

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50353674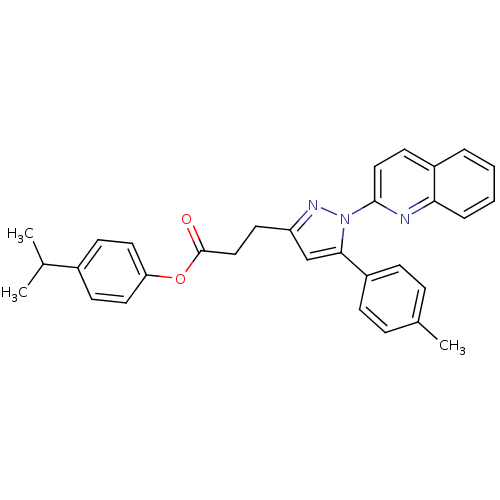 (CHEMBL1830480) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University Curated by ChEMBL | Assay Description Inhibition of COX1-mediated 12-HHT production in human platelet after 5 mins by HPLC analysis | Eur J Med Chem 46: 5021-33 (2011) Article DOI: 10.1016/j.ejmech.2011.08.009 BindingDB Entry DOI: 10.7270/Q2445MVS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50006805 (3-(1-(4-chlorobenzyl)-3-(tert-butylthio)-5-isoprop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University Curated by ChEMBL | Assay Description Inhibition of FLAP in A23187-stimulated human neutrophils assessed as 5-LO product formation preincubated for 15 mins measured after 10 mins | Bioorg Med Chem 20: 3728-41 (2012) Article DOI: 10.1016/j.bmc.2012.04.048 BindingDB Entry DOI: 10.7270/Q26H4JFM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM31134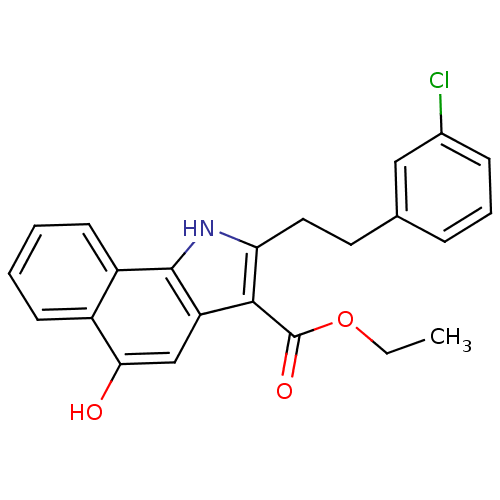 (5-hydroxy-1H-benzo[g]indole-3-carboxylate, 11m) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | 490 | n/a | n/a | n/a | n/a |
Friedrich Alexander University Erlangen | Assay Description For determination of the activity of 5-LO in 40000g supernatant (S40), aliquots of the supernatants were added to reaction mix, and were preincubated... | J Med Chem 52: 3474-83 (2009) Article DOI: 10.1021/jm900212y BindingDB Entry DOI: 10.7270/Q2HM56S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM22334 (BW A4C | BW4C | BWA4C | BWA4C, 10 | CHEMBL314360 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-Lipoxygenase expressed in Escherichia coli lysate after 10 mins by HPLC analysis | Eur J Med Chem 46: 5021-33 (2011) Article DOI: 10.1016/j.ejmech.2011.08.009 BindingDB Entry DOI: 10.7270/Q2445MVS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50353666 (CHEMBL1830471) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University Curated by ChEMBL | Assay Description Inhibition of COX1-mediated 12-HHT production in human platelet after 5 mins by HPLC analysis | Eur J Med Chem 46: 5021-33 (2011) Article DOI: 10.1016/j.ejmech.2011.08.009 BindingDB Entry DOI: 10.7270/Q2445MVS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM31132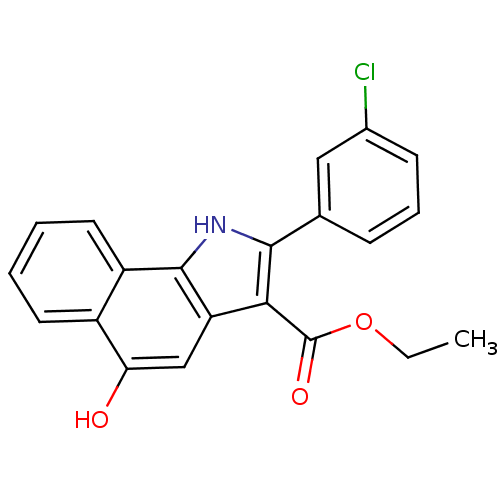 (5-hydroxy-1H-benzo[g]indole-3-carboxylate, 11k) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | 520 | n/a | n/a | 7.4 | 37 |
Friedrich Alexander University Erlangen | Assay Description For determination of the activity of 5-LO in 40000g supernatant (S40), aliquots of the supernatants were added to reaction mix, and were preincubated... | J Med Chem 52: 3474-83 (2009) Article DOI: 10.1021/jm900212y BindingDB Entry DOI: 10.7270/Q2HM56S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM31135 (5-hydroxy-1H-benzo[g]indole-3-carboxylate, 11n) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 49 | n/a | 2.80E+3 | n/a | n/a | n/a | n/a |
