

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18617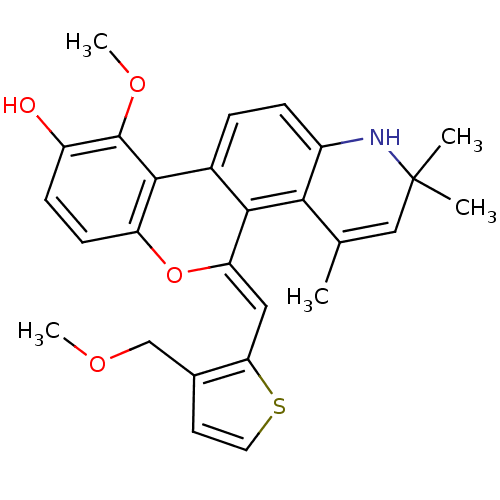 ((18Z)-12-methoxy-18-{[3-(methoxymethyl)thiophen-2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | -51.5 | 0.200 | n/a | 0.200 | n/a | n/a | 7.5 | 4 |
Ligand Pharmaceuticals Inc. | Assay Description Competitive Ligand Binding Assay- The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd) where [L] is the... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18161 ((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.200 | -57.6 | n/a | n/a | 5.10 | n/a | n/a | 7.4 | 37 |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | J Med Chem 50: 5049-52 (2007) Article DOI: 10.1021/jm070231h BindingDB Entry DOI: 10.7270/Q29Z935Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18161 ((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human androgen receptor expressed in monkey COS7 cells by whole cell binding assay | Bioorg Med Chem Lett 18: 3431-5 (2008) Article DOI: 10.1016/j.bmcl.2008.03.085 BindingDB Entry DOI: 10.7270/Q25M66J9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18161 ((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.200 | -57.6 | n/a | n/a | 5.10 | n/a | n/a | 7.4 | 37 |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | J Med Chem 49: 6143-6 (2006) Article DOI: 10.1021/jm060792t BindingDB Entry DOI: 10.7270/Q26971VV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18161 ((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.200 | n/a | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | Bioorg Med Chem Lett 17: 1523-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.007 BindingDB Entry DOI: 10.7270/Q22J6950 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18161 ((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.300 | -56.5 | n/a | n/a | 5.70 | n/a | n/a | 7.4 | 37 |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | J Med Chem 50: 2486-96 (2007) Article DOI: 10.1021/jm061329j BindingDB Entry DOI: 10.7270/Q20R9MNK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062432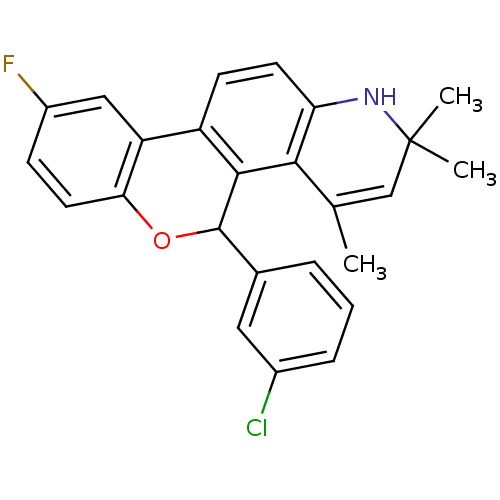 (5-(3-Chloro-phenyl)-9-fluoro-2,2,4-trimethyl-2,5-d...