

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50087713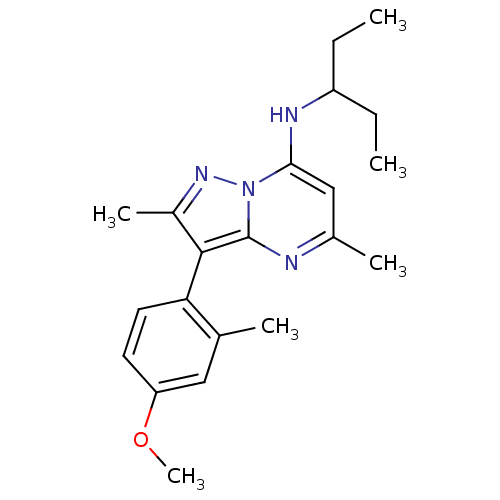 ((1-Ethyl-propyl)-[3-(4-methoxy-2-methyl-phenyl)-2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]CRF from human corticotropin-releasing factor receptor 1 expressed in CHO-K1 cells after 2 hrs by gamma counting | Bioorg Med Chem 19: 5432-45 (2011) Article DOI: 10.1016/j.bmc.2011.07.055 BindingDB Entry DOI: 10.7270/Q2Z038JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50330441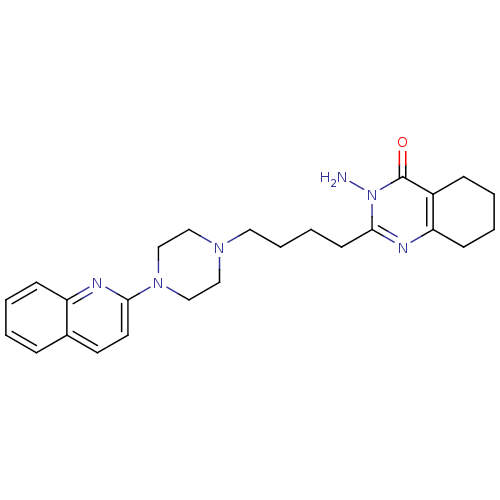 (3-amino-2-(4-(4-(quinolin-2-yl)piperazin-1-yl)buty...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ASKA Pharmaceutical Co, Ltd Curated by ChEMBL | Assay Description Binding affinity to human 5HT3 receptor | J Med Chem 53: 7549-63 (2010) Article DOI: 10.1021/jm1002292 BindingDB Entry DOI: 10.7270/Q27082D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50330444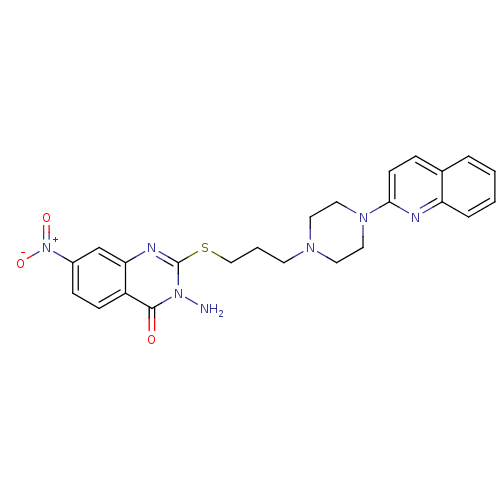 (3-Amino-7-nitro-2-[3-(4-quinolin-2-ylpiperazin-1-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
ASKA Pharmaceutical Co, Ltd Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 53: 7549-63 (2010) Article DOI: 10.1021/jm1002292 BindingDB Entry DOI: 10.7270/Q27082D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50330441 (3-amino-2-(4-(4-(quinolin-2-yl)piperazin-1-yl)buty...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
ASKA Pharmaceutical Co, Ltd Curated by ChEMBL | Assay Description Displacement of 8-hydroxy-[3H]DPAT from human recombinant 5HT1A receptor | J Med Chem 53: 7549-63 (2010) Article DOI: 10.1021/jm1002292 BindingDB Entry DOI: 10.7270/Q27082D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50330441 (3-amino-2-(4-(4-(quinolin-2-yl)piperazin-1-yl)buty...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
ASKA Pharmaceutical Co, Ltd Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 53: 7549-63 (2010) Article DOI: 10.1021/jm1002292 BindingDB Entry DOI: 10.7270/Q27082D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50330445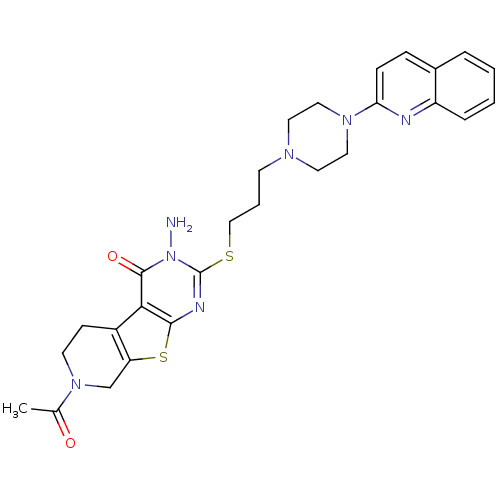 (7-Acetyl-3-amino-2-[3-(4-quinolin-2-ylpiperazin-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
