 Found 585 hits with Last Name = 'majellaro' and Initial = 'm'
Found 585 hits with Last Name = 'majellaro' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50146585
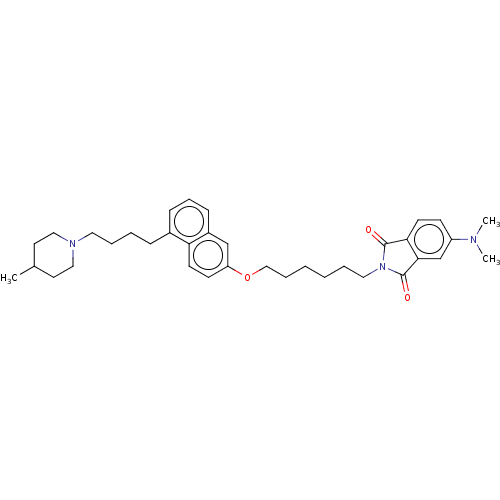
(CHEMBL3763396)Show SMILES CC1CCN(CCCCc2cccc3cc(OCCCCCCN4C(=O)c5ccc(cc5C4=O)N(C)C)ccc23)CC1 Show InChI InChI=1S/C36H47N3O3/c1-27-18-22-38(23-19-27)20-8-6-11-28-12-10-13-29-25-31(15-17-32(28)29)42-24-9-5-4-7-21-39-35(40)33-16-14-30(37(2)3)26-34(33)36(39)41/h10,12-17,25-27H,4-9,11,18-24H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50443101

(Cariprazine | RGH-188)Show SMILES CN(C)C(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc(Cl)c2Cl)CC1 |r,wU:6.5,wD:9.9,(-.77,5.69,;.57,4.92,;1.9,5.69,;.57,3.38,;-.77,2.61,;1.9,2.61,;1.9,1.07,;3.23,.3,;3.23,-1.24,;1.9,-2.01,;1.9,-3.55,;3.23,-4.32,;3.23,-5.86,;4.57,-6.63,;4.57,-8.17,;3.23,-8.94,;1.9,-8.17,;1.9,-6.63,;3.23,-10.48,;4.57,-11.25,;4.57,-12.79,;3.23,-13.56,;1.9,-12.79,;.57,-13.56,;1.9,-11.25,;.57,-10.48,;.57,-1.24,;.57,.3,)| Show InChI InChI=1S/C21H32Cl2N4O/c1-25(2)21(28)24-17-8-6-16(7-9-17)10-11-26-12-14-27(15-13-26)19-5-3-4-18(22)20(19)23/h3-5,16-17H,6-15H2,1-2H3,(H,24,28)/t16-,17- | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| | 0.0850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50161342
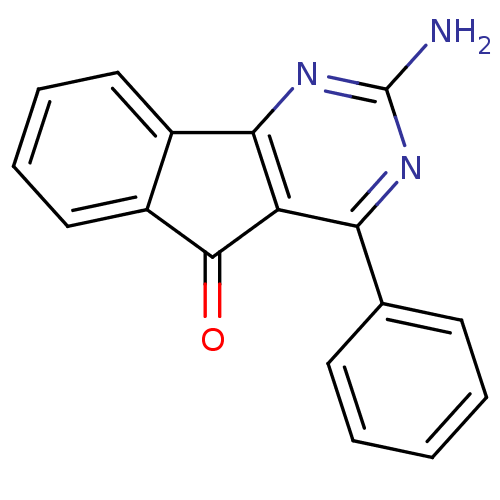
(2-Amino-4-phenyl-indeno[1,2-d]pyrimidin-5-one | 2-...)Show InChI InChI=1S/C17H11N3O/c18-17-19-14(10-6-2-1-3-7-10)13-15(20-17)11-8-4-5-9-12(11)16(13)21/h1-9H,(H2,18,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPCPX from A1 adenosine receptor (unknown origin) expressed in CHO cell membrane incubated for 60 mins at room temperature by NXT... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01636
BindingDB Entry DOI: 10.7270/Q2M61Q5K |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50130293

(7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...)Show SMILES Clc1cccc(N2CCN(CCCCOc3ccc4CCC(=O)Nc4c3)CC2)c1Cl Show InChI InChI=1S/C23H27Cl2N3O2/c24-19-4-3-5-21(23(19)25)28-13-11-27(12-14-28)10-1-2-15-30-18-8-6-17-7-9-22(29)26-20(17)16-18/h3-6,8,16H,1-2,7,9-15H2,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50048866

