

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM81811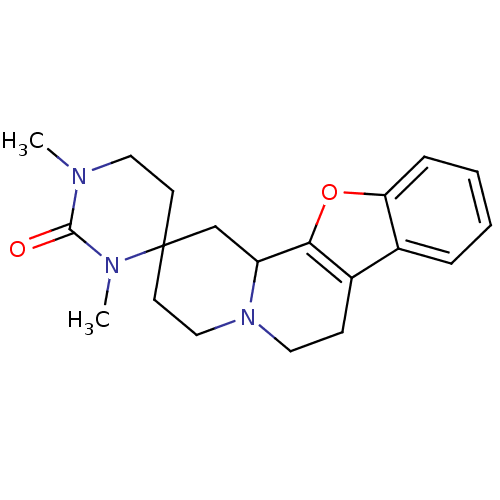 (CAS_123679 | L-657,743 | MK-912 | NSC_123679) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by PDSP Ki Database | Biochem Pharmacol 55: 1035-43 (1998) Article DOI: 10.1016/s0006-2952(97)00631-x BindingDB Entry DOI: 10.7270/Q2T72G01 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50297306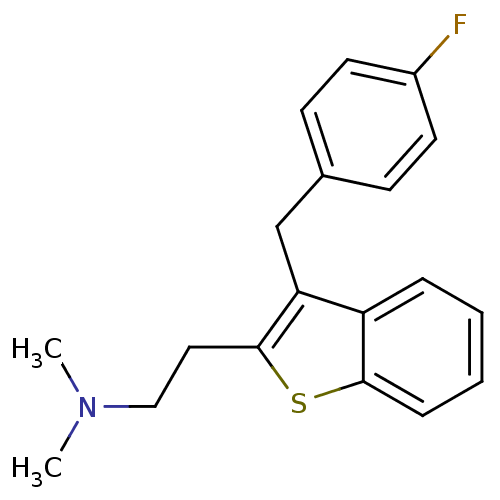 (CHEMBL540982 | {2-[3-(4-Fluoro-benzyl)-benzo[b]thi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2A receptor expressed in HEK293 Flp-In cells by liquid scintillation counting | J Med Chem 52: 5307-10 (2009) Article DOI: 10.1021/jm900933k BindingDB Entry DOI: 10.7270/Q2057G0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50518510 (CHEMBL4448325) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay | Bioorg Med Chem Lett 29: 491-495 (2019) Article DOI: 10.1016/j.bmcl.2018.12.015 BindingDB Entry DOI: 10.7270/Q21V5J93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50297304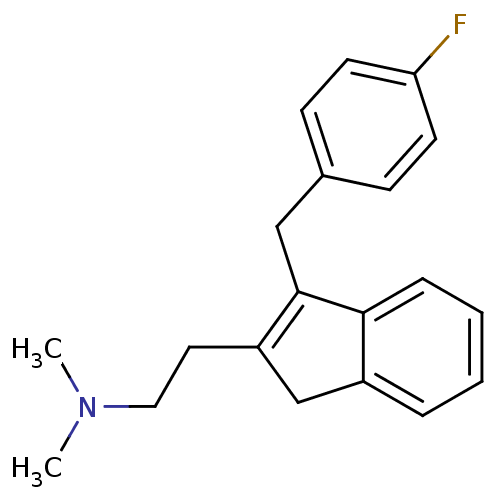 (CHEMBL560741 | {2-[3-(4-Fluoro-benzyl)-1H-inden-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2A receptor expressed in HEK293 Flp-In cells by liquid scintillation counting | J Med Chem 52: 5307-10 (2009) Article DOI: 10.1021/jm900933k BindingDB Entry DOI: 10.7270/Q2057G0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18161 ((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.200 | -57.6 | n/a | n/a | 5.10 | n/a | n/a | 7.4 | 37 |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | J Med Chem 49: 6143-6 (2006) Article DOI: 10.1021/jm060792t BindingDB Entry DOI: 10.7270/Q26971VV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50448583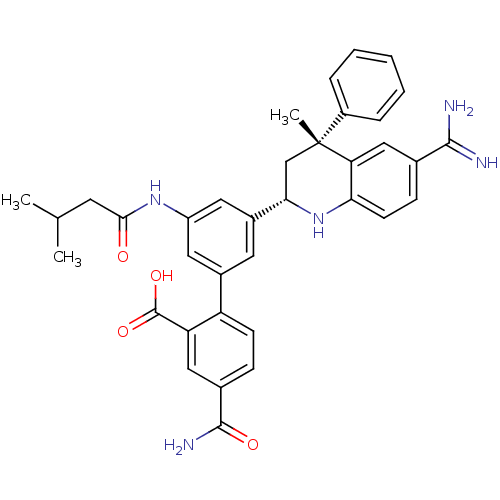 (CHEMBL3127491) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis by spectrophotometrically | J Med Chem 57: 9915-32 (2014) Article DOI: 10.1021/jm5010607 BindingDB Entry DOI: 10.7270/Q2RV0Q9S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18161 ((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.300 | -56.5 | n/a | n/a | 5.70 | n/a | n/a | 7.4 | 37 |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | J Med Chem 50: 2486-96 (2007) Article DOI: 10.1021/jm061329j BindingDB Entry DOI: 10.7270/Q20R9MNK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50315206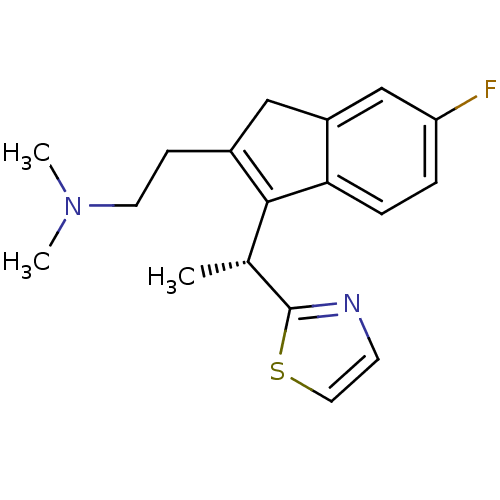 ((R)-2-(6-fluoro-3-(1-(thiazol-2-yl)ethyl)-1H-inden...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Binding affinity at histamine H1 receptor | Bioorg Med Chem Lett 20: 2629-33 (2010) Article DOI: 10.1016/j.bmcl.2010.02.055 BindingDB Entry DOI: 10.7270/Q2NG4QSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50032874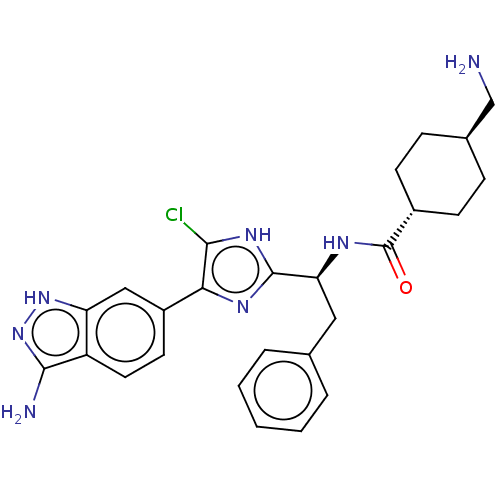 (CHEMBL3355683) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis by spectrophotometrically | J Med Chem 57: 9915-32 (2014) Article DOI: 10.1021/jm5010607 BindingDB Entry DOI: 10.7270/Q2RV0Q9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50032873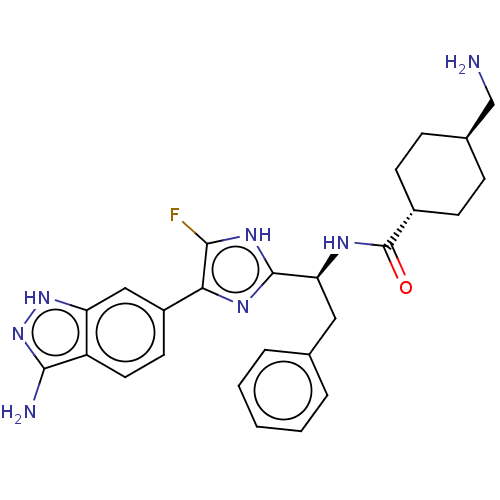 (CHEMBL3355684) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis by spectrophotometrically | J Med Chem 57: 9915-32 (2014) Article DOI: 10.1021/jm5010607 BindingDB Entry DOI: 10.7270/Q2RV0Q9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50297310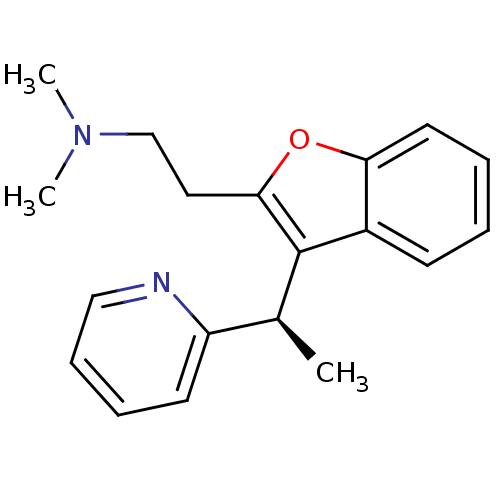 ((-)-Dimethyl-{2-[3-((R)-1-pyridin-2-yl-ethyl)-benz...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]N-methylscopolamine from human muscarinic M1 receptor expressed in CHO Flp-In cells by liquid scintillation counting | J Med Chem 52: 5307-10 (2009) Article DOI: 10.1021/jm900933k BindingDB Entry DOI: 10.7270/Q2057G0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50019492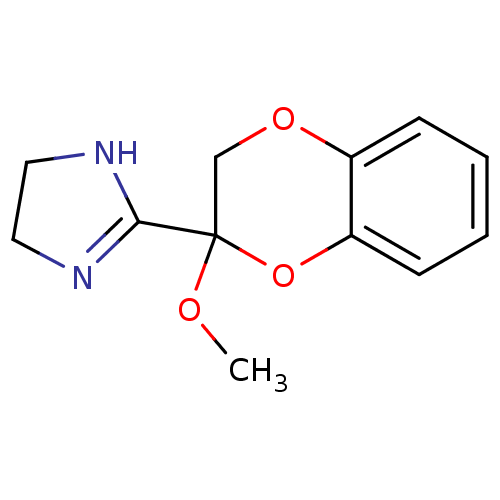 ((+)2-(2-Methoxy-2,3-dihydro-benzo[1,4]dioxin-2-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by PDSP Ki Database | Biochem Pharmacol 55: 1035-43 (1998) Article DOI: 10.1016/s0006-2952(97)00631-x BindingDB Entry DOI: 10.7270/Q2T72G01 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Flap endonuclease 1 (Homo sapiens (Human)) | BDBM167060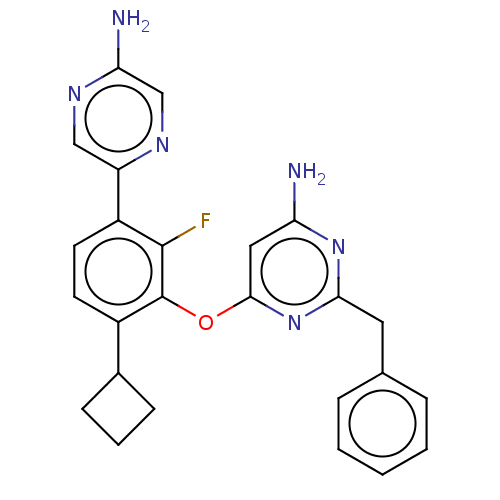 (US9073876, 146 | US9732093, Compound 146) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9732093 (2017) BindingDB Entry DOI: 10.7270/Q2NV9MCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM167060 (US9073876, 146 | US9732093, Compound 146) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9073876 (2015) BindingDB Entry DOI: 10.7270/Q28S4NPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Flap endonuclease 1 (Homo sapiens (Human)) | BDBM167060 (US9073876, 146 | US9732093, Compound 146) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9732093 (2017) BindingDB Entry DOI: 10.7270/Q2NV9MCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50518517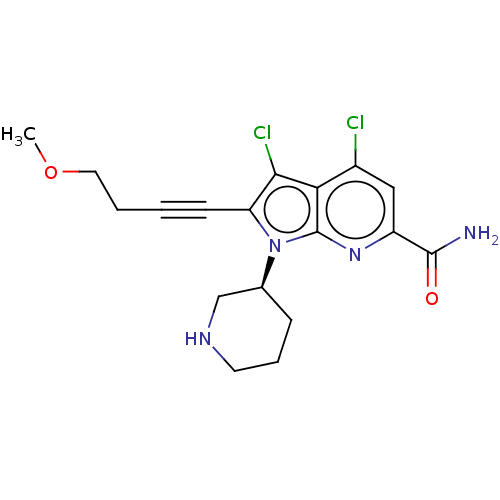 (CHEMBL4540910) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay | Bioorg Med Chem Lett 29: 491-495 (2019) Article DOI: 10.1016/j.bmcl.2018.12.015 BindingDB Entry DOI: 10.7270/Q21V5J93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50297307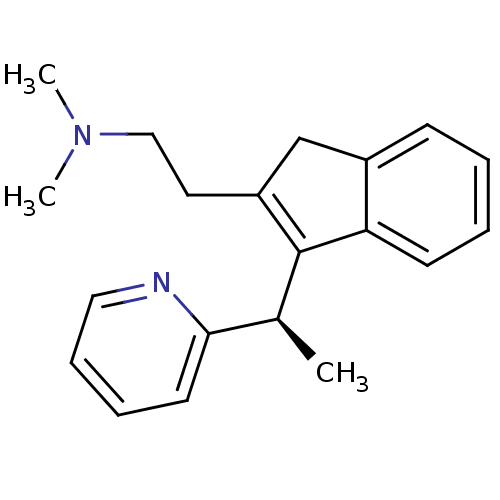 (CHEMBL564226 | R-dimethindene) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation counting | J Med Chem 52: 5307-10 (2009) Article DOI: 10.1021/jm900933k BindingDB Entry DOI: 10.7270/Q2057G0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Flap endonuclease 1 (Homo sapiens (Human)) | BDBM167191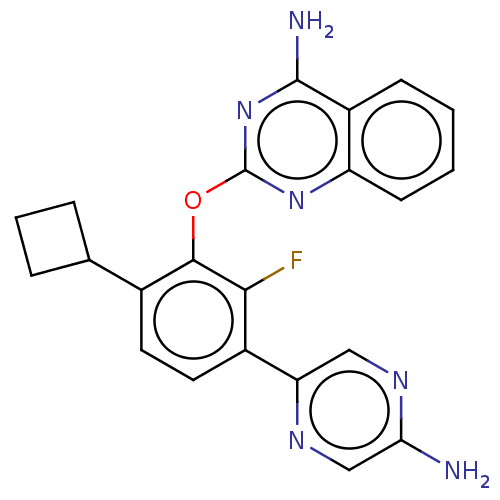 (US9073876, 272 | US9732093, Compound 272) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9732093 (2017) BindingDB Entry DOI: 10.7270/Q2NV9MCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Flap