ASKA Pharmaceutical Co, Ltd Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 53: 7549-63 (2010) Article DOI: 10.1021/jm1002292 BindingDB Entry DOI: 10.7270/Q27082D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50351393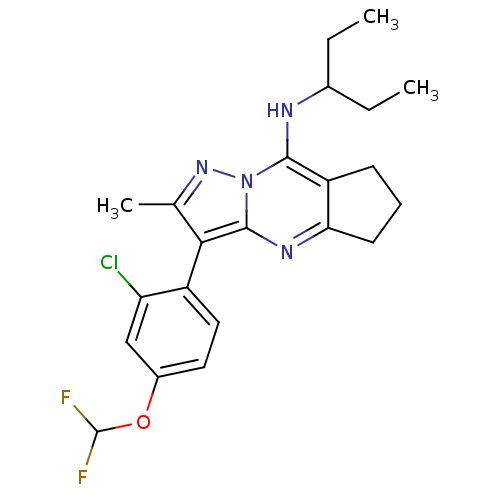 (CHEMBL1819083) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]CRF from human corticotropin-releasing factor receptor 1 expressed in CHO-K1 cells after 2 hrs by gamma counting | Bioorg Med Chem 19: 5432-45 (2011) Article DOI: 10.1016/j.bmc.2011.07.055 BindingDB Entry DOI: 10.7270/Q2Z038JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50003815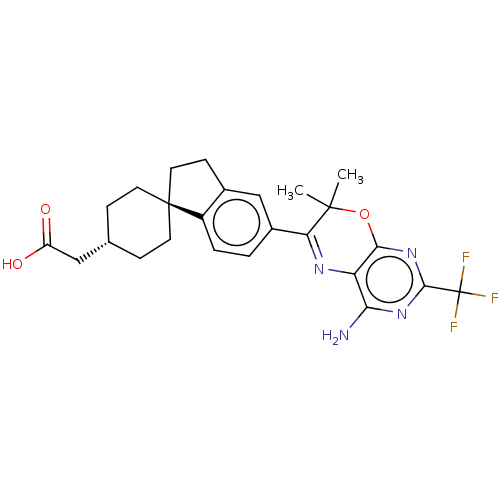 (CHEMBL3235321) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of DGAT1 (unknown origin) by cell-based assay | J Med Chem 57: 3464-83 (2014) Article DOI: 10.1021/jm500135c BindingDB Entry DOI: 10.7270/Q23X886W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM27947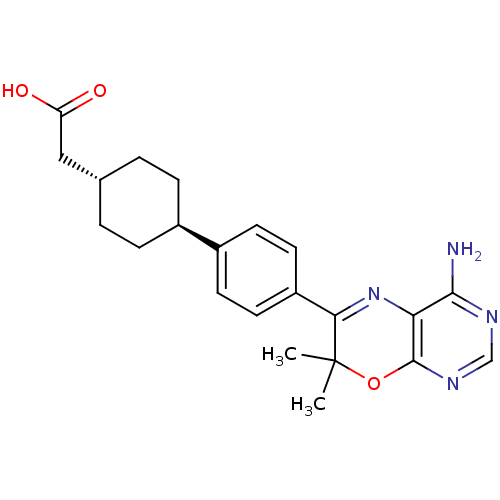 (2-[4-(4-{4-amino-7,7-dimethyl-7H-pyrimido[4,5-b][1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of DGAT1 (unknown origin) by cell-based assay | J Med Chem 57: 3464-83 (2014) Article DOI: 10.1021/jm500135c BindingDB Entry DOI: 10.7270/Q23X886W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50003815 (CHEMBL3235321) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of human liver flag-tagged DGAT1 expressed in baculovirus infected insect cells after 2 hrs by SPA | J Med Chem 57: 3464-83 (2014) Article DOI: 10.1021/jm500135c BindingDB Entry DOI: 10.7270/Q23X886W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50351387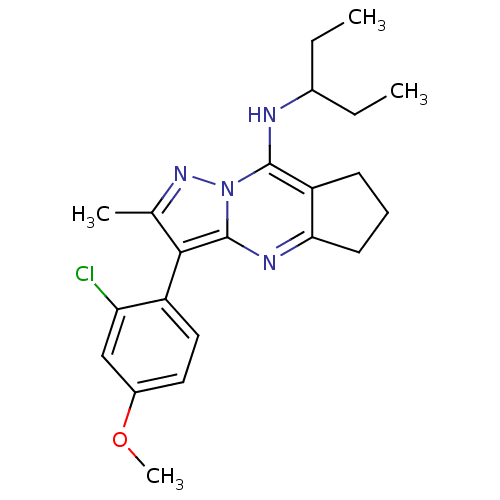 (CHEMBL1819077) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]CRF from human corticotropin-releasing factor receptor 1 expressed in CHO-K1 cells after 2 hrs by gamma counting | Bioorg Med Chem 19: 5432-45 (2011) Article DOI: 10.1016/j.bmc.2011.07.055 BindingDB Entry DOI: 10.7270/Q2Z038JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50330446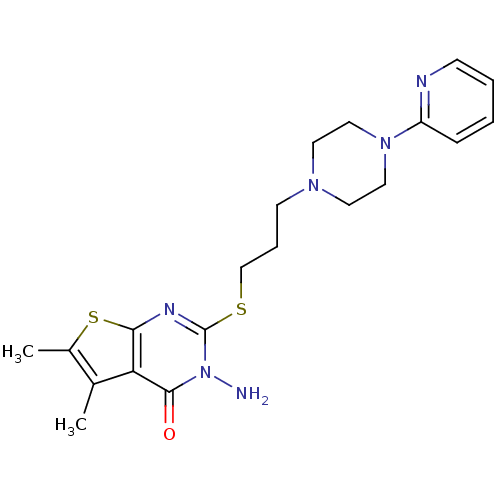 (3-Amino-5,6-dimethyl-2-[3-(4-pyridin-2-ylpiperazin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
ASKA Pharmaceutical Co, Ltd Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 53: 7549-63 (2010) Article DOI: 10.1021/jm1002292 BindingDB Entry DOI: 10.7270/Q27082D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50330447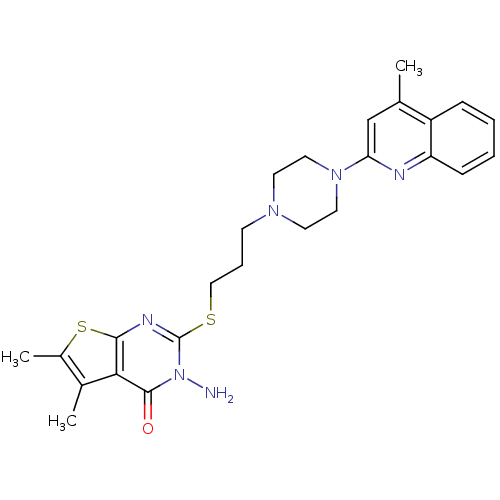 (3-Amino-5,6-dimethyl-2-[3-[4-(4-methylquinolin-2-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
