 Found 4982 hits with Last Name = 'maduskuie' and Initial = 't'
Found 4982 hits with Last Name = 'maduskuie' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50227363

(CHEMBL299837)Show SMILES COc1cc(ccc1Cc1nn(C)c2ccc(NC(=O)NC3CCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1Cl Show InChI InChI=1S/C28H28ClN5O5S/c1-34-24-13-12-20(31-28(36)30-19-6-5-7-19)16-21(24)23(32-34)14-17-10-11-18(15-25(17)39-2)27(35)33-40(37,38)26-9-4-3-8-22(26)29/h3-4,8-13,15-16,19H,5-7,14H2,1-2H3,(H,33,35)(H2,30,31,36) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. |
J Med Chem 33: 1781-90 (1990)
BindingDB Entry DOI: 10.7270/Q2862FDP |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50227366

(CHEMBL48927)Show SMILES COCCn1cc(Cc2ccc(cc2OC)C(=O)NS(=O)(=O)c2ccccc2)c2cc(NC(=O)OC3CCCC3)ccc12 Show InChI InChI=1S/C32H35N3O7S/c1-40-17-16-35-21-24(28-20-25(14-15-29(28)35)33-32(37)42-26-8-6-7-9-26)18-22-12-13-23(19-30(22)41-2)31(36)34-43(38,39)27-10-4-3-5-11-27/h3-5,10-15,19-21,26H,6-9,16-18H2,1-2H3,(H,33,37)(H,34,36) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. |
J Med Chem 33: 1781-90 (1990)
BindingDB Entry DOI: 10.7270/Q2862FDP |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50227361
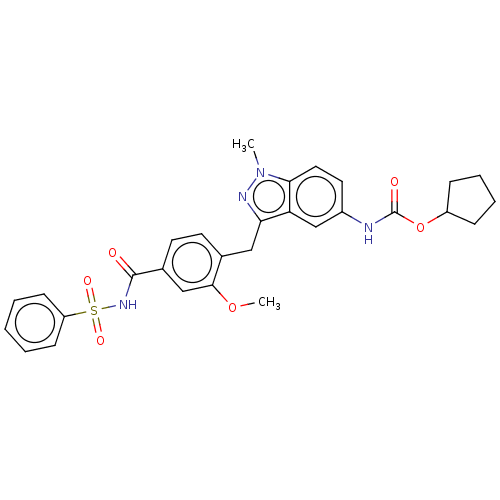
(CHEMBL51585)Show SMILES COc1cc(ccc1Cc1nn(C)c2ccc(NC(=O)OC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1 Show InChI InChI=1S/C29H30N4O6S/c1-33-26-15-14-21(30-29(35)39-22-8-6-7-9-22)18-24(26)25(31-33)16-19-12-13-20(17-27(19)38-2)28(34)32-40(36,37)23-10-4-3-5-11-23/h3-5,10-15,17-18,22H,6-9,16H2,1-2H3,(H,30,35)(H,32,34) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. |
J Med Chem 33: 1781-90 (1990)
BindingDB Entry DOI: 10.7270/Q2862FDP |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50227364
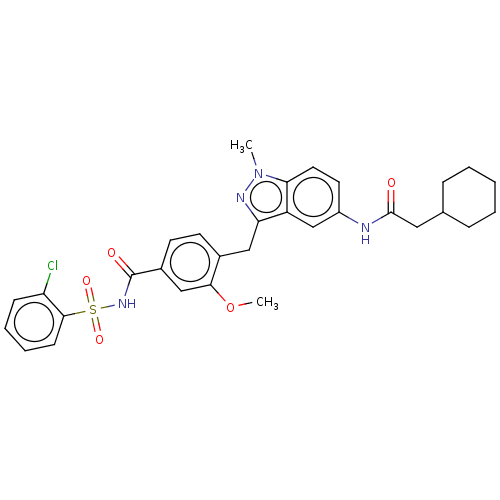
(CHEMBL299093)Show SMILES COc1cc(ccc1Cc1nn(C)c2ccc(NC(=O)CC3CCCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1Cl Show InChI InChI=1S/C31H33ClN4O5S/c1-36-27-15-14-23(33-30(37)16-20-8-4-3-5-9-20)19-24(27)26(34-36)17-21-12-13-22(18-28(21)41-2)31(38)35-42(39,40)29-11-7-6-10-25(29)32/h6-7,10-15,18-20H,3-5,8-9,16-17H2,1-2H3,(H,33,37)(H,35,38) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. |
J Med Chem 33: 1781-90 (1990)
BindingDB Entry DOI: 10.7270/Q2862FDP |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50015528

(CHEMBL50370 | N-[3-(4-Benzenesulfonylaminocarbonyl...)Show SMILES COc1cc(ccc1Cc1cn(C)c2ccc(NC(=O)CC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1 Show InChI InChI=1S/C31H33N3O5S/c1-34-20-24(27-19-25(14-15-28(27)34)32-30(35)16-21-8-6-7-9-21)17-22-12-13-23(18-29(22)39-2)31(36)33-40(37,38)26-10-4-3-5-11-26/h3-5,10-15,18-21H,6-9,16-17H2,1-2H3,(H,32,35)(H,33,36) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LTD4 on guinea pig lung parenchymal membranes |
J Med Chem 33: 1781-90 (1990)
BindingDB Entry DOI: 10.7270/Q2862FDP |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50227640

(CHEMBL297952)Show SMILES COc1cc(ccc1Cc1cn(C)c2ccc(NC(=O)CC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1Cl Show InChI InChI=1S/C31H32ClN3O5S/c1-35-19-23(25-18-24(13-14-27(25)35)33-30(36)15-20-7-3-4-8-20)16-21-11-12-22(17-28(21)40-2)31(37)34-41(38,39)29-10-6-5-9-26(29)32/h5-6,9-14,17-20H,3-4,7-8,15-16H2,1-2H3,(H,33,36)(H,34,37) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. |
J Med Chem 33: 1781-90 (1990)
BindingDB Entry DOI: 10.7270/Q2862FDP |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50227642

(CHEMBL50562)Show SMILES COc1cc(ccc1Cc1nn(C)c2ccc(NC(=O)CC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1C Show InChI InChI=1S/C31H34N4O5S/c1-20-8-4-7-11-29(20)41(38,39)34-31(37)23-13-12-22(28(18-23)40-3)17-26-25-19-24(14-15-27(25)35(2)33-26)32-30(36)16-21-9-5-6-10-21/h4,7-8,11-15,18-19,21H,5-6,9-10,16-17H2,1-3H3,(H,32,36)(H,34,37) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. |
J Med Chem 33: 1781-90 (1990)
BindingDB Entry DOI: 10.7270/Q2862FDP |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50227355

