 Found 530 hits with Last Name = 'möbitz' and Initial = 'h'
Found 530 hits with Last Name = 'möbitz' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50348537

(CHEMBL1801376)Show SMILES Fc1cccc(Nc2cc3c4[nH]c5CNC(=O)c5c4ccc3cn2)c1 Show InChI InChI=1S/C19H13FN4O/c20-11-2-1-3-12(6-11)23-16-7-14-10(8-21-16)4-5-13-17-15(24-18(13)14)9-22-19(17)25/h1-8,24H,9H2,(H,21,23)(H,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of JNK2 |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348545

(CHEMBL1801384)Show SMILES O=C1NCCc2[nH]c3c(ccc4cnc(\C=C\c5ccc(CN6CCOCC6)cc5)cc34)c12 Show InChI InChI=1S/C27H26N4O2/c32-27-25-22-8-6-20-16-29-21(15-23(20)26(22)30-24(25)9-10-28-27)7-5-18-1-3-19(4-2-18)17-31-11-13-33-14-12-31/h1-8,15-16,30H,9-14,17H2,(H,28,32)/b7-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET assay |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50348534
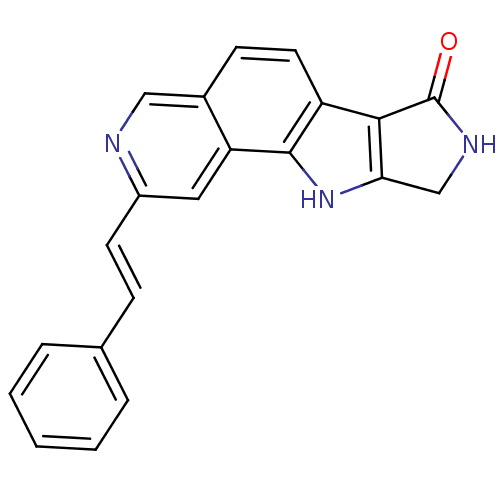
(CHEMBL1801373)Show SMILES O=C1NCc2[nH]c3c(ccc4cnc(\C=C\c5ccccc5)cc34)c12 Show InChI InChI=1S/C21H15N3O/c25-21-19-16-9-7-14-11-22-15(8-6-13-4-2-1-3-5-13)10-17(14)20(16)24-18(19)12-23-21/h1-11,24H,12H2,(H,23,25)/b8-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of JNK2 |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50386487
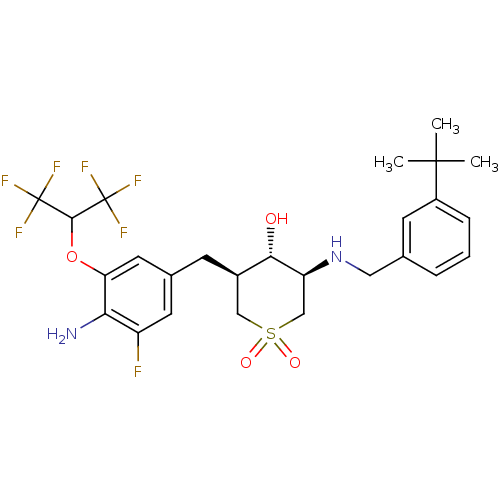
(CHEMBL2048047)Show SMILES CC(C)(C)c1cccc(CN[C@H]2CS(=O)(=O)C[C@@H](Cc3cc(F)c(N)c(OC(C(F)(F)F)C(F)(F)F)c3)[C@@H]2O)c1 |r| Show InChI InChI=1S/C26H31F7N2O4S/c1-24(2,3)17-6-4-5-14(8-17)11-35-19-13-40(37,38)12-16(22(19)36)7-15-9-18(27)21(34)20(10-15)39-23(25(28,29)30)26(31,32)33/h4-6,8-10,16,19,22-23,35-36H,7,11-13,34H2,1-3H3/t16-,19+,22+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE1 ectodomain after 1 hr by fluorescence analysis |
J Med Chem 55: 3364-86 (2012)
Article DOI: 10.1021/jm300069y
BindingDB Entry DOI: 10.7270/Q2P55PJW |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50386513
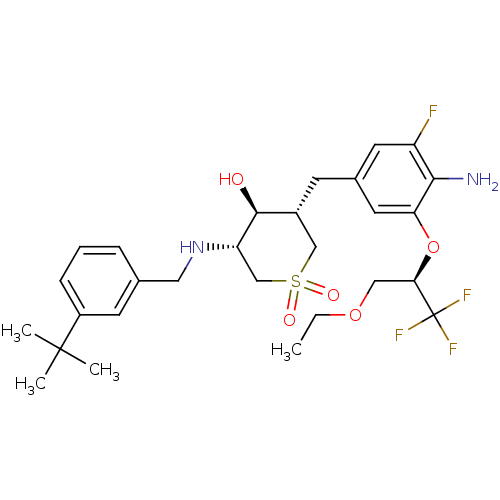
(CHEMBL2048051)Show SMILES CCOC[C@@H](Oc1cc(C[C@@H]2CS(=O)(=O)C[C@H](NCc3cccc(c3)C(C)(C)C)[C@H]2O)cc(F)c1N)C(F)(F)F |r| Show InChI InChI=1S/C28H38F4N2O5S/c1-5-38-14-24(28(30,31)32)39-23-12-18(11-21(29)25(23)33)9-19-15-40(36,37)16-22(26(19)35)34-13-17-7-6-8-20(10-17)27(2,3)4/h6-8,10-12,19,22,24,26,34-35H,5,9,13-16,33H2,1-4H3/t19-,22+,24-,26+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE1 ectodomain after 1 hr by fluorescence analysis |
J Med Chem 55: 3364-86 (2012)
Article DOI: 10.1021/jm300069y
BindingDB Entry DOI: 10.7270/Q2P55PJW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM16047

((4S)-4-[(2S)-2-[(2R,4S,5S)-5-[(2S)-2-[(2S)-2-[(4S)...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)CCC(O)=O)C(C)C)[C@@H](O)C[C@@H](C)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C41H64N8O14/c1-20(2)16-27(46-39(60)28(19-31(43)51)47-40(61)34(21(3)4)49-37(58)25(42)12-14-32(52)53)30(50)17-22(5)35(56)44-23(6)36(57)45-26(13-15-33(54)55)38(59)48-29(41(62)63)18-24-10-8-7-9-11-24/h7-11,20-23,25-30,34,50H,12-19,42H2,1-6H3,(H2,43,51)(H,44,56)(H,45,57)(H,46,60)(H,47,61)(H,48,59)(H,49,58)(H,52,53)(H,54,55)(H,62,63)/t22-,23+,25+,26+,27+,28+,29+,30+,34+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE1 ectodomain after 1 hr by fluorescence analysis |