ASKA Pharmaceutical Co, Ltd Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 53: 7549-63 (2010) Article DOI: 10.1021/jm1002292 BindingDB Entry DOI: 10.7270/Q27082D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50003860 (CHEMBL3235317) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of human liver flag-tagged DGAT1 expressed in baculovirus infected insect cells after 2 hrs by SPA | J Med Chem 57: 3464-83 (2014) Article DOI: 10.1021/jm500135c BindingDB Entry DOI: 10.7270/Q23X886W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50330448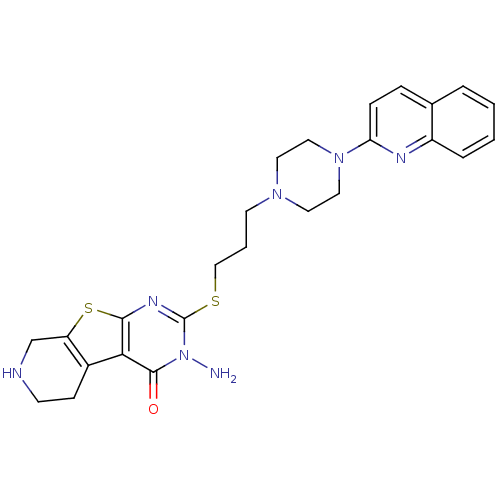 (3-Amino-2-[3-(4-quinolin-2-ylpiperazin-1-yl)propyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
ASKA Pharmaceutical Co, Ltd Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 53: 7549-63 (2010) Article DOI: 10.1021/jm1002292 BindingDB Entry DOI: 10.7270/Q27082D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50330440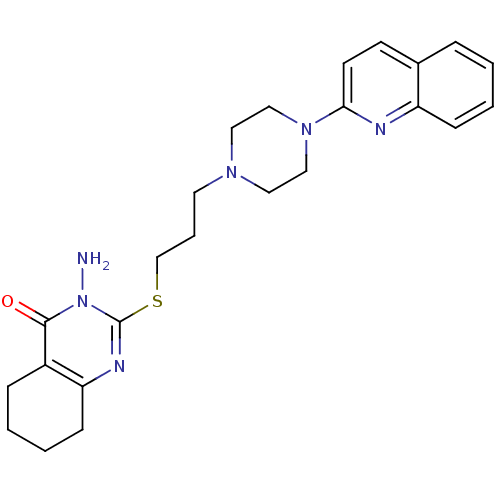 (3-Amino-2-[3-(4-quinolin-2-ylpiperazin-1-yl)propyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
ASKA Pharmaceutical Co, Ltd Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 53: 7549-63 (2010) Article DOI: 10.1021/jm1002292 BindingDB Entry DOI: 10.7270/Q27082D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50351394 (CHEMBL1819084) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]CRF from human corticotropin-releasing factor receptor 1 expressed in CHO-K1 cells after 2 hrs by gamma counting | Bioorg Med Chem 19: 5432-45 (2011) Article DOI: 10.1016/j.bmc.2011.07.055 BindingDB Entry DOI: 10.7270/Q2Z038JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM27947 (2-[4-(4-{4-amino-7,7-dimethyl-7H-pyrimido[4,5-b][1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of human liver flag-tagged DGAT1 expressed in baculovirus infected insect cells after 2 hrs by SPA | J Med Chem 57: 3464-83 (2014) Article DOI: 10.1021/jm500135c BindingDB Entry DOI: 10.7270/Q23X886W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50003857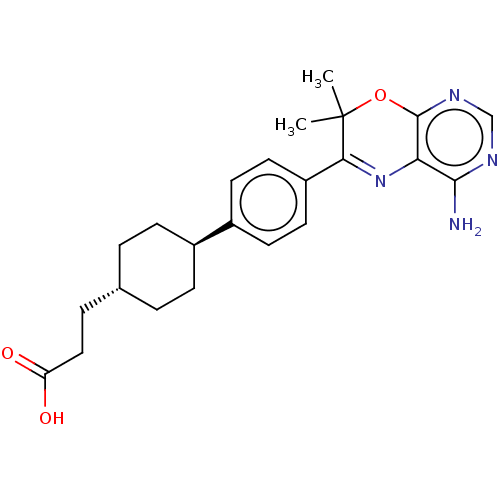 (CHEMBL3235314) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of human liver flag-tagged DGAT1 expressed in baculovirus infected insect cells after 2 hrs by SPA | J Med Chem 57: 3464-83 (2014) Article DOI: 10.1021/jm500135c BindingDB Entry DOI: 10.7270/Q23X886W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50003858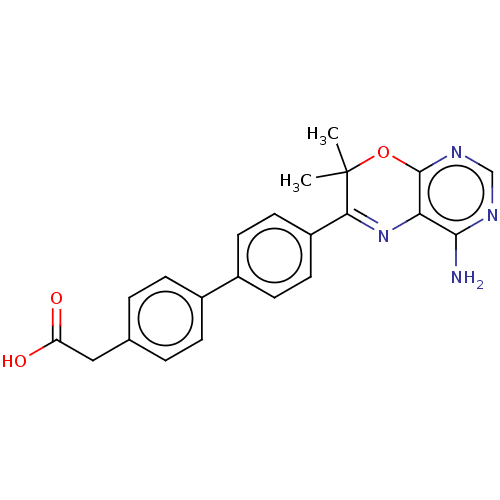 (CHEMBL3235315) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of human liver flag-tagged DGAT1 expressed in baculovirus infected insect cells after 2 hrs by SPA | J Med Chem 57: 3464-83 (2014) Article DOI: 10.1021/jm500135c BindingDB Entry DOI: 10.7270/Q23X886W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50330449 (3-Amino-5,6-dimethyl-2-[3-[4-(5,6,7,8-tetrahydroqu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