Friedrich Alexander University Erlangen | Assay Description For determination of the activity of 5-LO in 40000g supernatant (S40), aliquots of the supernatants were added to reaction mix, and were preincubated... | J Med Chem 52: 3474-83 (2009) Article DOI: 10.1021/jm900212y BindingDB Entry DOI: 10.7270/Q2HM56S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM22334 (BW A4C | BW4C | BWA4C | BWA4C, 10 | CHEMBL314360 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University Curated by ChEMBL | Assay Description Inhibition of 5-Lipoxygenase in arachidonic acid-stimulated human neutrophils after 15 mins by HPLC analysis in presence of A23187 | Eur J Med Chem 46: 5021-33 (2011) Article DOI: 10.1016/j.ejmech.2011.08.009 BindingDB Entry DOI: 10.7270/Q2445MVS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM31133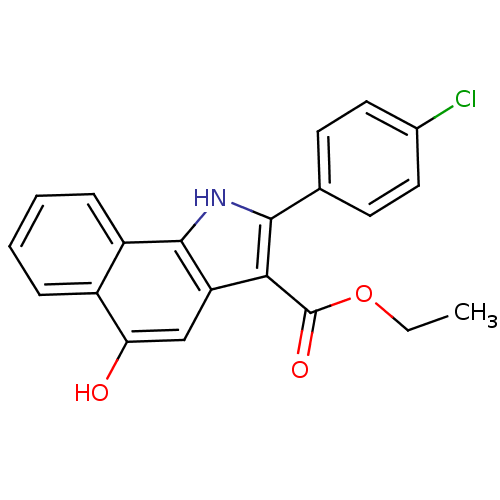 (5-hydroxy-1H-benzo[g]indole-3-carboxylate, 11l) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 67 | n/a | 320 | n/a | n/a | n/a | n/a |
Friedrich Alexander University Erlangen | Assay Description For determination of the activity of 5-LO in 40000g supernatant (S40), aliquots of the supernatants were added to reaction mix, and were preincubated... | J Med Chem 52: 3474-83 (2009) Article DOI: 10.1021/jm900212y BindingDB Entry DOI: 10.7270/Q2HM56S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University Curated by ChEMBL | Assay Description Inhibition of COX1-mediated 12-HHT production in human platelet after 5 mins by HPLC analysis | Eur J Med Chem 46: 5021-33 (2011) Article DOI: 10.1016/j.ejmech.2011.08.009 BindingDB Entry DOI: 10.7270/Q2445MVS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM31124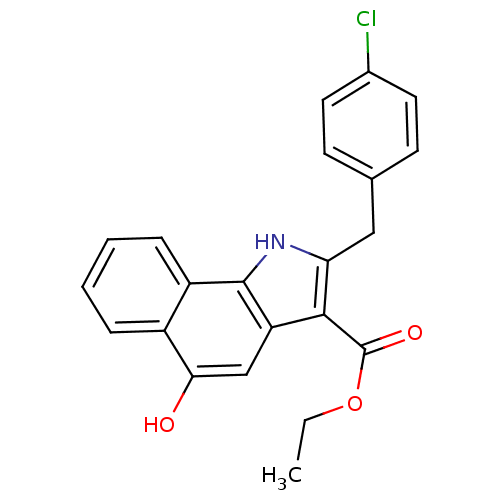 (5-hydroxy-1H-benzo[g]indole-3-carboxylate, 11c) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 84 | n/a | 1.20E+3 | n/a | n/a | 7.4 | 37 |
Friedrich Alexander University Erlangen | Assay Description For determination of the activity of 5-LO in 40000g supernatant (S40), aliquots of the supernatants were added to reaction mix, and were preincubated... | J Med Chem 52: 3474-83 (2009) Article DOI: 10.1021/jm900212y BindingDB Entry DOI: 10.7270/Q2HM56S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM31122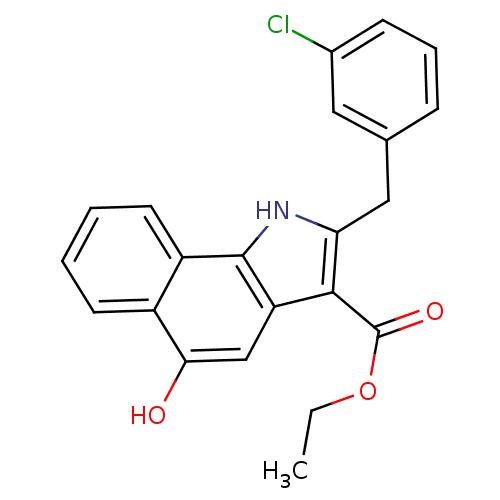 (5-hydroxy-1H-benzo[g]indole-3-carboxylate, 11a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 86 | n/a | 230 | n/a | n/a | 7.4 | 37 |
Friedrich Alexander University Erlangen | Assay Description For determination of the activity of 5-LO in 40000g supernatant (S40), aliquots of the supernatants were added to reaction mix, and were preincubated... | J Med Chem 52: 3474-83 (2009) Article DOI: 10.1021/jm900212y BindingDB Entry DOI: 10.7270/Q2HM56S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM31128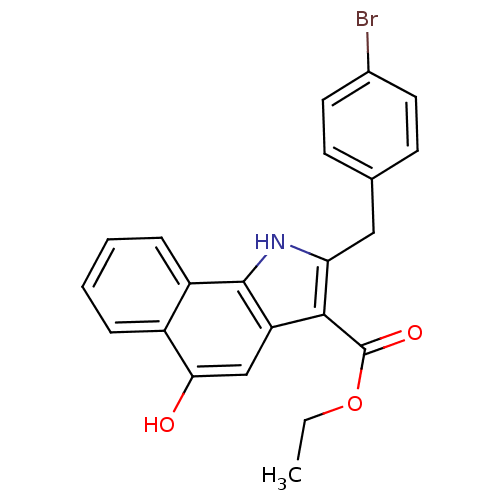 (5-hydroxy-1H-benzo[g]indole-3-carboxylate, 11g) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 95 | n/a | 600 | n/a | n/a | 7.4 | 37 |
