

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mitogen-activated protein kinase kinase kinase 14 (Homo sapiens (Human)) | BDBM312809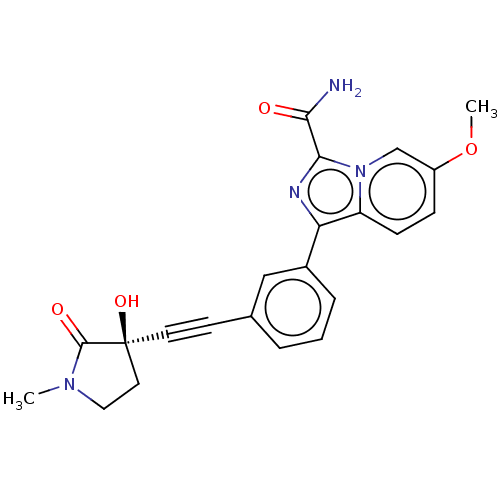 (1-[3-[2-[(3R)-3-hydroxy-1- methyl-2-oxo-pyrrolidin...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of NIK (unknown origin) expressed in baculovirus infected insect cells assessed as reduction in hydrolysis of ATP to ADP after 1 to 2 hrs ... | J Med Chem 61: 6801-6813 (2018) Article DOI: 10.1021/acs.jmedchem.8b00678 BindingDB Entry DOI: 10.7270/Q2PV6P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 14 (Homo sapiens (Human)) | BDBM50457816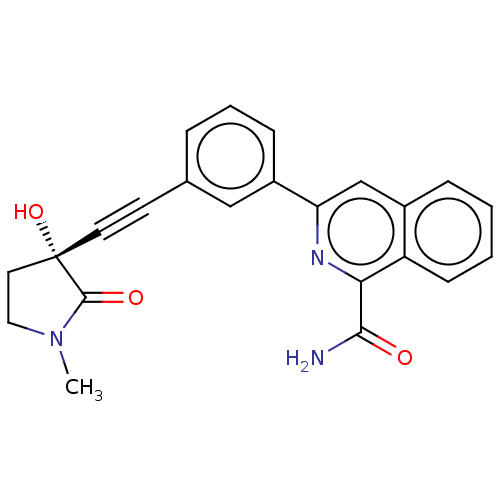 (CHEMBL4215425) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of NIK (unknown origin) expressed in baculovirus infected insect cells assessed as reduction in hydrolysis of ATP to ADP after 1 to 2 hrs ... | J Med Chem 61: 6801-6813 (2018) Article DOI: 10.1021/acs.jmedchem.8b00678 BindingDB Entry DOI: 10.7270/Q2PV6P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 14 (Homo sapiens (Human)) | BDBM50457820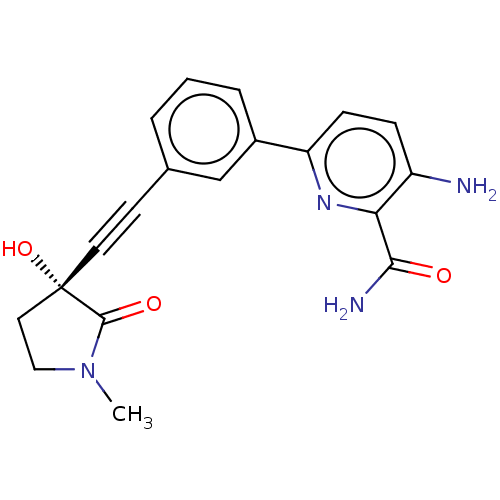 (CHEMBL4216289) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of NIK (unknown origin) expressed in baculovirus infected insect cells assessed as reduction in hydrolysis of ATP to ADP after 1 to 2 hrs ... | J Med Chem 61: 6801-6813 (2018) Article DOI: 10.1021/acs.jmedchem.8b00678 BindingDB Entry DOI: 10.7270/Q2PV6P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 14 (Homo sapiens (Human)) | BDBM312763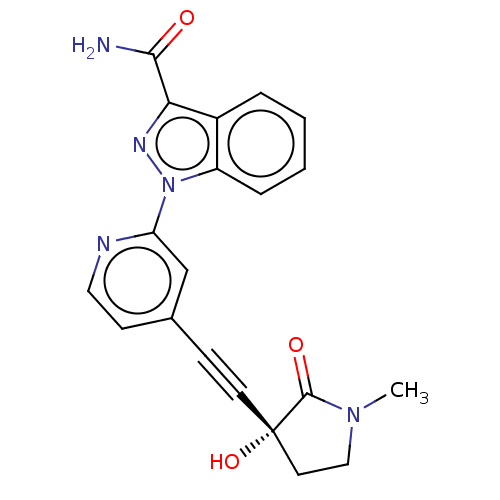 (1-[4-[2-[(3R)-3-hydroxy-1- methyl-2-oxo-pyrrolidin...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of NIK (unknown origin) expressed in baculovirus infected insect cells assessed as reduction in hydrolysis of ATP to ADP after 1 to 2 hrs ... | J Med Chem 61: 6801-6813 (2018) Article DOI: 10.1021/acs.jmedchem.8b00678 BindingDB Entry DOI: 10.7270/Q2PV6P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 14 (Homo sapiens (Human)) | BDBM312785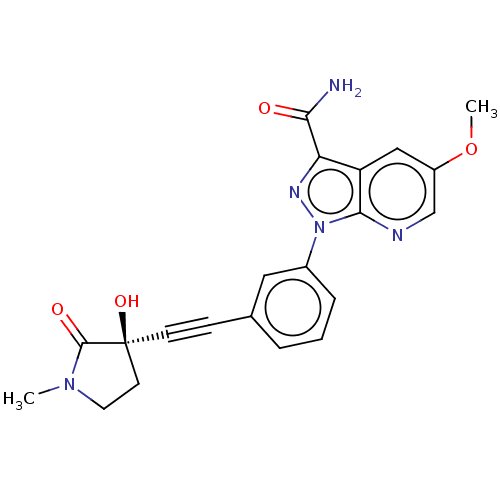 (1-[3-[2-[(3R)-3-hydroxy-1- methyl-2-oxo-pyrrolidin...