endonuclease 1 (Homo sapiens (Human)) | BDBM167191 (US9073876, 272 | US9732093, Compound 272) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9732093 (2017) BindingDB Entry DOI: 10.7270/Q2NV9MCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM167191 (US9073876, 272 | US9732093, Compound 272) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9073876 (2015) BindingDB Entry DOI: 10.7270/Q28S4NPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50028059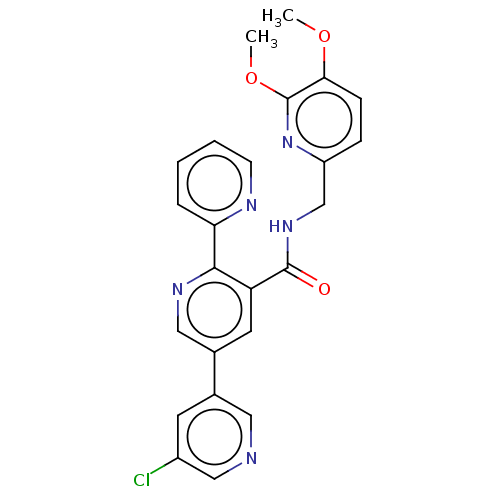 (CHEMBL3338866) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of (S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-((3H)-1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2 rec... | J Med Chem 58: 5620-36 (2015) Article DOI: 10.1021/acs.jmedchem.5b00742 BindingDB Entry DOI: 10.7270/Q2QR4ZWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50013515 ((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by PDSP Ki Database | Biochem Pharmacol 55: 1035-43 (1998) Article DOI: 10.1016/s0006-2952(97)00631-x BindingDB Entry DOI: 10.7270/Q2T72G01 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50297304 (CHEMBL560741 | {2-[3-(4-Fluoro-benzyl)-1H-inden-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation counting | J Med Chem 52: 5307-10 (2009) Article DOI: 10.1021/jm900933k BindingDB Entry DOI: 10.7270/Q2057G0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50518506 (CHEMBL4588948) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay | Bioorg Med Chem Lett 29: 491-495 (2019) Article DOI: 10.1016/j.bmcl.2018.12.015 BindingDB Entry DOI: 10.7270/Q21V5J93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50297305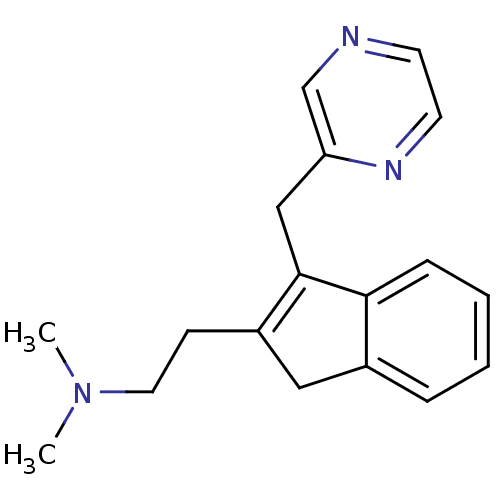 (CHEMBL559251 | Dimethyl-[2-(3-pyrazin-2ylmethyl-1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2A receptor expressed in HEK293 Flp-In cells by liquid scintillation counting | J Med Chem 52: 5307-10 (2009) Article DOI: 10.1021/jm900933k BindingDB Entry DOI: 10.7270/Q2057G0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (MOUSE) | BDBM50019492 ((+)2-(2-Methoxy-2,3-dihydro-benzo[1,4]dioxin-2-yl)...) | PDB Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by PDSP Ki Database | Biochem Pharmacol 55: 1035-43 (1998) Article DOI: 10.1016/s0006-2952(97)00631-x BindingDB Entry DOI: 10.7270/Q2T72G01 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50518520 (CHEMBL4593810) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay | Bioorg Med Chem Lett 29: 491-495 (2019) Article DOI: 10.1016/j.bmcl.2018.12.015 BindingDB Entry DOI: 10.7270/Q21V5J93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50089369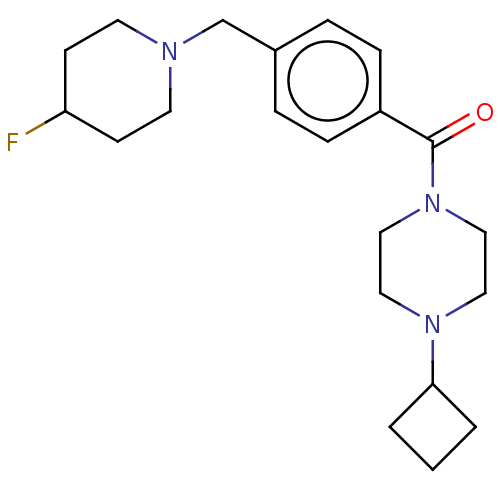 (CHEMBL3577959) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutical Company Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 mins | ACS Med Chem Lett 6: 450-4 (2015) Article DOI: 10.1021/ml5005156 BindingDB Entry DOI: 10.7270/Q22F7Q59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50315205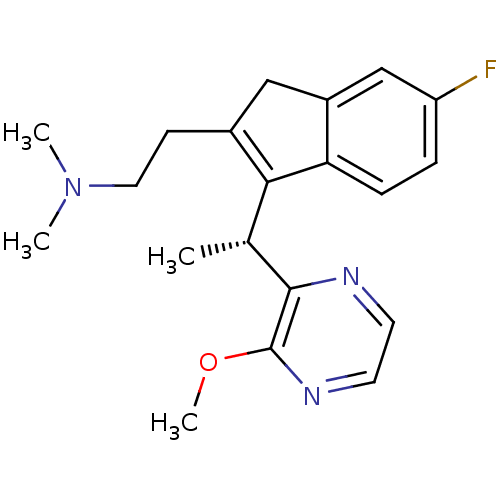 ((R)-2-(6-fluoro-3-(1-(3-methoxypyrazin-2-yl)ethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Inhibition of histamine H1 receptor | Bioorg Med Chem Lett 20: 5874-8 (2010) Article DOI: 10.1016/j.bmcl.2010.07.117 BindingDB Entry DOI: 10.7270/Q26W9BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50315205 ((R)-2-(6-fluoro-3-(1-(3-methoxypyrazin-2-yl)ethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Binding affinity at histamine H1 receptor | Bioorg Med Chem Lett 20: 2629-33 (2010) Article DOI: 10.1016/j.bmcl.2010.02.055 BindingDB Entry DOI: 10.7270/Q2NG4QSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50019492 ((+)2-(2-Methoxy-2,3-dihydro-benzo[1,4]dioxin-2-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by PDSP Ki Database | Biochem