Friedrich Alexander University Erlangen | Assay Description For determination of the activity of 5-LO in 40000g supernatant (S40), aliquots of the supernatants were added to reaction mix, and were preincubated... | J Med Chem 52: 3474-83 (2009) Article DOI: 10.1021/jm900212y BindingDB Entry DOI: 10.7270/Q2HM56S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM31126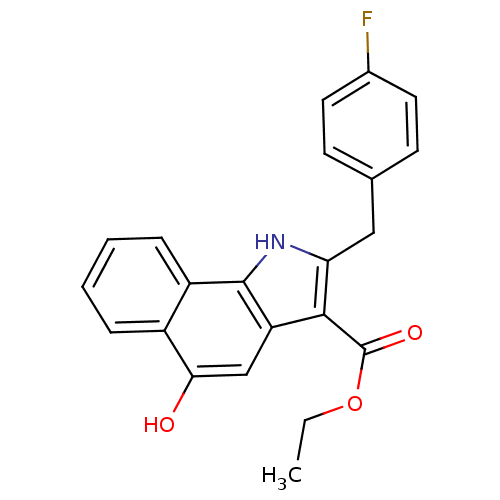 (5-hydroxy-1H-benzo[g]indole-3-carboxylate, 11e) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 96 | n/a | 500 | n/a | n/a | 7.4 | 37 |
Friedrich Alexander University Erlangen | Assay Description For determination of the activity of 5-LO in 40000g supernatant (S40), aliquots of the supernatants were added to reaction mix, and were preincubated... | J Med Chem 52: 3474-83 (2009) Article DOI: 10.1021/jm900212y BindingDB Entry DOI: 10.7270/Q2HM56S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM31123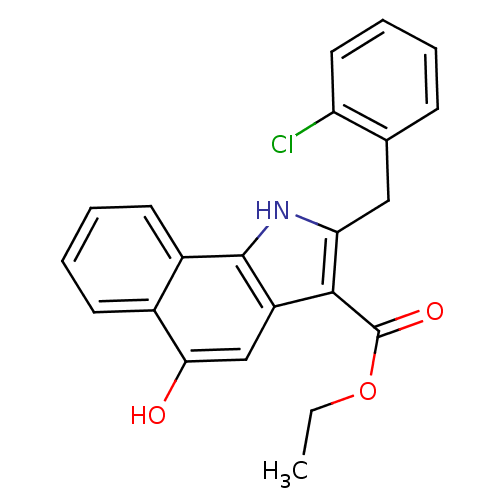 (5-hydroxy-1H-benzo[g]indole-3-carboxylate, 11b) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 97 | n/a | 1.20E+3 | n/a | n/a | 7.4 | 37 |
Friedrich Alexander University Erlangen | Assay Description For determination of the activity of 5-LO in 40000g supernatant (S40), aliquots of the supernatants were added to reaction mix, and were preincubated... | J Med Chem 52: 3474-83 (2009) Article DOI: 10.1021/jm900212y BindingDB Entry DOI: 10.7270/Q2HM56S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM22334 (BW A4C | BW4C | BWA4C | BWA4C, 10 | CHEMBL314360 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University Curated by ChEMBL | Assay Description Inhibition of FLAP in A23187-stimulated human neutrophils assessed as 5-LO product formation preincubated for 15 mins measured after 10 mins | Bioorg Med Chem 20: 3728-41 (2012) Article DOI: 10.1016/j.bmc.2012.04.048 BindingDB Entry DOI: 10.7270/Q26H4JFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50385376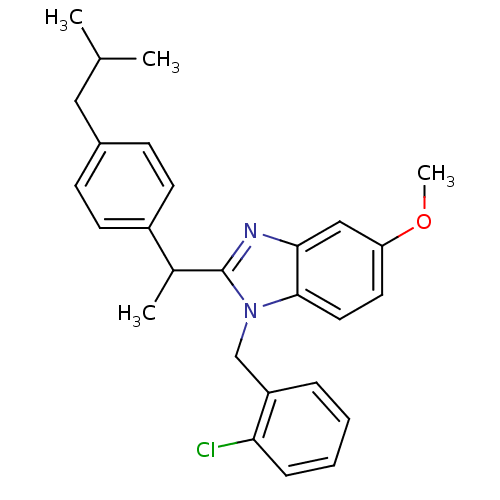 (CHEMBL2036377) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University Curated by ChEMBL | Assay Description Inhibition of FLAP in A23187-stimulated human neutrophils assessed as 5-LO product formation preincubated for 15 mins measured after 10 mins | Bioorg Med Chem 20: 3728-41 (2012) Article DOI: 10.1016/j.bmc.2012.04.048 BindingDB Entry DOI: 10.7270/Q26H4JFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM31138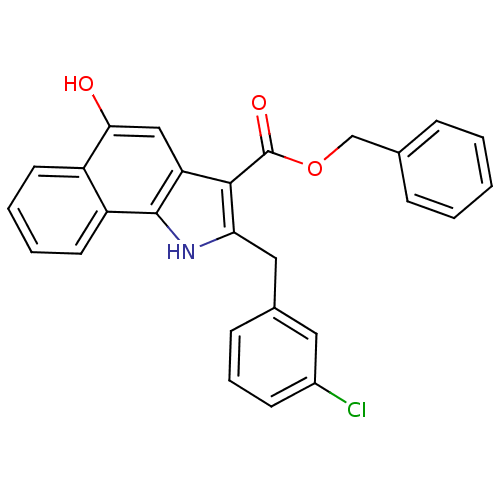 (5-hydroxy-1H-benzo[g]indole-3-carboxylate, 11q) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | 350 | n/a | n/a | n/a | n/a |