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against baculovirus expressed Progesterone receptor | J Med Chem 42: 1466-72 (1999) Article DOI: 10.1021/jm980723h BindingDB Entry DOI: 10.7270/Q2NK3FQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062443 (5-(4-Chloro-phenyl)-9-fluoro-2,2,4-trimethyl-2,5-d...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description The binding affinity measured using baculovirus-expressed hPR-A in sf21 cells. | J Med Chem 41: 303-10 (1998) Article DOI: 10.1021/jm9705770 BindingDB Entry DOI: 10.7270/Q2RV0PC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062432 (5-(3-Chloro-phenyl)-9-fluoro-2,2,4-trimethyl-2,5-d...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description The binding affinity measured using baculovirus-expressed hPR-A in sf21 cells. | J Med Chem 41: 303-10 (1998) Article DOI: 10.1021/jm9705770 BindingDB Entry DOI: 10.7270/Q2RV0PC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50129152 ((6S,8S,10R,14S,15S)-17-Acetyl-6,10-dimethyl-13-(R)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human progesterone receptor | Bioorg Med Chem Lett 13: 2071-4 (2003) BindingDB Entry DOI: 10.7270/Q2SX6DSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50067678 ((6alpha)-17-(Acetyloxy)-6-methylpreg-4-ene-3,20-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity was determined against hPR-A (human progesterone receptor) using progesterone radioligand in competitive binding assay | Bioorg Med Chem Lett 8: 3365-70 (1999) BindingDB Entry DOI: 10.7270/Q2BC402X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50067678 ((6alpha)-17-(Acetyloxy)-6-methylpreg-4-ene-3,20-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding affinity towards human progesterone receptor A isoform using progesterone as radioligand | J Med Chem 46: 4104-12 (2003) Article DOI: 10.1021/jm020477g BindingDB Entry DOI: 10.7270/Q2QZ29C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50067678 ((6alpha)-17-(Acetyloxy)-6-methylpreg-4-ene-3,20-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity against human progesterone receptor | J Med Chem 41: 4354-9 (1998) Article DOI: 10.1021/jm980366a BindingDB Entry DOI: 10.7270/Q2V40TB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50067678 ((6alpha)-17-(Acetyloxy)-6-methylpreg-4-ene-3,20-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description The binding affinity measured using baculovirus-expressed hPR-A in sf21 cells. | J Med Chem 41: 303-10 (1998) Article DOI: 10.1021/jm9705770 BindingDB Entry DOI: 10.7270/Q2RV0PC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062444 (9-Chloro-5-(3-fluoro-phenyl)-2,2,4-trimethyl-2,5-d...