 Found 135 hits with Last Name = 'mccoy' and Initial = 'ma'
Found 135 hits with Last Name = 'mccoy' and Initial = 'ma' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Genome polyprotein
(Hepatitis C virus) | BDBM50145828
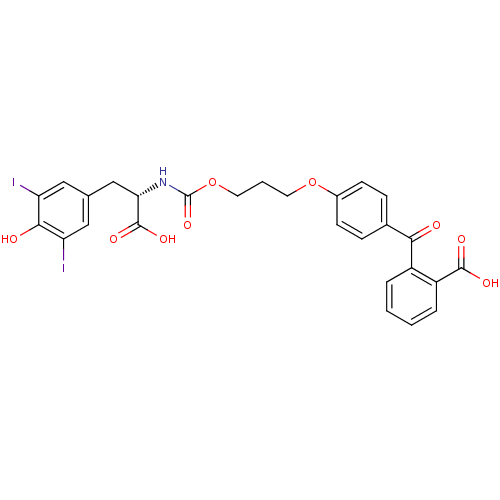
(2-(4-{3-[(S)-1-Carboxy-2-(4-hydroxy-3,5-diiodo-phe...)Show SMILES OC(=O)[C@H](Cc1cc(I)c(O)c(I)c1)NC(=O)OCCCOc1ccc(cc1)C(=O)c1ccccc1C(O)=O Show InChI InChI=1S/C27H23I2NO9/c28-20-12-15(13-21(29)24(20)32)14-22(26(35)36)30-27(37)39-11-3-10-38-17-8-6-16(7-9-17)23(31)18-4-1-2-5-19(18)25(33)34/h1-2,4-9,12-13,22,32H,3,10-11,14H2,(H,30,37)(H,33,34)(H,35,36)/t22-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition constant for HCV NS3 protease substrate binding site |
J Med Chem 47: 2486-98 (2004)
Article DOI: 10.1021/jm0305117
BindingDB Entry DOI: 10.7270/Q2FF3RSP |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50145844
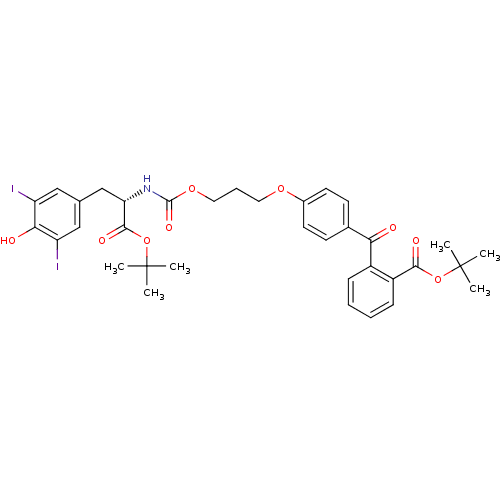
(2-(4-{3-[(S)-1-tert-Butoxycarbonyl-2-(4-hydroxy-3,...)Show SMILES CC(C)(C)OC(=O)[C@H](Cc1cc(I)c(O)c(I)c1)NC(=O)OCCCOc1ccc(cc1)C(=O)c1ccccc1C(=O)OC(C)(C)C Show InChI InChI=1S/C35H39I2NO9/c1-34(2,3)46-31(41)25-11-8-7-10-24(25)29(39)22-12-14-23(15-13-22)44-16-9-17-45-33(43)38-28(32(42)47-35(4,5)6)20-21-18-26(36)30(40)27(37)19-21/h7-8,10-15,18-19,28,40H,9,16-17,20H2,1-6H3,(H,38,43)/t28-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition constant for HCV NS3 protease substrate binding site |
J Med Chem 47: 2486-98 (2004)
Article DOI: 10.1021/jm0305117
BindingDB Entry DOI: 10.7270/Q2FF3RSP |
More data for this
Ligand-Target Pair | |
B-cell CLL/lymphoma 9 protein
(Homo sapiens) | BDBM50371232
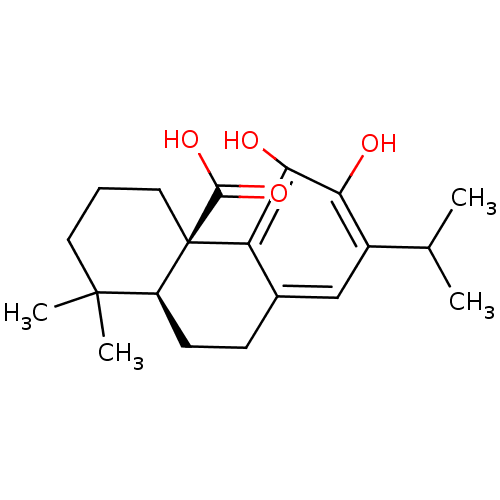