Friedrich Alexander University Erlangen | Assay Description For determination of the activity of 5-LO in 40000g supernatant (S40), aliquots of the supernatants were added to reaction mix, and were preincubated... | J Med Chem 52: 3474-83 (2009) Article DOI: 10.1021/jm900212y BindingDB Entry DOI: 10.7270/Q2HM56S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM31136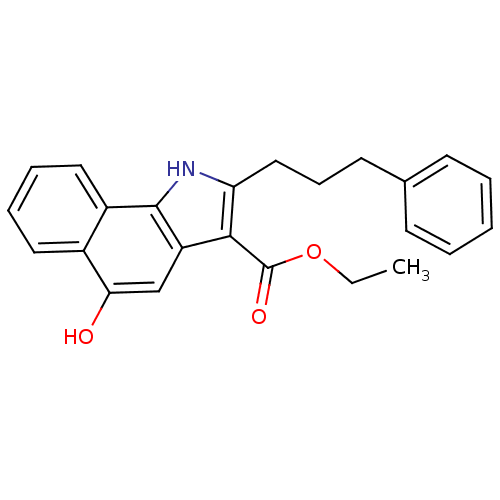 (5-hydroxy-1H-benzo[g]indole-3-carboxylate, 11o) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | 440 | n/a | n/a | n/a | n/a |
Friedrich Alexander University Erlangen | Assay Description For determination of the activity of 5-LO in 40000g supernatant (S40), aliquots of the supernatants were added to reaction mix, and were preincubated... | J Med Chem 52: 3474-83 (2009) Article DOI: 10.1021/jm900212y BindingDB Entry DOI: 10.7270/Q2HM56S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM31129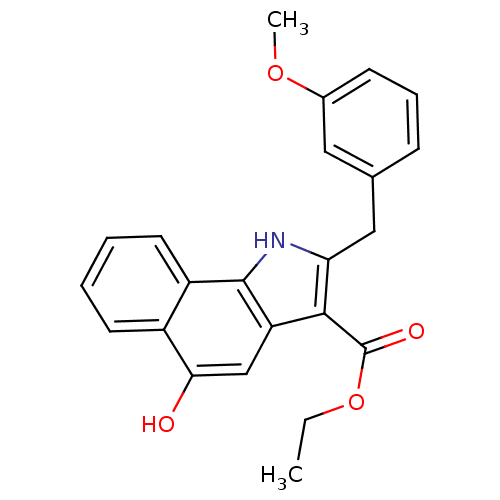 (5-hydroxy-1H-benzo[g]indole-3-carboxylate, 11h) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | 520 | n/a | n/a | 7.4 | 37 |
Friedrich Alexander University Erlangen | Assay Description For determination of the activity of 5-LO in 40000g supernatant (S40), aliquots of the supernatants were added to reaction mix, and were preincubated... | J Med Chem 52: 3474-83 (2009) Article DOI: 10.1021/jm900212y BindingDB Entry DOI: 10.7270/Q2HM56S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM31125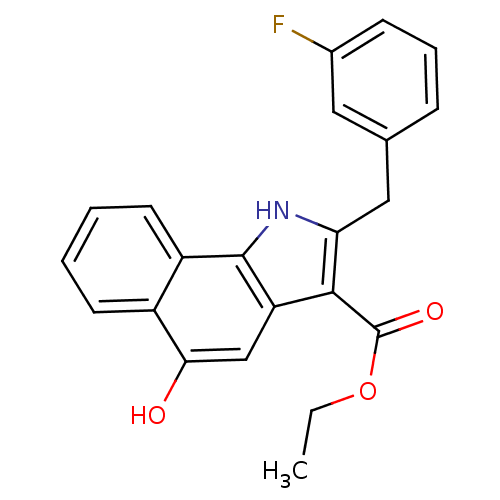 (5-hydroxy-1H-benzo[g]indole-3-carboxylate, 11d) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | 340 | n/a | n/a | 7.4 | 37 |
Friedrich Alexander University Erlangen | Assay Description For determination of the activity of 5-LO in 40000g supernatant (S40), aliquots of the supernatants were added to reaction mix, and were preincubated... | J Med Chem 52: 3474-83 (2009) Article DOI: 10.1021/jm900212y BindingDB Entry DOI: 10.7270/Q2HM56S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM31127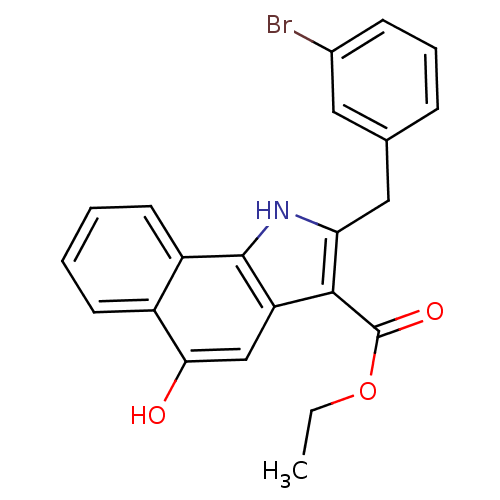 (5-hydroxy-1H-benzo[g]indole-3-carboxylate, 11f) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | 450 | n/a | n/a | 7.4 | 37 |
Friedrich Alexander University Erlangen | Assay Description For determination of the activity of 5-LO in 40000g supernatant (S40), aliquots of the supernatants were added to reaction mix, and were preincubated... | J Med Chem 52: 3474-83 (2009) Article DOI: 10.1021/jm900212y BindingDB Entry DOI: 10.7270/Q2HM56S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM31130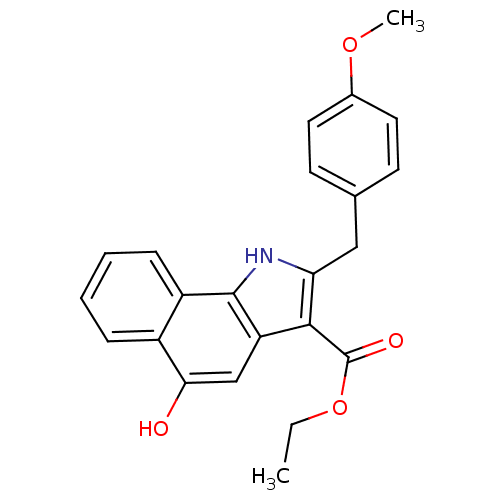 (5-hydroxy-1H-benzo[g]indole-3-carboxylate, 11i) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | 650 | n/a | n/a | 7.4 | 37 |