Pharmacol 55: 1035-43 (1998) Article DOI: 10.1016/s0006-2952(97)00631-x BindingDB Entry DOI: 10.7270/Q2T72G01 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50315198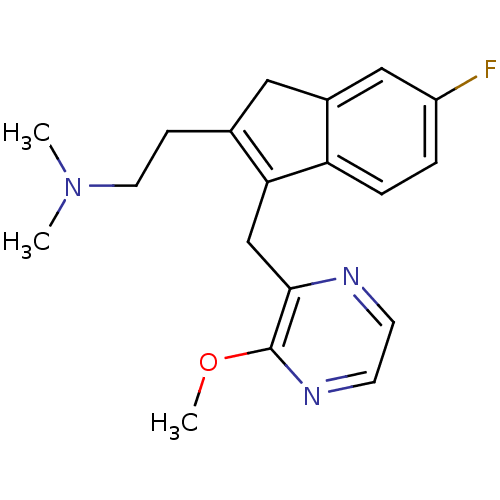 (2-(6-fluoro-3-((3-methoxypyrazin-2-yl)methyl)-1H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Binding affinity at histamine H1 receptor | Bioorg Med Chem Lett 20: 2629-33 (2010) Article DOI: 10.1016/j.bmcl.2010.02.055 BindingDB Entry DOI: 10.7270/Q2NG4QSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50032874 (CHEMBL3355683) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis at 37 degC by spectrophotometrically | J Med Chem 57: 9915-32 (2014) Article DOI: 10.1021/jm5010607 BindingDB Entry DOI: 10.7270/Q2RV0Q9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50315191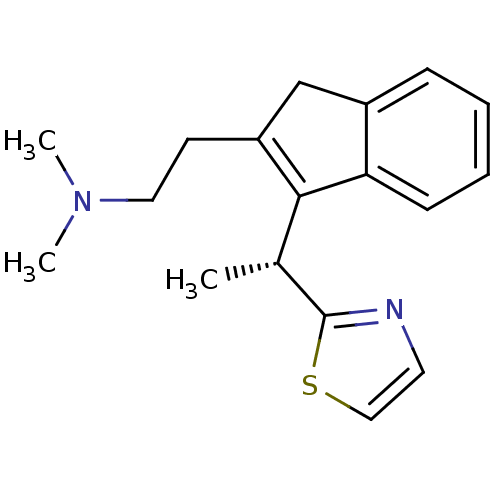 ((R)-N,N-dimethyl-2-(3-(1-(thiazol-2-yl)ethyl)-1H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Binding affinity at histamine H1 receptor | Bioorg Med Chem Lett 20: 2629-33 (2010) Article DOI: 10.1016/j.bmcl.2010.02.055 BindingDB Entry DOI: 10.7270/Q2NG4QSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Flap endonuclease 1 (Homo sapiens (Human)) | BDBM167005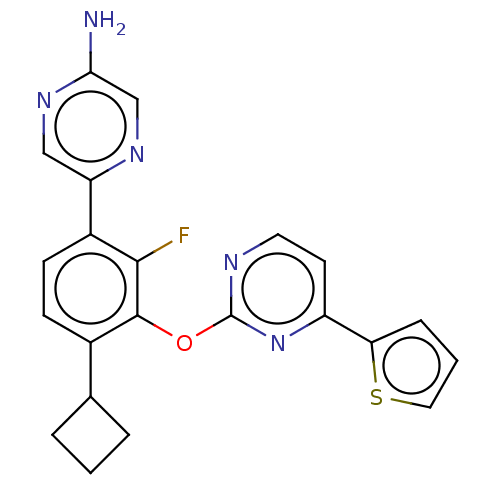 (US9073876, 91 | US9732093, Compound 91) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9732093 (2017) BindingDB Entry DOI: 10.7270/Q2NV9MCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM81811 (CAS_123679 | L-657,743 | MK-912 | NSC_123679) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by PDSP Ki Database | Biochem Pharmacol 55: 1035-43 (1998) Article DOI: 10.1016/s0006-2952(97)00631-x BindingDB Entry DOI: 10.7270/Q2T72G01 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2B adrenergic receptor (Homo sapiens (Human)) | BDBM81811 (CAS_123679 | L-657,743 | MK-912 | NSC_123679) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by