(1-Cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydro-na...)Show InChI InChI=1S/C24H38N2O/c1-27-24-14-6-12-22-20(8-5-13-23(22)24)9-7-15-25-16-18-26(19-17-25)21-10-3-2-4-11-21/h6,12,14,20-21H,2-5,7-11,13,15-19H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Rattus norvegicus (Rat)) | BDBM50613242
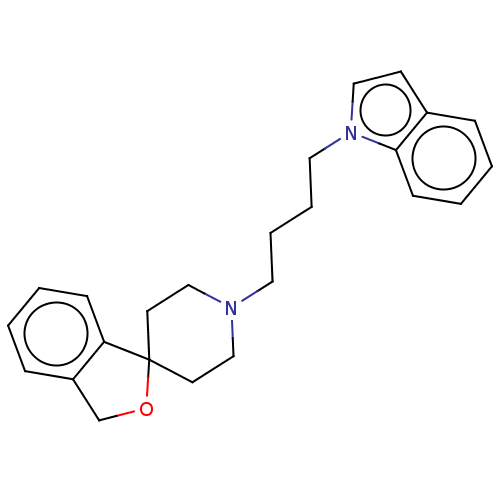
(CHEMBL5271751) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50268107
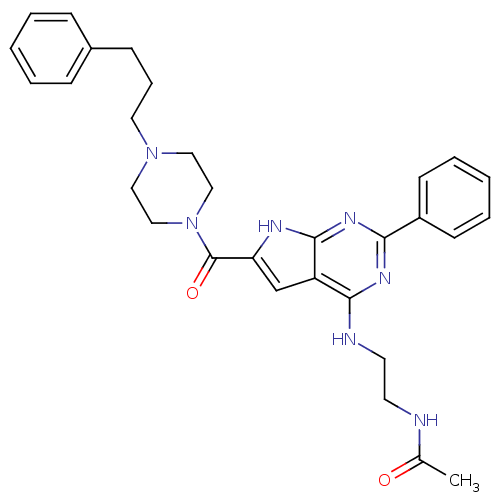
(CHEMBL485862 | CHEMBL500634 | N-(2-(2-phenyl-6-(4-...)Show SMILES CC(=O)NCCNc1nc(nc2[nH]c(cc12)C(=O)N1CCN(CCCc2ccccc2)CC1)-c1ccccc1 Show InChI InChI=1S/C30H35N7O2/c1-22(38)31-14-15-32-28-25-21-26(33-29(25)35-27(34-28)24-12-6-3-7-13-24)30(39)37-19-17-36(18-20-37)16-8-11-23-9-4-2-5-10-23/h2-7,9-10,12-13,21H,8,11,14-20H2,1H3,(H,31,38)(H2,32,33,34,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant A2B receptor expressed in HEK293 cell membranes |
J Med Chem 62: 9315-9330 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01340
BindingDB Entry DOI: 10.7270/Q29C71VG |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50268107

(CHEMBL485862 | CHEMBL500634 | N-(2-(2-phenyl-6-(4-...)Show SMILES CC(=O)NCCNc1nc(nc2[nH]c(cc12)C(=O)N1CCN(CCCc2ccccc2)CC1)-c1ccccc1 Show InChI InChI=1S/C30H35N7O2/c1-22(38)31-14-15-32-28-25-21-26(33-29(25)35-27(34-28)24-12-6-3-7-13-24)30(39)37-19-17-36(18-20-37)16-8-11-23-9-4-2-5-10-23/h2-7,9-10,12-13,21H,8,11,14-20H2,1H3,(H,31,38)(H2,32,33,34,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant A2B receptor expressed in HEK293 cell membranes |
J Med Chem 62: 9315-9330 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01340
BindingDB Entry DOI: 10.7270/Q29C71VG |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50268107

(CHEMBL485862 | CHEMBL500634 | N-(2-(2-phenyl-6-(4-...)Show SMILES CC(=O)NCCNc1nc(nc2[nH]c(cc12)C(=O)N1CCN(CCCc2ccccc2)CC1)-c1ccccc1 Show InChI InChI=1S/C30H35N7O2/c1-22(38)31-14-15-32-28-25-21-26(33-29(25)35-27(34-28)24-12-6-3-7-13-24)30(39)37-19-17-36(18-20-37)16-8-11-23-9-4-2-5-10-23/h2-7,9-10,12-13,21H,8,11,14-20H2,1H3,(H,31,38)(H2,32,33,34,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human A2B receptor |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01431
BindingDB Entry DOI: 10.7270/Q2JQ14S5 |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Homo sapiens (Human)) | BDBM50613240
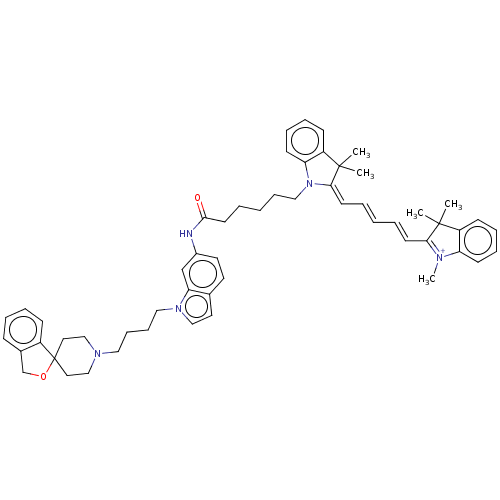
(CHEMBL5278003)Show SMILES [I-].C[N+]1=C(\C=C\C=C\C=C2\N(CCCCCC(=O)Nc3ccc4ccn(CCCCN5CCC6(CC5)OCc5ccccc65)c4c3)c3ccccc3C2(C)C)C(C)(C)c2ccccc12 |c:1| | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Homo sapiens (Human)) | BDBM50048866

(1-Cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydro-na...)Show InChI InChI=1S/C24H38N2O/c1-27-24-14-6-12-22-20(8-5-13-23(22)24)9-7-15-25-16-18-26(19-17-25)21-10-3-2-4-11-21/h6,12,14,20-21H,2-5,7-11,13,15-19H2,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50210854
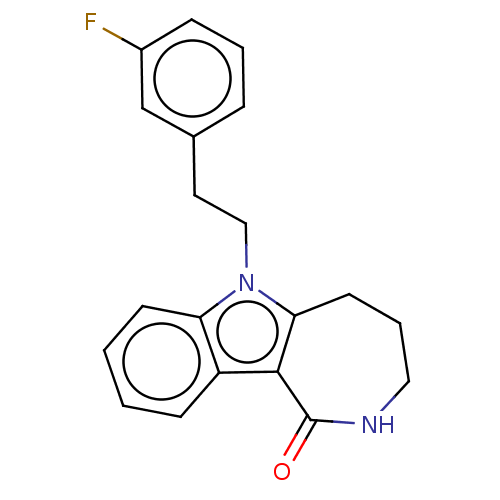
(CHEMBL3960040)Show InChI InChI=1S/C20H19FN2O/c21-15-6-3-5-14(13-15)10-12-23-17-8-2-1-7-16(17)19-18(23)9-4-11-22-20(19)24/h1-3,5-8,13H,4,9-12H2,(H,22,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari"A. Moro"
Curated by ChEMBL
| Assay Description
Competitive inhibition of horse serum BChE in presence of varying levels of butyrylthiocholine iodide substrate by Lineweaver-burk plot method |
Eur J Med Chem 125: 288-298 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.037
BindingDB Entry DOI: 10.7270/Q2G44S9D |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50130293

