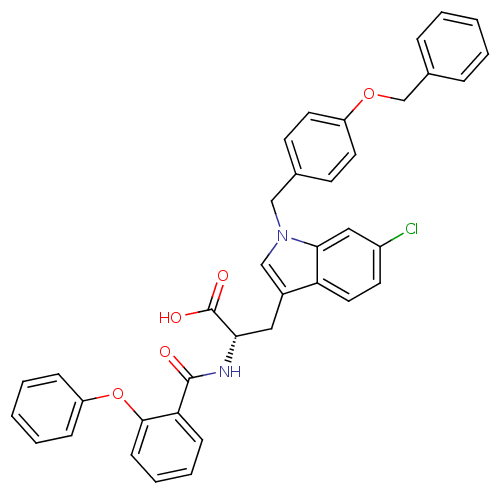 (2-phenoxybenzoyltryptophan derivative, R2C4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | -34.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 23 |
Schering-Plough Research Institute | Assay Description Ki values were determined from a competition experiment in which serial dilutions of inhibitor were added to compete against a fixed concentration (1... | Anal Biochem 331: 138-46 (2004) Article DOI: 10.1016/j.ab.2004.03.009 BindingDB Entry DOI: 10.7270/Q2VX0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM31218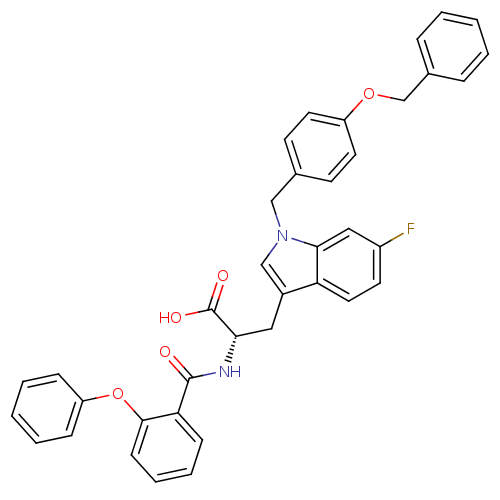 (2-phenoxybenzoyltryptophan derivative, R3C4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | -32.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 23 |
Schering-Plough Research Institute | Assay Description Ki values were determined from a competition experiment in which serial dilutions of inhibitor were added to compete against a fixed concentration (1... | Anal Biochem 331: 138-46 (2004) Article DOI: 10.1016/j.ab.2004.03.009 BindingDB Entry DOI: 10.7270/Q2VX0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM31207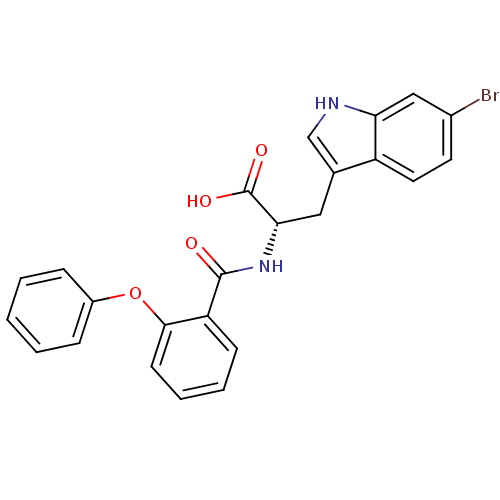 (2-phenoxybenzoyltryptophan derivative, R1C1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.00E+3 | -31.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 23 |
Schering-Plough Research Institute | Assay Description Ki values were determined from a competition experiment in which serial dilutions of inhibitor were added to compete against a fixed concentration (1... | Anal Biochem 331: 138-46 (2004) Article DOI: 10.1016/j.ab.2004.03.009 BindingDB Entry DOI: 10.7270/Q2VX0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM31211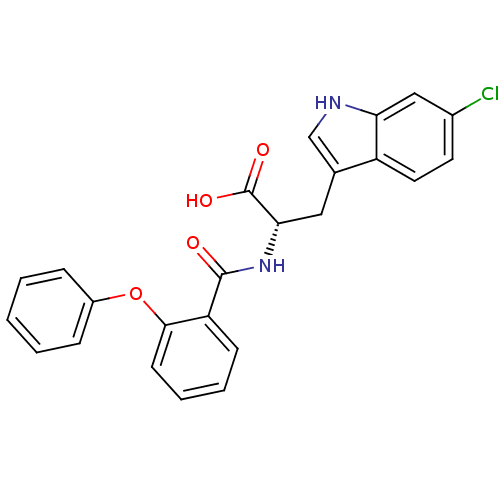 (2-phenoxybenzoyltryptophan derivative, R2C1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.00E+3 | -29.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 23 |