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of NIK (unknown origin) expressed in baculovirus infected insect cells assessed as reduction in hydrolysis of ATP to ADP after 1 to 2 hrs ... | J Med Chem 61: 6801-6813 (2018) Article DOI: 10.1021/acs.jmedchem.8b00678 BindingDB Entry DOI: 10.7270/Q2PV6P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 14 (Homo sapiens (Human)) | BDBM312765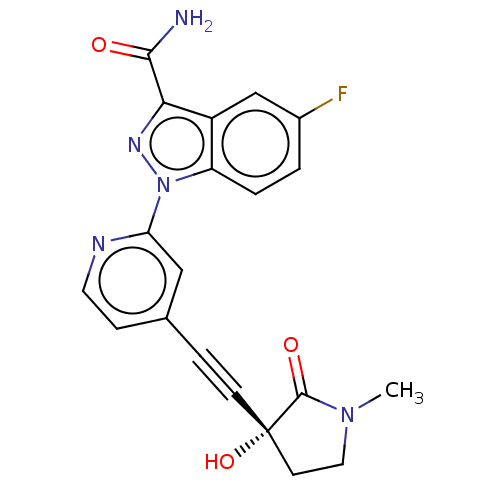 (5-fluoro-1-[4-[2-[(3R)-3-hy- droxy-1-methyl-2-oxo-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of NIK (unknown origin) expressed in baculovirus infected insect cells assessed as reduction in hydrolysis of ATP to ADP after 1 to 2 hrs ... | J Med Chem 61: 6801-6813 (2018) Article DOI: 10.1021/acs.jmedchem.8b00678 BindingDB Entry DOI: 10.7270/Q2PV6P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 14 (Homo sapiens (Human)) | BDBM312788 (1-[3-[2-[(3R)-3-hydroxy-1- methyl-2-oxo-pyrrolidin...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of NIK (unknown origin) expressed in baculovirus infected insect cells assessed as reduction in hydrolysis of ATP to ADP after 1 to 2 hrs ... | J Med Chem 61: 6801-6813 (2018) Article DOI: 10.1021/acs.jmedchem.8b00678 BindingDB Entry DOI: 10.7270/Q2PV6P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 14 (Homo sapiens (Human)) | BDBM312764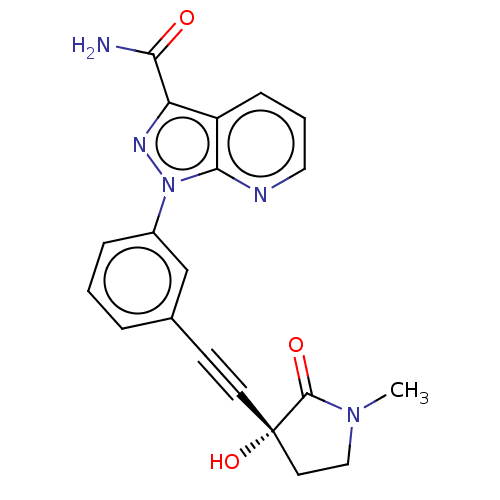 (1-[3-[2-[(3R)-3-hydroxy-1- methyl-2-oxo-pyrrolidin...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of NIK (unknown origin) expressed in baculovirus infected insect cells assessed as reduction in hydrolysis of ATP to ADP after 1 to 2 hrs ... | J Med Chem 61: 6801-6813 (2018) Article DOI: 10.1021/acs.jmedchem.8b00678 BindingDB Entry DOI: 10.7270/Q2PV6P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 14 (Homo sapiens (Human)) | BDBM50457815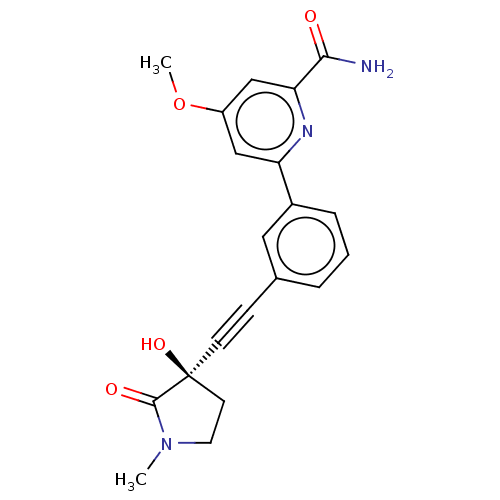 (CHEMBL4211840) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of NIK (unknown origin) expressed in baculovirus infected insect cells assessed as reduction in hydrolysis of ATP to ADP after 1 to 2 hrs ... | J Med Chem 61: 6801-6813 (2018) Article DOI: 10.1021/acs.jmedchem.8b00678 BindingDB Entry DOI: 10.7270/Q2PV6P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 14 (Homo sapiens (Human)) | BDBM312711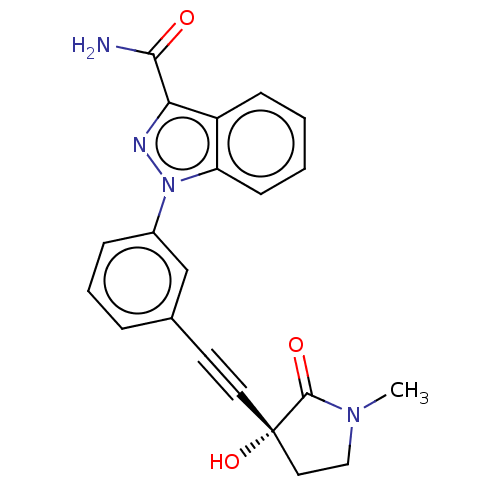 (1-[3-[2-[(3R)-3-hydroxy-1- methyl-2-oxo-pyrrolidin...