(7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...)Show SMILES Clc1cccc(N2CCN(CCCCOc3ccc4CCC(=O)Nc4c3)CC2)c1Cl Show InChI InChI=1S/C23H27Cl2N3O2/c24-19-4-3-5-21(23(19)25)28-13-11-27(12-14-28)10-1-2-15-30-18-8-6-17-7-9-22(29)26-20(17)16-18/h3-6,8,16H,1-2,7,9-15H2,(H,26,29) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50584560
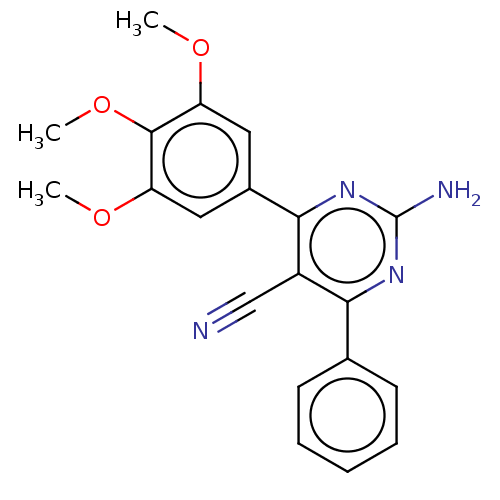
(CHEMBL5077370)Show SMILES COc1cc(cc(OC)c1OC)-c1nc(N)nc(-c2ccccc2)c1C#N | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]HZ241385 from human A2AAR expressed in HeLa cell membrane incubated for 30 mins by microbeta trilux scintillation counter analysi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01636
BindingDB Entry DOI: 10.7270/Q2M61Q5K |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50207816
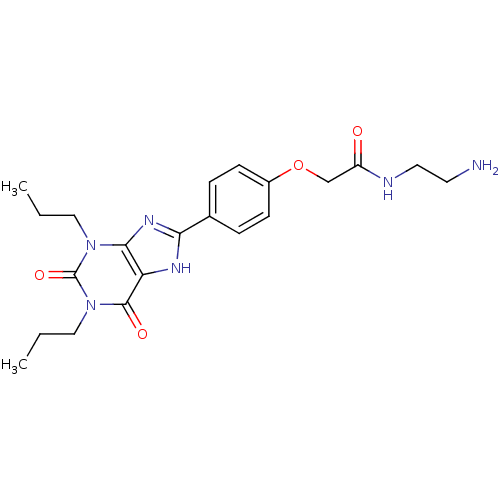
(CHEMBL273094 | N-(2-Amino-ethyl)-2-[4-(2,6-dioxo-1...)Show SMILES CCCn1c2nc([nH]c2c(=O)n(CCC)c1=O)-c1ccc(OCC(=O)NCCN)cc1 Show InChI InChI=1S/C21H28N6O4/c1-3-11-26-19-17(20(29)27(12-4-2)21(26)30)24-18(25-19)14-5-7-15(8-6-14)31-13-16(28)23-10-9-22/h5-8H,3-4,9-13,22H2,1-2H3,(H,23,28)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]HZ241385 from human A2AAR expressed in HeLa cell membrane incubated for 30 mins by microbeta trilux scintillation counter analysi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01636
BindingDB Entry DOI: 10.7270/Q2M61Q5K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50542172
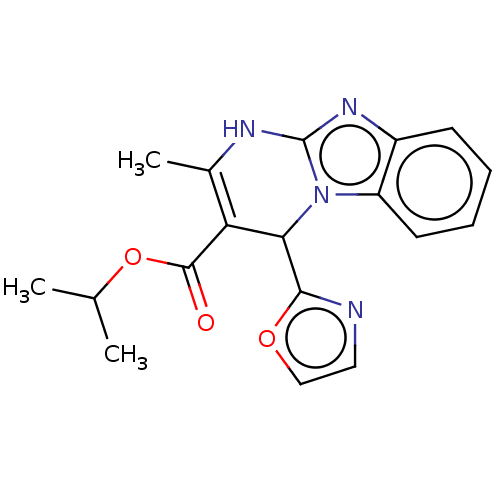
(CHEMBL4632760)Show SMILES CC(C)OC(=O)C1=C(C)Nc2nc3ccccc3n2C1c1ncco1 |c:6| Show InChI InChI=1S/C18H18N4O3/c1-10(2)25-17(23)14-11(3)20-18-21-12-6-4-5-7-13(12)22(18)15(14)16-19-8-9-24-16/h4-10,15H,1-3H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement [3H]ZM241385 from adenosine A2A receptor in human HeLa cell membranes incubated for 30 mins by scintillation counting method |
J Med Chem 63: 7721-7739 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00564
BindingDB Entry DOI: 10.7270/Q2WD444S |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50161342