Schering-Plough Research Institute | Assay Description Ki values were determined from a competition experiment in which serial dilutions of inhibitor were added to compete against a fixed concentration (1... | Anal Biochem 331: 138-46 (2004) Article DOI: 10.1016/j.ab.2004.03.009 BindingDB Entry DOI: 10.7270/Q2VX0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM31223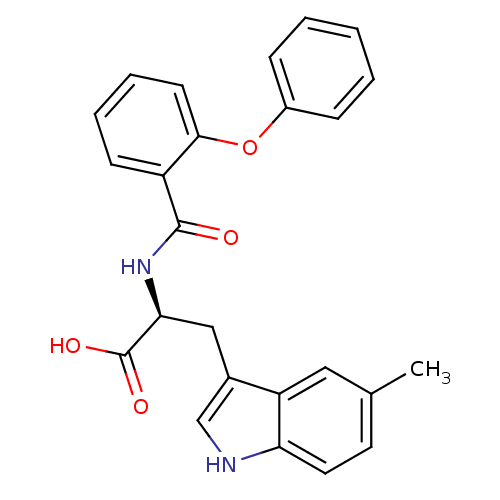 (2-phenoxybenzoyltryptophan derivative, R5C1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.00E+4 | -28.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 23 |
Schering-Plough Research Institute | Assay Description Ki values were determined from a competition experiment in which serial dilutions of inhibitor were added to compete against a fixed concentration (1... | Anal Biochem 331: 138-46 (2004) Article DOI: 10.1016/j.ab.2004.03.009 BindingDB Entry DOI: 10.7270/Q2VX0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM31219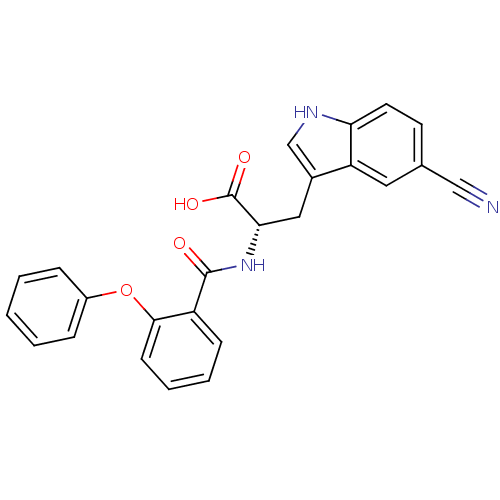 (2-phenoxybenzoyltryptophan derivative, R4C1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.00E+4 | -28.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 23 |
Schering-Plough Research Institute | Assay Description Ki values were determined from a competition experiment in which serial dilutions of inhibitor were added to compete against a fixed concentration (1... | Anal Biochem 331: 138-46 (2004) Article DOI: 10.1016/j.ab.2004.03.009 BindingDB Entry DOI: 10.7270/Q2VX0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM31215 (2-phenoxybenzoyltryptophan derivative, R3C1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.00E+4 | -26.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 23 |
Schering-Plough Research Institute | Assay Description Ki values were determined from a competition experiment in which serial dilutions of inhibitor were added to compete against a fixed concentration (1... | Anal Biochem 331: 138-46 (2004) Article DOI: 10.1016/j.ab.2004.03.009 BindingDB Entry DOI: 10.7270/Q2VX0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50063416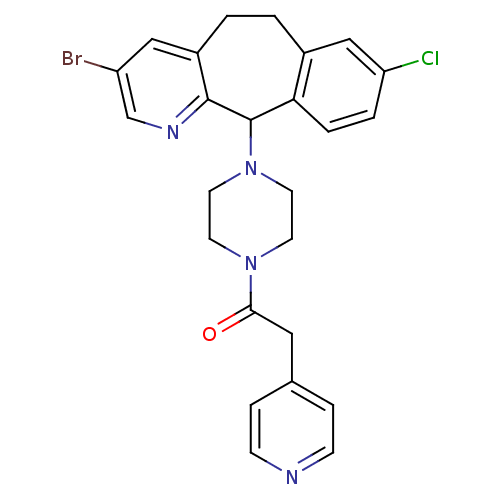 (1-[4-(3-Bromo-8-chloro-6,11-dihydro-5H-benzo[5,6]c...