ASKA Pharmaceutical Co, Ltd Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 53: 7549-63 (2010) Article DOI: 10.1021/jm1002292 BindingDB Entry DOI: 10.7270/Q27082D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50330450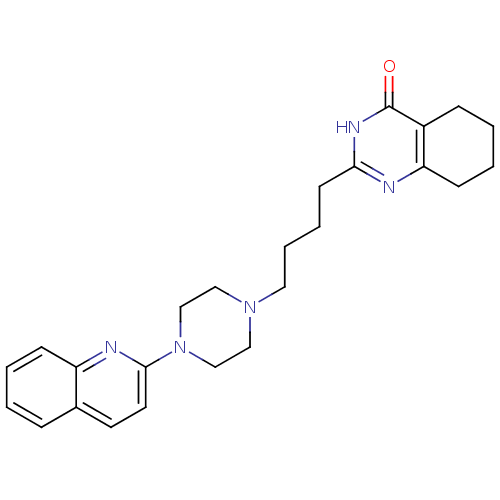 (2-[4-(4-Quinolin-2-ylpiperazin-1-yl)butyl]-5,6,7,8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
ASKA Pharmaceutical Co, Ltd Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 53: 7549-63 (2010) Article DOI: 10.1021/jm1002292 BindingDB Entry DOI: 10.7270/Q27082D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50351396 (CHEMBL1819086) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]CRF from human corticotropin-releasing factor receptor 1 expressed in CHO-K1 cells after 2 hrs by gamma counting | Bioorg Med Chem 19: 5432-45 (2011) Article DOI: 10.1016/j.bmc.2011.07.055 BindingDB Entry DOI: 10.7270/Q2Z038JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50351387 (CHEMBL1819077) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]CRF from corticotropin-releasing factor receptor 1 in rat brain membranes after 2 hrs by gamma counting | Bioorg Med Chem 19: 5432-45 (2011) Article DOI: 10.1016/j.bmc.2011.07.055 BindingDB Entry DOI: 10.7270/Q2Z038JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50003862 (CHEMBL3235319) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of human liver flag-tagged DGAT1 expressed in baculovirus infected insect cells after 2 hrs by SPA | J Med Chem 57: 3464-83 (2014) Article DOI: 10.1021/jm500135c BindingDB Entry DOI: 10.7270/Q23X886W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50330451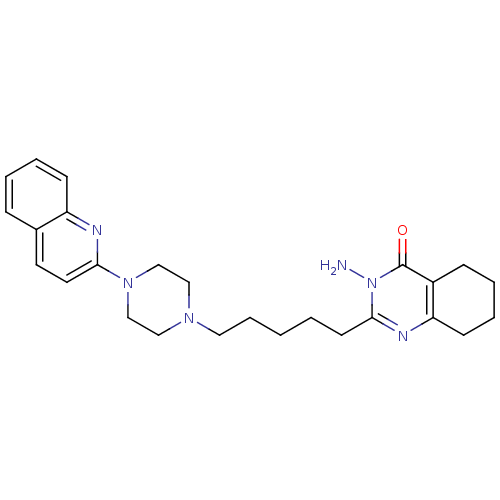 (5-Amino-2-[5-(4-quinolin-2-ylpiperazin-1-yl)pentyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