Friedrich Alexander University Erlangen | Assay Description For determination of the activity of 5-LO in 40000g supernatant (S40), aliquots of the supernatants were added to reaction mix, and were preincubated... | J Med Chem 52: 3474-83 (2009) Article DOI: 10.1021/jm900212y BindingDB Entry DOI: 10.7270/Q2HM56S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50397228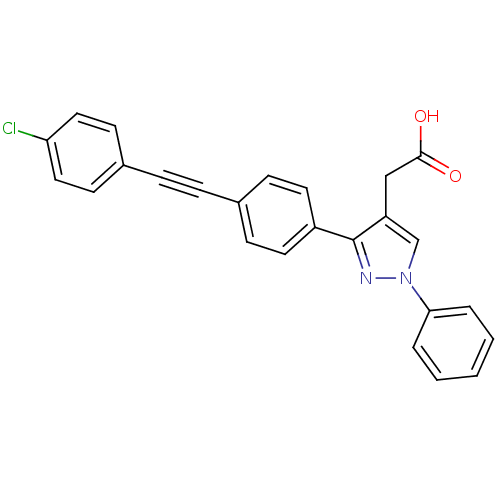 (CHEMBL2172775) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as inhibition of PGE2 formation from PGH2 after 15 mins by RP-HPLC a... | J Med Chem 55: 8958-62 (2012) Article DOI: 10.1021/jm3010543 BindingDB Entry DOI: 10.7270/Q20Z74FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50385380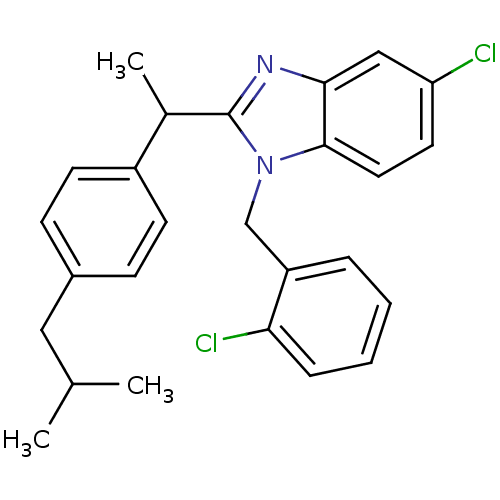 (CHEMBL2036382) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University Curated by ChEMBL | Assay Description Inhibition of FLAP in A23187-stimulated human neutrophils assessed as 5-LO product formation preincubated for 15 mins measured after 10 mins | Bioorg Med Chem 20: 3728-41 (2012) Article DOI: 10.1016/j.bmc.2012.04.048 BindingDB Entry DOI: 10.7270/Q26H4JFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM22334 (BW A4C | BW4C | BWA4C | BWA4C, 10 | CHEMBL314360 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in A23187-stimulated human neutrophils assessed as inhibition of enzyme product formation by RP-HPLC analysis | J Med Chem 55: 8958-62 (2012) Article DOI: 10.1021/jm3010543 BindingDB Entry DOI: 10.7270/Q20Z74FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM31139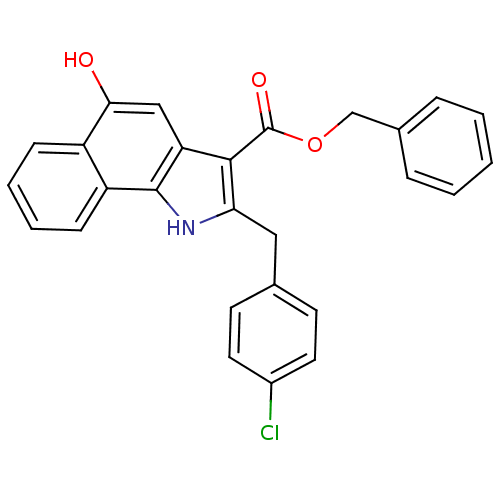 (5-hydroxy-1H-benzo[g]indole-3-carboxylate, 11r) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | 480 | n/a | n/a | n/a | n/a |
Friedrich Alexander University Erlangen | Assay Description For determination of the activity of 5-LO in 40000g supernatant (S40), aliquots of the supernatants were added to reaction mix, and were preincubated... | J Med Chem 52: 3474-83 (2009) Article DOI: 10.1021/jm900212y BindingDB Entry DOI: 10.7270/Q2HM56S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50397227 (CHEMBL2172776) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as inhibition of PGE2 formation from PGH2 after 15 mins by RP-HPLC a... | J Med Chem 55: 8958-62 (2012) Article DOI: 10.1021/jm3010543 BindingDB Entry DOI: 10.7270/Q20Z74FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50385378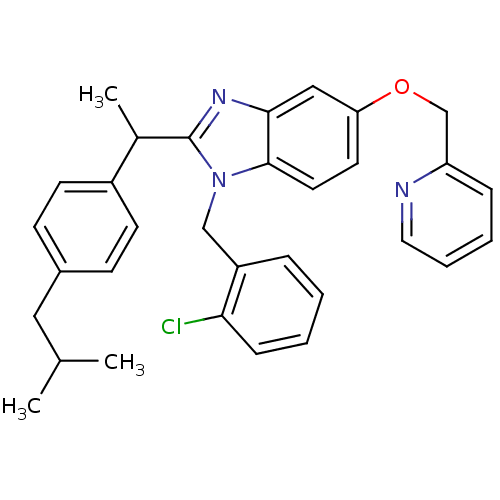 (CHEMBL2036379) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University Curated by ChEMBL | Assay Description Inhibition of FLAP in A23187-stimulated human neutrophils assessed as 5-LO product formation preincubated for 15 mins measured after 10 mins | Bioorg Med Chem 20: 3728-41 (2012) Article DOI: 10.1016/j.bmc.2012.04.048 BindingDB Entry DOI: 10.7270/Q26H4JFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50385377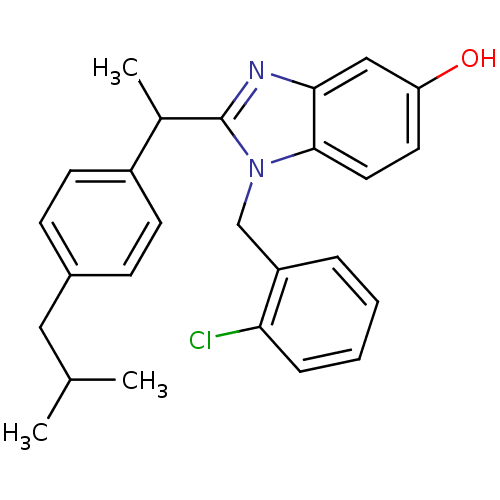 (CHEMBL2036378) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University Curated by ChEMBL | Assay Description Inhibition of FLAP in A23187-stimulated human neutrophils assessed as 5-LO product formation preincubated for 15 mins measured after 10 mins | Bioorg Med Chem 20: 3728-41 (2012) Article DOI: 10.1016/j.bmc.2012.04.048 BindingDB Entry DOI: 