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of farnesyltransferase farnesylation of the H-ras oncogene | J Med Chem 41: 877-93 (1998) Article DOI: 10.1021/jm970462w BindingDB Entry DOI: 10.7270/Q2M61JDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50063372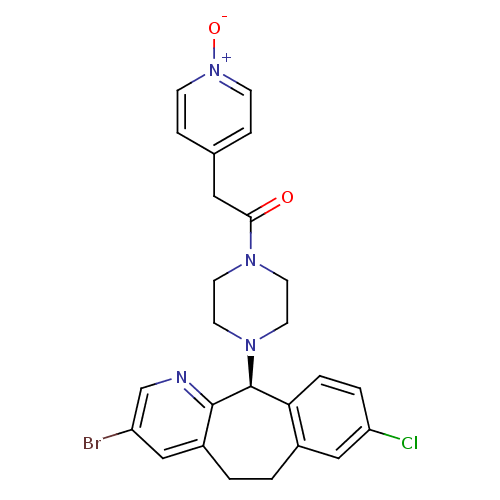 (1-[4-((S)-3-Bromo-8-chloro-6,11-dihydro-5H-benzo[5...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of farnesyltransferase farnesylation of the H-ras oncogene | J Med Chem 41: 877-93 (1998) Article DOI: 10.1021/jm970462w BindingDB Entry DOI: 10.7270/Q2M61JDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50063359 (1-[4-((R)-3-Bromo-8-chloro-6,11-dihydro-5H-benzo[5...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of farnesyltransferase farnesylation of the H-ras oncogene | J Med Chem 41: 877-93 (1998) Article DOI: 10.1021/jm970462w BindingDB Entry DOI: 10.7270/Q2M61JDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50063353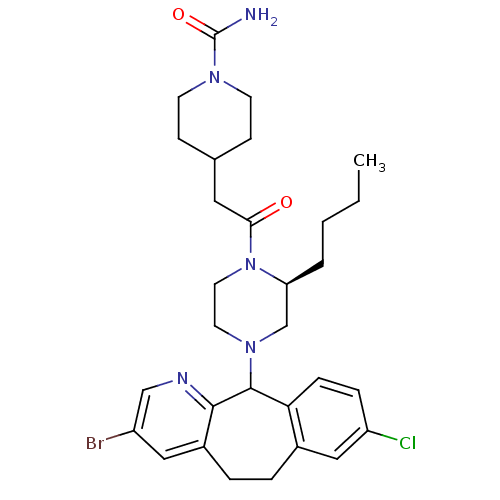 (4-{2-[(S)-4-(3-Bromo-8-chloro-6,11-dihydro-5H-benz...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of farnesyltransferase farnesylation of the H-ras oncogene | J Med Chem 41: 877-93 (1998) Article DOI: 10.1021/jm970462w BindingDB Entry DOI: 10.7270/Q2M61JDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50063455 (1-[4-(3-Bromo-8-chloro-11H-benzo[5,6]cyclohepta[1,...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of farnesyltransferase farnesylation of the H-ras oncogene | J Med Chem 41: 877-93 (1998) Article DOI: 10.1021/jm970462w BindingDB Entry DOI: 10.7270/Q2M61JDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50063402 (1-[(S)-4-(3-Bromo-8-chloro-6,11-dihydro-5H-benzo[5...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of farnesyltransferase farnesylation of the H-ras oncogene | J Med Chem 41: 877-93 (1998) Article DOI: 10.1021/jm970462w BindingDB Entry DOI: 10.7270/Q2M61JDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50061469 (1-[4-(8-CHLORO-3-METHYL-5,6-DIHYDRO-BENZO[5,6]CYCL...