(CHEMBL296821)Show SMILES COc1cc(ccc1Cc1nn(C)c2ccc(NC(=O)OC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1Br Show InChI InChI=1S/C29H29BrN4O6S/c1-34-25-14-13-20(31-29(36)40-21-7-3-4-8-21)17-22(25)24(32-34)15-18-11-12-19(16-26(18)39-2)28(35)33-41(37,38)27-10-6-5-9-23(27)30/h5-6,9-14,16-17,21H,3-4,7-8,15H2,1-2H3,(H,31,36)(H,33,35) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. |
J Med Chem 33: 1781-90 (1990)
BindingDB Entry DOI: 10.7270/Q2862FDP |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50227356

(CHEMBL49944)Show SMILES COc1cc(ccc1Cc1nn(C)c2ccc(NC(=O)OC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1Cl Show InChI InChI=1S/C29H29ClN4O6S/c1-34-25-14-13-20(31-29(36)40-21-7-3-4-8-21)17-22(25)24(32-34)15-18-11-12-19(16-26(18)39-2)28(35)33-41(37,38)27-10-6-5-9-23(27)30/h5-6,9-14,16-17,21H,3-4,7-8,15H2,1-2H3,(H,31,36)(H,33,35) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. |
J Med Chem 33: 1781-90 (1990)
BindingDB Entry DOI: 10.7270/Q2862FDP |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50227358

(CHEMBL412056)Show SMILES COc1cc(ccc1Cc1nn(C)c2ccc(NC(=O)OC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1C Show InChI InChI=1S/C30H32N4O6S/c1-19-8-4-7-11-28(19)41(37,38)33-29(35)21-13-12-20(27(17-21)39-3)16-25-24-18-22(14-15-26(24)34(2)32-25)31-30(36)40-23-9-5-6-10-23/h4,7-8,11-15,17-18,23H,5-6,9-10,16H2,1-3H3,(H,31,36)(H,33,35) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. |
J Med Chem 33: 1781-90 (1990)
BindingDB Entry DOI: 10.7270/Q2862FDP |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50227365
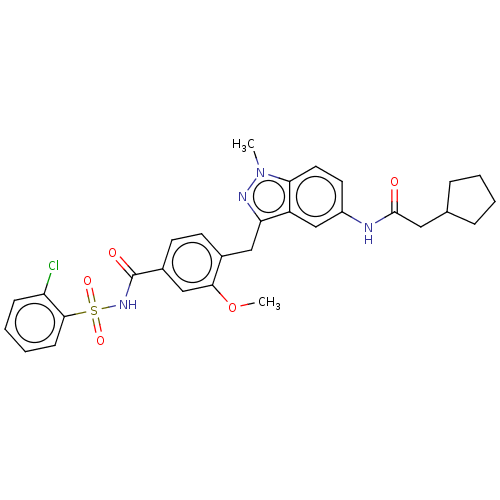
(CHEMBL300096)Show SMILES COc1cc(ccc1Cc1nn(C)c2ccc(NC(=O)CC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1Cl Show InChI InChI=1S/C30H31ClN4O5S/c1-35-26-14-13-22(32-29(36)15-19-7-3-4-8-19)18-23(26)25(33-35)16-20-11-12-21(17-27(20)40-2)30(37)34-41(38,39)28-10-6-5-9-24(28)31/h5-6,9-14,17-19H,3-4,7-8,15-16H2,1-2H3,(H,32,36)(H,34,37) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. |
J Med Chem 33: 1781-90 (1990)
BindingDB Entry DOI: 10.7270/Q2862FDP |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50009073

(4-(5-cyclopentyloxycarbonylamino-1-methyl-1H-indol...)Show SMILES COc1cc(ccc1Cc1cn(C)c2ccc(NC(=O)OC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1C Show InChI InChI=1S/C31H33N3O6S/c1-20-8-4-7-11-29(20)41(37,38)33-30(35)22-13-12-21(28(17-22)39-3)16-23-19-34(2)27-15-14-24(18-26(23)27)32-31(36)40-25-9-5-6-10-25/h4,7-8,11-15,17-19,25H,5-6,9-10,16H2,1-3H3,(H,32,36)(H,33,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LTD4 on guinea pig lung parenchymal membranes |
J Med Chem 33: 1781-90 (1990)
BindingDB Entry DOI: 10.7270/Q2862FDP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Disintegrin and metalloproteinase domain-containing protein 17
(Sus scrofa (pig)) | BDBM50183715
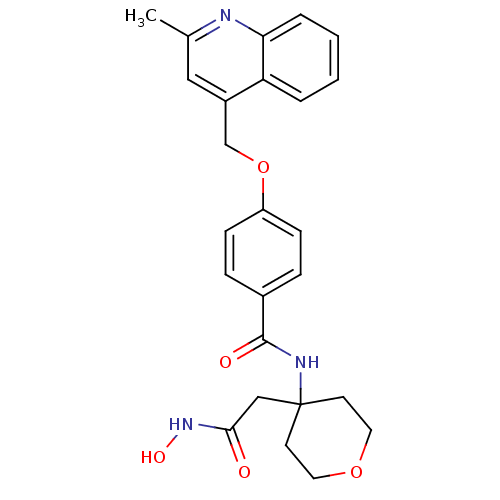
(CHEMBL207305 | N-(4-(2-(hydroxyamino)-2-oxoethyl)-...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NC2(CC(=O)NO)CCOCC2)c2ccccc2n1 Show InChI InChI=1S/C25H27N3O5/c1-17-14-19(21-4-2-3-5-22(21)26-17)16-33-20-8-6-18(7-9-20)24(30)27-25(15-23(29)28-31)10-12-32-13-11-25/h2-9,14,31H,10-13,15-16H2,1H3,(H,27,30)(H,28,29) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of porcine TACE |
Bioorg Med Chem Lett 16: 2699-704 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.015
BindingDB Entry DOI: 10.7270/Q2TB16H3 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50227390

(CHEMBL301616)Show SMILES COc1cc(ccc1Cc1cn(C)c2ccc(NC(=O)CC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1Br Show InChI InChI=1S/C31H32BrN3O5S/c1-35-19-23(25-18-24(13-14-27(25)35)33-30(36)15-20-7-3-4-8-20)16-21-11-12-22(17-28(21)40-2)31(37)34-41(38,39)29-10-6-5-9-26(29)32/h5-6,9-14,17-20H,3-4,7-8,15-16H2,1-2H3,(H,33,36)(H,34,37) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. |
J Med Chem 33: 1781-90 (1990)
BindingDB Entry DOI: 10.7270/Q2862FDP |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50015523