J Med Chem 55: 3364-86 (2012)
Article DOI: 10.1021/jm300069y
BindingDB Entry DOI: 10.7270/Q2P55PJW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50386482
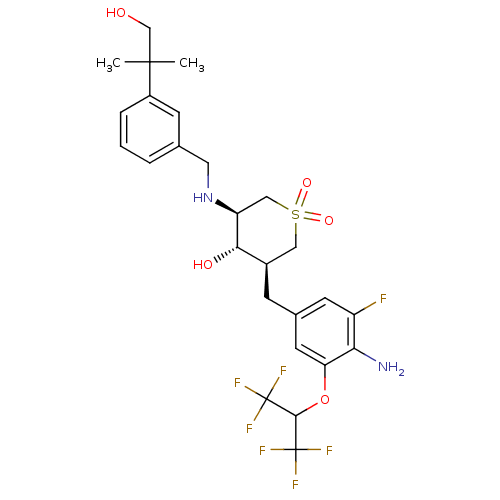
(CHEMBL2048059)Show SMILES CC(C)(CO)c1cccc(CN[C@H]2CS(=O)(=O)C[C@@H](Cc3cc(F)c(N)c(OC(C(F)(F)F)C(F)(F)F)c3)[C@@H]2O)c1 |r| Show InChI InChI=1S/C26H31F7N2O5S/c1-24(2,13-36)17-5-3-4-14(7-17)10-35-19-12-41(38,39)11-16(22(19)37)6-15-8-18(27)21(34)20(9-15)40-23(25(28,29)30)26(31,32)33/h3-5,7-9,16,19,22-23,35-37H,6,10-13,34H2,1-2H3/t16-,19+,22+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE1 ectodomain after 1 hr by fluorescence analysis |
J Med Chem 55: 3364-86 (2012)
Article DOI: 10.1021/jm300069y
BindingDB Entry DOI: 10.7270/Q2P55PJW |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50386481
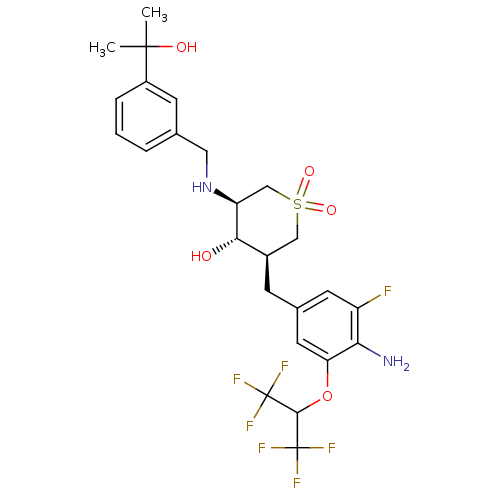
(CHEMBL2048058)Show SMILES CC(C)(O)c1cccc(CN[C@H]2CS(=O)(=O)C[C@@H](Cc3cc(F)c(N)c(OC(C(F)(F)F)C(F)(F)F)c3)[C@@H]2O)c1 |r| Show InChI InChI=1S/C25H29F7N2O5S/c1-23(2,36)16-5-3-4-13(7-16)10-34-18-12-40(37,38)11-15(21(18)35)6-14-8-17(26)20(33)19(9-14)39-22(24(27,28)29)25(30,31)32/h3-5,7-9,15,18,21-22,34-36H,6,10-12,33H2,1-2H3/t15-,18+,21+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE1 ectodomain after 1 hr by fluorescence analysis |
J Med Chem 55: 3364-86 (2012)
Article DOI: 10.1021/jm300069y
BindingDB Entry DOI: 10.7270/Q2P55PJW |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50386515
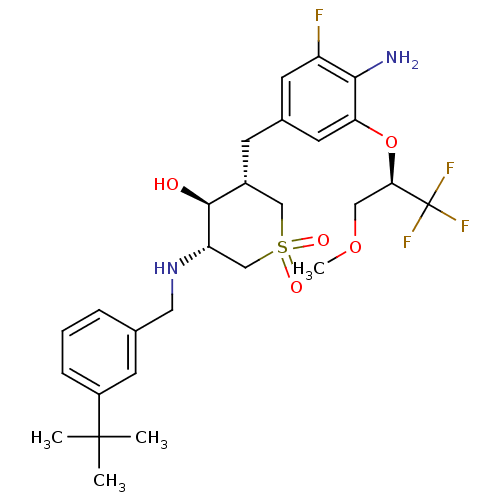
(CHEMBL2048053)Show SMILES COC[C@@H](Oc1cc(C[C@@H]2CS(=O)(=O)C[C@H](NCc3cccc(c3)C(C)(C)C)[C@H]2O)cc(F)c1N)C(F)(F)F |r| Show InChI InChI=1S/C27H36F4N2O5S/c1-26(2,3)19-7-5-6-16(9-19)12-33-21-15-39(35,36)14-18(25(21)34)8-17-10-20(28)24(32)22(11-17)38-23(13-37-4)27(29,30)31/h5-7,9-11,18,21,23,25,33-34H,8,12-15,32H2,1-4H3/t18-,21+,23-,25+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE1 ectodomain after 1 hr by fluorescence analysis |
J Med Chem 55: 3364-86 (2012)
Article DOI: 10.1021/jm300069y
BindingDB Entry DOI: 10.7270/Q2P55PJW |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348515
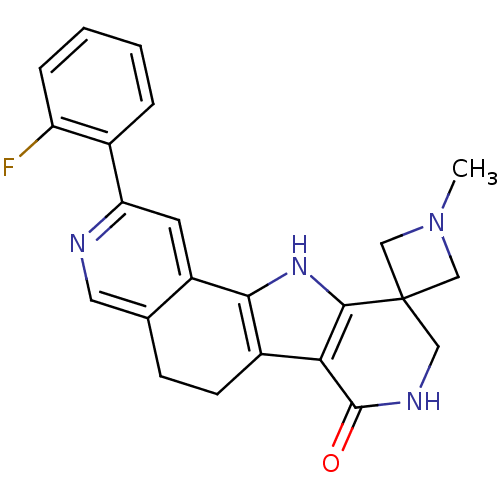
(CHEMBL1233942)Show SMILES CN1CC2(C1)CNC(=O)c1c3CCc4cnc(cc4-c3[nH]c21)-c1ccccc1F Show InChI InChI=1S/C23H21FN4O/c1-28-11-23(12-28)10-26-22(29)19-15-7-6-13-9-25-18(14-4-2-3-5-17(14)24)8-16(13)20(15)27-21(19)23/h2-5,8-9,27H,6-7,10-12H2,1H3,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of MK2 mediated anisomycin-stimulated hsp27 phosphorylation in human THP-1 cells by fluorometric analysis |
Bioorg Med Chem Lett 20: 4719-23 (2010)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.04.023
BindingDB Entry DOI: 10.7270/Q2BZ66C2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50386512

(CHEMBL2048050)Show SMILES C[C@@H](Oc1cc(C[C@@H]2CS(=O)(=O)C[C@H](NCc3cccc(c3)C(C)(C)C)[C@H]2O)cc(F)c1N)C(F)(F)F |r| Show InChI InChI=1S/C26H34F4N2O4S/c1-15(26(28,29)30)36-22-11-17(10-20(27)23(22)31)8-18-13-37(34,35)14-21(24(18)33)32-12-16-6-5-7-19(9-16)25(2,3)4/h5-7,9-11,15,18,21,24,32-33H,8,12-14,31H2,1-4H3/t15-,18-,21+,24+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE1 ectodomain after 1 hr by fluorescence analysis |