ASKA Pharmaceutical Co, Ltd Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 53: 7549-63 (2010) Article DOI: 10.1021/jm1002292 BindingDB Entry DOI: 10.7270/Q27082D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50003859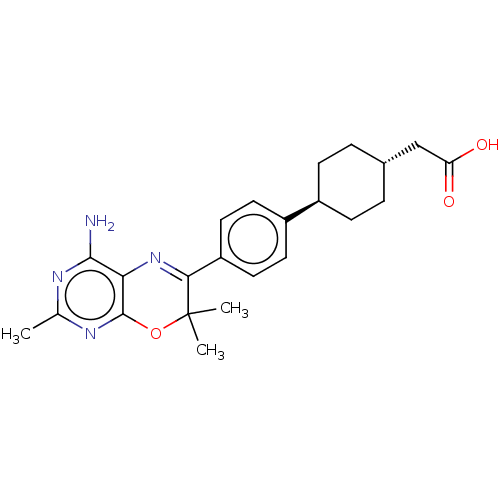 (CHEMBL3235316) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of human liver flag-tagged DGAT1 expressed in baculovirus infected insect cells after 2 hrs by SPA | J Med Chem 57: 3464-83 (2014) Article DOI: 10.1021/jm500135c BindingDB Entry DOI: 10.7270/Q23X886W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50003864 (CHEMBL3235322) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of human liver flag-tagged DGAT1 expressed in baculovirus infected insect cells after 2 hrs by SPA | J Med Chem 57: 3464-83 (2014) Article DOI: 10.1021/jm500135c BindingDB Entry DOI: 10.7270/Q23X886W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50330441 (3-amino-2-(4-(4-(quinolin-2-yl)piperazin-1-yl)buty...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
ASKA Pharmaceutical Co, Ltd Curated by ChEMBL | Assay Description Displacement of [3H]BRL-43694 from human 5HT3 receptor expressed in HEK293 cells by liquid scintillation counting | J Med Chem 53: 7549-63 (2010) Article DOI: 10.1021/jm1002292 BindingDB Entry DOI: 10.7270/Q27082D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50330441 (3-amino-2-(4-(4-(quinolin-2-yl)piperazin-1-yl)buty...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >8.93 | n/a | n/a | n/a | n/a | n/a | n/a |
ASKA Pharmaceutical Co, Ltd Curated by ChEMBL | Assay Description Displacement of [3H]BRL-43694 from human recombinant 5HT3 receptor | J Med Chem 53: 7549-63 (2010) Article DOI: 10.1021/jm1002292 BindingDB Entry DOI: 10.7270/Q27082D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50003713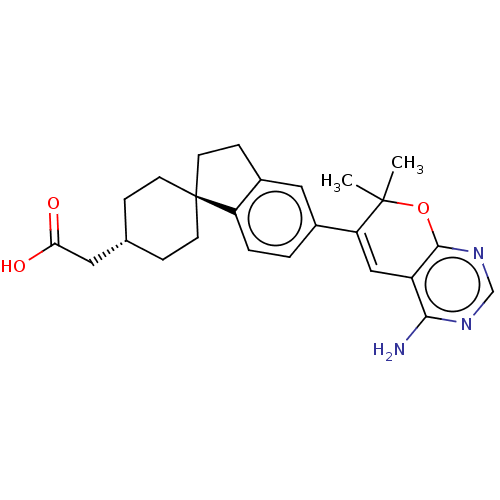 (CHEMBL3235323) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of DGAT1 (unknown origin) by cell-based assay | J Med Chem 57: 3464-83 (2014) Article DOI: 10.1021/jm500135c BindingDB Entry DOI: 10.7270/Q23X886W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50351386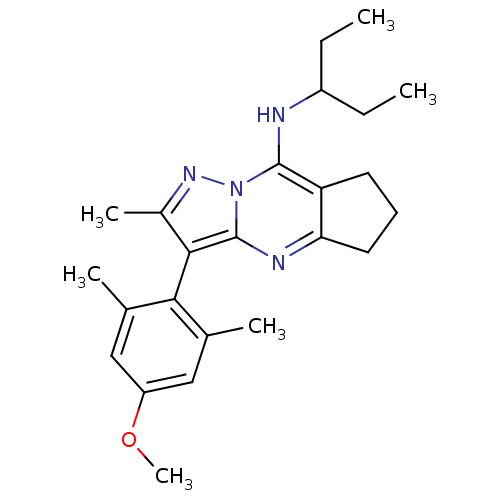 (CHEMBL1819076) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]CRF from human corticotropin-releasing factor receptor 1 expressed in CHO-K1 cells after 2 hrs by gamma counting | Bioorg Med Chem 19: 5432-45 (2011) Article DOI: 10.1016/j.bmc.2011.07.055 BindingDB Entry DOI: 10.7270/Q2Z038JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50003856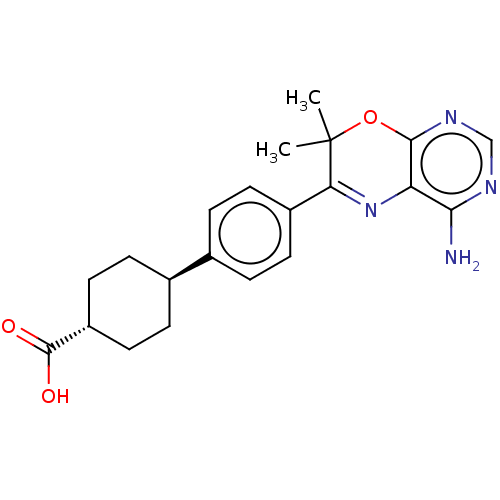 (CHEMBL3235313) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of human liver flag-tagged