(CHEMBL52492 | N-{4-[5-(3-Cyclopentyl-ureido)-1-pro...)Show SMILES CCCn1cc(Cc2ccc(cc2OC)C(=O)NS(=O)(=O)c2ccccc2)c2cc(NC(=O)NC3CCCC3)ccc12 Show InChI InChI=1S/C32H36N4O5S/c1-3-17-36-21-24(28-20-26(15-16-29(28)36)34-32(38)33-25-9-7-8-10-25)18-22-13-14-23(19-30(22)41-2)31(37)35-42(39,40)27-11-5-4-6-12-27/h4-6,11-16,19-21,25H,3,7-10,17-18H2,1-2H3,(H,35,37)(H2,33,34,38) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LTD4 on guinea pig lung parenchymal membranes |
J Med Chem 33: 1781-90 (1990)
BindingDB Entry DOI: 10.7270/Q2862FDP |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50000819

(2-Cyclopentyl-N-{3-[2-methoxy-4-(toluene-2-sulfony...)Show SMILES COc1cc(ccc1Cc1cn(C)c2ccc(NC(=O)CC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1C Show InChI InChI=1S/C32H35N3O5S/c1-21-8-4-7-11-30(21)41(38,39)34-32(37)24-13-12-23(29(18-24)40-3)17-25-20-35(2)28-15-14-26(19-27(25)28)33-31(36)16-22-9-5-6-10-22/h4,7-8,11-15,18-20,22H,5-6,9-10,16-17H2,1-3H3,(H,33,36)(H,34,37) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LTD4 on guinea pig lung parenchymal membranes |
J Med Chem 33: 1781-90 (1990)
BindingDB Entry DOI: 10.7270/Q2862FDP |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50227389
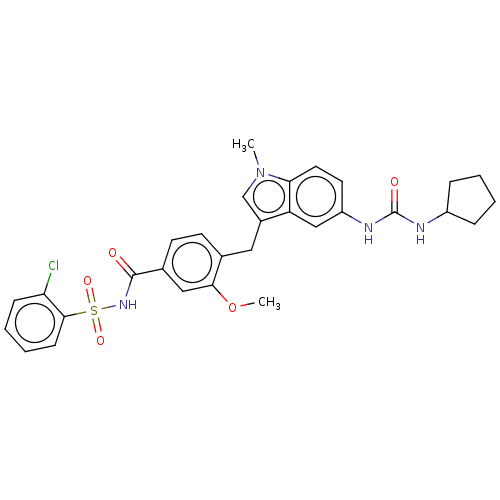
(CHEMBL51580)Show SMILES COc1cc(ccc1Cc1cn(C)c2ccc(NC(=O)NC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1Cl Show InChI InChI=1S/C30H31ClN4O5S/c1-35-18-21(24-17-23(13-14-26(24)35)33-30(37)32-22-7-3-4-8-22)15-19-11-12-20(16-27(19)40-2)29(36)34-41(38,39)28-10-6-5-9-25(28)31/h5-6,9-14,16-18,22H,3-4,7-8,15H2,1-2H3,(H,34,36)(H2,32,33,37) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. |
J Med Chem 33: 1781-90 (1990)
BindingDB Entry DOI: 10.7270/Q2862FDP |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50015551

(CHEMBL301498 | N-{4-[5-(3-Cyclopentyl-ureido)-1-me...)Show SMILES COc1cc(ccc1Cc1cn(C)c2ccc(NC(=O)NC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1 Show InChI InChI=1S/C30H32N4O5S/c1-34-19-22(26-18-24(14-15-27(26)34)32-30(36)31-23-8-6-7-9-23)16-20-12-13-21(17-28(20)39-2)29(35)33-40(37,38)25-10-4-3-5-11-25/h3-5,10-15,17-19,23H,6-9,16H2,1-2H3,(H,33,35)(H2,31,32,36) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LTD4 on guinea pig lung parenchymal membranes |
J Med Chem 33: 1781-90 (1990)
BindingDB Entry DOI: 10.7270/Q2862FDP |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50227367
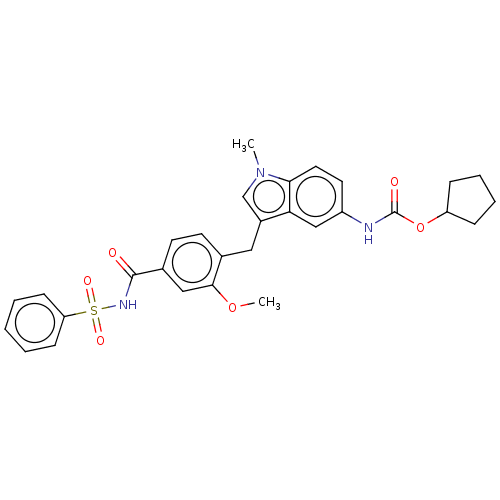
(CHEMBL48435)Show SMILES COc1cc(ccc1Cc1cn(C)c2ccc(NC(=O)OC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1 Show InChI InChI=1S/C30H31N3O6S/c1-33-19-22(26-18-23(14-15-27(26)33)31-30(35)39-24-8-6-7-9-24)16-20-12-13-21(17-28(20)38-2)29(34)32-40(36,37)25-10-4-3-5-11-25/h3-5,10-15,17-19,24H,6-9,16H2,1-2H3,(H,31,35)(H,32,34) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. |
J Med Chem 33: 1781-90 (1990)
BindingDB Entry DOI: 10.7270/Q2862FDP |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50015517

(2-Cyclopentyl-N-{3-[2-methoxy-4-(toluene-2-sulfony...)Show SMILES COc1cc(ccc1Cc1c[nH]c2ccc(NC(=O)CC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1C Show InChI InChI=1S/C31H33N3O5S/c1-20-7-3-6-10-29(20)40(37,38)34-31(36)23-12-11-22(28(17-23)39-2)16-24-19-32-27-14-13-25(18-26(24)27)33-30(35)15-21-8-4-5-9-21/h3,6-7,10-14,17-19,21,32H,4-5,8-9,15-16H2,1-2H3,(H,33,35)(H,34,36) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LTD4 on guinea pig lung parenchymal membranes |
J Med Chem 33: 1781-90 (1990)
BindingDB Entry DOI: 10.7270/Q2862FDP |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50227638