J Med Chem 55: 3364-86 (2012)
Article DOI: 10.1021/jm300069y
BindingDB Entry DOI: 10.7270/Q2P55PJW |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348492
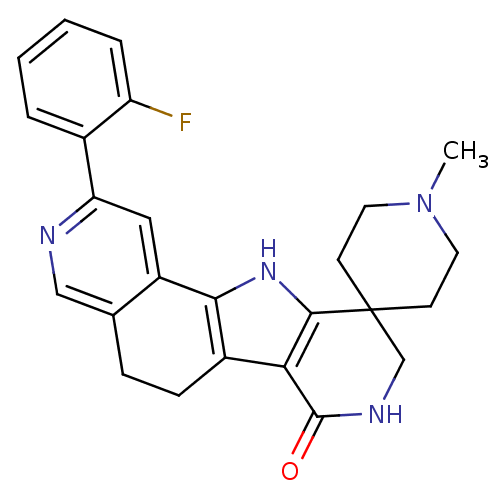
(CHEMBL1801296)Show SMILES CN1CCC2(CC1)CNC(=O)c1c3CCc4cnc(cc4-c3[nH]c21)-c1ccccc1F Show InChI InChI=1S/C25H25FN4O/c1-30-10-8-25(9-11-30)14-28-24(31)21-17-7-6-15-13-27-20(16-4-2-3-5-19(16)26)12-18(15)22(17)29-23(21)25/h2-5,12-13,29H,6-11,14H2,1H3,(H,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET analysis |
Bioorg Med Chem Lett 20: 4719-23 (2010)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.04.023
BindingDB Entry DOI: 10.7270/Q2BZ66C2 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50386516
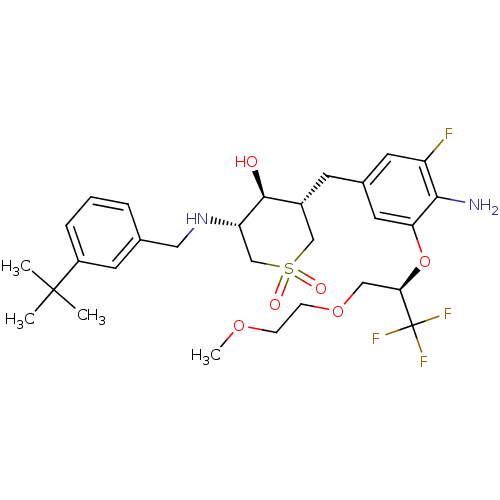
(CHEMBL2048054)Show SMILES COCCOC[C@@H](Oc1cc(C[C@@H]2CS(=O)(=O)C[C@H](NCc3cccc(c3)C(C)(C)C)[C@H]2O)cc(F)c1N)C(F)(F)F |r| Show InChI InChI=1S/C29H40F4N2O6S/c1-28(2,3)21-7-5-6-18(11-21)14-35-23-17-42(37,38)16-20(27(23)36)10-19-12-22(30)26(34)24(13-19)41-25(29(31,32)33)15-40-9-8-39-4/h5-7,11-13,20,23,25,27,35-36H,8-10,14-17,34H2,1-4H3/t20-,23+,25-,27+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE1 ectodomain after 1 hr by fluorescence analysis |
J Med Chem 55: 3364-86 (2012)
Article DOI: 10.1021/jm300069y
BindingDB Entry DOI: 10.7270/Q2P55PJW |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348542
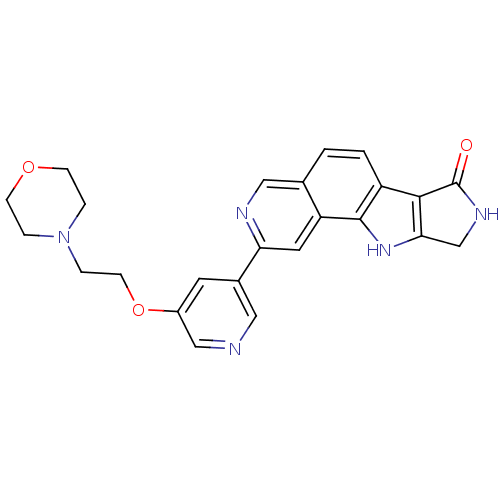
(CHEMBL1801381)Show SMILES O=C1NCc2[nH]c3c(ccc4cnc(cc34)-c3cncc(OCCN4CCOCC4)c3)c12 Show InChI InChI=1S/C24H23N5O3/c30-24-22-18-2-1-15-12-26-20(10-19(15)23(18)28-21(22)14-27-24)16-9-17(13-25-11-16)32-8-5-29-3-6-31-7-4-29/h1-2,9-13,28H,3-8,14H2,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET assay |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348546
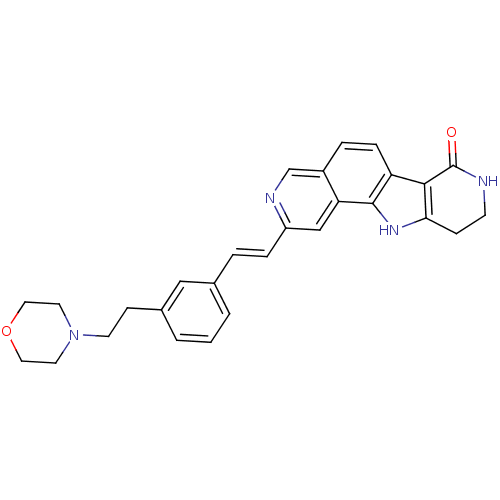
(CHEMBL1801385)Show SMILES O=C1NCCc2[nH]c3c(ccc4cnc(\C=C\c5cccc(CCN6CCOCC6)c5)cc34)c12 Show InChI InChI=1S/C28H28N4O2/c33-28-26-23-7-5-21-18-30-22(17-24(21)27(23)31-25(26)8-10-29-28)6-4-19-2-1-3-20(16-19)9-11-32-12-14-34-15-13-32/h1-7,16-18,31H,8-15H2,(H,29,33)/b6-4+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET assay |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348543

(CHEMBL1801382)Show SMILES COCCOc1cncc(c1)-c1cc2c3[nH]c4CNC(=O)c4c3ccc2cn1 Show InChI InChI=1S/C21H18N4O3/c1-27-4-5-28-14-6-13(8-22-10-14)17-7-16-12(9-23-17)2-3-15-19-18(25-20(15)16)11-24-21(19)26/h2-3,6-10,25H,4-5,11H2,1H3,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET assay |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50386508
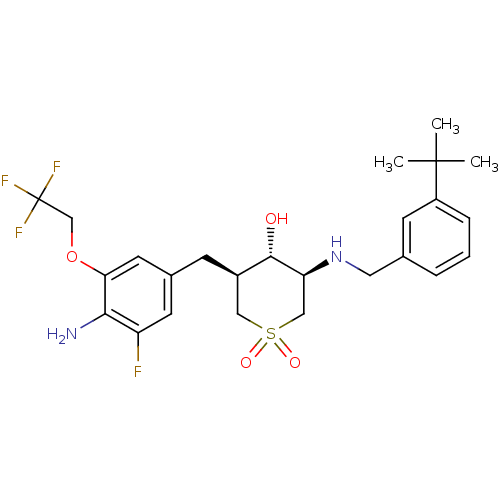
(CHEMBL2048045)Show SMILES CC(C)(C)c1cccc(CN[C@H]2CS(=O)(=O)C[C@@H](Cc3cc(F)c(N)c(OCC(F)(F)F)c3)[C@@H]2O)c1 |r| Show InChI InChI=1S/C25H32F4N2O4S/c1-24(2,3)18-6-4-5-15(8-18)11-31-20-13-36(33,34)12-17(23(20)32)7-16-9-19(26)22(30)21(10-16)35-14-25(27,28)29/h4-6,8-10,17,20,23,31-32H,7,11-14,30H2,1-3H3/t17-,20+,23+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE1 ectodomain after 1 hr by fluorescence analysis |
J Med Chem 55: 3364-86 (2012)
Article DOI: 10.1021/jm300069y
BindingDB Entry DOI: 10.7270/Q2P55PJW |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50386507