(2-Amino-4-phenyl-indeno[1,2-d]pyrimidin-5-one | 2-...)Show InChI InChI=1S/C17H11N3O/c18-17-19-14(10-6-2-1-3-7-10)13-15(20-17)11-8-4-5-9-12(11)16(13)21/h1-9H,(H2,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]SCH-58261 from A2A adenosine receptor (unknown origin) expressed in HEK cell membrane incubated for 60 mins at room temperature b... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01636
BindingDB Entry DOI: 10.7270/Q2M61Q5K |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50613231
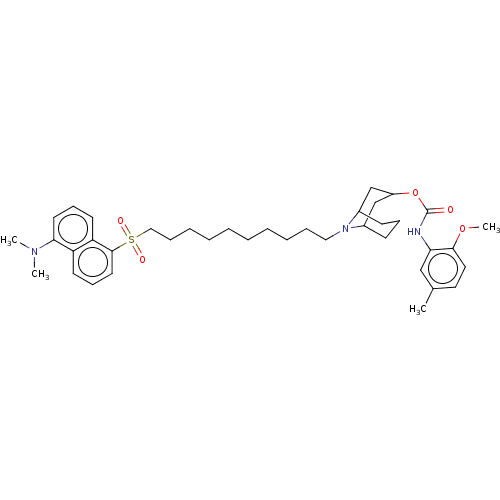
(CHEMBL5275443)Show SMILES COc1ccc(C)cc1NC(=O)OC1CC2CCCC(C1)N2CCCCCCCCCCS(=O)(=O)c1cccc2c(cccc12)N(C)C |TLB:12:13:21:16.17.18| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50375499
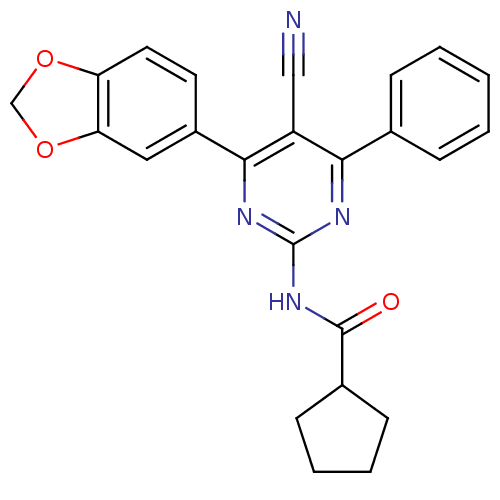
(CHEMBL259319)Show SMILES O=C(Nc1nc(-c2ccccc2)c(C#N)c(n1)-c1ccc2OCOc2c1)C1CCCC1 Show InChI InChI=1S/C24H20N4O3/c25-13-18-21(15-6-2-1-3-7-15)26-24(28-23(29)16-8-4-5-9-16)27-22(18)17-10-11-19-20(12-17)31-14-30-19/h1-3,6-7,10-12,16H,4-5,8-9,14H2,(H,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPCPX from A1 adenosine receptor (unknown origin) expressed in CHO cell membrane incubated for 60 mins at room temperature by NXT... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01636
BindingDB Entry DOI: 10.7270/Q2M61Q5K |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50584555
(CHEMBL5084351) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPCPX from human A1AR expressed in CHO cell membrane incubated for 60 mins by microbeta trilux scintillation counter analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01636
BindingDB Entry DOI: 10.7270/Q2M61Q5K |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50584559
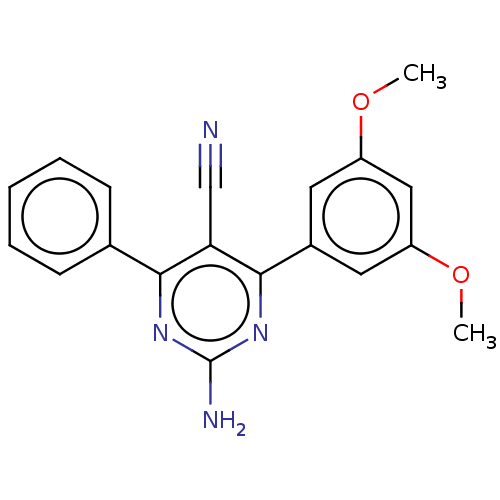
(CHEMBL5088876) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]HZ241385 from human A2AAR expressed in HeLa cell membrane incubated for 30 mins by microbeta trilux scintillation counter analysi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01636
BindingDB Entry DOI: 10.7270/Q2M61Q5K |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50375500
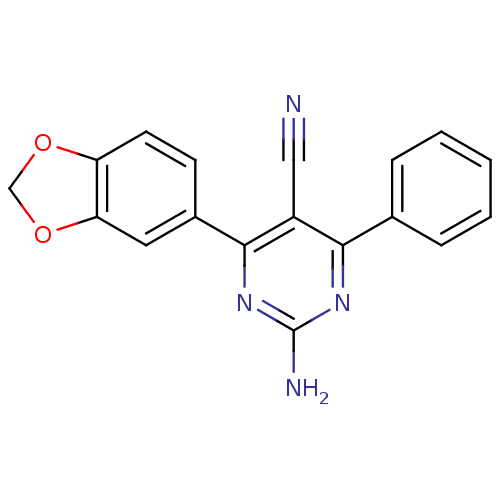
(CHEMBL259607)Show InChI InChI=1S/C18H12N4O2/c19-9-13-16(11-4-2-1-3-5-11)21-18(20)22-17(13)12-6-7-14-15(8-12)24-10-23-14/h1-8H,10H2,(H2,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPCPX from human A1AR expressed in CHO cell membrane incubated for 60 mins by microbeta trilux scintillation counter analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01636
BindingDB Entry DOI: 10.7270/Q2M61Q5K |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM21190