PDSP Ki Database | Biochem Pharmacol 55: 1035-43 (1998) Article DOI: 10.1016/s0006-2952(97)00631-x BindingDB Entry DOI: 10.7270/Q2T72G01 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50297310 ((-)-Dimethyl-{2-[3-((R)-1-pyridin-2-yl-ethyl)-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation counting | J Med Chem 52: 5307-10 (2009) Article DOI: 10.1021/jm900933k BindingDB Entry DOI: 10.7270/Q2057G0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM167188 (US9073876, 269 | US9732093, Compound 269) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9073876 (2015) BindingDB Entry DOI: 10.7270/Q28S4NPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Flap endonuclease 1 (Homo sapiens (Human)) | BDBM167188 (US9073876, 269 | US9732093, Compound 269) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9732093 (2017) BindingDB Entry DOI: 10.7270/Q2NV9MCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50518507 (CHEMBL4583118) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay | Bioorg Med Chem Lett 29: 491-495 (2019) Article DOI: 10.1016/j.bmcl.2018.12.015 BindingDB Entry DOI: 10.7270/Q21V5J93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Flap endonuclease 1 (Homo sapiens (Human)) | BDBM167188 (US9073876, 269 | US9732093, Compound 269) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9732093 (2017) BindingDB Entry DOI: 10.7270/Q2NV9MCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM167001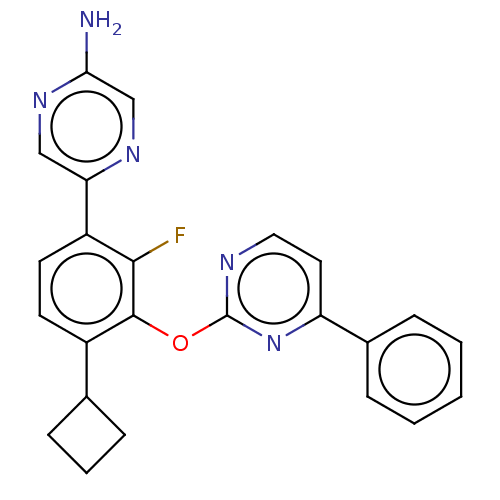 (US9073876, 87 | US9732093, Compound 87) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9073876 (2015) BindingDB Entry DOI: 10.7270/Q28S4NPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM167005 (US9073876, 91 | US9732093, Compound 91) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9073876 (2015) BindingDB Entry DOI: 10.7270/Q28S4NPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Flap endonuclease 