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description The binding affinity measured using baculovirus-expressed hPR-A in sf21 cells. | J Med Chem 41: 303-10 (1998) Article DOI: 10.1021/jm9705770 BindingDB Entry DOI: 10.7270/Q2RV0PC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50067678 ((6alpha)-17-(Acetyloxy)-6-methylpreg-4-ene-3,20-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against baculovirus expressed Progesterone receptor | J Med Chem 42: 1466-72 (1999) Article DOI: 10.1021/jm980723h BindingDB Entry DOI: 10.7270/Q2NK3FQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50067678 ((6alpha)-17-(Acetyloxy)-6-methylpreg-4-ene-3,20-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description The binding affinity of the compound was determined on Human progesterone receptor (hPR-A) using progesterone as radioligand. | J Med Chem 41: 291-302 (1998) Article DOI: 10.1021/jm9705768 BindingDB Entry DOI: 10.7270/Q29W0DMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062427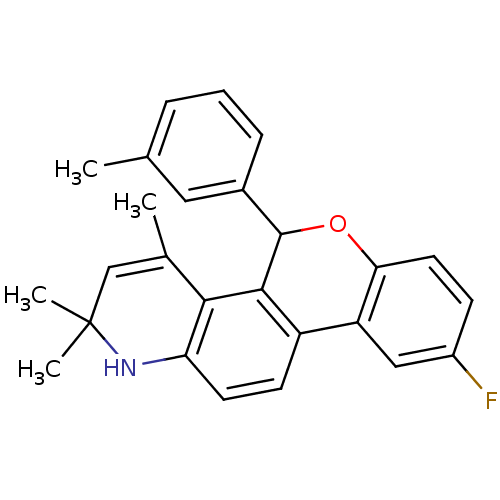 (9-Fluoro-2,2,4-trimethyl-5-m-tolyl-2,5-dihydro-1H-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description The binding affinity measured using baculovirus-expressed hPR-A in sf21 cells. | J Med Chem 41: 303-10 (1998) Article DOI: 10.1021/jm9705770 BindingDB Entry DOI: 10.7270/Q2RV0PC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50340671 (5,7-difluoro-6-(1H-indol-7-yl)-2,2-dimethyl-2,3-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of radiolabeled dexamethasone from glucocorticoid receptor expressed in baculovirus | Bioorg Med Chem Lett 21: 1654-7 (2011) Article DOI: 10.1016/j.bmcl.2011.01.104 BindingDB Entry DOI: 10.7270/Q21V5F8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18605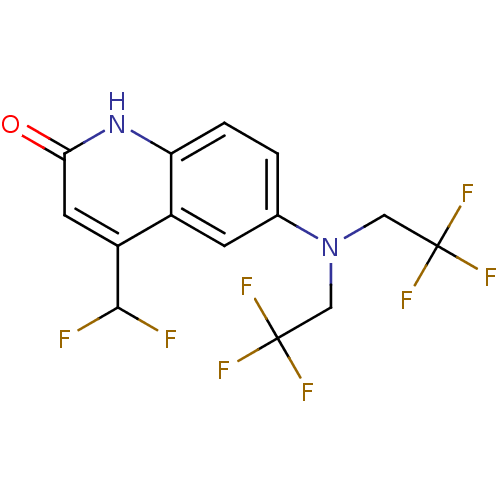 (6-[bis(2,2,2-trifluoroethyl)amino]-4-(difluorometh...