(CHEMBL49566)Show SMILES COc1cc(ccc1Cc1c[nH]c2ccc(NC(=O)OC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1 Show InChI InChI=1S/C29H29N3O6S/c1-37-27-16-20(28(33)32-39(35,36)24-9-3-2-4-10-24)12-11-19(27)15-21-18-30-26-14-13-22(17-25(21)26)31-29(34)38-23-7-5-6-8-23/h2-4,9-14,16-18,23,30H,5-8,15H2,1H3,(H,31,34)(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. |
J Med Chem 33: 1781-90 (1990)
BindingDB Entry DOI: 10.7270/Q2862FDP |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50015540

(CHEMBL431348 | N-{4-[5-(3-Cyclopentyl-ureido)-1-me...)Show SMILES COc1cc(ccc1Cc1cn(C)c2ccc(NC(=O)NC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1C Show InChI InChI=1S/C31H34N4O5S/c1-20-8-4-7-11-29(20)41(38,39)34-30(36)22-13-12-21(28(17-22)40-3)16-23-19-35(2)27-15-14-25(18-26(23)27)33-31(37)32-24-9-5-6-10-24/h4,7-8,11-15,17-19,24H,5-6,9-10,16H2,1-3H3,(H,34,36)(H2,32,33,37) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LTD4 on guinea pig lung parenchymal membranes |
J Med Chem 33: 1781-90 (1990)
BindingDB Entry DOI: 10.7270/Q2862FDP |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50182403

((2R,3S)-N1-((S)-1-(3-phenoxybenzyl)-2-oxoazepan-3-...)Show SMILES CCC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H]1CCCCN(Cc2cccc(Oc3ccccc3)c2)C1=O)C(=O)NO Show InChI InChI=1S/C30H41N3O5/c1-4-11-25(29(35)32-37)26(18-21(2)3)28(34)31-27-16-8-9-17-33(30(27)36)20-22-12-10-15-24(19-22)38-23-13-6-5-7-14-23/h5-7,10,12-15,19,21,25-27,37H,4,8-9,11,16-18,20H2,1-3H3,(H,31,34)(H,32,35)/t25-,26+,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 16: 2357-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.055
BindingDB Entry DOI: 10.7270/Q2WD405K |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50009070
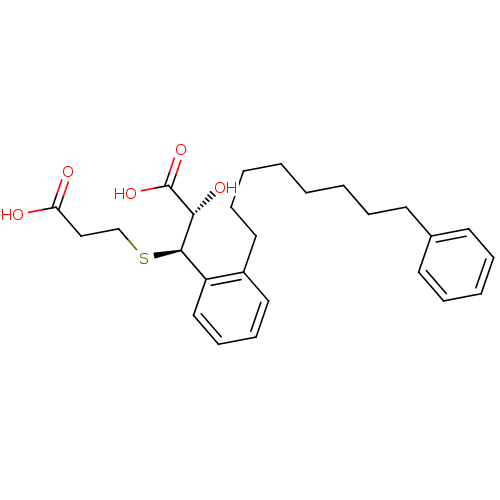
((2S,3R)-3-(2-Carboxy-ethylsulfanyl)-2-hydroxy-3-[2...)Show SMILES O[C@H]([C@H](SCCC(O)=O)c1ccccc1CCCCCCCCc1ccccc1)C(O)=O Show InChI InChI=1S/C26H34O5S/c27-23(28)18-19-32-25(24(29)26(30)31)22-17-11-10-16-21(22)15-9-4-2-1-3-6-12-20-13-7-5-8-14-20/h5,7-8,10-11,13-14,16-17,24-25,29H,1-4,6,9,12,15,18-19H2,(H,27,28)(H,30,31)/t24-,25-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
Inhibition constant for displasement of [3H]-LTD4 on guinea pig lung parenchymal membranes. |
J Med Chem 33: 1781-90 (1990)
BindingDB Entry DOI: 10.7270/Q2862FDP |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50182403

((2R,3S)-N1-((S)-1-(3-phenoxybenzyl)-2-oxoazepan-3-...)Show SMILES CCC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H]1CCCCN(Cc2cccc(Oc3ccccc3)c2)C1=O)C(=O)NO Show InChI InChI=1S/C30H41N3O5/c1-4-11-25(29(35)32-37)26(18-21(2)3)28(34)31-27-16-8-9-17-33(30(27)36)20-22-12-10-15-24(19-22)38-23-13-6-5-7-14-23/h5-7,10,12-15,19,21,25-27,37H,4,8-9,11,16-18,20H2,1-3H3,(H,31,34)(H,32,35)/t25-,26+,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 16: 2357-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.055
BindingDB Entry DOI: 10.7270/Q2WD405K |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50227354

(CHEMBL51289)Show SMILES COc1cc(ccc1Cc1cn(Cc2cccc(c2)C#N)c2ccc(NC(=O)CC3CCCC3)cc12)C(=O)NS(C)(=O)=O Show InChI InChI=1S/C33H34N4O5S/c1-42-31-17-26(33(39)36-43(2,40)41)11-10-25(31)16-27-21-37(20-24-9-5-8-23(14-24)19-34)30-13-12-28(18-29(27)30)35-32(38)15-22-6-3-4-7-22/h5,8-14,17-18,21-22H,3-4,6-7,15-16,20H2,1-2H3,(H,35,38)(H,36,39) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. |
J Med Chem 33: 1781-90 (1990)
BindingDB Entry DOI: 10.7270/Q2862FDP |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50227353
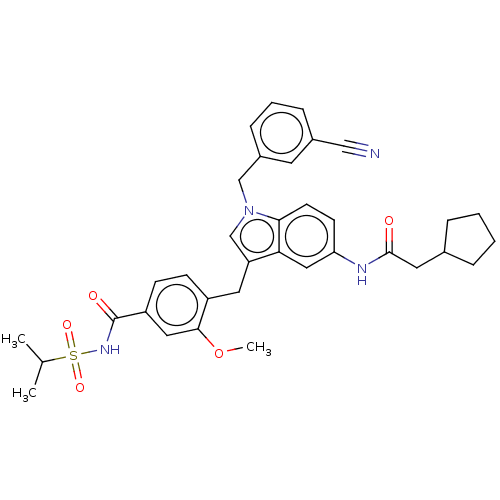
(CHEMBL51239)Show SMILES COc1cc(ccc1Cc1cn(Cc2cccc(c2)C#N)c2ccc(NC(=O)CC3CCCC3)cc12)C(=O)NS(=O)(=O)C(C)C Show InChI InChI=1S/C35H38N4O5S/c1-23(2)45(42,43)38-35(41)28-12-11-27(33(18-28)44-3)17-29-22-39(21-26-10-6-9-25(15-26)20-36)32-14-13-30(19-31(29)32)37-34(40)16-24-7-4-5-8-24/h6,9-15,18-19,22-24H,4-5,7-8,16-17,21H2,1-3H3,(H,37,40)(H,38,41) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. |
J Med Chem 33: 1781-90 (1990)
BindingDB Entry DOI: 10.7270/Q2862FDP |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50182403