(4-(2-{[5-amino-2-(furan-2-yl)-[1,2,4]triazolo[1,5-...)Show InChI InChI=1S/C16H15N7O2/c17-14-20-15(18-8-7-10-3-5-11(24)6-4-10)21-16-19-13(22-23(14)16)12-2-1-9-25-12/h1-6,9,24H,7-8H2,(H3,17,18,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement [3H]ZM241385 from adenosine A2A receptor in human HeLa cell membranes incubated for 30 mins by scintillation counting method |
J Med Chem 63: 7721-7739 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00564
BindingDB Entry DOI: 10.7270/Q2WD444S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM21190

(4-(2-{[5-amino-2-(furan-2-yl)-[1,2,4]triazolo[1,5-...)Show InChI InChI=1S/C16H15N7O2/c17-14-20-15(18-8-7-10-3-5-11(24)6-4-10)21-16-19-13(22-23(14)16)12-2-1-9-25-12/h1-6,9,24H,7-8H2,(H3,17,18,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from human recombinant A2A receptor expressed in human HeLa cell membranes incubated for 30 mins by radioligand binding ... |
J Med Chem 62: 9315-9330 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01340
BindingDB Entry DOI: 10.7270/Q29C71VG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM21190

(4-(2-{[5-amino-2-(furan-2-yl)-[1,2,4]triazolo[1,5-...)Show InChI InChI=1S/C16H15N7O2/c17-14-20-15(18-8-7-10-3-5-11(24)6-4-10)21-16-19-13(22-23(14)16)12-2-1-9-25-12/h1-6,9,24H,7-8H2,(H3,17,18,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from human recombinant A2A receptor expressed in human HeLa cell membranes incubated for 30 mins by radioligand binding ... |
J Med Chem 62: 9315-9330 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01340
BindingDB Entry DOI: 10.7270/Q29C71VG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM21190

(4-(2-{[5-amino-2-(furan-2-yl)-[1,2,4]triazolo[1,5-...)Show InChI InChI=1S/C16H15N7O2/c17-14-20-15(18-8-7-10-3-5-11(24)6-4-10)21-16-19-13(22-23(14)16)12-2-1-9-25-12/h1-6,9,24H,7-8H2,(H3,17,18,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]HZ241385 from human A2AAR expressed in HeLa cell membrane incubated for 30 mins by microbeta trilux scintillation counter analysi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01636
BindingDB Entry DOI: 10.7270/Q2M61Q5K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM21190

(4-(2-{[5-amino-2-(furan-2-yl)-[1,2,4]triazolo[1,5-...)Show InChI InChI=1S/C16H15N7O2/c17-14-20-15(18-8-7-10-3-5-11(24)6-4-10)21-16-19-13(22-23(14)16)12-2-1-9-25-12/h1-6,9,24H,7-8H2,(H3,17,18,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]ZM2421385 from adenosine A2A receptor expressed in human HeLa cell membranes incubated for 30 mins by scintillation counting meth... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01431
BindingDB Entry DOI: 10.7270/Q2JQ14S5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50159488

(CHEMBL3787197)Show SMILES CC(C)OC(=O)C1=C(C)Nc2nc3ccccc3n2C1c1ccco1 |c:6| Show InChI InChI=1S/C19H19N3O3/c1-11(2)25-18(23)16-12(3)20-19-21-13-7-4-5-8-14(13)22(19)17(16)15-9-6-10-24-15/h4-11,17H,1-3H3,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [3H]NECA from human A3 receptor expressed in human HeLa cell membranes incubated for 180 mins by radioligand binding competition assa... |
J Med Chem 62: 9315-9330 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01340
BindingDB Entry DOI: 10.7270/Q29C71VG |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50159488