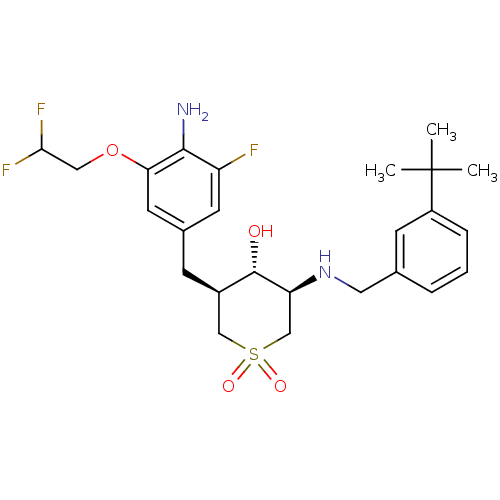
(CHEMBL2048044)Show SMILES CC(C)(C)c1cccc(CN[C@H]2CS(=O)(=O)C[C@@H](Cc3cc(F)c(N)c(OCC(F)F)c3)[C@@H]2O)c1 |r| Show InChI InChI=1S/C25H33F3N2O4S/c1-25(2,3)18-6-4-5-15(8-18)11-30-20-14-35(32,33)13-17(24(20)31)7-16-9-19(26)23(29)21(10-16)34-12-22(27)28/h4-6,8-10,17,20,22,24,30-31H,7,11-14,29H2,1-3H3/t17-,20+,24+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE1 ectodomain after 1 hr by fluorescence analysis |
J Med Chem 55: 3364-86 (2012)
Article DOI: 10.1021/jm300069y
BindingDB Entry DOI: 10.7270/Q2P55PJW |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348491

(CHEMBL1801305)Show SMILES CN1CC2(C1)CNC(=O)c1c3CCc4cnc(nc4-c3[nH]c21)-c1cccc(F)c1 Show InChI InChI=1S/C22H20FN5O/c1-28-10-22(11-28)9-25-21(29)16-15-6-5-13-8-24-20(12-3-2-4-14(23)7-12)27-17(13)18(15)26-19(16)22/h2-4,7-8,26H,5-6,9-11H2,1H3,(H,25,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET analysis |
Bioorg Med Chem Lett 20: 4719-23 (2010)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.04.023
BindingDB Entry DOI: 10.7270/Q2BZ66C2 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348493
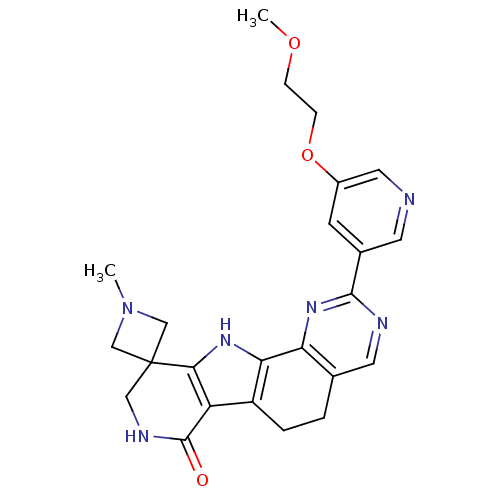
(CHEMBL1801304)Show SMILES COCCOc1cncc(c1)-c1ncc2CCc3c([nH]c4c3C(=O)NCC43CN(C)C3)-c2n1 Show InChI InChI=1S/C24H26N6O3/c1-30-12-24(13-30)11-27-23(31)18-17-4-3-14-9-26-22(29-19(14)20(17)28-21(18)24)15-7-16(10-25-8-15)33-6-5-32-2/h7-10,28H,3-6,11-13H2,1-2H3,(H,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET analysis |
Bioorg Med Chem Lett 20: 4719-23 (2010)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.04.023
BindingDB Entry DOI: 10.7270/Q2BZ66C2 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50386483
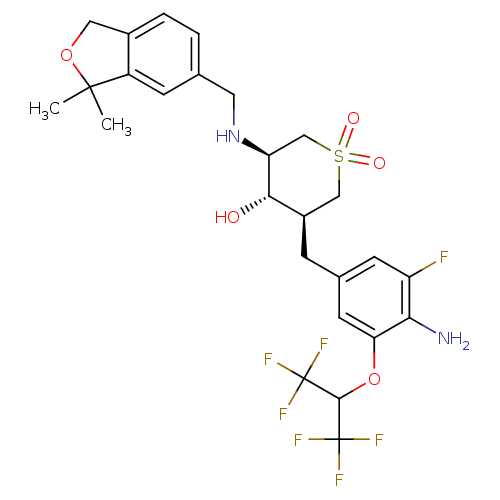
(CHEMBL2048060)Show SMILES CC1(C)OCc2ccc(CN[C@H]3CS(=O)(=O)C[C@@H](Cc4cc(F)c(N)c(OC(C(F)(F)F)C(F)(F)F)c4)[C@@H]3O)cc12 |r| Show InChI InChI=1S/C26H29F7N2O5S/c1-24(2)17-6-13(3-4-15(17)10-39-24)9-35-19-12-41(37,38)11-16(22(19)36)5-14-7-18(27)21(34)20(8-14)40-23(25(28,29)30)26(31,32)33/h3-4,6-8,16,19,22-23,35-36H,5,9-12,34H2,1-2H3/t16-,19+,22+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE1 ectodomain after 1 hr by fluorescence analysis |
J Med Chem 55: 3364-86 (2012)
Article DOI: 10.1021/jm300069y
BindingDB Entry DOI: 10.7270/Q2P55PJW |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348481
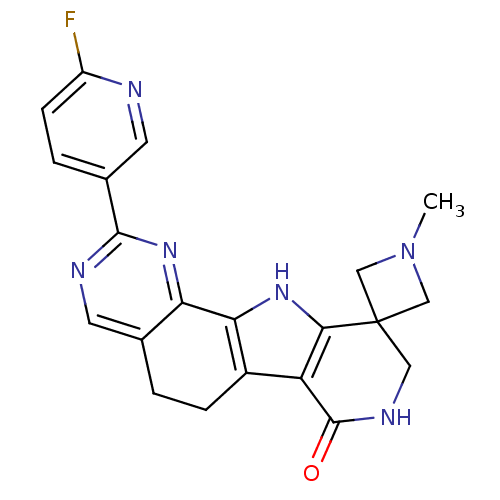
(CHEMBL1801307)Show SMILES CN1CC2(C1)CNC(=O)c1c3CCc4cnc(nc4-c3[nH]c21)-c1ccc(F)nc1 Show InChI InChI=1S/C21H19FN6O/c1-28-9-21(10-28)8-25-20(29)15-13-4-2-11-6-24-19(12-3-5-14(22)23-7-12)27-16(11)17(13)26-18(15)21/h3,5-7,26H,2,4,8-10H2,1H3,(H,25,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET analysis |
Bioorg Med Chem Lett 20: 4719-23 (2010)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.04.023
BindingDB Entry DOI: 10.7270/Q2BZ66C2 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50386486
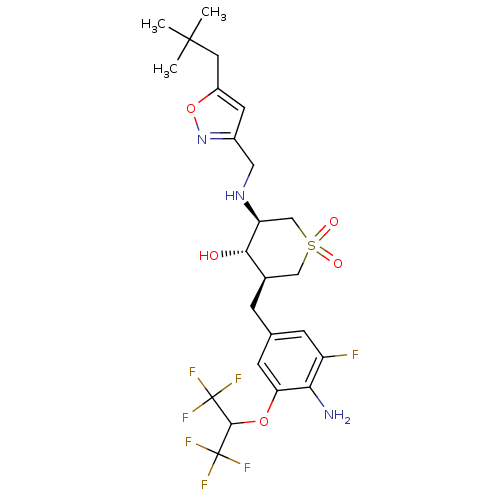
(CHEMBL2048063)Show SMILES CC(C)(C)Cc1cc(CN[C@H]2CS(=O)(=O)C[C@@H](Cc3cc(F)c(N)c(OC(C(F)(F)F)C(F)(F)F)c3)[C@@H]2O)no1 |r| Show InChI InChI=1S/C24H30F7N3O5S/c1-22(2,3)8-15-7-14(34-39-15)9-33-17-11-40(36,37)10-13(20(17)35)4-12-5-16(25)19(32)18(6-12)38-21(23(26,27)28)24(29,30)31/h5-7,13,17,20-21,33,35H,4,8-11,32H2,1-3H3/t13-,17+,20+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE1 ectodomain after 1 hr by fluorescence analysis |