((2R,3S)-N1-((S)-1-(3-phenoxybenzyl)-2-oxoazepan-3-...)Show SMILES CCC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H]1CCCCN(Cc2cccc(Oc3ccccc3)c2)C1=O)C(=O)NO Show InChI InChI=1S/C30H41N3O5/c1-4-11-25(29(35)32-37)26(18-21(2)3)28(34)31-27-16-8-9-17-33(30(27)36)20-22-12-10-15-24(19-22)38-23-13-6-5-7-14-23/h5-7,10,12-15,19,21,25-27,37H,4,8-9,11,16-18,20H2,1-3H3,(H,31,34)(H,32,35)/t25-,26+,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 16: 2357-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.055
BindingDB Entry DOI: 10.7270/Q2WD405K |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50062027
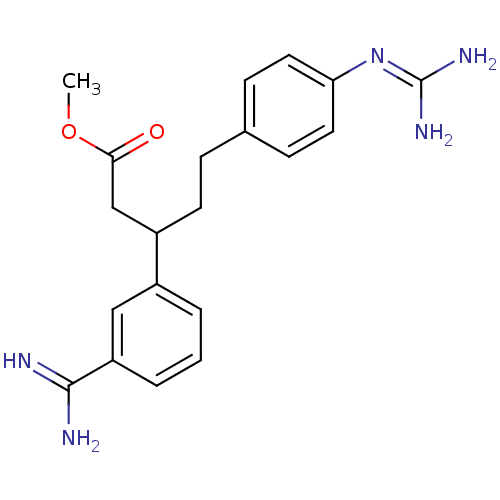
(3-(3-Carbamimidoyl-phenyl)-5-(4-guanidino-phenyl)-...)Show SMILES [#6]-[#8]-[#6](=O)-[#6]-[#6](-[#6]-[#6]-c1ccc(cc1)\[#7]=[#6](/[#7])-[#7])-c1cccc(c1)-[#6](-[#7])=[#7] Show InChI InChI=1S/C20H25N5O2/c1-27-18(26)12-15(14-3-2-4-16(11-14)19(21)22)8-5-13-6-9-17(10-7-13)25-20(23)24/h2-4,6-7,9-11,15H,5,8,12H2,1H3,(H3,21,22)(H4,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
In vitro inhibition of Coagulation factor X |
J Med Chem 41: 53-62 (1998)
Article DOI: 10.1021/jm970485a
BindingDB Entry DOI: 10.7270/Q2R49PW5 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50227359
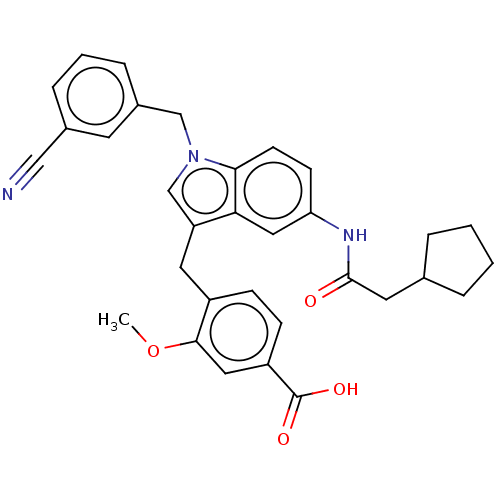
(CHEMBL51061)Show SMILES COc1cc(ccc1Cc1cn(Cc2cccc(c2)C#N)c2ccc(NC(=O)CC3CCCC3)cc12)C(O)=O Show InChI InChI=1S/C32H31N3O4/c1-39-30-16-25(32(37)38)10-9-24(30)15-26-20-35(19-23-8-4-7-22(13-23)18-33)29-12-11-27(17-28(26)29)34-31(36)14-21-5-2-3-6-21/h4,7-13,16-17,20-21H,2-3,5-6,14-15,19H2,1H3,(H,34,36)(H,37,38) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. |
J Med Chem 33: 1781-90 (1990)
BindingDB Entry DOI: 10.7270/Q2862FDP |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50085518

(Cyclic urea 2,4-diazepin-3-one analogue | bis(2,2,...)Show SMILES NC(=[NH2+])c1cccc(c1)N1CCCCN(C2CCN(CC2)S(=O)(=O)Cc2ccccn2)C1=O Show InChI InChI=1S/C23H30N6O3S/c24-22(25)18-6-5-8-21(16-18)29-13-4-3-12-28(23(29)30)20-9-14-27(15-10-20)33(31,32)17-19-7-1-2-11-26-19/h1-2,5-8,11,16,20H,3-4,9-10,12-15,17H2,(H3,24,25)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Inhibition of coagulation factor Xa |
Bioorg Med Chem Lett 10: 301-4 (2000)
BindingDB Entry DOI: 10.7270/Q2639Q7R |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50182403