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | Bioorg Med Chem Lett 17: 1527-31 (2007) Article DOI: 10.1016/j.bmcl.2007.01.001 BindingDB Entry DOI: 10.7270/Q2XS5SP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062415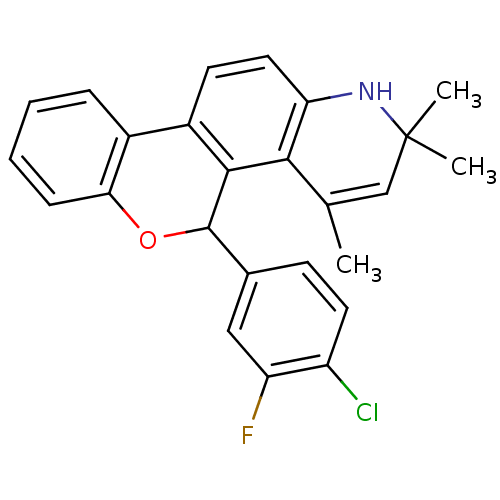 (5-(4-Chloro-3-fluoro-phenyl)-2,2,4-trimethyl-2,5-d...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description The binding affinity of the compound was determined on Human progesterone receptor (hPR-A) using progesterone as radioligand. | J Med Chem 41: 291-302 (1998) Article DOI: 10.1021/jm9705768 BindingDB Entry DOI: 10.7270/Q29W0DMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50133141 (9-Fluoro-2,2,4-trimethyl-5-[1-(3-methyl-pyridin-2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding affinity towards human progesterone receptor A isoform using progesterone as radioligand | J Med Chem 46: 4104-12 (2003) Article DOI: 10.1021/jm020477g BindingDB Entry DOI: 10.7270/Q2QZ29C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062406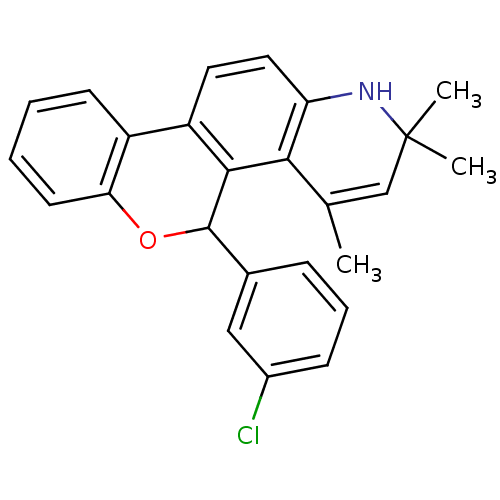 (5-(3-Chloro-phenyl)-2,2,4-trimethyl-2,5-dihydro-1H...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description The binding affinity of the compound was determined on Human progesterone receptor (hPR-A) using progesterone as radioligand. | J Med Chem 41: 291-302 (1998) Article DOI: 10.1021/jm9705768 BindingDB Entry DOI: 10.7270/Q29W0DMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50072701 (9-Chloro-2,2,4,5-tetramethyl-2,5-dihydro-1H-6-oxa-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against baculovirus expressed Progesterone receptor | J Med Chem 42: 1466-72 (1999) Article DOI: 10.1021/jm980723h BindingDB Entry DOI: 10.7270/Q2NK3FQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50072701 (9-Chloro-2,2,4,5-tetramethyl-2,5-dihydro-1H-6-oxa-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity was determined against hPR-A (human progesterone receptor) using progesterone radioligand in competitive binding assay | Bioorg Med Chem Lett 8: 3365-70 (1999) BindingDB Entry DOI: 10.7270/Q2BC402X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062430 (9-Chloro-5-(3-chloro-phenyl)-2,2,4-trimethyl-2,5-d...