DGAT1 expressed in baculovirus infected insect cells after 2 hrs by SPA | J Med Chem 57: 3464-83 (2014) Article DOI: 10.1021/jm500135c BindingDB Entry DOI: 10.7270/Q23X886W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50087713 ((1-Ethyl-propyl)-[3-(4-methoxy-2-methyl-phenyl)-2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human corticotropin-releasing factor receptor 1 expressed in CHO-K1 cells assessed as inhibition of CRF-induced cAMP productio... | Bioorg Med Chem 19: 5432-45 (2011) Article DOI: 10.1016/j.bmc.2011.07.055 BindingDB Entry DOI: 10.7270/Q2Z038JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50003713 (CHEMBL3235323) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of human liver flag-tagged DGAT1 expressed in baculovirus infected insect cells after 2 hrs by SPA | J Med Chem 57: 3464-83 (2014) Article DOI: 10.1021/jm500135c BindingDB Entry DOI: 10.7270/Q23X886W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50330452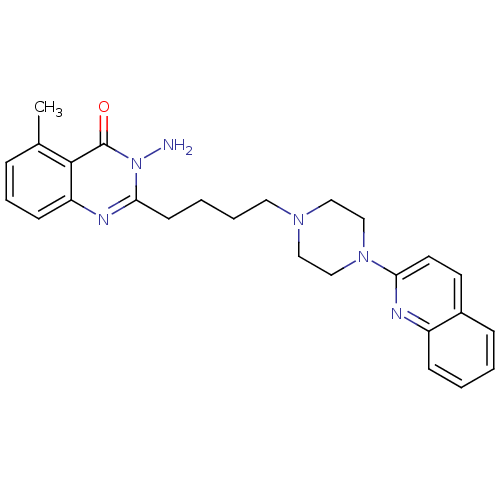 (3-Amino-5-methyl-2-[4-(4-quinolin-2-ylpiperazin-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13.5 | n/a | n/a | n/a | n/a | n/a | n/a |
ASKA Pharmaceutical Co, Ltd Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 53: 7549-63 (2010) Article DOI: 10.1021/jm1002292 BindingDB Entry DOI: 10.7270/Q27082D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50351391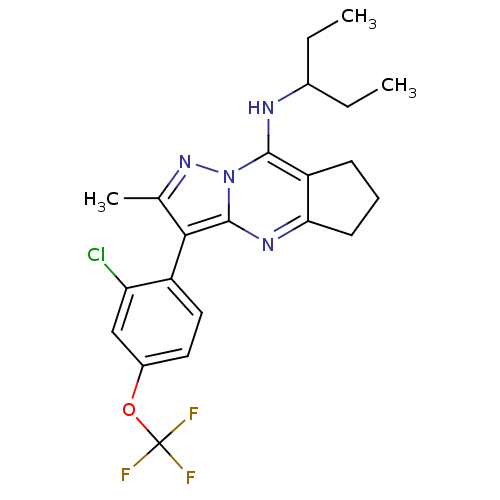 (CHEMBL1819081) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]CRF from human corticotropin-releasing factor receptor 1 expressed in CHO-K1 cells after 2 hrs by gamma counting | Bioorg Med Chem 19: 5432-45 (2011) Article DOI: 10.1016/j.bmc.2011.07.055 BindingDB Entry DOI: 10.7270/Q2Z038JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50003861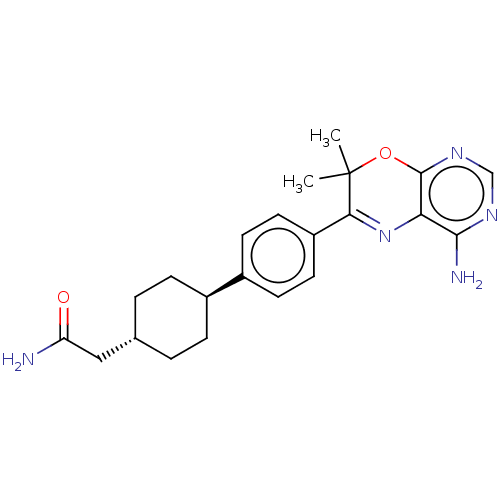 (CHEMBL3235318) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of human liver flag-tagged DGAT1 expressed in baculovirus infected insect cells after 2 hrs by SPA | J Med Chem 57: 3464-83 (2014) Article DOI: 10.1021/jm500135c BindingDB Entry DOI: 10.7270/Q23X886W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50330454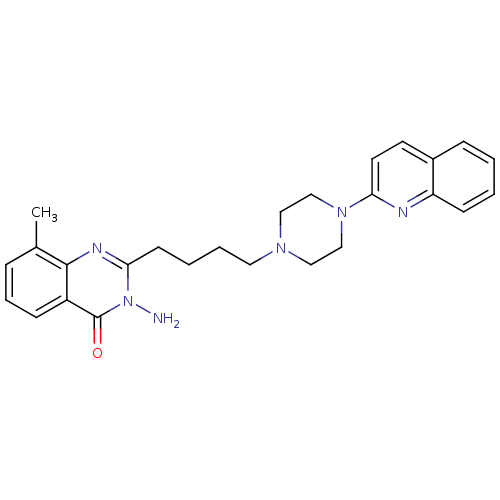 (3-Amino-8-methyl-2-[4-(4-quinolin-2-ylpiperazin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15.6 | n/a | n/a | n/a | n/a | n/a | n/a |