(CHEMBL3787197)Show SMILES CC(C)OC(=O)C1=C(C)Nc2nc3ccccc3n2C1c1ccco1 |c:6| Show InChI InChI=1S/C19H19N3O3/c1-11(2)25-18(23)16-12(3)20-19-21-13-7-4-5-8-14(13)22(19)17(16)15-9-6-10-24-15/h4-11,17H,1-3H3,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [3H]NECA from human A3 receptor expressed in human HeLa cell membranes incubated for 180 mins by radioligand binding competition assa... |
J Med Chem 62: 9315-9330 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01340
BindingDB Entry DOI: 10.7270/Q29C71VG |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50542171
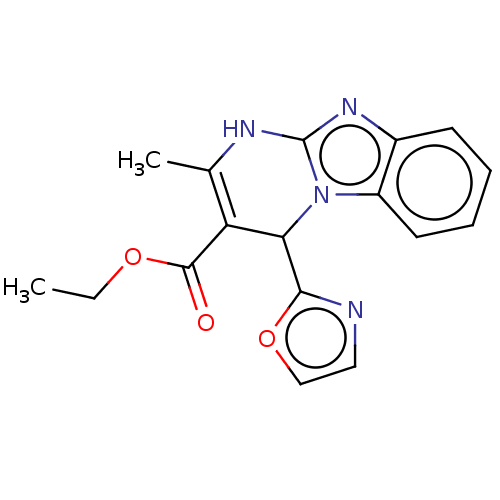
(CHEMBL4645754)Show SMILES CCOC(=O)C1=C(C)Nc2nc3ccccc3n2C1c1ncco1 |c:5| Show InChI InChI=1S/C17H16N4O3/c1-3-23-16(22)13-10(2)19-17-20-11-6-4-5-7-12(11)21(17)14(13)15-18-8-9-24-15/h4-9,14H,3H2,1-2H3,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement [3H]ZM241385 from adenosine A2A receptor in human HeLa cell membranes incubated for 30 mins by scintillation counting method |
J Med Chem 63: 7721-7739 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00564
BindingDB Entry DOI: 10.7270/Q2WD444S |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50086170
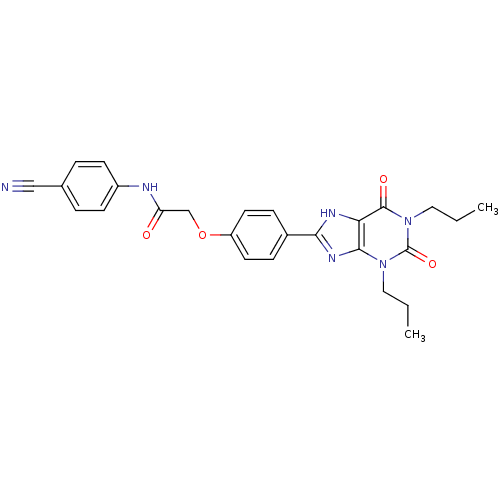
((4-Cyano-phenyl)-carbamic acid 4-(2,6-dioxo-1,3-di...)Show SMILES CCCn1c2nc([nH]c2c(=O)n(CCC)c1=O)-c1ccc(OCC(=O)Nc2ccc(cc2)C#N)cc1 Show InChI InChI=1S/C26H26N6O4/c1-3-13-31-24-22(25(34)32(14-4-2)26(31)35)29-23(30-24)18-7-11-20(12-8-18)36-16-21(33)28-19-9-5-17(15-27)6-10-19/h5-12H,3-4,13-14,16H2,1-2H3,(H,28,33)(H,29,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human A2B receptor |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01431
BindingDB Entry DOI: 10.7270/Q2JQ14S5 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50584556
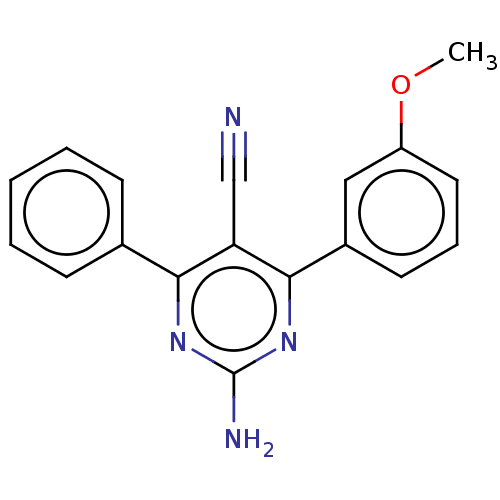
(CHEMBL5086406) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPCPX from human A1AR expressed in CHO cell membrane incubated for 60 mins by microbeta trilux scintillation counter analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01636
BindingDB Entry DOI: 10.7270/Q2M61Q5K |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM21173

(1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...)Show InChI InChI=1S/C16H24N4O2/c1-3-9-19-14-12(15(21)20(10-4-2)16(19)22)17-13(18-14)11-7-5-6-8-11/h11H,3-10H2,1-2H3,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPCPX from human A1AR expressed in CHO cell membrane incubated for 60 mins by microbeta trilux scintillation counter analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01636
BindingDB Entry DOI: 10.7270/Q2M61Q5K |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM21173

(1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...)Show InChI InChI=1S/C16H24N4O2/c1-3-9-19-14-12(15(21)20(10-4-2)16(19)22)17-13(18-14)11-7-5-6-8-11/h11H,3-10H2,1-2H3,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in CHO cell membranes incubated for 60 mins by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01431
BindingDB Entry DOI: 10.7270/Q2JQ14S5 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50584608
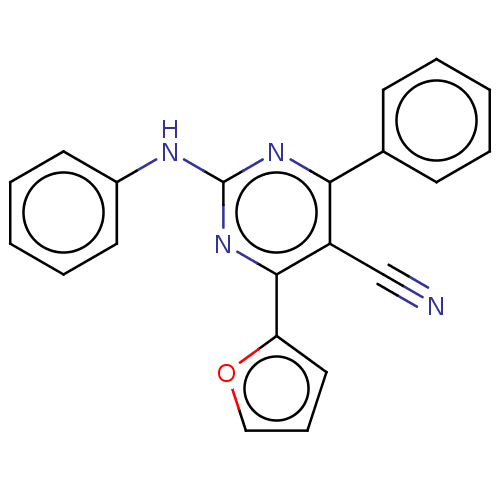
(CHEMBL5071174) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]HZ241385 from human A2AAR expressed in HeLa cell membrane incubated for 30 mins by microbeta trilux scintillation counter analysi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01636
BindingDB Entry DOI: 10.7270/Q2M61Q5K |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM21173

(1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...)Show InChI InChI=1S/C16H24N4O2/c1-3-9-19-14-12(15(21)20(10-4-2)16(19)22)17-13(18-14)11-7-5-6-8-11/h11H,3-10H2,1-2H3,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement [3H]DPCPX from human adenosine A1 receptor expressed in CHO cell membranes incubated for 60 mins by scintillation counting method |
J Med Chem 63: 7721-7739 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00564
BindingDB Entry DOI: 10.7270/Q2WD444S |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50004589