((2R,3S)-N1-((S)-1-(3-phenoxybenzyl)-2-oxoazepan-3-...)Show SMILES CCC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H]1CCCCN(Cc2cccc(Oc3ccccc3)c2)C1=O)C(=O)NO Show InChI InChI=1S/C30H41N3O5/c1-4-11-25(29(35)32-37)26(18-21(2)3)28(34)31-27-16-8-9-17-33(30(27)36)20-22-12-10-15-24(19-22)38-23-13-6-5-7-14-23/h5-7,10,12-15,19,21,25-27,37H,4,8-9,11,16-18,20H2,1-3H3,(H,31,34)(H,32,35)/t25-,26+,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 16: 2357-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.055
BindingDB Entry DOI: 10.7270/Q2WD405K |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50085511
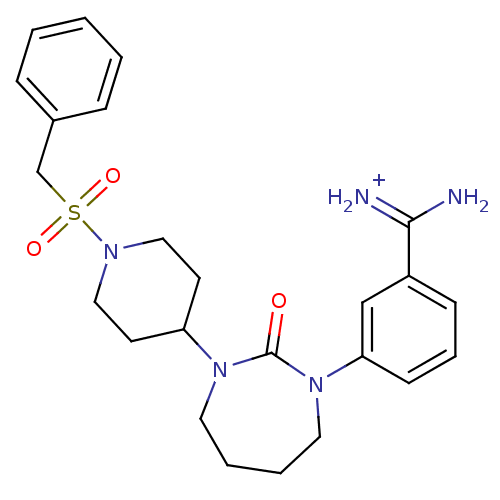
(CHEMBL103993 | Cyclic urea 2,4-diazepin-3-one anal...)Show SMILES NC(=[NH2+])c1cccc(c1)N1CCCCN(C2CCN(CC2)S(=O)(=O)Cc2ccccc2)C1=O Show InChI InChI=1S/C24H31N5O3S/c25-23(26)20-9-6-10-22(17-20)29-14-5-4-13-28(24(29)30)21-11-15-27(16-12-21)33(31,32)18-19-7-2-1-3-8-19/h1-3,6-10,17,21H,4-5,11-16,18H2,(H3,25,26)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Inhibition of coagulation factor Xa |
Bioorg Med Chem Lett 10: 301-4 (2000)
BindingDB Entry DOI: 10.7270/Q2639Q7R |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50085509
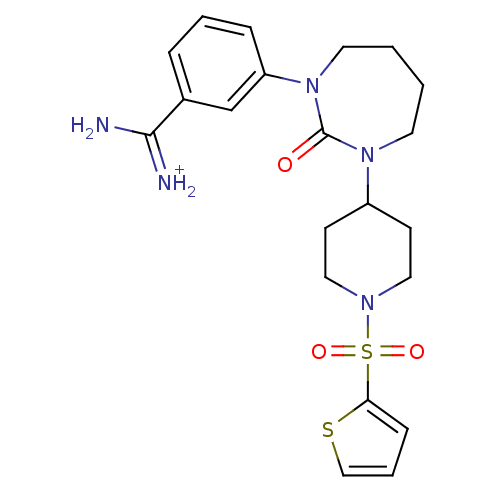
(CHEMBL104995 | Cyclic urea 2,4-diazepin-3-one anal...)Show SMILES NC(=[NH2+])c1cccc(c1)N1CCCCN(C2CCN(CC2)S(=O)(=O)c2cccs2)C1=O Show InChI InChI=1S/C21H27N5O3S2/c22-20(23)16-5-3-6-18(15-16)26-11-2-1-10-25(21(26)27)17-8-12-24(13-9-17)31(28,29)19-7-4-14-30-19/h3-7,14-15,17H,1-2,8-13H2,(H3,22,23)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Inhibition of coagulation factor Xa |
Bioorg Med Chem Lett 10: 301-4 (2000)
BindingDB Entry DOI: 10.7270/Q2639Q7R |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50085523
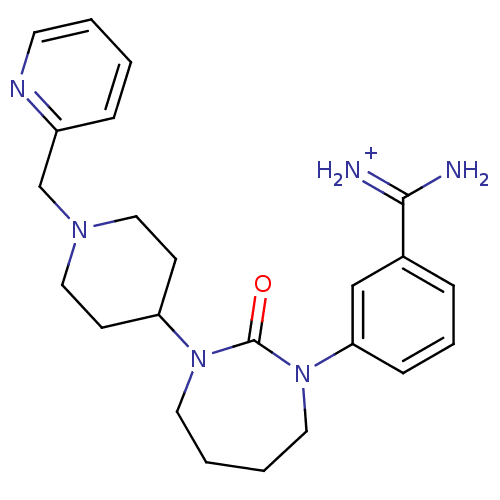
(Cyclic urea 2,4-diazepin-3-one analogue | bis(2,2,...)Show SMILES NC(=[NH2+])c1cccc(c1)N1CCCCN(C2CCN(Cc3ccccn3)CC2)C1=O Show InChI InChI=1S/C23H30N6O/c24-22(25)18-6-5-8-21(16-18)29-13-4-3-12-28(23(29)30)20-9-14-27(15-10-20)17-19-7-1-2-11-26-19/h1-2,5-8,11,16,20H,3-4,9-10,12-15,17H2,(H3,24,25)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Inhibition of coagulation factor Xa |
Bioorg Med Chem Lett 10: 301-4 (2000)
BindingDB Entry DOI: 10.7270/Q2639Q7R |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50183711
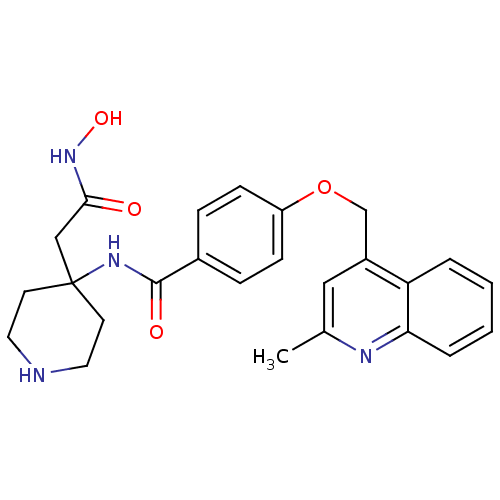
(CHEMBL208009 | N-(4-(2-(hydroxyamino)-2-oxoethyl)p...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NC2(CC(=O)NO)CCNCC2)c2ccccc2n1 Show InChI InChI=1S/C25H28N4O4/c1-17-14-19(21-4-2-3-5-22(21)27-17)16-33-20-8-6-18(7-9-20)24(31)28-25(15-23(30)29-32)10-12-26-13-11-25/h2-9,14,26,32H,10-13,15-16H2,1H3,(H,28,31)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to MMP7 |
Bioorg Med Chem Lett 16: 2699-704 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.015
BindingDB Entry DOI: 10.7270/Q2TB16H3 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50182403

((2R,3S)-N1-((S)-1-(3-phenoxybenzyl)-2-oxoazepan-3-...)Show SMILES CCC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H]1CCCCN(Cc2cccc(Oc3ccccc3)c2)C1=O)C(=O)NO Show InChI InChI=1S/C30H41N3O5/c1-4-11-25(29(35)32-37)26(18-21(2)3)28(34)31-27-16-8-9-17-33(30(27)36)20-22-12-10-15-24(19-22)38-23-13-6-5-7-14-23/h5-7,10,12-15,19,21,25-27,37H,4,8-9,11,16-18,20H2,1-3H3,(H,31,34)(H,32,35)/t25-,26+,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP1 |
Bioorg Med Chem Lett 16: 2357-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.055
BindingDB Entry DOI: 10.7270/Q2WD405K |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50085519