ASKA Pharmaceutical Co, Ltd Curated by ChEMBL | Assay Description Displacement of [3H]BRL-43694 from human 5HT3 receptor expressed in HEK293 cells by liquid scintillation counting | J Med Chem 53: 7549-63 (2010) Article DOI: 10.1021/jm1002292 BindingDB Entry DOI: 10.7270/Q27082D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50330455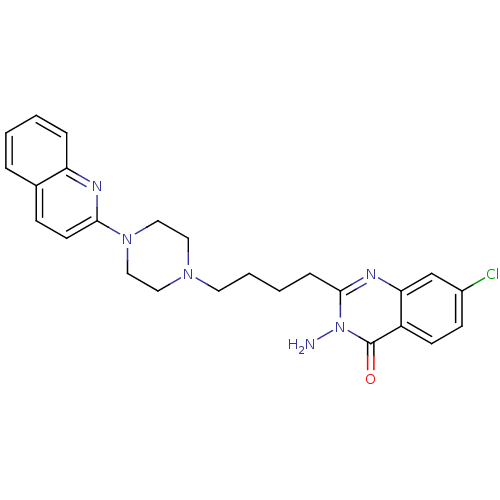 (3-Amino-7-chloro-2-[4-(4-quinolin-2-ylpiperazin-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
ASKA Pharmaceutical Co, Ltd Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 53: 7549-63 (2010) Article DOI: 10.1021/jm1002292 BindingDB Entry DOI: 10.7270/Q27082D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50003863 (CHEMBL3235320) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of human liver flag-tagged DGAT1 expressed in baculovirus infected insect cells after 2 hrs by SPA | J Med Chem 57: 3464-83 (2014) Article DOI: 10.1021/jm500135c BindingDB Entry DOI: 10.7270/Q23X886W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50351397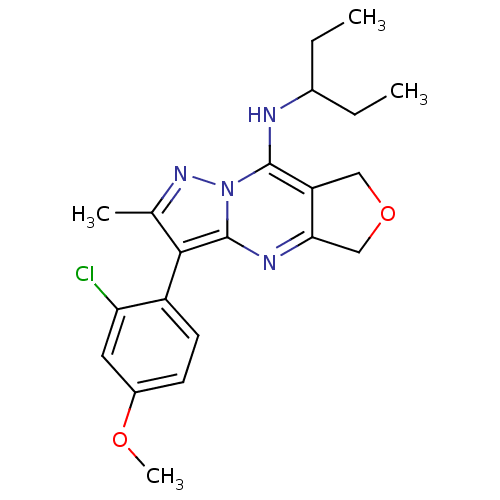 (CHEMBL1819087) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]CRF from human corticotropin-releasing factor receptor 1 expressed in CHO-K1 cells after 2 hrs by gamma counting | Bioorg Med Chem 19: 5432-45 (2011) Article DOI: 10.1016/j.bmc.2011.07.055 BindingDB Entry DOI: 10.7270/Q2Z038JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50351385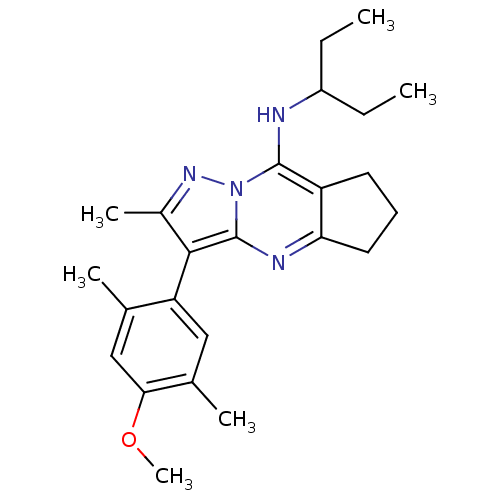 (CHEMBL1819075) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]CRF from human corticotropin-releasing factor receptor 1 expressed in CHO-K1 cells after 2 hrs by gamma counting | Bioorg Med Chem 19: 5432-45 (2011) Article DOI: 10.1016/j.bmc.2011.07.055 BindingDB Entry DOI: 10.7270/Q2Z038JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50330456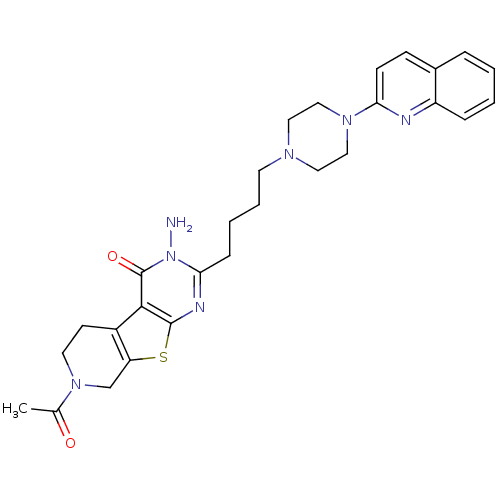 (7-Acetyl-3-amino-2-[4-(4-quinolin-2-ylpiperazin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22.1 | n/a | n/a | n/a | n/a | n/a | n/a |
ASKA Pharmaceutical Co, Ltd Curated by ChEMBL | Assay Description Displacement of [3H]BRL-43694 from human 5HT3 receptor expressed in HEK293 cells by liquid scintillation counting | J Med Chem 53: 7549-63 (2010) Article DOI: 10.1021/jm1002292 BindingDB Entry DOI: 10.7270/Q27082D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50330457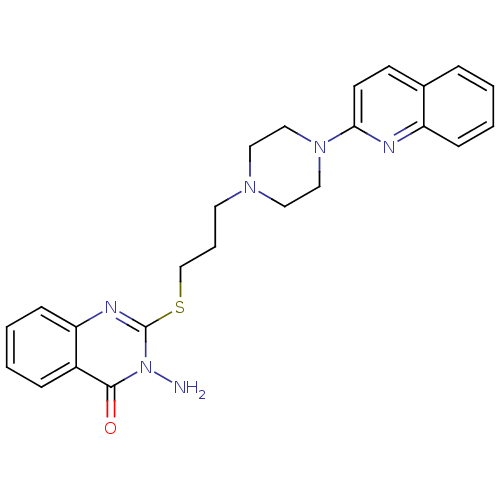 (3-Amino-2-[3-(4-quinolin-2-ylpiperazin-1-yl)propyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24.4 | n/a | n/a | n/a | n/a | n/a | n/a |