1 (Homo sapiens (Human)) | BDBM167014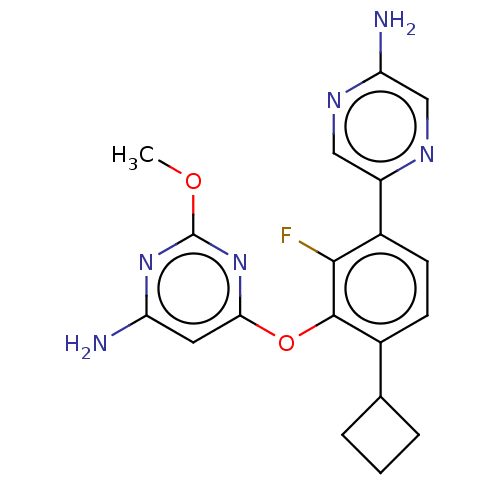 (US9073876, 100 | US9732093, Compound 100) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9732093 (2017) BindingDB Entry DOI: 10.7270/Q2NV9MCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Flap endonuclease 1 (Homo sapiens (Human)) | BDBM167005 (US9073876, 91 | US9732093, Compound 91) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9732093 (2017) BindingDB Entry DOI: 10.7270/Q2NV9MCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Flap endonuclease 1 (Homo sapiens (Human)) | BDBM167001 (US9073876, 87 | US9732093, Compound 87) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9732093 (2017) BindingDB Entry DOI: 10.7270/Q2NV9MCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Rattus norvegicus (Rat)) | BDBM254320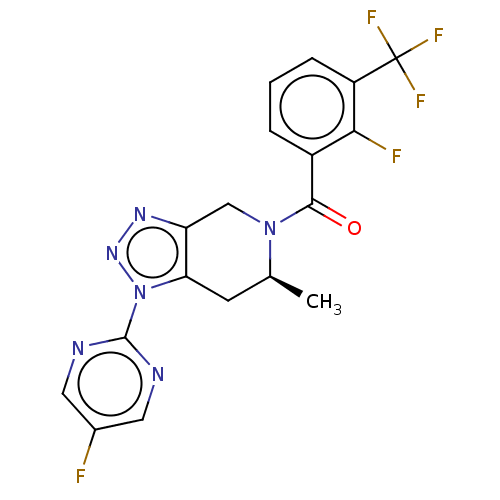 (US10112937, Example 220 | US10150765, Example 220 ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description uman or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according t... | US Patent US10112937 (2018) BindingDB Entry DOI: 10.7270/Q25H7J9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50089375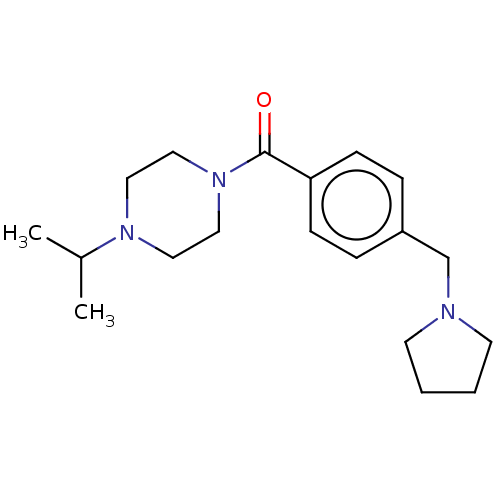 (CHEMBL3577953) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutical Company Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 mins | ACS Med Chem Lett 6: 450-4 (2015) Article DOI: 10.1021/ml5005156 BindingDB Entry DOI: 10.7270/Q22F7Q59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50089374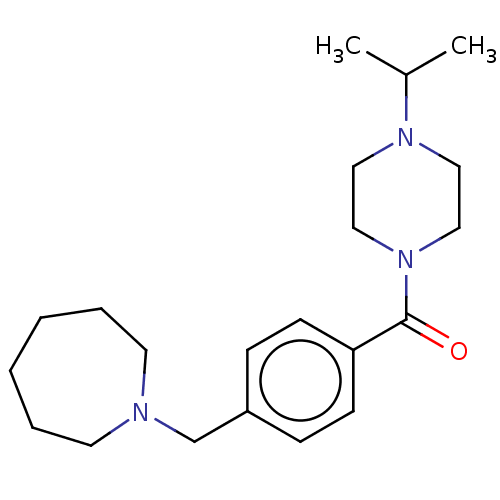 (CHEMBL3577954) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutical Company Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 mins | ACS Med Chem Lett 6: 450-4 (2015) Article DOI: 10.1021/ml5005156 BindingDB Entry DOI: 10.7270/Q22F7Q59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 14064 total ) | Next | Last >> |