(CHEMBL102502 | Cyclic urea 2,4-diazepin-3-one anal...)Show SMILES NC(=[NH2+])c1cccc(c1)N1CCCCN(C2CCN(CC2)S(=O)(=O)c2ccccc2)C1=O Show InChI InChI=1S/C23H29N5O3S/c24-22(25)18-7-6-8-20(17-18)28-14-5-4-13-27(23(28)29)19-11-15-26(16-12-19)32(30,31)21-9-2-1-3-10-21/h1-3,6-10,17,19H,4-5,11-16H2,(H3,24,25)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Inhibition of coagulation factor Xa |
Bioorg Med Chem Lett 10: 301-4 (2000)
BindingDB Entry DOI: 10.7270/Q2639Q7R |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50183715

(CHEMBL207305 | N-(4-(2-(hydroxyamino)-2-oxoethyl)-...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NC2(CC(=O)NO)CCOCC2)c2ccccc2n1 Show InChI InChI=1S/C25H27N3O5/c1-17-14-19(21-4-2-3-5-22(21)26-17)16-33-20-8-6-18(7-9-20)24(30)27-25(15-23(29)28-31)10-12-32-13-11-25/h2-9,14,31H,10-13,15-16H2,1H3,(H,27,30)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to MMP7 |
Bioorg Med Chem Lett 16: 2699-704 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.015
BindingDB Entry DOI: 10.7270/Q2TB16H3 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50071948
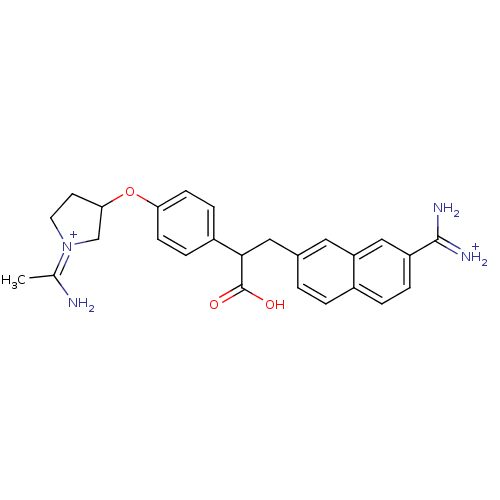
(CHEMBL314535 | Urea analogue)Show SMILES C\C(N)=[N+]1/CCC(C1)Oc1ccc(cc1)C(Cc1ccc2ccc(cc2c1)C(N)=[NH2+])C(O)=O Show InChI InChI=1S/C26H28N4O3/c1-16(27)30-11-10-23(15-30)33-22-8-6-19(7-9-22)24(26(31)32)13-17-2-3-18-4-5-20(25(28)29)14-21(18)12-17/h2-9,12,14,23-24,27H,10-11,13,15H2,1H3,(H4,28,29,31,32)/p+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of blood coagulation protein factor Xa |
Bioorg Med Chem Lett 8: 2705-10 (1999)
BindingDB Entry DOI: 10.7270/Q23R0S13 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50062035

(5-(4-Carbamimidoyl-phenyl)-3-(3-carbamimidoyl-phen...)Show SMILES COC(=O)CC(CCc1ccc(cc1)C(N)=N)c1cccc(c1)C(N)=N Show InChI InChI=1S/C20H24N4O2/c1-26-18(25)12-16(15-3-2-4-17(11-15)20(23)24)10-7-13-5-8-14(9-6-13)19(21)22/h2-6,8-9,11,16H,7,10,12H2,1H3,(H3,21,22)(H3,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
In vitro inhibition of Coagulation factor X |
J Med Chem 41: 53-62 (1998)
Article DOI: 10.1021/jm970485a
BindingDB Entry DOI: 10.7270/Q2R49PW5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50085515