J Med Chem 55: 3364-86 (2012)
Article DOI: 10.1021/jm300069y
BindingDB Entry DOI: 10.7270/Q2P55PJW |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348535

(CHEMBL1801374)Show SMILES O=C1NCc2[nH]c3c(ccc4cnc(\C=C\c5ccc(CN6CCOCC6)cc5)cc34)c12 Show InChI InChI=1S/C26H24N4O2/c31-26-24-21-8-6-19-14-27-20(13-22(19)25(21)29-23(24)15-28-26)7-5-17-1-3-18(4-2-17)16-30-9-11-32-12-10-30/h1-8,13-14,29H,9-12,15-16H2,(H,28,31)/b7-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET assay |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348494
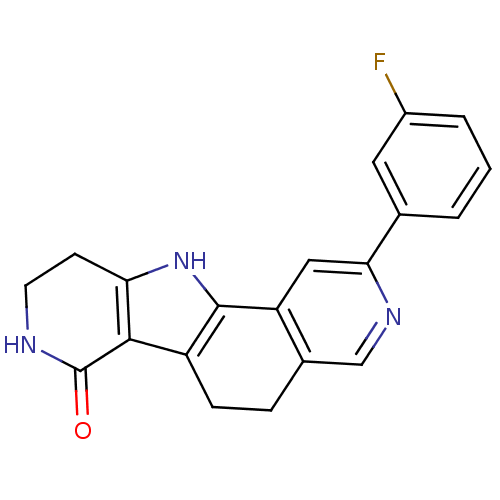
(CHEMBL1801274)Show SMILES Fc1cccc(c1)-c1cc2-c3[nH]c4CCNC(=O)c4c3CCc2cn1 Show InChI InChI=1S/C20H16FN3O/c21-13-3-1-2-11(8-13)17-9-15-12(10-23-17)4-5-14-18-16(24-19(14)15)6-7-22-20(18)25/h1-3,8-10,24H,4-7H2,(H,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET analysis |
Bioorg Med Chem Lett 20: 4719-23 (2010)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.04.023
BindingDB Entry DOI: 10.7270/Q2BZ66C2 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50386490
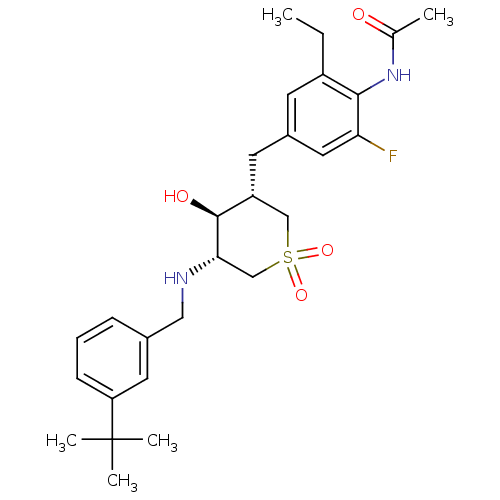
(CHEMBL2047905)Show SMILES CCc1cc(C[C@@H]2CS(=O)(=O)C[C@H](NCc3cccc(c3)C(C)(C)C)[C@H]2O)cc(F)c1NC(C)=O |r| Show InChI InChI=1S/C27H37FN2O4S/c1-6-20-10-19(13-23(28)25(20)30-17(2)31)11-21-15-35(33,34)16-24(26(21)32)29-14-18-8-7-9-22(12-18)27(3,4)5/h7-10,12-13,21,24,26,29,32H,6,11,14-16H2,1-5H3,(H,30,31)/t21-,24+,26+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE1 ectodomain after 1 hr by fluorescence analysis |
J Med Chem 55: 3364-86 (2012)
Article DOI: 10.1021/jm300069y
BindingDB Entry DOI: 10.7270/Q2P55PJW |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348534

(CHEMBL1801373)Show SMILES O=C1NCc2[nH]c3c(ccc4cnc(\C=C\c5ccccc5)cc34)c12 Show InChI InChI=1S/C21H15N3O/c25-21-19-16-9-7-14-11-22-15(8-6-13-4-2-1-3-5-13)10-17(14)20(16)24-18(19)12-23-21/h1-11,24H,12H2,(H,23,25)/b8-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET assay |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348536
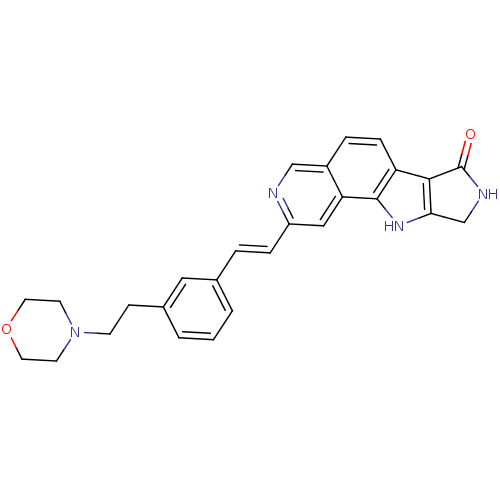
(CHEMBL1801375)Show SMILES O=C1NCc2[nH]c3c(ccc4cnc(\C=C\c5cccc(CCN6CCOCC6)c5)cc34)c12 Show InChI InChI=1S/C27H26N4O2/c32-27-25-22-7-5-20-16-28-21(15-23(20)26(22)30-24(25)17-29-27)6-4-18-2-1-3-19(14-18)8-9-31-10-12-33-13-11-31/h1-7,14-16,30H,8-13,17H2,(H,29,32)/b6-4+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET assay |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348495

(CHEMBL1614995)Show SMILES COCCOc1cncc(c1)-c1cc2c3[nH]c4CCNC(=O)c4c3ccc2cn1 Show InChI InChI=1S/C22H20N4O3/c1-28-6-7-29-15-8-14(10-23-12-15)19-9-17-13(11-25-19)2-3-16-20-18(26-21(16)17)4-5-24-22(20)27/h2-3,8-12,26H,4-7H2,1H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET analysis |
Bioorg Med Chem Lett 20: 4719-23 (2010)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.04.023
BindingDB Entry DOI: 10.7270/Q2BZ66C2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50386524
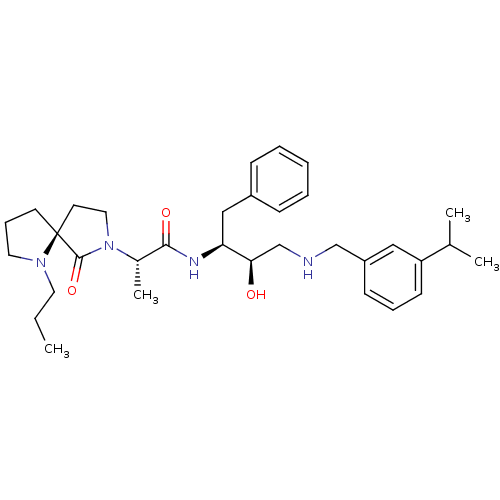