(8-Cyclopentyl-1,3-diethyl-3,7-dihydro-purine-2,6-d...)Show InChI InChI=1S/C14H20N4O2/c1-3-17-12-10(13(19)18(4-2)14(17)20)15-11(16-12)9-7-5-6-8-9/h9H,3-8H2,1-2H3,(H,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from human recombinant A1 receptor expressed in CHOA1 cell membranes incubated for 60 mins by radioligand binding competiti... |
J Med Chem 62: 9315-9330 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01340
BindingDB Entry DOI: 10.7270/Q29C71VG |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50004589

(8-Cyclopentyl-1,3-diethyl-3,7-dihydro-purine-2,6-d...)Show InChI InChI=1S/C14H20N4O2/c1-3-17-12-10(13(19)18(4-2)14(17)20)15-11(16-12)9-7-5-6-8-9/h9H,3-8H2,1-2H3,(H,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from human recombinant A1 receptor expressed in CHOA1 cell membranes incubated for 60 mins by radioligand binding competiti... |
J Med Chem 62: 9315-9330 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01340
BindingDB Entry DOI: 10.7270/Q29C71VG |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50584557
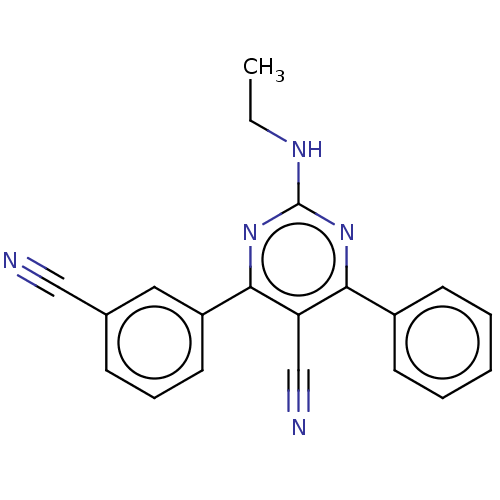
(CHEMBL5086487)Show SMILES CCNc1nc(-c2ccccc2)c(C#N)c(n1)-c1cccc(c1)C#N | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPCPX from human A1AR expressed in CHO cell membrane incubated for 60 mins by microbeta trilux scintillation counter analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01636
BindingDB Entry DOI: 10.7270/Q2M61Q5K |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50584558
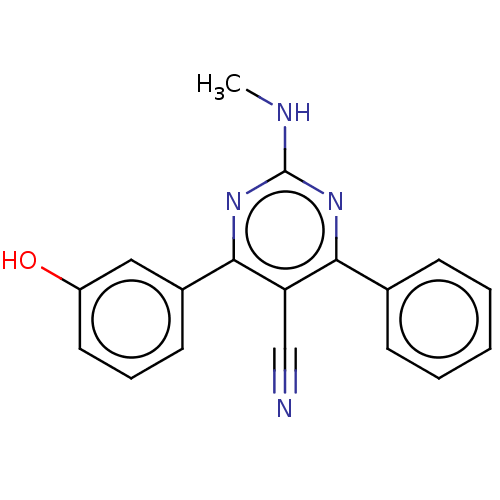
(CHEMBL5072557) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPCPX from human A1AR expressed in CHO cell membrane incubated for 60 mins by microbeta trilux scintillation counter analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01636
BindingDB Entry DOI: 10.7270/Q2M61Q5K |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50584559

(CHEMBL5088876) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPCPX from human A1AR expressed in CHO cell membrane incubated for 60 mins by microbeta trilux scintillation counter analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01636
BindingDB Entry DOI: 10.7270/Q2M61Q5K |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50584560

(CHEMBL5077370)Show SMILES COc1cc(cc(OC)c1OC)-c1nc(N)nc(-c2ccccc2)c1C#N | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPCPX from human A1AR expressed in CHO cell membrane incubated for 60 mins by microbeta trilux scintillation counter analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01636
BindingDB Entry DOI: 10.7270/Q2M61Q5K |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50258891
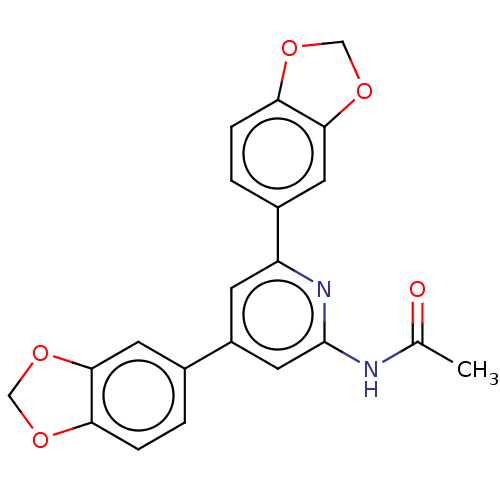
(CHEMBL4067466)Show SMILES CC(=O)Nc1cc(cc(n1)-c1ccc2OCOc2c1)-c1ccc2OCOc2c1 Show InChI InChI=1S/C21H16N2O5/c1-12(24)22-21-9-15(13-2-4-17-19(7-13)27-10-25-17)6-16(23-21)14-3-5-18-20(8-14)28-11-26-18/h2-9H,10-11H2,1H3,(H,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [3H]NECA from human adenosine A3 receptor expressed in human HeLa cell membranes after 180 mins |
J Med Chem 60: 7502-7511 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00860
BindingDB Entry DOI: 10.7270/Q2ST7S8S |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50542173
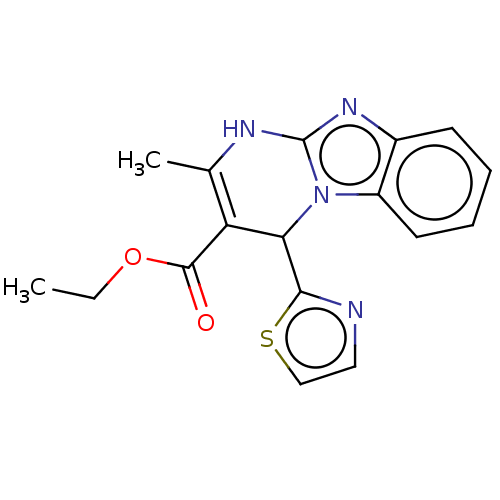
(CHEMBL4634105)Show SMILES CCOC(=O)C1=C(C)Nc2nc3ccccc3n2C1c1nccs1 |c:5| Show InChI InChI=1S/C17H16N4O2S/c1-3-23-16(22)13-10(2)19-17-20-11-6-4-5-7-12(11)21(17)14(13)15-18-8-9-24-15/h4-9,14H,3H2,1-2H3,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement [3H]ZM241385 from adenosine A2A receptor in human HeLa cell membranes incubated for 30 mins by scintillation counting method |
J Med Chem 63: 7721-7739 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00564
BindingDB Entry DOI: 10.7270/Q2WD444S |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50584561