10.7270/Q26H4JFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50397226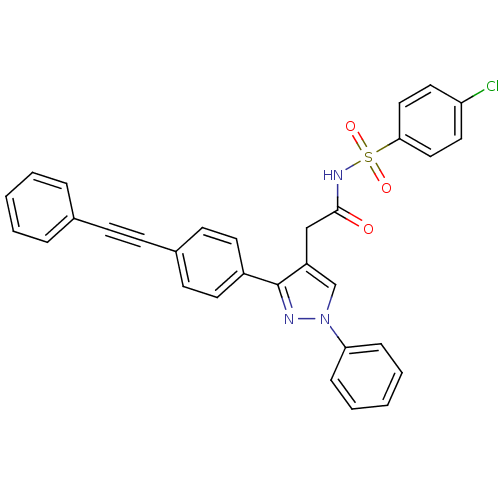 (CHEMBL2172777) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as inhibition of PGE2 formation from PGH2 after 15 mins by RP-HPLC a... | J Med Chem 55: 8958-62 (2012) Article DOI: 10.1021/jm3010543 BindingDB Entry DOI: 10.7270/Q20Z74FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM22334 (BW A4C | BW4C | BWA4C | BWA4C, 10 | CHEMBL314360 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of 5-LO (unknown origin) | Eur J Med Chem 67: 269-79 (2013) Article DOI: 10.1016/j.ejmech.2013.06.039 BindingDB Entry DOI: 10.7270/Q2XW4M9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM31121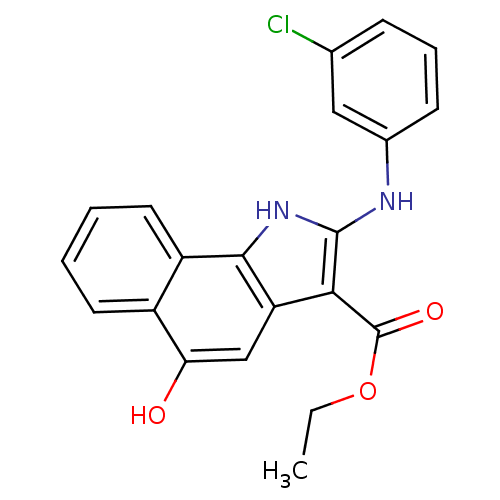 (5-hydroxy-1H-benzo[g]indole-3-carboxylate, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | 710 | n/a | n/a | 7.4 | 37 |
Friedrich Alexander University Erlangen | Assay Description For determination of the activity of 5-LO in 40000g supernatant (S40), aliquots of the supernatants were added to reaction mix, and were preincubated... | J Med Chem 52: 3474-83 (2009) Article DOI: 10.1021/jm900212y BindingDB Entry DOI: 10.7270/Q2HM56S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50385355 (CHEMBL2036180) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University Curated by ChEMBL | Assay Description Inhibition of FLAP in A23187-stimulated human neutrophils assessed as 5-LO product formation preincubated for 15 mins measured after 10 mins | Bioorg Med Chem 20: 3728-41 (2012) Article DOI: 10.1016/j.bmc.2012.04.048 BindingDB Entry DOI: 10.7270/Q26H4JFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50385357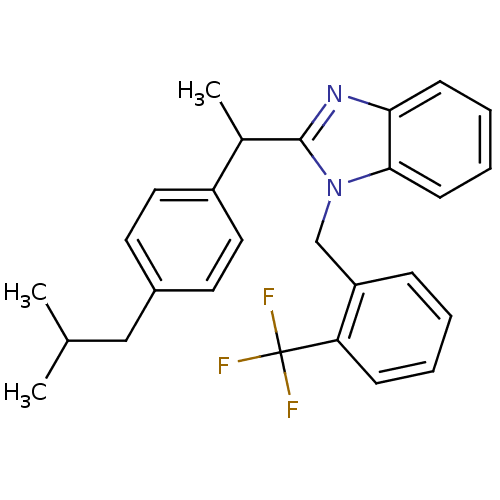 (CHEMBL2036182) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University Curated by ChEMBL | Assay Description Inhibition of FLAP in A23187-stimulated human neutrophils assessed as 5-LO product formation preincubated for 15 mins measured after 10 mins | Bioorg Med Chem 20: 3728-41 (2012) Article DOI: 10.1016/j.bmc.2012.04.048 BindingDB Entry DOI: 10.7270/Q26H4JFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM31131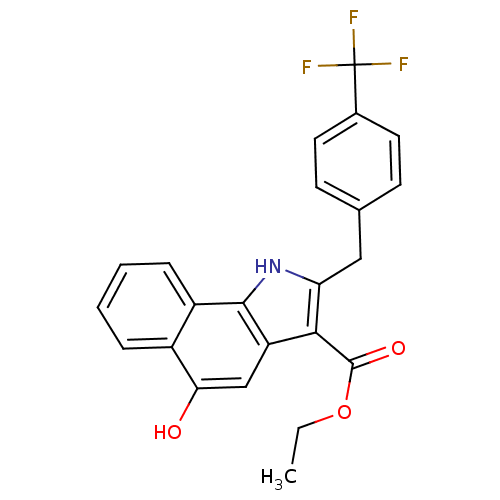 (5-hydroxy-1H-benzo[g]indole-3-carboxylate, 11j) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | 1.70E+3 | n/a | n/a | 7.4 | 37 |
Friedrich Alexander University Erlangen | Assay Description For determination of the activity of 5-LO in 40000g supernatant (S40), aliquots of the supernatants were added to reaction mix, and were preincubated... | J Med Chem 52: 3474-83 (2009) Article DOI: 10.1021/jm900212y BindingDB Entry DOI: 10.7270/Q2HM56S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50446419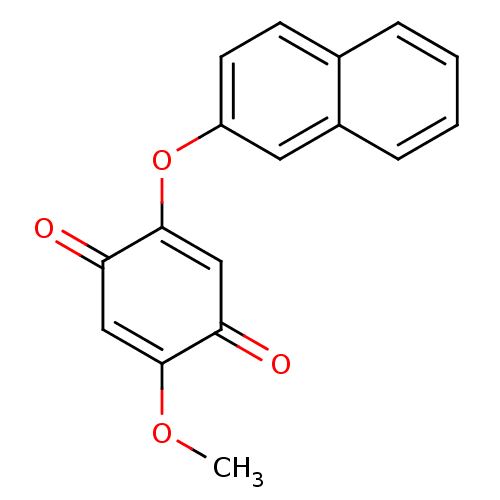 (CHEMBL3109719) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LO expressed in Escherichia coli BL21 using arachidonic acid as substrate after 10 mins | Eur J Med Chem 67: 269-79 (2013) Article DOI: 10.1016/j.ejmech.2013.06.039 BindingDB Entry DOI: 10.7270/Q2XW4M9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM31103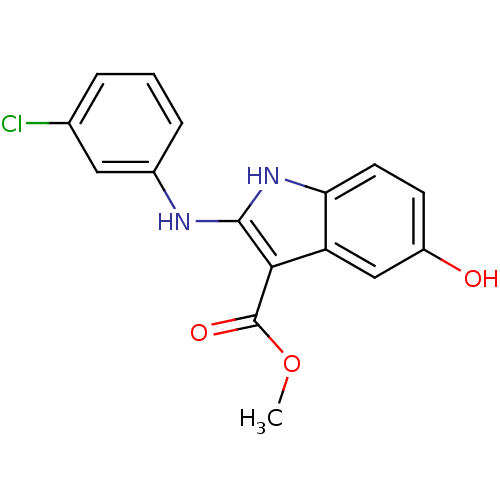 (5-hydroxy-1H-indole-3-carboxylate, 37) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | 2.40E+3 | n/a | n/a | 7.4 | 37 |