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Compound was tested for the inhibition of Farnesyltransferase | J Med Chem 40: 4290-301 (1998) Article DOI: 10.1021/jm970464g BindingDB Entry DOI: 10.7270/Q2NC60B2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50063358 (4-(3-Bromo-8-chloro-6,11-dihydro-5H-benzo[5,6]cycl...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of farnesyltransferase farnesylation of the H-ras oncogene | J Med Chem 41: 877-93 (1998) Article DOI: 10.1021/jm970462w BindingDB Entry DOI: 10.7270/Q2M61JDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50063412 (4-{2-[4-((R)-3-Bromo-8-chloro-6,11-dihydro-5H-benz...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of farnesyltransferase farnesylation of the H-ras oncogene | J Med Chem 41: 877-93 (1998) Article DOI: 10.1021/jm970462w BindingDB Entry DOI: 10.7270/Q2M61JDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50063384 (4-{2-[4-(3-Bromo-8-chloro-11H-benzo[5,6]cyclohepta...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of farnesyltransferase farnesylation of the H-ras oncogene | J Med Chem 41: 877-93 (1998) Article DOI: 10.1021/jm970462w BindingDB Entry DOI: 10.7270/Q2M61JDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50063366 (1-[4-(3-Bromo-8-chloro-6,11-dihydro-5H-benzo[5,6]c...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of farnesyltransferase farnesylation of the H-ras oncogene | J Med Chem 41: 877-93 (1998) Article DOI: 10.1021/jm970462w BindingDB Entry DOI: 10.7270/Q2M61JDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50063339 (4-{2-[4-((S)-3-Bromo-8-chloro-6,11-dihydro-5H-benz...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of farnesyltransferase farnesylation of the H-ras oncogene | J Med Chem 41: 877-93 (1998) Article DOI: 10.1021/jm970462w BindingDB Entry DOI: 10.7270/Q2M61JDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM14456 (4-N-carboxamidopiperidinylacetyl 2 | 4-[2-(4-{6-br...