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of NIK (unknown origin) expressed in baculovirus infected insect cells assessed as reduction in hydrolysis of ATP to ADP after 1 to 2 hrs ... | J Med Chem 61: 6801-6813 (2018) Article DOI: 10.1021/acs.jmedchem.8b00678 BindingDB Entry DOI: 10.7270/Q2PV6P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50406713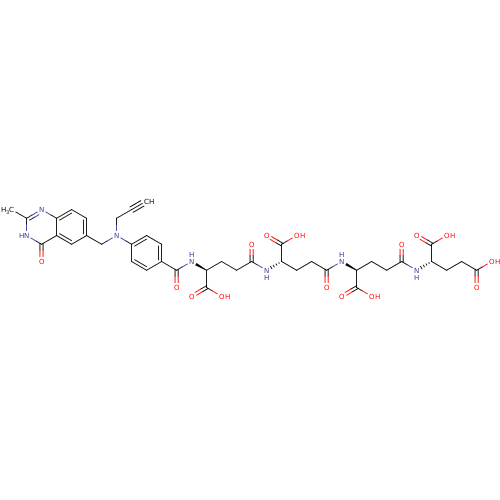 (CHEMBL1202139) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase, partially purified from L1210 mouse leukemia cells overexpressing TS | J Med Chem 35: 859-66 (1992) BindingDB Entry DOI: 10.7270/Q2G44RG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 14 (Homo sapiens (Human)) | BDBM312723 (5-fluoro-1-[3-[2-[(3R)-3-hy- droxy-1-methyl-2-oxo-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of NIK (unknown origin) expressed in baculovirus infected insect cells assessed as reduction in hydrolysis of ATP to ADP after 1 to 2 hrs ... | J Med Chem 61: 6801-6813 (2018) Article DOI: 10.1021/acs.jmedchem.8b00678 BindingDB Entry DOI: 10.7270/Q2PV6P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50406714 (CHEMBL264807) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase, partially purified from L1210 mouse leukemia cells overexpressing TS | J Med Chem 35: 859-66 (1992) BindingDB Entry DOI: 10.7270/Q2G44RG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50406717 (CHEMBL1202137) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase, partially purified from L1210 mouse leukemia cells overexpressing TS | J Med Chem 35: 859-66 (1992) BindingDB Entry DOI: 10.7270/Q2G44RG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 14 (Homo sapiens (Human)) | BDBM50457818 (CHEMBL4211560) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of NIK (unknown origin) expressed in baculovirus infected insect cells assessed as reduction in hydrolysis of ATP to ADP after 1 to 2 hrs ... | J Med Chem 61: 6801-6813 (2018) Article DOI: 10.1021/acs.jmedchem.8b00678 BindingDB Entry DOI: 10.7270/Q2PV6P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50436682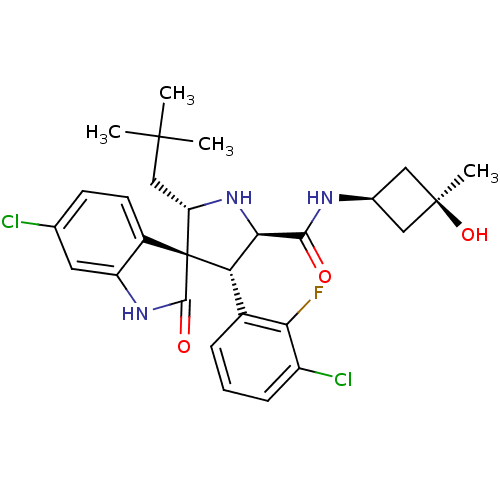 (CHEMBL2396674) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50205275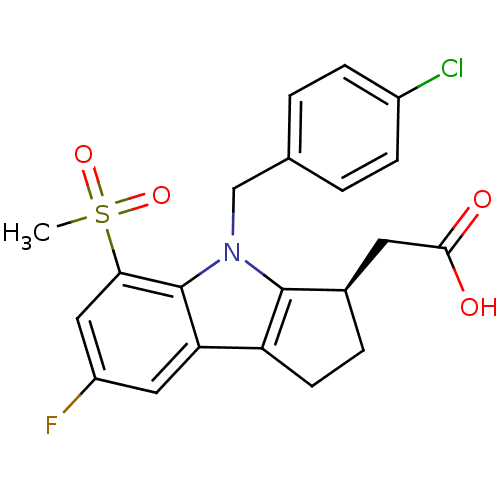 (CHEMBL426559 | [(3R)-4-(4-chlorobenzyl)-7-fluoro-5...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada & Co. Curated by ChEMBL | Assay Description Binding affinity to human DP receptor expressed in HEK293 cells | J Med Chem 50: 794-806 (2007) Article DOI: 10.1021/jm0603668 BindingDB Entry DOI: 10.7270/Q20P0ZP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM50006906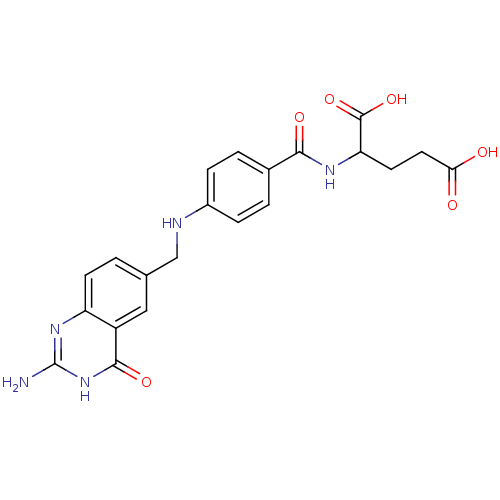 (2-{4-[(2-Amino-4-hydroxy-quinazolin-6-ylmethyl)-am...