Friedrich Alexander University Erlangen | Assay Description For determination of the activity of 5-LO in 40000g supernatant (S40), aliquots of the supernatants were added to reaction mix, and were preincubated... | J Med Chem 52: 3474-83 (2009) Article DOI: 10.1021/jm900212y BindingDB Entry DOI: 10.7270/Q2HM56S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM22334 (BW A4C | BW4C | BWA4C | BWA4C, 10 | CHEMBL314360 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of 5-LO (unknown origin) by cell based assay | Eur J Med Chem 67: 269-79 (2013) Article DOI: 10.1016/j.ejmech.2013.06.039 BindingDB Entry DOI: 10.7270/Q2XW4M9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50353672 (CHEMBL1830478) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University Curated by ChEMBL | Assay Description Inhibition of COX1-mediated 12-HHT production in human platelet after 5 mins by HPLC analysis | Eur J Med Chem 46: 5021-33 (2011) Article DOI: 10.1016/j.ejmech.2011.08.009 BindingDB Entry DOI: 10.7270/Q2445MVS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50385390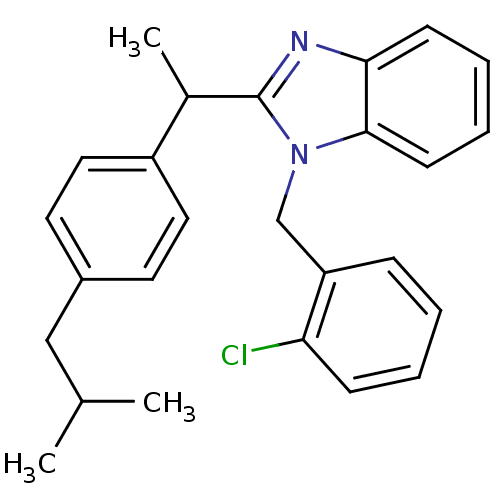 (CHEMBL2036163) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University Curated by ChEMBL | Assay Description Inhibition of FLAP in A23187-stimulated human neutrophils assessed as 5-LO product formation preincubated for 15 mins measured after 10 mins | Bioorg Med Chem 20: 3728-41 (2012) Article DOI: 10.1016/j.bmc.2012.04.048 BindingDB Entry DOI: 10.7270/Q26H4JFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50446413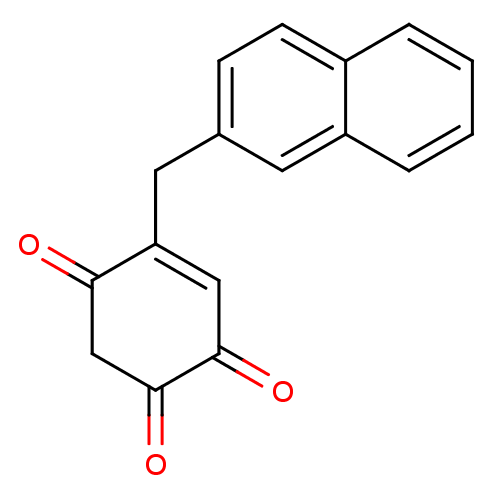 (CHEMBL1668217) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant 5-LO expressed in Escherichia coli BL21 using 40 uM of arachidonic acid as substrate preincubated for 15 ... | Eur J Med Chem 67: 269-79 (2013) Article DOI: 10.1016/j.ejmech.2013.06.039 BindingDB Entry DOI: 10.7270/Q2XW4M9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM31143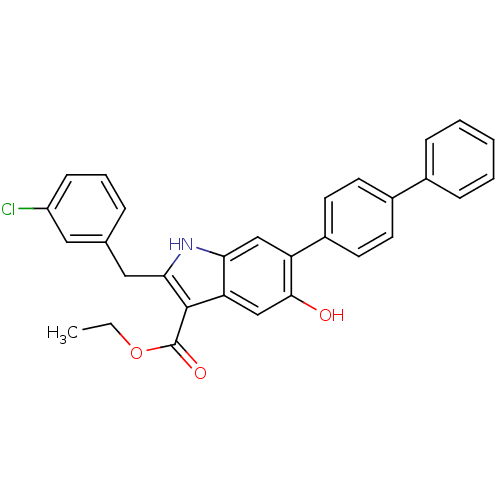 (5-hydroxy-1H-indole-3-carboxylate, 22) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 330 | n/a | 2.80E+3 | n/a | n/a | n/a | n/a |
Friedrich Alexander University Erlangen | Assay Description For determination of the activity of 5-LO in 40000g supernatant (S40), aliquots of the supernatants were added to reaction mix, and were preincubated... | J Med Chem 52: 3474-83 (2009) Article DOI: 10.1021/jm900212y BindingDB Entry DOI: 10.7270/Q2HM56S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50397225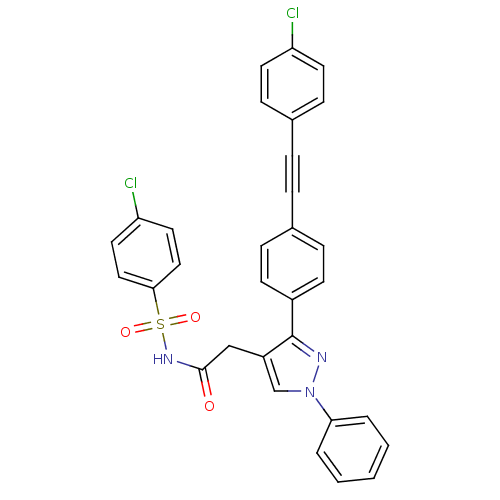 (CHEMBL2172778) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as