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description The binding affinity measured using baculovirus-expressed hPR-A in sf21 cells. | J Med Chem 41: 303-10 (1998) Article DOI: 10.1021/jm9705770 BindingDB Entry DOI: 10.7270/Q2RV0PC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062429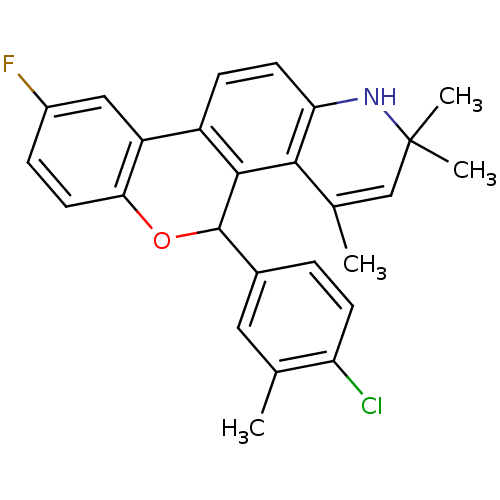 (5-(4-Chloro-3-methyl-phenyl)-9-fluoro-2,2,4-trimet...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description The binding affinity measured using baculovirus-expressed hPR-A in sf21 cells. | J Med Chem 41: 303-10 (1998) Article DOI: 10.1021/jm9705770 BindingDB Entry DOI: 10.7270/Q2RV0PC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50214923 ((Z)-5-(4-fluoro-2-(hydroxymethyl)benzylidene)-10-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of radiolabeled dexamethasone from glucocorticoid receptor | Bioorg Med Chem Lett 17: 4158-62 (2007) Article DOI: 10.1016/j.bmcl.2007.05.062 BindingDB Entry DOI: 10.7270/Q2DF6QXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18607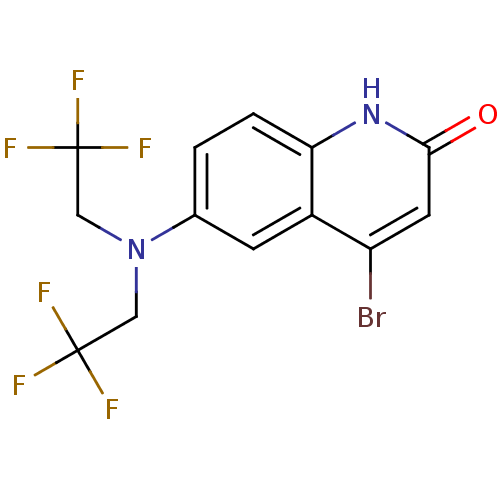 (6-[bis(2,2,2-trifluoroethyl)amino]-4-bromo-1,2-dih...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | Bioorg Med Chem Lett 17: 1527-31 (2007) Article DOI: 10.1016/j.bmcl.2007.01.001 BindingDB Entry DOI: 10.7270/Q2XS5SP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18606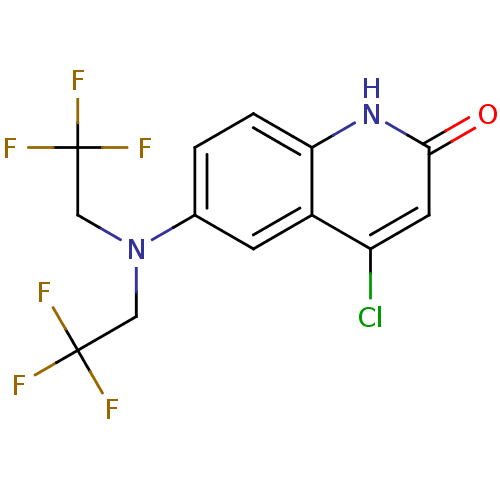 (6-[bis(2,2,2-trifluoroethyl)amino]-4-chloro-1,2-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | Bioorg Med Chem Lett 17: 1527-31 (2007) Article DOI: 10.1016/j.bmcl.2007.01.001 BindingDB Entry DOI: 10.7270/Q2XS5SP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18578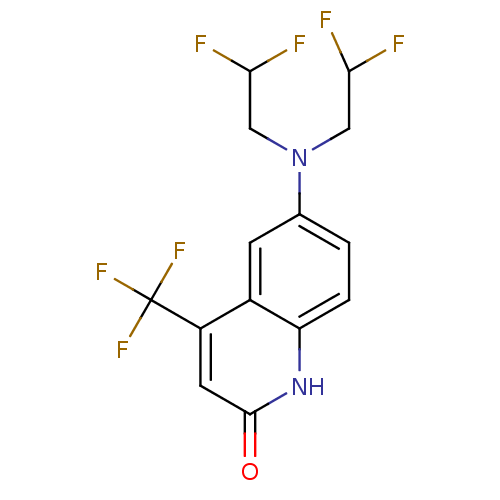 (6-[bis(2,2-difluoroethyl)amino]-4-(trifluoromethyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.5 | -55.2 | n/a | n/a | 0.400 | n/a | n/a | 7.4 | 37 |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | Bioorg Med Chem Lett 17: 1527-31 (2007) Article DOI: 10.1016/j.bmcl.2007.01.001 BindingDB Entry DOI: 10.7270/Q2XS5SP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062439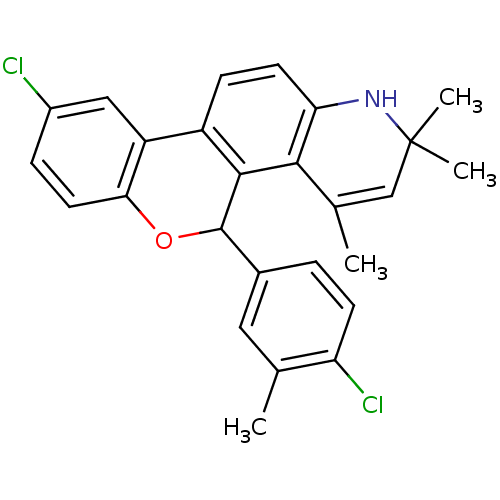 (9-Chloro-5-(4-chloro-3-methyl-phenyl)-2,2,4-trimet...