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of partially purified rat liver Dihydrofolate reductase (DHFR) enzyme. | J Med Chem 32: 847-52 (1989) BindingDB Entry DOI: 10.7270/Q2BP01SB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 14 (Homo sapiens (Human)) | BDBM50457817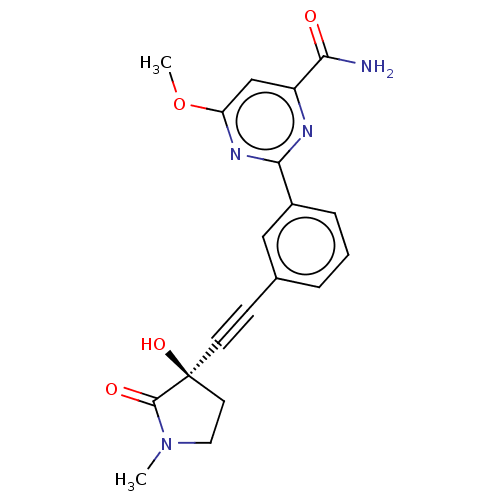 (CHEMBL4214032) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of NIK (unknown origin) expressed in baculovirus infected insect cells assessed as reduction in hydrolysis of ATP to ADP after 1 to 2 hrs ... | J Med Chem 61: 6801-6813 (2018) Article DOI: 10.1021/acs.jmedchem.8b00678 BindingDB Entry DOI: 10.7270/Q2PV6P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50406721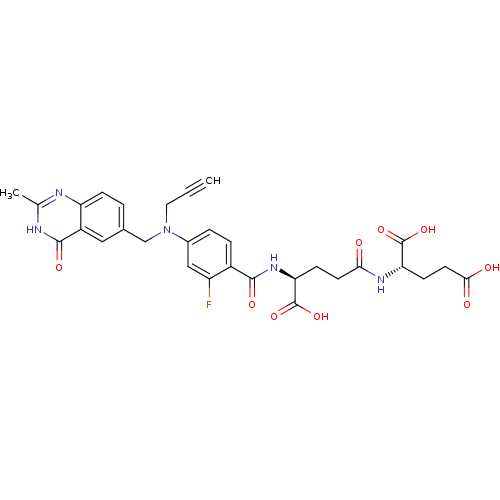 (CHEMBL171226) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase, partially purified from L1210 mouse leukemia cells overexpressing TS | J Med Chem 35: 859-66 (1992) BindingDB Entry DOI: 10.7270/Q2G44RG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50406716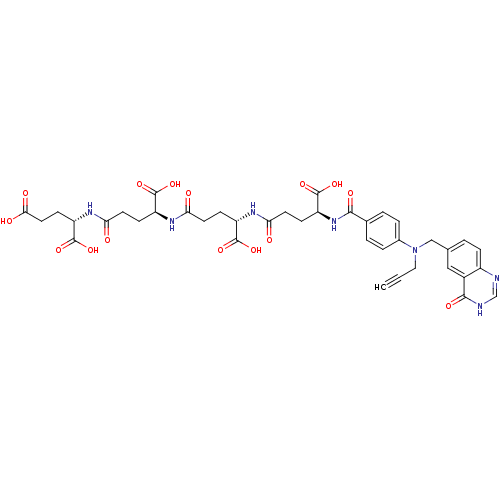 (CHEMBL1202138) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase, partially purified from L1210 mouse leukemia cells overexpressing TS | J Med Chem 35: 859-66 (1992) BindingDB Entry DOI: 10.7270/Q2G44RG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50205274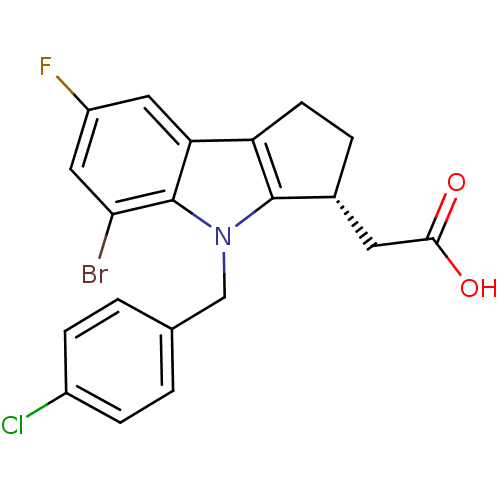 (CHEMBL426387 | [(3R)-5-bromo-4-(4-chlorobenzyl)-7-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada & Co. Curated by ChEMBL | Assay Description Binding affinity to human TP receptor expressed in HEK293 cells | J Med Chem 50: 794-806 (2007) Article DOI: 10.1021/jm0603668 BindingDB Entry DOI: 10.7270/Q20P0ZP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 14 (Homo sapiens (Human)) | BDBM312762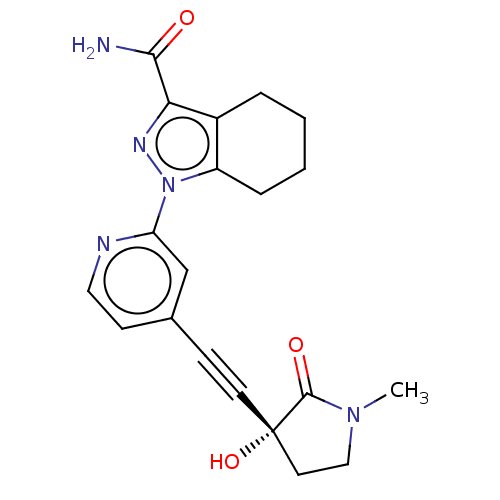 (1-[4-[2-[(3R)-3-hydroxy-1- methyl-2-oxo-pyrrolidin...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of NIK (unknown origin) expressed in baculovirus infected insect cells assessed as reduction in hydrolysis of ATP to ADP after 1 to 2 hrs ... | J Med Chem 61: 6801-6813 (2018) Article DOI: 10.1021/acs.jmedchem.8b00678 BindingDB Entry DOI: 10.7270/Q2PV6P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50406718 (CHEMBL1202140) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase, partially