(CHEMBL5088749) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPCPX from human A1AR expressed in CHO cell membrane incubated for 60 mins by microbeta trilux scintillation counter analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01636
BindingDB Entry DOI: 10.7270/Q2M61Q5K |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50032403
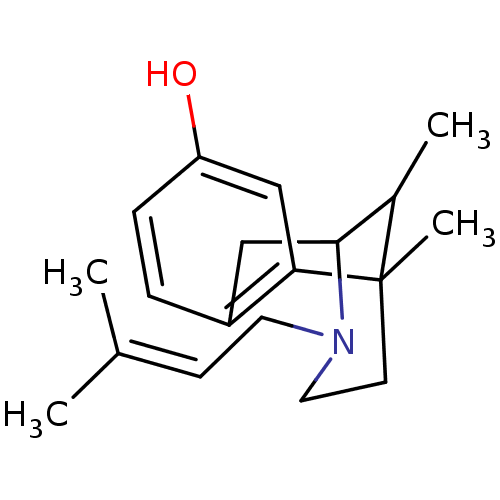
((Pentazocine) 6,11-Dimethyl-3-(3-methyl-but-2-enyl...)Show SMILES [#6]-[#6]1-[#6]-2-[#6]-c3ccc(-[#8])cc3C1([#6])[#6]-[#6]-[#7]-2-[#6]\[#6]=[#6](\[#6])-[#6] |TLB:16:15:1:10.4.3,9:10:1:15.13.14| Show InChI InChI=1S/C19H27NO/c1-13(2)7-9-20-10-8-19(4)14(3)18(20)11-15-5-6-16(21)12-17(15)19/h5-7,12,14,18,21H,8-11H2,1-4H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sigma intracellular receptor 2
(Homo sapiens (Human)) | BDBM50613236
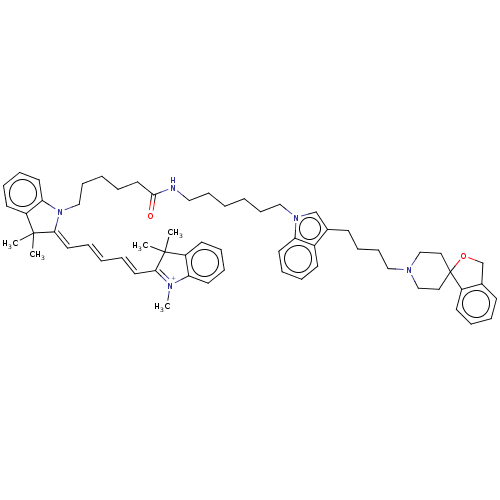
(CHEMBL5290452)Show SMILES [I-].C[N+]1=C(\C=C\C=C\C=C2/N(CCCCCC(=O)NCCCCCCn3cc(CCCCN4CCC5(CC4)OCc4ccccc54)c4ccccc34)c3ccccc3C2(C)C)C(C)(C)c2ccccc12 |c:1| | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50609164

(CHEMBL5289063)Show SMILES Oc1ccc(N2CCCN(CCCCOc3ccc4ccnn4c3)CC2)c2ccc(=O)[nH]c12 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50159488

(CHEMBL3787197)Show SMILES CC(C)OC(=O)C1=C(C)Nc2nc3ccccc3n2C1c1ccco1 |c:6| Show InChI InChI=1S/C19H19N3O3/c1-11(2)25-18(23)16-12(3)20-19-21-13-7-4-5-8-14(13)22(19)17(16)15-9-6-10-24-15/h4-11,17H,1-3H3,(H,20,21) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from human recombinant A2B receptor expressed in human HEK293 cell membranes incubated for 30 mins by radioligand binding c... |
J Med Chem 62: 9315-9330 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01340
BindingDB Entry DOI: 10.7270/Q29C71VG |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50159488

(CHEMBL3787197)Show SMILES CC(C)OC(=O)C1=C(C)Nc2nc3ccccc3n2C1c1ccco1 |c:6| Show InChI InChI=1S/C19H19N3O3/c1-11(2)25-18(23)16-12(3)20-19-21-13-7-4-5-8-14(13)22(19)17(16)15-9-6-10-24-15/h4-11,17H,1-3H3,(H,20,21) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement [3H]DPCPX from human adenosine A2B receptor expressed in HEK293 cell membranes incubated for 60 mins by scintillation counting method |
J Med Chem 63: 7721-7739 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00564
BindingDB Entry DOI: 10.7270/Q2WD444S |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data