inhibition of PGE2 formation from PGH2 after 15 mins by RP-HPLC a... | J Med Chem 55: 8958-62 (2012) Article DOI: 10.1021/jm3010543 BindingDB Entry DOI: 10.7270/Q20Z74FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50397229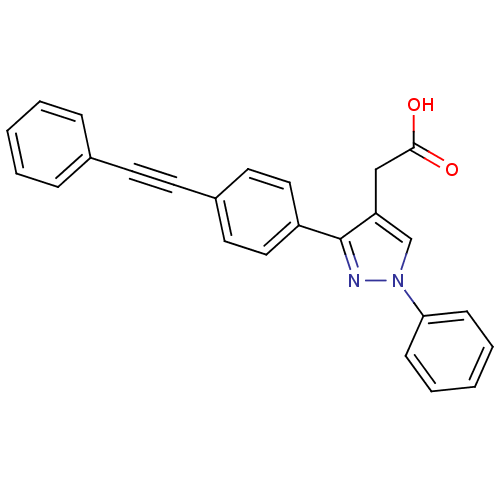 (CHEMBL2172774) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as inhibition of PGE2 formation from PGH2 after 15 mins by RP-HPLC a... | J Med Chem 55: 8958-62 (2012) Article DOI: 10.1021/jm3010543 BindingDB Entry DOI: 10.7270/Q20Z74FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50385356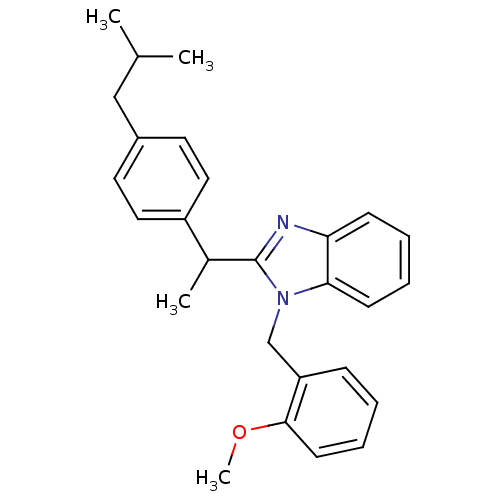 (CHEMBL2036181) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University Curated by ChEMBL | Assay Description Inhibition of FLAP in A23187-stimulated human neutrophils assessed as 5-LO product formation preincubated for 15 mins measured after 10 mins | Bioorg Med Chem 20: 3728-41 (2012) Article DOI: 10.1016/j.bmc.2012.04.048 BindingDB Entry DOI: 10.7270/Q26H4JFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50385353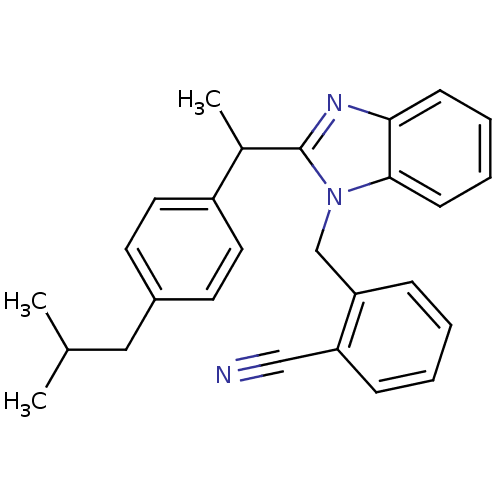 (CHEMBL2036178) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University Curated by ChEMBL | Assay Description Inhibition of FLAP in A23187-stimulated human neutrophils assessed as 5-LO product formation preincubated for 15 mins measured after 10 mins | Bioorg Med Chem 20: 3728-41 (2012) Article DOI: 10.1016/j.bmc.2012.04.048 BindingDB Entry DOI: 10.7270/Q26H4JFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50385372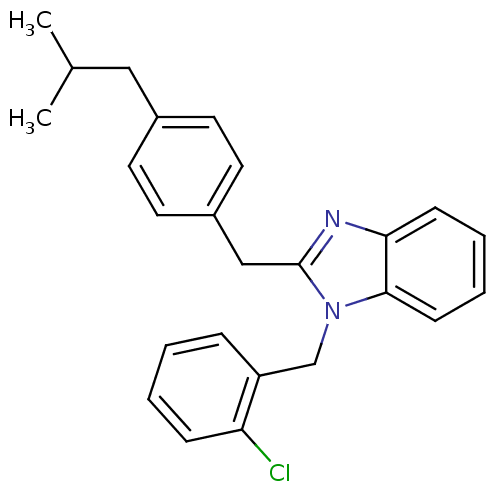 (CHEMBL2036372) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University Curated by ChEMBL | Assay Description Inhibition of FLAP in A23187-stimulated human neutrophils assessed as 5-LO product formation preincubated for 15 mins measured after 10 mins | Bioorg Med Chem 20: 3728-41 (2012) Article DOI: 10.1016/j.bmc.2012.04.048 BindingDB Entry DOI: 10.7270/Q26H4JFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50385351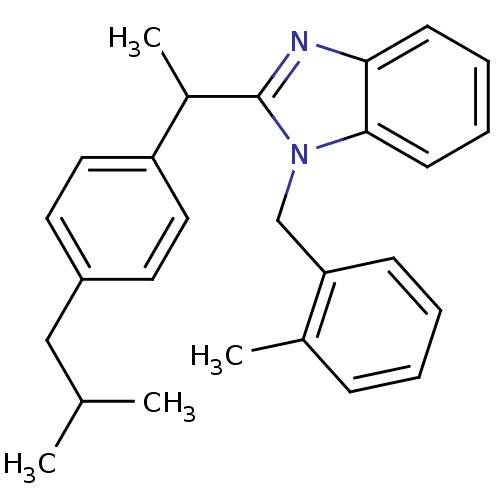 (CHEMBL2036176) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University Curated by ChEMBL | Assay Description Inhibition of FLAP in A23187-stimulated human neutrophils assessed as 5-LO product formation preincubated for 15 mins measured after 10 mins | Bioorg Med Chem 20: 3728-41 (2012) Article DOI: 10.1016/j.bmc.2012.04.048 BindingDB Entry DOI: 10.7270/Q26H4JFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50385359 (CHEMBL2036186) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University Curated by ChEMBL | Assay Description Inhibition of FLAP in A23187-stimulated human neutrophils assessed as 5-LO product formation preincubated for 15 mins measured after 10 mins | Bioorg Med Chem 20: 3728-41 (2012) Article DOI: 10.1016/j.bmc.2012.04.048 BindingDB Entry DOI: 10.7270/Q26H4JFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 265 total ) | Next | Last >> |