purified from L1210 mouse leukemia cells overexpressing TS | J Med Chem 35: 859-66 (1992) BindingDB Entry DOI: 10.7270/Q2G44RG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50406719 (CHEMBL436448) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase, partially purified from L1210 mouse leukemia cells overexpressing TS | J Med Chem 35: 859-66 (1992) BindingDB Entry DOI: 10.7270/Q2G44RG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50205276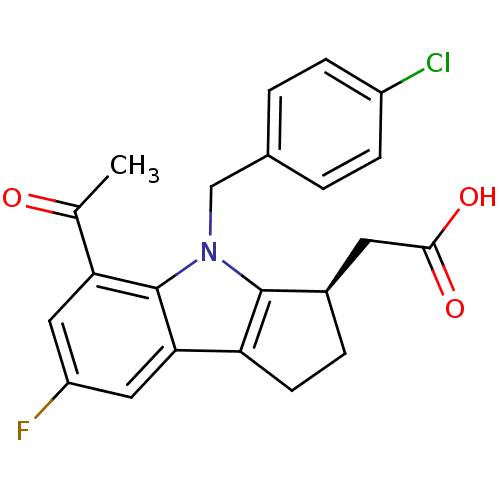 (CHEMBL385126 | [(3R)-4-(4-chlorobenzyl)-7-fluoro-5...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada & Co. Curated by ChEMBL | Assay Description Binding affinity to human DP receptor expressed in HEK293 cells | J Med Chem 50: 794-806 (2007) Article DOI: 10.1021/jm0603668 BindingDB Entry DOI: 10.7270/Q20P0ZP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50049164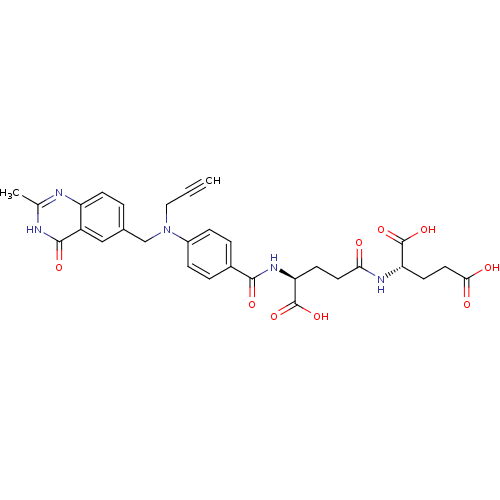 ((S)-2-((S)-4-Carboxy-4-{4-[(2-methyl-4-oxo-3,4-dih...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase, partially purified from L1210 mouse leukemia cells overexpressing TS | J Med Chem 35: 859-66 (1992) BindingDB Entry DOI: 10.7270/Q2G44RG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Homo sapiens (Human)) | BDBM50184825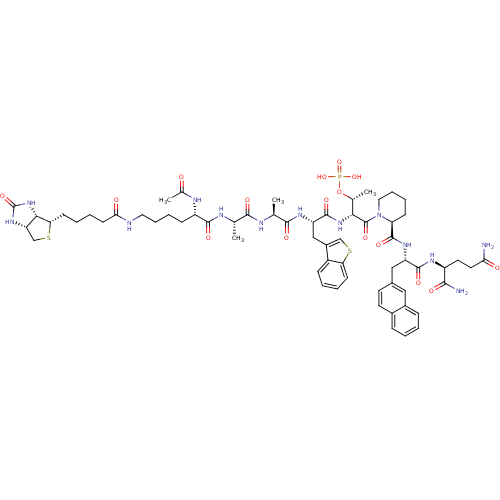 (Ac-Lys(N-epsilon-biotinoyl)-Ala-Ala-Bth-D-Thr(PO3H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Research Unit for Enzymology of Protein Folding Curated by ChEMBL | Assay Description Inhibition of human Pin1 PPIase Activity by protease free PPIase assay | J Med Chem 49: 2147-50 (2006) Article DOI: 10.1021/jm060036n BindingDB Entry DOI: 10.7270/Q2S46RJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50093383 (2-(4-Trifluoromethyl-phenyl)-1H-benzoimidazole-4-c...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University Curated by ChEMBL | Assay Description In vitro inhibition against human full length PARP protein | J Med Chem 43: 4084-97 (2000) BindingDB Entry DOI: 10.7270/Q2MP52HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 14 (Homo sapiens (Human)) | BDBM50457812 (CHEMBL4217923) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of NIK (unknown origin) expressed in baculovirus infected insect cells assessed as reduction in hydrolysis of ATP to ADP after 1 to 2 hrs ... | J Med Chem 61: 6801-6813 (2018) Article