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description The binding affinity measured using baculovirus-expressed hPR-A in sf21 cells. | J Med Chem 41: 303-10 (1998) Article DOI: 10.1021/jm9705770 BindingDB Entry DOI: 10.7270/Q2RV0PC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062409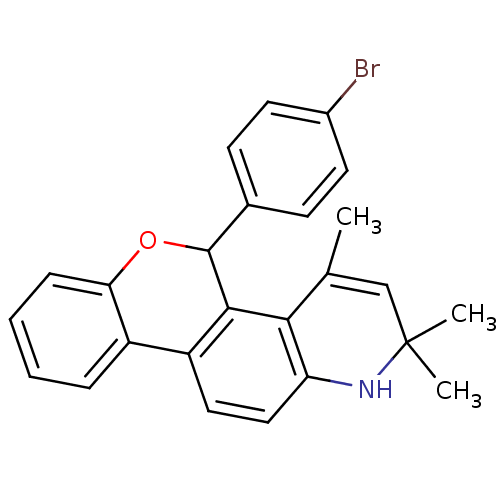 (5-(4-Bromo-phenyl)-2,2,4-trimethyl-2,5-dihydro-1H-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description The binding affinity of the compound was determined on Human progesterone receptor (hPR-A) using progesterone as radioligand. | J Med Chem 41: 291-302 (1998) Article DOI: 10.1021/jm9705768 BindingDB Entry DOI: 10.7270/Q29W0DMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50133131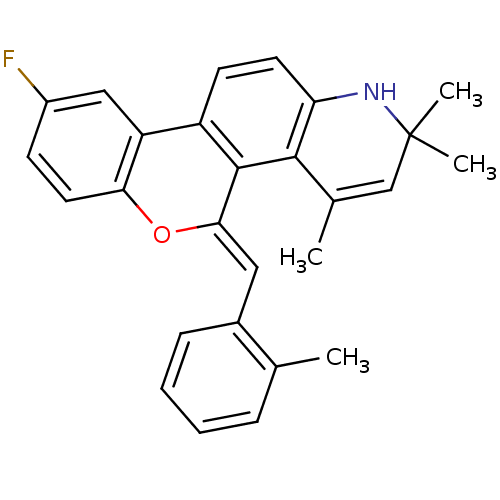 (9-Fluoro-2,2,4-trimethyl-5-[1-o-tolyl-meth-(Z)-yli...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding affinity towards human progesterone receptor A isoform using progesterone as radioligand | J Med Chem 46: 4104-12 (2003) Article DOI: 10.1021/jm020477g BindingDB Entry DOI: 10.7270/Q2QZ29C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062438 (9-Chloro-2,2,4-trimethyl-5-m-tolyl-2,5-dihydro-1H-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description The binding affinity measured using baculovirus-expressed hPR-A in sf21 cells. | J Med Chem 41: 303-10 (1998) Article DOI: 10.1021/jm9705770 BindingDB Entry DOI: 10.7270/Q2RV0PC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062445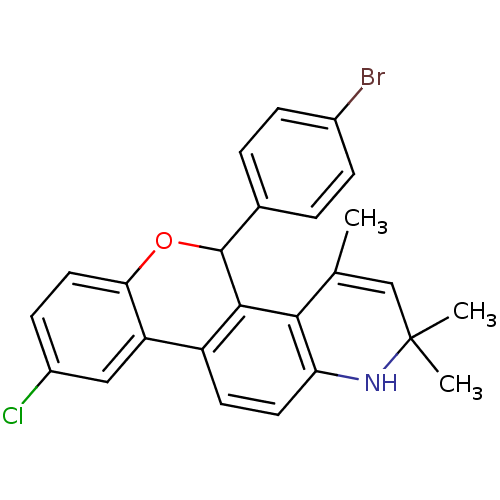 (5-(4-Bromo-phenyl)-9-chloro-2,2,4-trimethyl-2,5-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description The binding affinity measured using baculovirus-expressed hPR-A in sf21 cells. | J Med Chem 41: 303-10 (1998) Article DOI: 10.1021/jm9705770 BindingDB Entry DOI: 10.7270/Q2RV0PC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062442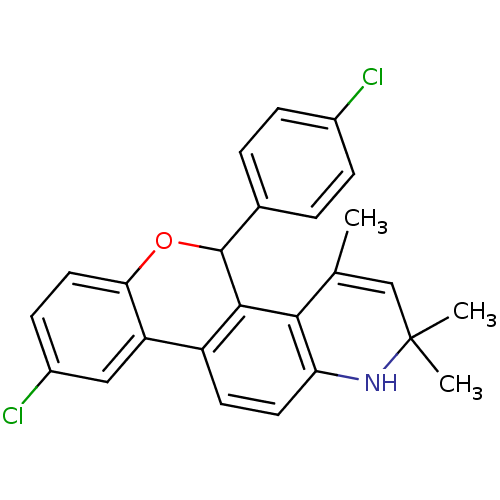 (9-Chloro-5-(4-chloro-phenyl)-2,2,4-trimethyl-2,5-d...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description The binding affinity measured using baculovirus-expressed hPR-A in sf21 cells. | J Med Chem 41: 303-10 (1998) Article DOI: 10.1021/jm9705770 BindingDB Entry DOI: 10.7270/Q2RV0PC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50133135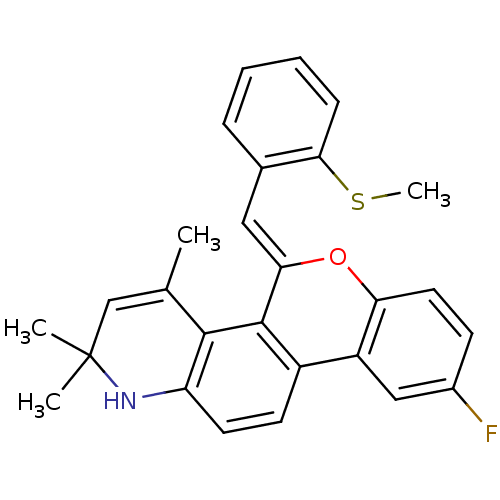 (9-Fluoro-2,2,4-trimethyl-5-[1-(2-methylsulfanyl-ph...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding affinity towards human progesterone receptor A isoform using progesterone as radioligand | J Med Chem 46: 4104-12 (2003) Article DOI: 10.1021/jm020477g BindingDB Entry DOI: 10.7270/Q2QZ29C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18622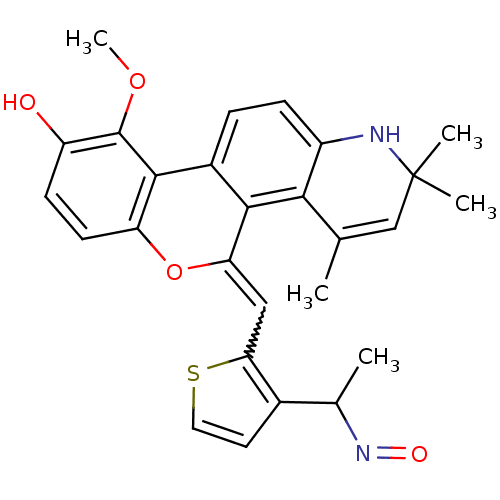 ((18Z)-18-({3-[(1E)-1-(hydroxyimino)ethyl]thiophen-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | -48.9 | 1.10 | n/a | 5 | n/a | n/a | 7.5 | 4 |