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of farnesyltransferase farnesylation of the H-ras oncogene | J Med Chem 41: 877-93 (1998) Article DOI: 10.1021/jm970462w BindingDB Entry DOI: 10.7270/Q2M61JDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50063415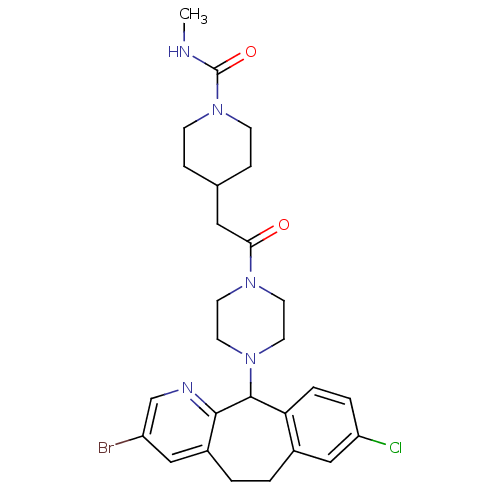 (4-{2-[4-(3-Bromo-8-chloro-6,11-dihydro-5H-benzo[5,...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of farnesyltransferase farnesylation of the H-ras oncogene | J Med Chem 41: 877-93 (1998) Article DOI: 10.1021/jm970462w BindingDB Entry DOI: 10.7270/Q2M61JDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50063379 (1-[4-((S)-3-Bromo-8-chloro-6,11-dihydro-5H-benzo[5...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of farnesyltransferase farnesylation of the H-ras oncogene | J Med Chem 41: 877-93 (1998) Article DOI: 10.1021/jm970462w BindingDB Entry DOI: 10.7270/Q2M61JDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50063457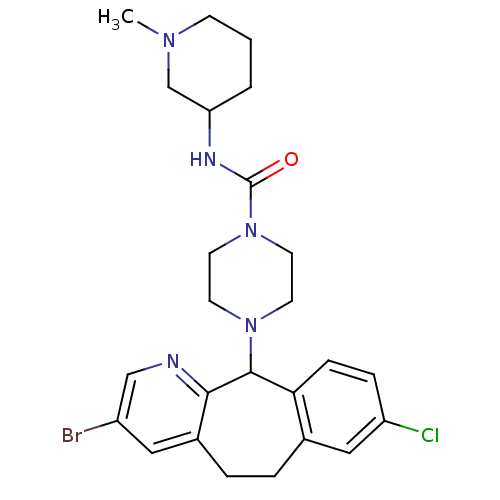 (4-(3-Bromo-8-chloro-6,11-dihydro-5H-benzo[5,6]cycl...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of farnesyltransferase farnesylation of the H-ras oncogene | J Med Chem 41: 877-93 (1998) Article DOI: 10.1021/jm970462w BindingDB Entry DOI: 10.7270/Q2M61JDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50063342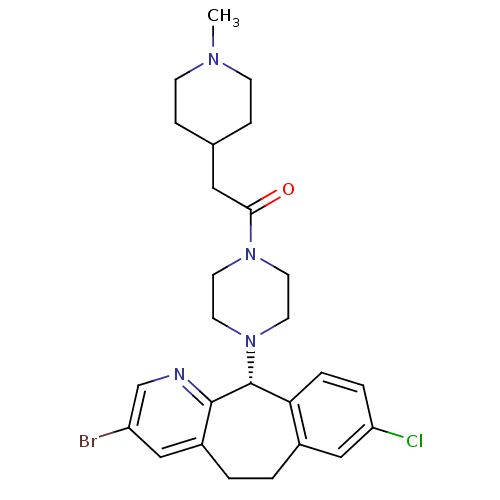 (1-[4-((R)-3-Bromo-8-chloro-6,11-dihydro-5H-benzo[5...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of farnesyltransferase farnesylation of the H-ras oncogene | J Med Chem 41: 877-93 (1998) Article DOI: 10.1021/jm970462w BindingDB Entry DOI: 10.7270/Q2M61JDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50063351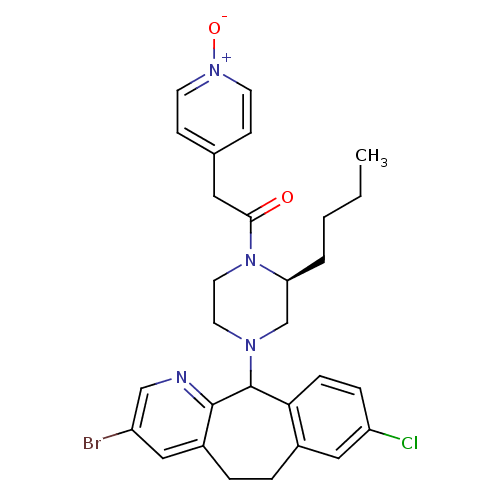 (1-[(S)-4-(3-Bromo-8-chloro-6,11-dihydro-5H-benzo[5...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of farnesyltransferase farnesylation of the H-ras oncogene | J Med Chem 41: 877-93 (1998) Article DOI: 10.1021/jm970462w BindingDB Entry DOI: 10.7270/Q2M61JDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50061819 (1-[4-(3-Bromo-8-chloro-5,6-dihydro-benzo[5,6]cyclo...