DOI: 10.1021/acs.jmedchem.8b00678 BindingDB Entry DOI: 10.7270/Q2PV6P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50205274 (CHEMBL426387 | [(3R)-5-bromo-4-(4-chlorobenzyl)-7-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada & Co. Curated by ChEMBL | Assay Description Binding affinity to human DP receptor expressed in HEK293 cells | J Med Chem 50: 794-806 (2007) Article DOI: 10.1021/jm0603668 BindingDB Entry DOI: 10.7270/Q20P0ZP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50093359 (2-(4-Hydroxymethyl-phenyl)-1H-benzoimidazole-4-car...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University Curated by ChEMBL | Assay Description In vitro inhibition against human full length PARP protein | J Med Chem 43: 4084-97 (2000) BindingDB Entry DOI: 10.7270/Q2MP52HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50184217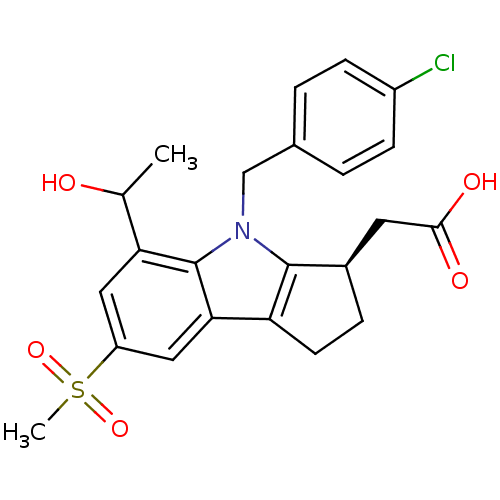 (2-((R)-4-(4-chlorobenzyl)-5-(1-hydroxyethyl)-7-(me...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada & Co. Curated by ChEMBL | Assay Description Binding affinity to human DP receptor expressed in HEK293 cells | J Med Chem 50: 794-806 (2007) Article DOI: 10.1021/jm0603668 BindingDB Entry DOI: 10.7270/Q20P0ZP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50093384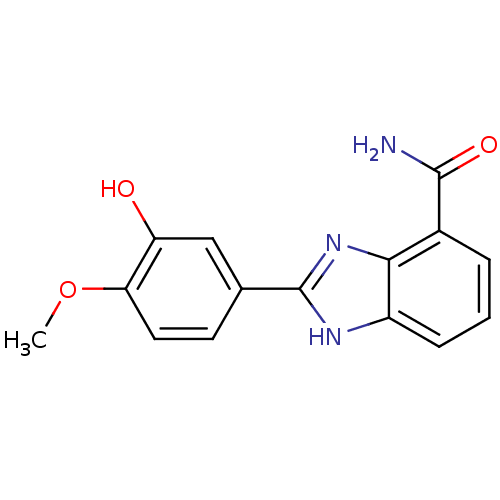 (2-(3-Hydroxy-4-methoxy-phenyl)-1H-benzoimidazole-4...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University Curated by ChEMBL | Assay Description In vitro inhibition against human full length PARP protein | J Med Chem 43: 4084-97 (2000) BindingDB Entry DOI: 10.7270/Q2MP52HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 14 (Homo sapiens (Human)) | BDBM50457811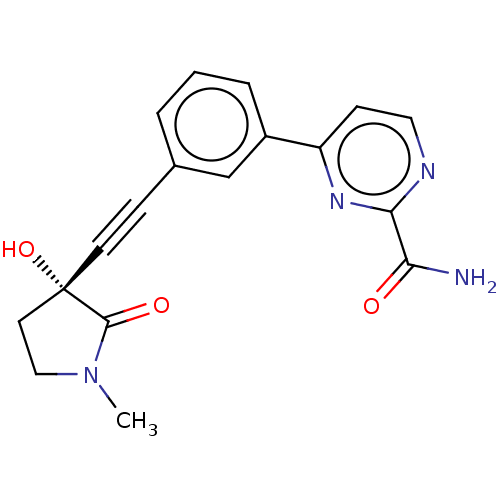 (CHEMBL4210461) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of NIK (unknown origin) expressed in baculovirus infected insect cells assessed as reduction in hydrolysis of ATP to ADP after 1 to 2 hrs ... | J Med Chem 61: 6801-6813 (2018) Article DOI: 10.1021/acs.jmedchem.8b00678 BindingDB Entry DOI: 10.7270/Q2PV6P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50184212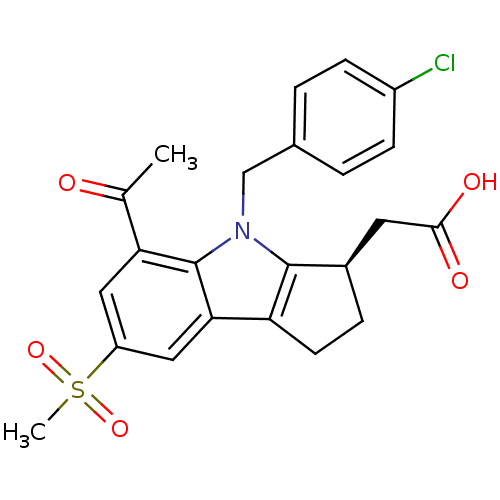 ((R)-2-(4-(4-chlorobenzyl)-5-acetyl-7-(methylsulfon...