Ligand Pharmaceuticals Inc. | Assay Description Competitive Ligand Binding Assay- The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd) where [L] is the... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50214929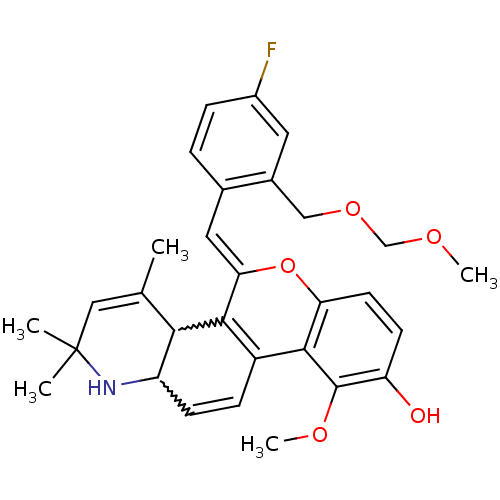 ((Z)-5-(4-fluoro-2-((methoxymethoxy)methyl)benzylid...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of radiolabeled dexamethasone from glucocorticoid receptor | Bioorg Med Chem Lett 17: 4158-62 (2007) Article DOI: 10.1016/j.bmcl.2007.05.062 BindingDB Entry DOI: 10.7270/Q2DF6QXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50334048 ((-)-5-chloro-6-(1H-indol-7-yl)-2,2,4,8-tetramethyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of radiolabeled Dexamethasone from glucocorticoid receptor | Bioorg Med Chem Lett 18: 3504-8 (2008) Article DOI: 10.1016/j.bmcl.2008.05.029 BindingDB Entry DOI: 10.7270/Q2CR5V8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50334048 ((-)-5-chloro-6-(1H-indol-7-yl)-2,2,4,8-tetramethyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of radiolabeled dexamethasone from glucocorticoid receptor expressed in baculovirus | Bioorg Med Chem Lett 21: 168-71 (2010) Article DOI: 10.1016/j.bmcl.2010.11.047 BindingDB Entry DOI: 10.7270/Q2VX0GRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50334048 ((-)-5-chloro-6-(1H-indol-7-yl)-2,2,4,8-tetramethyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of radiolabeled Dexamethasone from glucocorticoid receptor | Bioorg Med Chem Lett 18: 3504-8 (2008) Article DOI: 10.1016/j.bmcl.2008.05.029 BindingDB Entry DOI: 10.7270/Q2CR5V8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50340660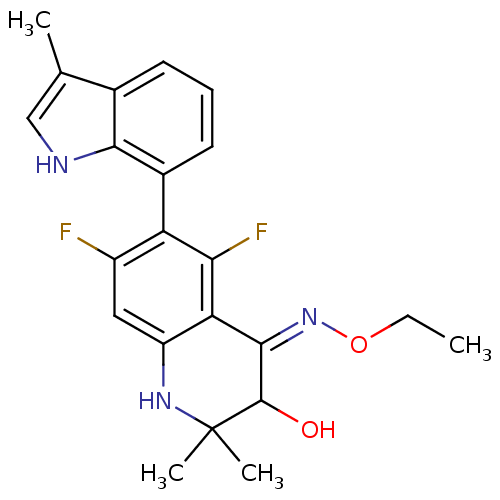 (CHEMBL1762210 | rac-5,7-difluoro-3-hydroxy-2,2-dim...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of radiolabeled dexamethasone from glucocorticoid receptor expressed in baculovirus | Bioorg Med Chem Lett 21: 1654-7 (2011) Article DOI: 10.1016/j.bmcl.2011.01.104 BindingDB Entry DOI: 10.7270/Q21V5F8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50133146 (7-Fluoro-2,2,4-trimethyl-5-[1-phenyl-meth-(Z)-ylid...