(CHEMBL2047887)Show SMILES CCCN1CCC[C@@]11CCN([C@@H](C)C(=O)N[C@@H](Cc2ccccc2)[C@H](O)CNCc2cccc(c2)C(C)C)C1=O |r| Show InChI InChI=1S/C33H48N4O3/c1-5-17-36-18-10-15-33(36)16-19-37(32(33)40)25(4)31(39)35-29(21-26-11-7-6-8-12-26)30(38)23-34-22-27-13-9-14-28(20-27)24(2)3/h6-9,11-14,20,24-25,29-30,34,38H,5,10,15-19,21-23H2,1-4H3,(H,35,39)/t25-,29-,30+,33-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of human BACE-1 mediated amyloid beta 40 release in human HEK293 cells |
J Med Chem 55: 3364-86 (2012)
Article DOI: 10.1021/jm300069y
BindingDB Entry DOI: 10.7270/Q2P55PJW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348524
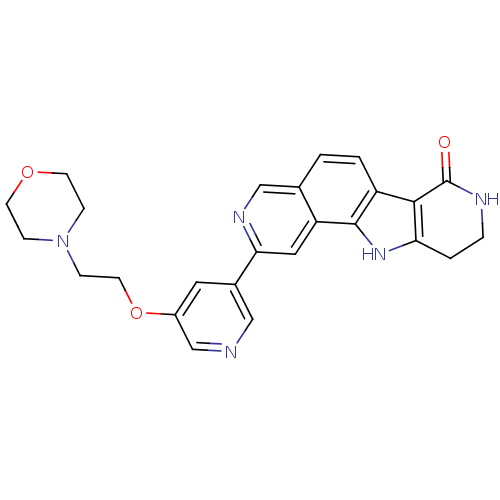
(CHEMBL1801388)Show SMILES O=C1NCCc2[nH]c3c(ccc4cnc(cc34)-c3cncc(OCCN4CCOCC4)c3)c12 Show InChI InChI=1S/C25H25N5O3/c31-25-23-19-2-1-16-14-28-22(12-20(16)24(19)29-21(23)3-4-27-25)17-11-18(15-26-13-17)33-10-7-30-5-8-32-9-6-30/h1-2,11-15,29H,3-10H2,(H,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET assay |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50386503

(CHEMBL2048040)Show SMILES CC(C)(C)c1cccc(CN[C@H]2CS(=O)(=O)C[C@@H](Cc3cc(F)c(N)c(OC4CCC4)c3)[C@@H]2O)c1 |r| Show InChI InChI=1S/C27H37FN2O4S/c1-27(2,3)20-7-4-6-17(11-20)14-30-23-16-35(32,33)15-19(26(23)31)10-18-12-22(28)25(29)24(13-18)34-21-8-5-9-21/h4,6-7,11-13,19,21,23,26,30-31H,5,8-10,14-16,29H2,1-3H3/t19-,23+,26+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE1 ectodomain after 1 hr by fluorescence analysis |
J Med Chem 55: 3364-86 (2012)
Article DOI: 10.1021/jm300069y
BindingDB Entry DOI: 10.7270/Q2P55PJW |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50348541
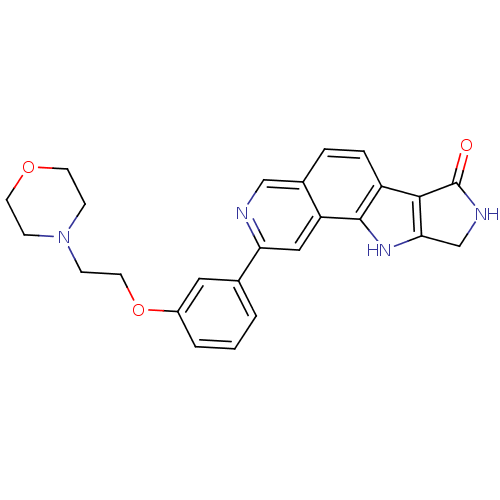
(CHEMBL1801380)Show SMILES O=C1NCc2[nH]c3c(ccc4cnc(cc34)-c3cccc(OCCN4CCOCC4)c3)c12 Show InChI InChI=1S/C25H24N4O3/c30-25-23-19-5-4-17-14-26-21(13-20(17)24(19)28-22(23)15-27-25)16-2-1-3-18(12-16)32-11-8-29-6-9-31-10-7-29/h1-5,12-14,28H,6-11,15H2,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of JNK2 |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348486
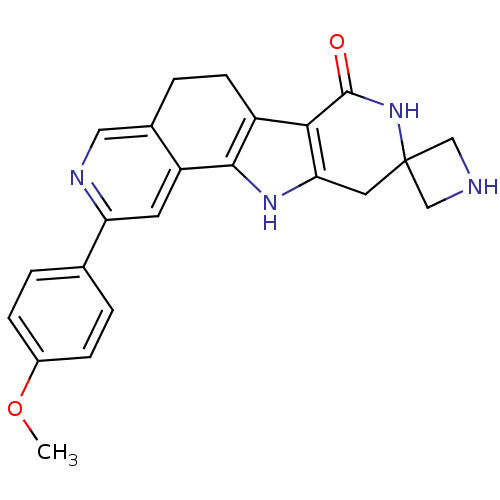
(CHEMBL1801294)Show SMILES COc1ccc(cc1)-c1cc2-c3[nH]c4CC5(CNC5)NC(=O)c4c3CCc2cn1 Show InChI InChI=1S/C23H22N4O2/c1-29-15-5-2-13(3-6-15)18-8-17-14(10-25-18)4-7-16-20-19(26-21(16)17)9-23(11-24-12-23)27-22(20)28/h2-3,5-6,8,10,24,26H,4,7,9,11-12H2,1H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET analysis |
Bioorg Med Chem Lett 20: 4719-23 (2010)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.04.023
BindingDB Entry DOI: 10.7270/Q2BZ66C2 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348495

(CHEMBL1614995)Show SMILES COCCOc1cncc(c1)-c1cc2c3[nH]c4CCNC(=O)c4c3ccc2cn1 Show InChI InChI=1S/C22H20N4O3/c1-28-6-7-29-15-8-14(10-23-12-15)19-9-17-13(11-25-19)2-3-16-20-18(26-21(16)17)4-5-24-22(20)27/h2-3,8-12,26H,4-7H2,1H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET assay |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348480
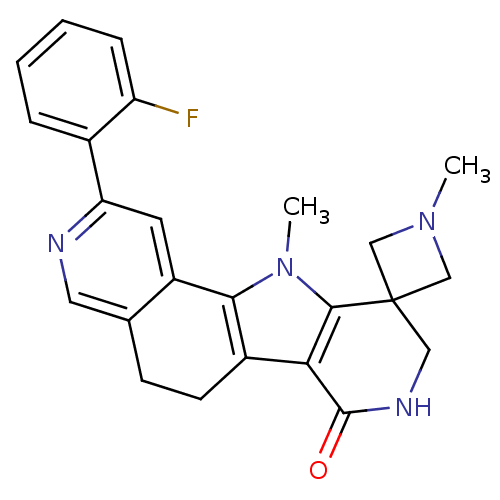
(CHEMBL1801312)Show SMILES CN1CC2(C1)CNC(=O)c1c3CCc4cnc(cc4-c3n(C)c21)-c1ccccc1F Show InChI InChI=1S/C24H23FN4O/c1-28-12-24(13-28)11-27-23(30)20-16-8-7-14-10-26-19(15-5-3-4-6-18(15)25)9-17(14)21(16)29(2)22(20)24/h3-6,9-10H,7-8,11-13H2,1-2H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET analysis |