(Cyclic urea 2,4-diazepin-3-one analogue | bis(2,2,...)Show SMILES NC(=[NH2+])c1cccc(c1)N1CCCCN(C2CCN(CC2)S(=O)(=O)c2ccccc2N)C1=O Show InChI InChI=1S/C23H30N6O3S/c24-20-8-1-2-9-21(20)33(31,32)27-14-10-18(11-15-27)28-12-3-4-13-29(23(28)30)19-7-5-6-17(16-19)22(25)26/h1-2,5-9,16,18H,3-4,10-15,24H2,(H3,25,26)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Inhibition of coagulation factor Xa |
Bioorg Med Chem Lett 10: 301-4 (2000)
BindingDB Entry DOI: 10.7270/Q2639Q7R |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50015542
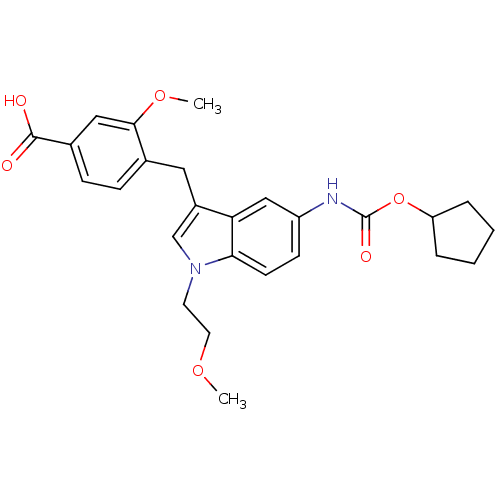
(4-[5-Cyclopentyloxycarbonylamino-1-(2-methoxy-ethy...)Show SMILES COCCn1cc(Cc2ccc(cc2OC)C(O)=O)c2cc(NC(=O)OC3CCCC3)ccc12 Show InChI InChI=1S/C26H30N2O6/c1-32-12-11-28-16-19(13-17-7-8-18(25(29)30)14-24(17)33-2)22-15-20(9-10-23(22)28)27-26(31)34-21-5-3-4-6-21/h7-10,14-16,21H,3-6,11-13H2,1-2H3,(H,27,31)(H,29,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
Inhibition constant for displacement of [3H]-LTD4 on guinea pig lung parenchymal membranes. |
J Med Chem 33: 1781-90 (1990)
BindingDB Entry DOI: 10.7270/Q2862FDP |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50062033
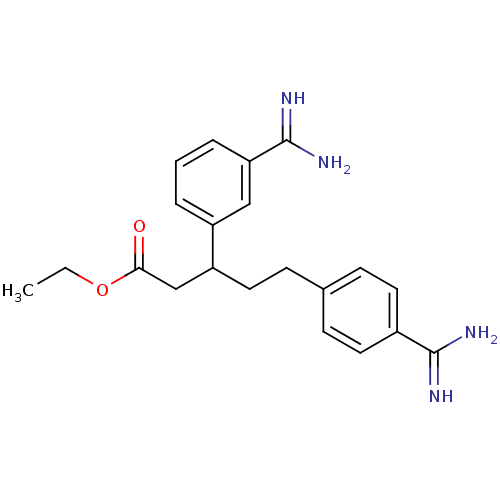
(5-(4-Carbamimidoyl-phenyl)-3-(3-carbamimidoyl-phen...)Show SMILES CCOC(=O)CC(CCc1ccc(cc1)C(N)=N)c1cccc(c1)C(N)=N Show InChI InChI=1S/C21H26N4O2/c1-2-27-19(26)13-17(16-4-3-5-18(12-16)21(24)25)11-8-14-6-9-15(10-7-14)20(22)23/h3-7,9-10,12,17H,2,8,11,13H2,1H3,(H3,22,23)(H3,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
In vitro inhibition of Coagulation factor X |
J Med Chem 41: 53-62 (1998)
Article DOI: 10.1021/jm970485a
BindingDB Entry DOI: 10.7270/Q2R49PW5 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50015516
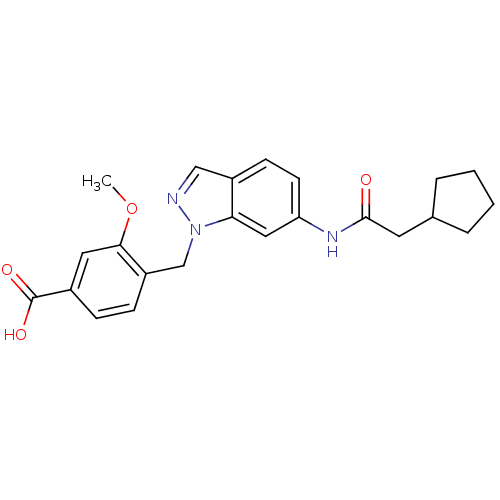
(4-[6-(2-Cyclopentyl-acetylamino)-indazol-1-ylmethy...)Show SMILES COc1cc(ccc1Cn1ncc2ccc(NC(=O)CC3CCCC3)cc12)C(O)=O Show InChI InChI=1S/C23H25N3O4/c1-30-21-11-16(23(28)29)6-7-18(21)14-26-20-12-19(9-8-17(20)13-24-26)25-22(27)10-15-4-2-3-5-15/h6-9,11-13,15H,2-5,10,14H2,1H3,(H,25,27)(H,28,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LTD4 on guinea pig lung parenchymal membranes |
J Med Chem 33: 1781-90 (1990)
BindingDB Entry DOI: 10.7270/Q2862FDP |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50085509

(CHEMBL104995 | Cyclic urea 2,4-diazepin-3-one anal...)Show SMILES NC(=[NH2+])c1cccc(c1)N1CCCCN(C2CCN(CC2)S(=O)(=O)c2cccs2)C1=O Show InChI InChI=1S/C21H27N5O3S2/c22-20(23)16-5-3-6-18(15-16)26-11-2-1-10-25(21(26)27)17-8-12-24(13-9-17)31(28,29)19-7-4-14-30-19/h3-7,14-15,17H,1-2,8-13H2,(H3,22,23)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against trypsin |
Bioorg Med Chem Lett 10: 301-4 (2000)
BindingDB Entry DOI: 10.7270/Q2639Q7R |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50227357

(CHEMBL49981)Show SMILES COc1cc(ccc1Cc1cn(C2CCCC2)c2ccc(NC(=O)OC3CCCC3)cc12)C(O)=O Show InChI InChI=1S/C28H32N2O5/c1-34-26-15-19(27(31)32)11-10-18(26)14-20-17-30(22-6-2-3-7-22)25-13-12-21(16-24(20)25)29-28(33)35-23-8-4-5-9-23/h10-13,15-17,22-23H,2-9,14H2,1H3,(H,29,33)(H,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. |
J Med Chem 33: 1781-90 (1990)
BindingDB Entry DOI: 10.7270/Q2862FDP |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50062038

(3-(3-Carbamimidoyl-phenyl)-7-(4-guanidino-phenyl)-...)Show SMILES [#6]-[#8]-[#6](=O)-[#6]-[#6](-[#6]-[#6]-[#6]-[#6]-c1ccc(cc1)\[#7]=[#6](/[#7])-[#7])-c1cccc(c1)-[#6](-[#7])=[#7] Show InChI InChI=1S/C22H29N5O2/c1-29-20(28)14-17(16-7-4-8-18(13-16)21(23)24)6-3-2-5-15-9-11-19(12-10-15)27-22(25)26/h4,7-13,17H,2-3,5-6,14H2,1H3,(H3,23,24)(H4,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
In vitro inhibition of Coagulation factor X |
J Med Chem 41: 53-62 (1998)
Article DOI: 10.1021/jm970485a
BindingDB Entry DOI: 10.7270/Q2R49PW5 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50085518

(Cyclic urea 2,4-diazepin-3-one analogue | bis(2,2,...)Show SMILES NC(=[NH2+])c1cccc(c1)N1CCCCN(C2CCN(CC2)S(=O)(=O)Cc2ccccn2)C1=O Show InChI InChI=1S/C23H30N6O3S/c24-22(25)18-6-5-8-21(16-18)29-13-4-3-12-28(23(29)30)20-9-14-27(15-10-20)33(31,32)17-19-7-1-2-11-26-19/h1-2,5-8,11,16,20H,3-4,9-10,12-15,17H2,(H3,24,25)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against trypsin |
Bioorg Med Chem Lett 10: 301-4 (2000)
BindingDB Entry DOI: 10.7270/Q2639Q7R |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50227360

(CHEMBL50364)Show SMILES COc1cc(ccc1Cc1cn(CC2CC2)c2ccc(NC(=O)OC3CCCC3)cc12)C(O)=O Show InChI InChI=1S/C27H30N2O5/c1-33-25-13-19(26(30)31)9-8-18(25)12-20-16-29(15-17-6-7-17)24-11-10-21(14-23(20)24)28-27(32)34-22-4-2-3-5-22/h8-11,13-14,16-17,22H,2-7,12,15H2,1H3,(H,28,32)(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. |
J Med Chem 33: 1781-90 (1990)
BindingDB Entry DOI: 10.7270/Q2862FDP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data