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Compound was tested for the inhibition of Farnesyltransferase | J Med Chem 40: 4290-301 (1998) Article DOI: 10.1021/jm970464g BindingDB Entry DOI: 10.7270/Q2NC60B2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50061811 (1-[4-(8-Chloro-3-iodo-5,6-dihydro-benzo[5,6]cycloh...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Compound was tested for the inhibition of Farnesyltransferase | J Med Chem 40: 4290-301 (1998) Article DOI: 10.1021/jm970464g BindingDB Entry DOI: 10.7270/Q2NC60B2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50063433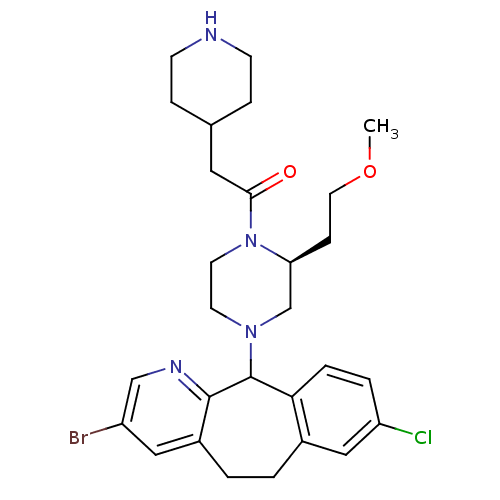 (1-[(S)-4-(3-Bromo-8-chloro-6,11-dihydro-5H-benzo[5...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of farnesyltransferase farnesylation of the H-ras oncogene | J Med Chem 41: 877-93 (1998) Article DOI: 10.1021/jm970462w BindingDB Entry DOI: 10.7270/Q2M61JDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50063373 (1-[4-(3-Bromo-8-chloro-6,11-dihydro-5H-benzo[5,6]c...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of farnesyltransferase farnesylation of the H-ras oncogene | J Med Chem 41: 877-93 (1998) Article DOI: 10.1021/jm970462w BindingDB Entry DOI: 10.7270/Q2M61JDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50061818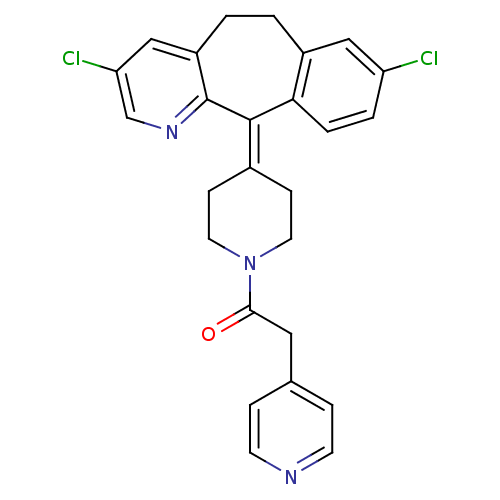 (1-[4-(3,8-Dichloro-5,6-dihydro-benzo[5,6]cyclohept...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Compound was tested for the inhibition of Farnesyltransferase | J Med Chem 40: 4290-301 (1998) Article DOI: 10.1021/jm970464g BindingDB Entry DOI: 10.7270/Q2NC60B2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50063410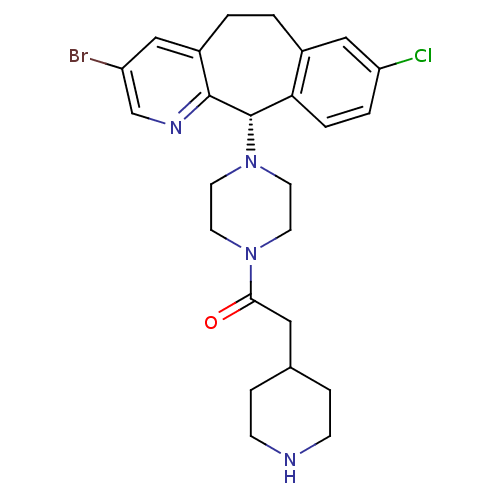 (1-[4-((S)-3-Bromo-8-chloro-6,11-dihydro-5H-benzo[5...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of farnesyltransferase farnesylation of the H-ras oncogene | J Med Chem 41: 877-93 (1998) Article DOI: 10.1021/jm970462w BindingDB Entry DOI: 10.7270/Q2M61JDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50063349 (1-[4-(3-Bromo-8-chloro-11H-benzo[5,6]cyclohepta[1,...