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada & Co. Curated by ChEMBL | Assay Description Binding affinity to human DP receptor expressed in HEK293 cells | J Med Chem 50: 794-806 (2007) Article DOI: 10.1021/jm0603668 BindingDB Entry DOI: 10.7270/Q20P0ZP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50028408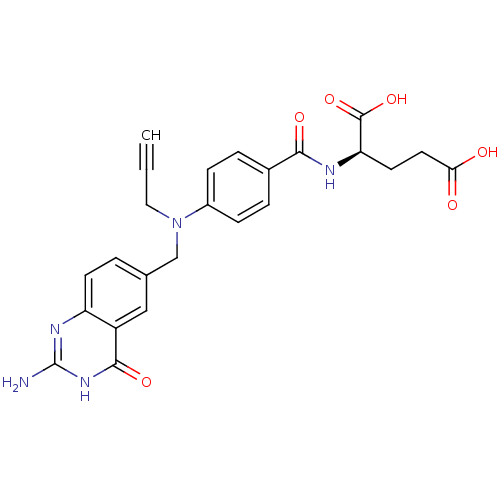 ((R)-2-{4-[(2-Amino-4-oxo-1,4-dihydro-quinazolin-6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Tested for binding affinity against thymidylate synthase(TS) | J Med Chem 37: 3294-302 (1994) BindingDB Entry DOI: 10.7270/Q2QV3N50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50093372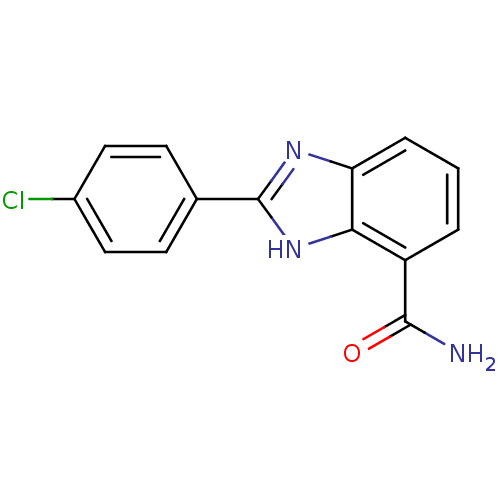 (2-(4-Chloro-phenyl)-1H-benzoimidazole-4-carboxylic...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University Curated by ChEMBL | Assay Description In vitro inhibition against human full length PARP protein | J Med Chem 43: 4084-97 (2000) BindingDB Entry DOI: 10.7270/Q2MP52HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50205275 (CHEMBL426559 | [(3R)-4-(4-chlorobenzyl)-7-fluoro-5...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada & Co. Curated by ChEMBL | Assay Description Binding affinity to human TP receptor expressed in HEK293 cells | J Med Chem 50: 794-806 (2007) Article DOI: 10.1021/jm0603668 BindingDB Entry DOI: 10.7270/Q20P0ZP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM50600091 (CHEMBL5191406) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116700 BindingDB Entry DOI: 10.7270/Q2FN1B89 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM18771 ((2S)-2-[(4-{[(2-amino-4-oxo-1,4-dihydroquinazolin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Mereside Curated by ChEMBL | Assay Description Binding affinity against Thymidylate synthase was measured in vitro | J Med Chem 33: 3060-7 (1990) BindingDB Entry DOI: 10.7270/Q2ZW1JWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50406711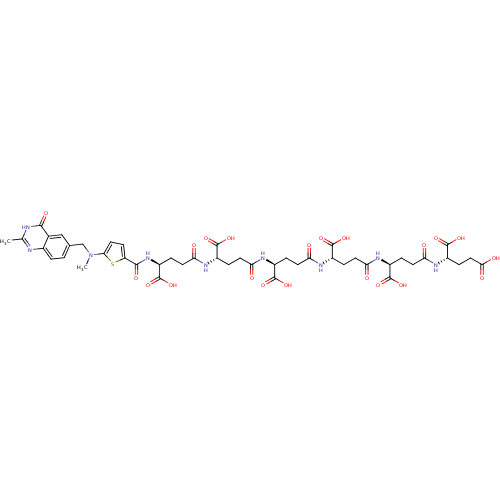 (CHEMBL405513) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase, partially purified from L1210 mouse leukemia cells overexpressing TS | J Med Chem 35: 859-66 (1992) BindingDB Entry DOI: 10.7270/Q2G44RG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50154756 (6-(4-Methylaminomethyl-phenyl)-3,4-dihydro-2H-[1,4...