Bioorg Med Chem Lett 20: 4719-23 (2010)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.04.023
BindingDB Entry DOI: 10.7270/Q2BZ66C2 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348530
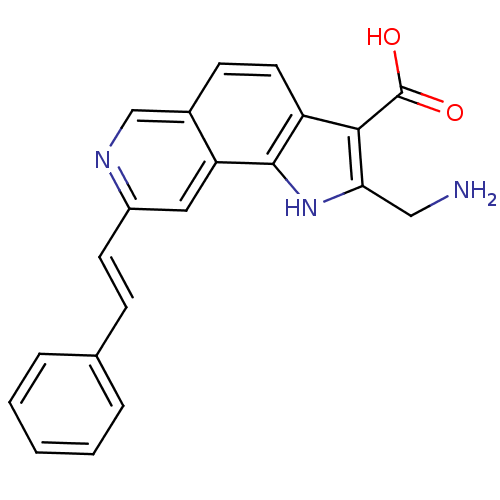
(CHEMBL1801313)Show SMILES NCc1[nH]c2c(ccc3cnc(\C=C\c4ccccc4)cc23)c1C(O)=O Show InChI InChI=1S/C21H17N3O2/c22-11-18-19(21(25)26)16-9-7-14-12-23-15(10-17(14)20(16)24-18)8-6-13-4-2-1-3-5-13/h1-10,12,24H,11,22H2,(H,25,26)/b8-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET assay |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348547
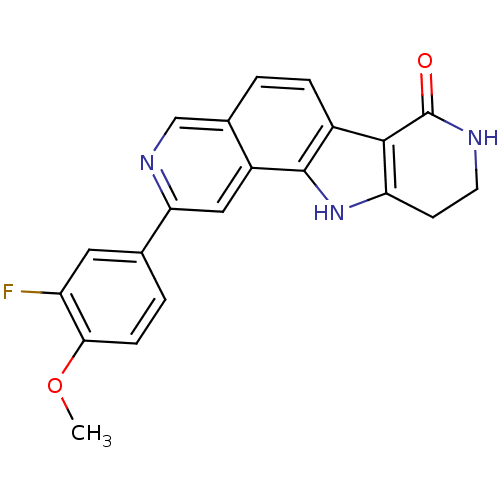
(CHEMBL1801386)Show SMILES COc1ccc(cc1F)-c1cc2c3[nH]c4CCNC(=O)c4c3ccc2cn1 Show InChI InChI=1S/C21H16FN3O2/c1-27-18-5-3-11(8-15(18)22)17-9-14-12(10-24-17)2-4-13-19-16(25-20(13)14)6-7-23-21(19)26/h2-5,8-10,25H,6-7H2,1H3,(H,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET assay |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348548
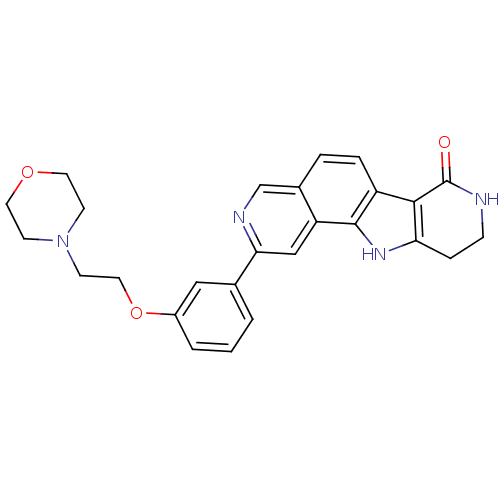
(CHEMBL1801387)Show SMILES O=C1NCCc2[nH]c3c(ccc4cnc(cc34)-c3cccc(OCCN4CCOCC4)c3)c12 Show InChI InChI=1S/C26H26N4O3/c31-26-24-20-5-4-18-16-28-23(15-21(18)25(20)29-22(24)6-7-27-26)17-2-1-3-19(14-17)33-13-10-30-8-11-32-12-9-30/h1-5,14-16,29H,6-13H2,(H,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET assay |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
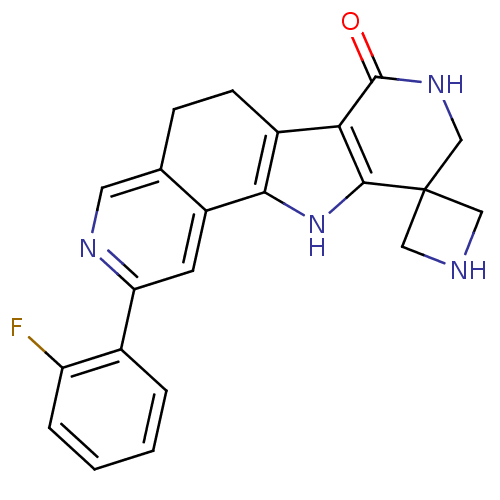
(Homo sapiens (Human)) | BDBM50348496
(CHEMBL1801284)Show SMILES Fc1ccccc1-c1cc2-c3[nH]c4c(c3CCc2cn1)C(=O)NCC41CNC1 Show InChI InChI=1S/C22H19FN4O/c23-16-4-2-1-3-13(16)17-7-15-12(8-25-17)5-6-14-18-20(27-19(14)15)22(9-24-10-22)11-26-21(18)28/h1-4,7-8,24,27H,5-6,9-11H2,(H,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET analysis |
Bioorg Med Chem Lett 20: 4719-23 (2010)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.04.023
BindingDB Entry DOI: 10.7270/Q2BZ66C2 |
More data for this
Ligand-Target Pair | |
Bifunctional 3'-5' exonuclease/ATP-dependent helicase WRN [517-1238]
(Homo sapiens (Human)) | BDBM646432
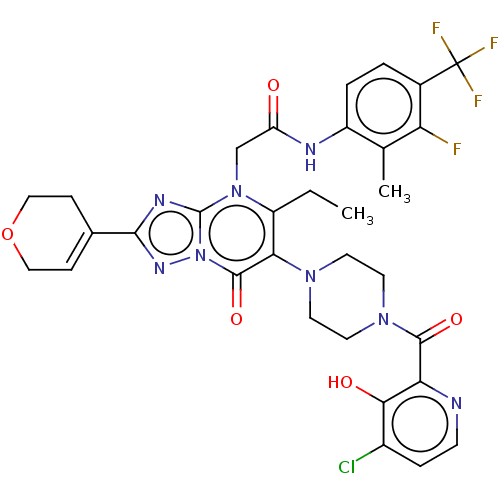
(US11878973, Example 37)Show SMILES CCc1c(N2CCN(CC2)C(=O)c2nccc(Cl)c2O)c(=O)n2nc(nc2n1CC(=O)Nc1ccc(c(F)c1C)C(F)(F)F)C1=CCOCC1 |t:49| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348497
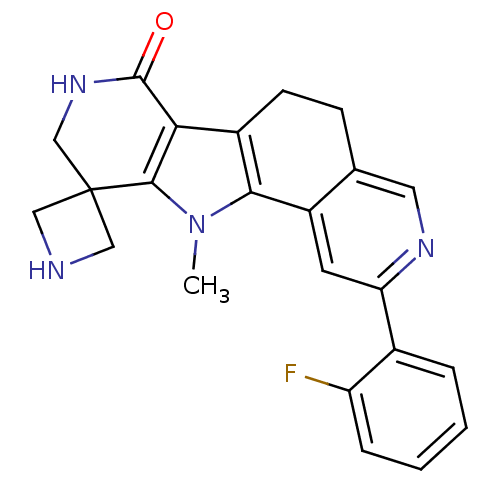
(CHEMBL1801311)Show SMILES Cn1c-2c(CCc3cnc(cc-23)-c2ccccc2F)c2c1C1(CNC1)CNC2=O Show InChI InChI=1S/C23H21FN4O/c1-28-20-15(19-21(28)23(10-25-11-23)12-27-22(19)29)7-6-13-9-26-18(8-16(13)20)14-4-2-3-5-17(14)24/h2-5,8-9,25H,6-7,10-12H2,1H3,(H,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET analysis |
Bioorg Med Chem Lett 20: 4719-23 (2010)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.04.023
BindingDB Entry DOI: 10.7270/Q2BZ66C2 |
More data for this
Ligand-Target Pair | |
Bifunctional 3'-5' exonuclease/ATP-dependent helicase WRN [517-1238]
(Homo sapiens (Human)) | BDBM646438

(US11878973, Example 40)Show SMILES CCc1c(N2CCN(CC2)C(=O)c2nccc(F)c2O)c(=O)n2nc(nc2n1CC(=O)Nc1ccc(cc1Cl)C(F)(F)F)C1=CCOCC1 |t:48| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50348535