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding affinity towards human progesterone receptor A isoform using progesterone as radioligand | J Med Chem 46: 4104-12 (2003) Article DOI: 10.1021/jm020477g BindingDB Entry DOI: 10.7270/Q2QZ29C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50133152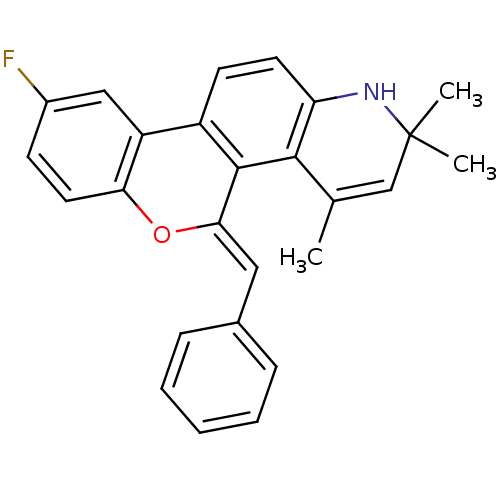 (9-Fluoro-2,2,4-trimethyl-5-[1-phenyl-meth-(Z)-ylid...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding affinity towards human progesterone receptor A isoform using progesterone as radioligand | J Med Chem 46: 4104-12 (2003) Article DOI: 10.1021/jm020477g BindingDB Entry DOI: 10.7270/Q2QZ29C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50133139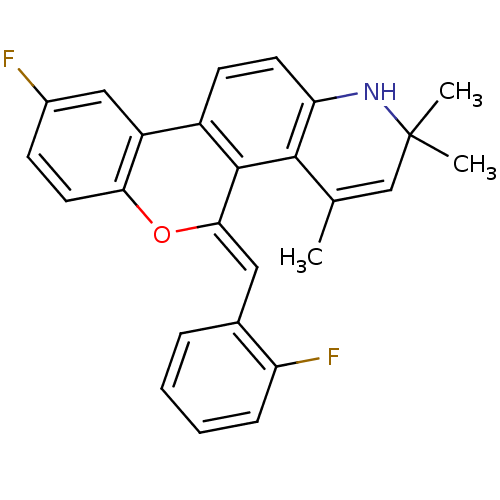 (9-Fluoro-5-[1-(2-fluoro-phenyl)-meth-(Z)-ylidene]-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding affinity towards human progesterone receptor A isoform using progesterone as radioligand | J Med Chem 46: 4104-12 (2003) Article DOI: 10.1021/jm020477g BindingDB Entry DOI: 10.7270/Q2QZ29C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50067684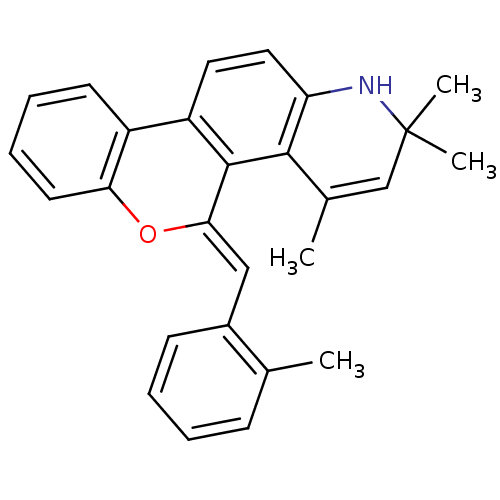 (2,2,4-Trimethyl-5-[1-o-tolyl-meth-(Z)-ylidene]-2,5...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity against human progesterone receptor | J Med Chem 41: 4354-9 (1998) Article DOI: 10.1021/jm980366a BindingDB Entry DOI: 10.7270/Q2V40TB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50067684 (2,2,4-Trimethyl-5-[1-o-tolyl-meth-(Z)-ylidene]-2,5...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding affinity towards human progesterone receptor A isoform using progesterone as radioligand | J Med Chem 46: 4104-12 (2003) Article DOI: 10.1021/jm020477g BindingDB Entry DOI: 10.7270/Q2QZ29C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50067684 (2,2,4-Trimethyl-5-[1-o-tolyl-meth-(Z)-ylidene]-2,5...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human progesterone receptor | Bioorg Med Chem Lett 13: 2071-4 (2003) BindingDB Entry DOI: 10.7270/Q2SX6DSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2750 total ) | Next | Last >> |