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D--La Jolla Laboratories Curated by ChEMBL | Assay Description Inhibition of human Poly (ADP-ribose) polymerase 1 enzyme | J Med Chem 47: 5467-81 (2004) Article DOI: 10.1021/jm030513r BindingDB Entry DOI: 10.7270/Q2PK0FM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50406712 (CHEMBL268593) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase, partially purified from L1210 mouse leukemia cells overexpressing TS | J Med Chem 35: 859-66 (1992) BindingDB Entry DOI: 10.7270/Q2G44RG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM50017876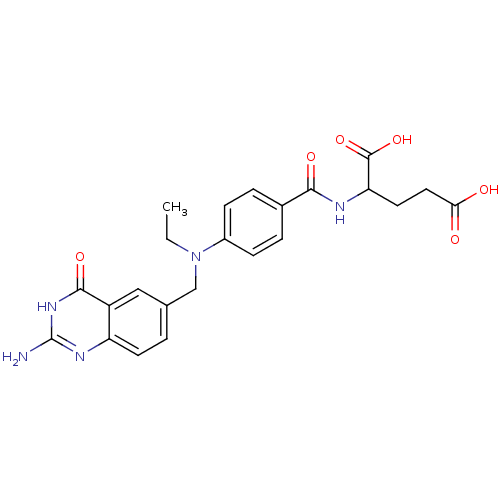 (2-{4-[(2-Amino-4-oxo-3,4-dihydro-quinazolin-6-ylme...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of partially purified rat liver Dihydrofolate reductase (DHFR) enzyme. | J Med Chem 32: 847-52 (1989) BindingDB Entry DOI: 10.7270/Q2BP01SB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50093379 (2-(4-Cyano-phenyl)-1H-benzoimidazole-4-carboxylic ...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University Curated by ChEMBL | Assay Description In vitro inhibition against human full length PARP protein | J Med Chem 43: 4084-97 (2000) BindingDB Entry DOI: 10.7270/Q2MP52HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50122777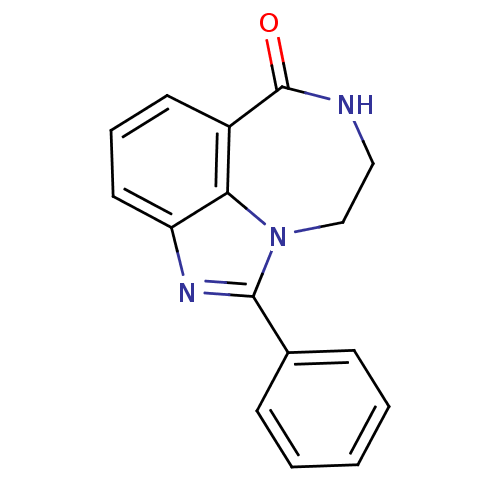 (1-Phenyl-8,9-dihydro-7H-2,7,9a-triaza-benzo[cd]azu...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human full length Poly (ADP-ribose) polymerase 1 | J Med Chem 46: 210-3 (2003) Article DOI: 10.1021/jm0255769 BindingDB Entry DOI: 10.7270/Q2154GDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50122775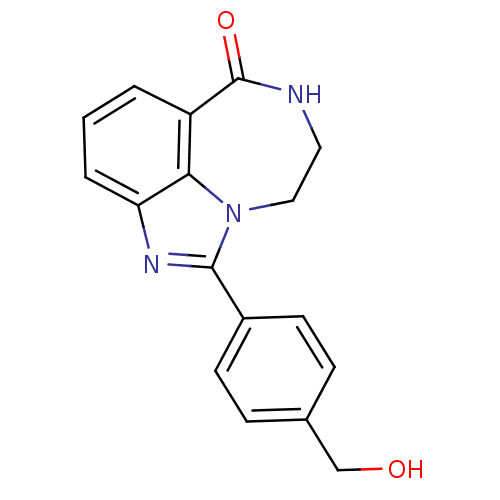 (1-(4-Hydroxymethyl-phenyl)-8,9-dihydro-7H-2,7,9a-t...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human full length Poly (ADP-ribose) polymerase 1 | J Med Chem 46: 210-3 (2003) Article DOI: 10.1021/jm0255769 BindingDB Entry DOI: 10.7270/Q2154GDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50008294 (2-(4-(((2-amino-4-oxo-1,4-dihydroquinazolin-6-yl)m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity was evaluated against thymidylate synthase | J Med Chem 28: 1468-76 (1985) BindingDB Entry DOI: 10.7270/Q2348JDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50406720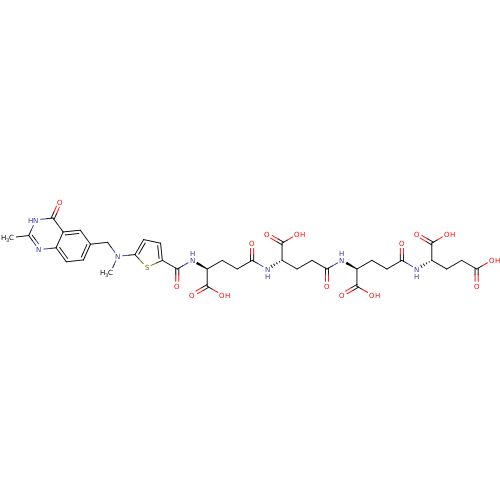 (CHEMBL172160) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase, partially purified from L1210 mouse leukemia cells overexpressing TS | J Med Chem 35: 859-66 (1992) BindingDB Entry DOI: 10.7270/Q2G44RG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2264 total ) | Next | Last >> |