ASKA Pharmaceutical Co, Ltd Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 53: 7549-63 (2010) Article DOI: 10.1021/jm1002292 BindingDB Entry DOI: 10.7270/Q27082D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50330450 (2-[4-(4-Quinolin-2-ylpiperazin-1-yl)butyl]-5,6,7,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24.6 | n/a | n/a | n/a | n/a | n/a | n/a |
ASKA Pharmaceutical Co, Ltd Curated by ChEMBL | Assay Description Displacement of [3H]BRL-43694 from human 5HT3 receptor expressed in HEK293 cells by liquid scintillation counting | J Med Chem 53: 7549-63 (2010) Article DOI: 10.1021/jm1002292 BindingDB Entry DOI: 10.7270/Q27082D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50330458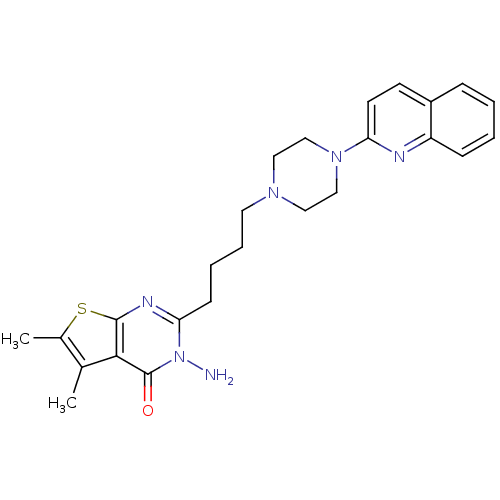 (3-Amino-5,6-dimethyl-2-[4-(4-quinolin-2-ylpiperazi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26.7 | n/a | n/a | n/a | n/a | n/a | n/a |
ASKA Pharmaceutical Co, Ltd Curated by ChEMBL | Assay Description Displacement of [3H]BRL-43694 from human 5HT3 receptor expressed in HEK293 cells by liquid scintillation counting | J Med Chem 53: 7549-63 (2010) Article DOI: 10.1021/jm1002292 BindingDB Entry DOI: 10.7270/Q27082D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50003859 (CHEMBL3235316) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of DGAT1 (unknown origin) by cell-based assay | J Med Chem 57: 3464-83 (2014) Article DOI: 10.1021/jm500135c BindingDB Entry DOI: 10.7270/Q23X886W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50351371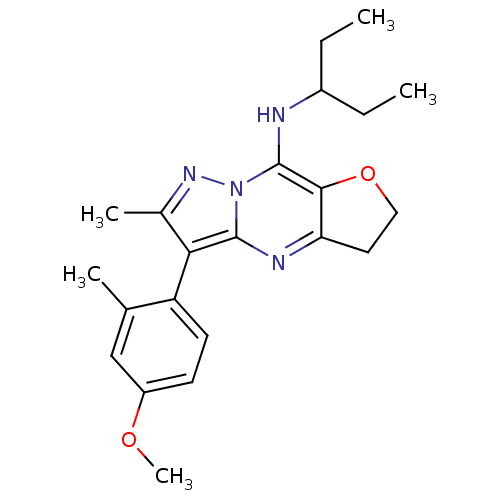 (CHEMBL1819061) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]CRF from human corticotropin-releasing factor receptor 1 expressed in CHO-K1 cells after 2 hrs by gamma counting | Bioorg Med Chem 19: 5432-45 (2011) Article DOI: 10.1016/j.bmc.2011.07.055 BindingDB Entry DOI: 10.7270/Q2Z038JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50351374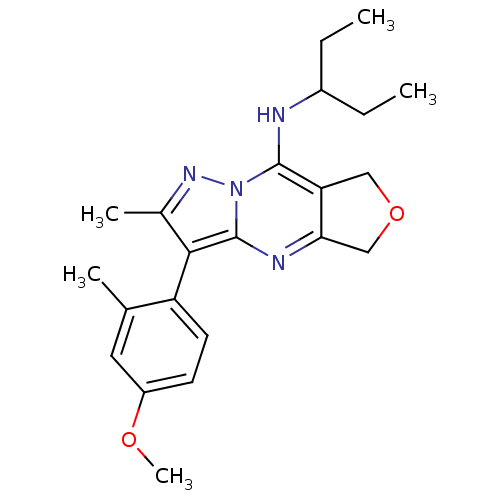 (CHEMBL1819064) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]CRF from human corticotropin-releasing factor receptor 1 expressed in CHO-K1 cells after 2 hrs by gamma counting | Bioorg Med Chem 19: 5432-45 (2011) Article DOI: 10.1016/j.bmc.2011.07.055 BindingDB Entry DOI: 10.7270/Q2Z038JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 595 total ) | Next | Last >> |