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of farnesyltransferase farnesylation of the H-ras oncogene | J Med Chem 41: 877-93 (1998) Article DOI: 10.1021/jm970462w BindingDB Entry DOI: 10.7270/Q2M61JDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50063461 (1-[4-((R)-3-Bromo-8-chloro-6,11-dihydro-5H-benzo[5...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of farnesyltransferase farnesylation of the H-ras oncogene | J Med Chem 41: 877-93 (1998) Article DOI: 10.1021/jm970462w BindingDB Entry DOI: 10.7270/Q2M61JDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM14455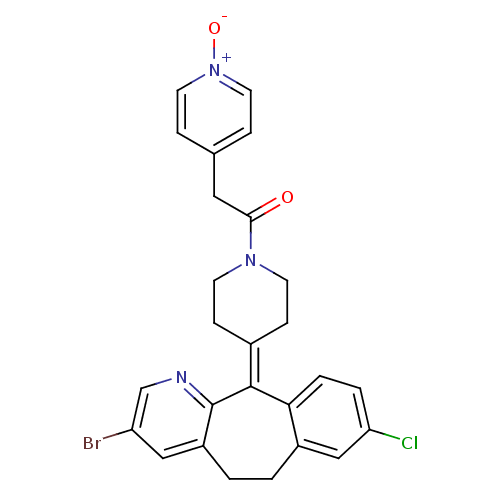 (3-bromo-4-pyridylacetyl N-oxide 1 | 4-[2-(4-{6-bro...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of Farnesyltransferase | J Med Chem 40: 4290-301 (1998) Article DOI: 10.1021/jm970464g BindingDB Entry DOI: 10.7270/Q2NC60B2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50063418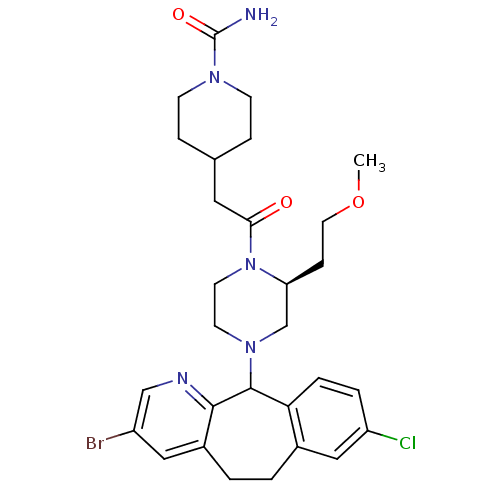 (4-{2-[(S)-4-(3-Bromo-8-chloro-6,11-dihydro-5H-benz...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of farnesyltransferase farnesylation of the H-ras oncogene | J Med Chem 41: 877-93 (1998) Article DOI: 10.1021/jm970462w BindingDB Entry DOI: 10.7270/Q2M61JDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50063362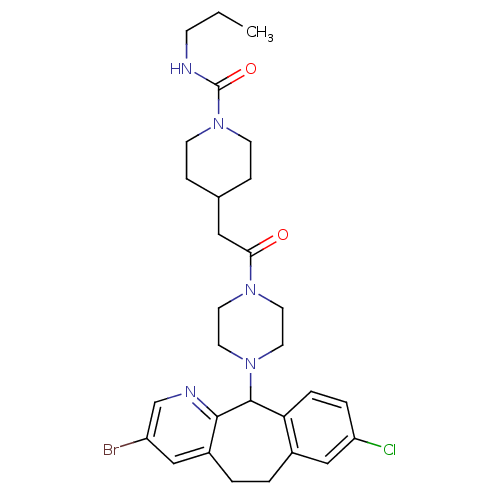 (4-{2-[4-(3-Bromo-8-chloro-6,11-dihydro-5H-benzo[5,...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of farnesyltransferase farnesylation of the H-ras oncogene | J Med Chem 41: 877-93 (1998) Article DOI: 10.1021/jm970462w BindingDB Entry DOI: 10.7270/Q2M61JDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50063368 (1-[4-(3-Bromo-8-chloro-6,11-dihydro-5H-benzo[5,6]c...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of Farnesyltransferase from Ras-CVLS or Ras-CVLL in Cos-7 monkey kidney cells | J Med Chem 41: 877-93 (1998) Article DOI: 10.1021/jm970462w BindingDB Entry DOI: 10.7270/Q2M61JDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 314 total ) | Next | Last >> |