(CHEMBL1801374)Show SMILES O=C1NCc2[nH]c3c(ccc4cnc(\C=C\c5ccc(CN6CCOCC6)cc5)cc34)c12 Show InChI InChI=1S/C26H24N4O2/c31-26-24-21-8-6-19-14-27-20(13-22(19)25(21)29-23(24)15-28-26)7-5-17-1-3-18(4-2-17)16-30-9-11-32-12-10-30/h1-8,13-14,29H,9-12,15-16H2,(H,28,31)/b7-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of JNK2 |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348533
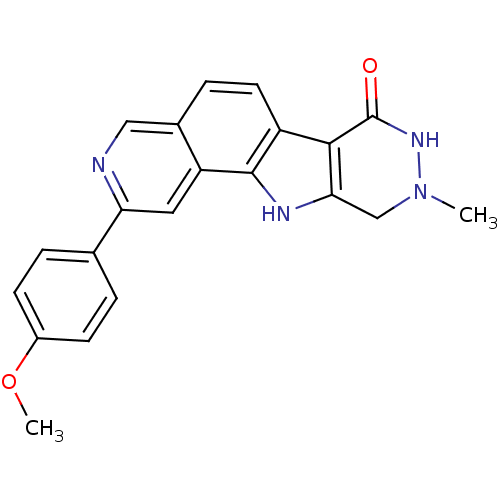
(CHEMBL1801316)Show SMILES COc1ccc(cc1)-c1cc2c3[nH]c4CN(C)NC(=O)c4c3ccc2cn1 Show InChI InChI=1S/C21H18N4O2/c1-25-11-18-19(21(26)24-25)15-8-5-13-10-22-17(9-16(13)20(15)23-18)12-3-6-14(27-2)7-4-12/h3-10,23H,11H2,1-2H3,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET assay |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50386502
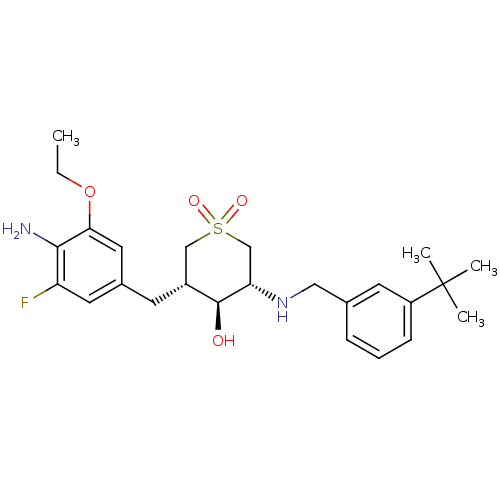
(CHEMBL2048039)Show SMILES CCOc1cc(C[C@@H]2CS(=O)(=O)C[C@H](NCc3cccc(c3)C(C)(C)C)[C@H]2O)cc(F)c1N |r| Show InChI InChI=1S/C25H35FN2O4S/c1-5-32-22-12-17(11-20(26)23(22)27)9-18-14-33(30,31)15-21(24(18)29)28-13-16-7-6-8-19(10-16)25(2,3)4/h6-8,10-12,18,21,24,28-29H,5,9,13-15,27H2,1-4H3/t18-,21+,24+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE1 ectodomain after 1 hr by fluorescence analysis |
J Med Chem 55: 3364-86 (2012)
Article DOI: 10.1021/jm300069y
BindingDB Entry DOI: 10.7270/Q2P55PJW |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50386504
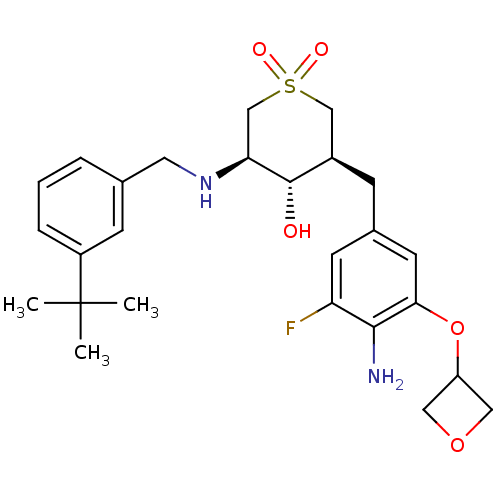
(CHEMBL2048041)Show SMILES CC(C)(C)c1cccc(CN[C@H]2CS(=O)(=O)C[C@@H](Cc3cc(F)c(N)c(OC4COC4)c3)[C@@H]2O)c1 |r| Show InChI InChI=1S/C26H35FN2O5S/c1-26(2,3)19-6-4-5-16(8-19)11-29-22-15-35(31,32)14-18(25(22)30)7-17-9-21(27)24(28)23(10-17)34-20-12-33-13-20/h4-6,8-10,18,20,22,25,29-30H,7,11-15,28H2,1-3H3/t18-,22+,25+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE1 ectodomain after 1 hr by fluorescence analysis |
J Med Chem 55: 3364-86 (2012)
Article DOI: 10.1021/jm300069y
BindingDB Entry DOI: 10.7270/Q2P55PJW |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348532

(CHEMBL1801315)Show SMILES OC1=NOCc2[nH]c3c(ccc4cnc(C=Cc5ccccc5)cc34)c12 |w:15.14,t:1| Show InChI InChI=1S/C21H15N3O2/c25-21-19-16-9-7-14-11-22-15(8-6-13-4-2-1-3-5-13)10-17(14)20(16)23-18(19)12-26-24-21/h1-11,23H,12H2,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET assay |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348498
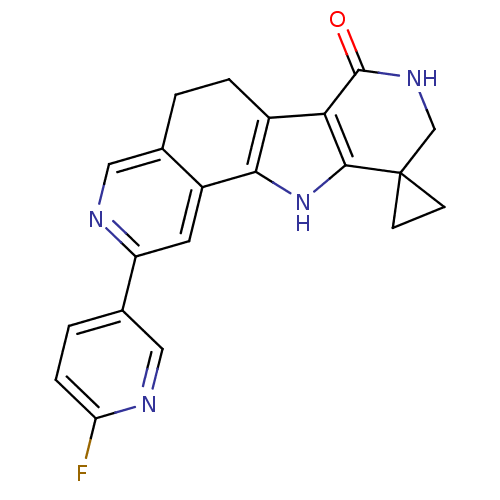
(CHEMBL1801283)Show SMILES Fc1ccc(cn1)-c1cc2-c3[nH]c4c(c3CCc2cn1)C(=O)NCC41CC1 Show InChI InChI=1S/C21H17FN4O/c22-16-4-2-12(9-24-16)15-7-14-11(8-23-15)1-3-13-17-19(26-18(13)14)21(5-6-21)10-25-20(17)27/h2,4,7-9,26H,1,3,5-6,10H2,(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET analysis |
Bioorg Med Chem Lett 20: 4719-23 (2010)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.04.023
BindingDB Entry DOI: 10.7270/Q2BZ66C2 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348538
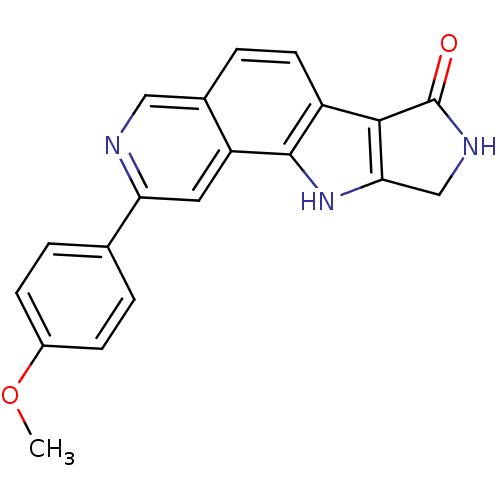
(CHEMBL1801377)Show SMILES COc1ccc(cc1)-c1cc2c3[nH]c4CNC(=O)c4c3ccc2cn1 Show InChI InChI=1S/C20H15N3O2/c1-25-13-5-2-11(3-6-13)16-8-15-12(9-21-16)4-7-14-18-17(23-19(14)15)10-22-20(18)24/h2-9,23H,10H2,1H3,(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET assay |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data