(CARNOSIC ACID)Show SMILES CC(C)c1cc2CC[C@H]3C(C)(C)CCC[C@]3(C(O)=O)c2c(O)c1O |r| Show InChI InChI=1S/C20H28O4/c1-11(2)13-10-12-6-7-14-19(3,4)8-5-9-20(14,18(23)24)15(12)17(22)16(13)21/h10-11,14,21-22H,5-9H2,1-4H3,(H,23,24)/t14-,20+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00228
BindingDB Entry DOI: 10.7270/Q2GQ72Q3 |
More data for this
Ligand-Target Pair | |
Catenin beta-1
(Homo sapiens (Human)) | BDBM50014132
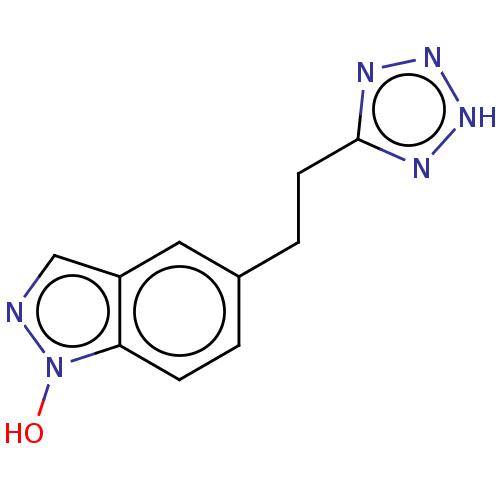
(CHEMBL2323032)Show InChI InChI=1S/C10H10N6O/c17-16-9-3-1-7(5-8(9)6-11-16)2-4-10-12-14-15-13-10/h1,3,5-6,17H,2,4H2,(H,12,13,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00228
BindingDB Entry DOI: 10.7270/Q2GQ72Q3 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50145820
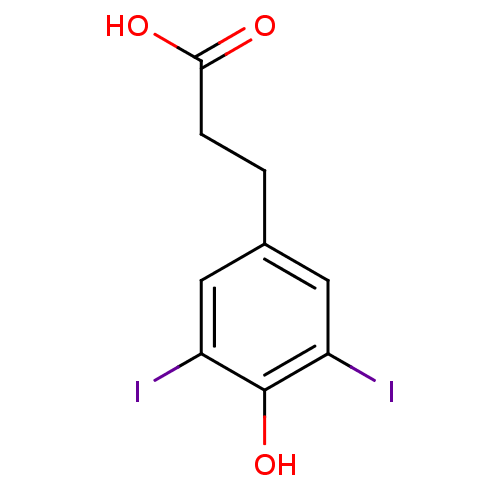
(3-(4-Hydroxy-3,5-diiodo-phenyl)-propionic acid | C...)Show InChI InChI=1S/C9H8I2O3/c10-6-3-5(1-2-8(12)13)4-7(11)9(6)14/h3-4,14H,1-2H2,(H,12,13) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition constant for HCV NS3 protease substrate binding site |
J Med Chem 47: 2486-98 (2004)
Article DOI: 10.1021/jm0305117
BindingDB Entry DOI: 10.7270/Q2FF3RSP |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50145823
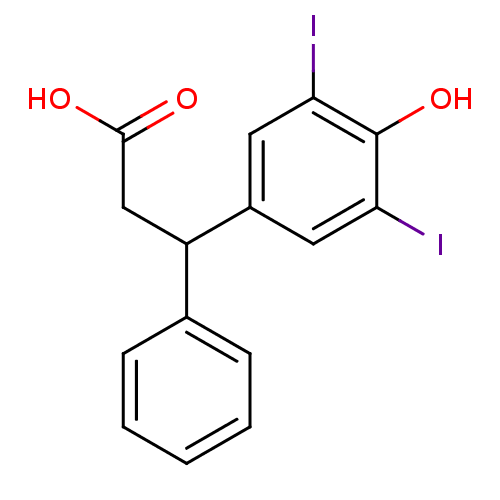
(3-(4-Hydroxy-3,5-diiodo-phenyl)-3-phenyl-propionic...)Show InChI InChI=1S/C15H12I2O3/c16-12-6-10(7-13(17)15(12)20)11(8-14(18)19)9-4-2-1-3-5-9/h1-7,11,20H,8H2,(H,18,19) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition constant for HCV NS3 protease substrate binding site |
J Med Chem 47: 2486-98 (2004)
Article DOI: 10.1021/jm0305117
BindingDB Entry DOI: 10.7270/Q2FF3RSP |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50145804
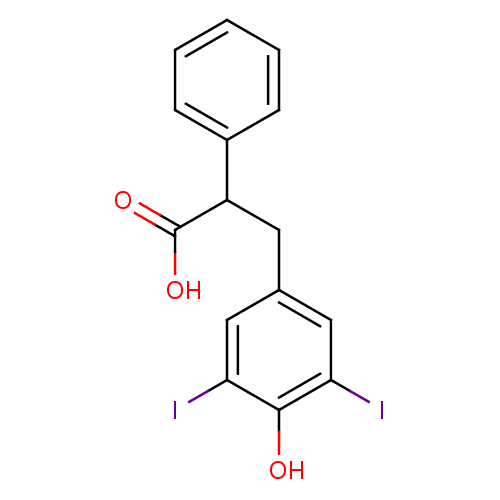
(3-(4-Hydroxy-3,5-diiodo-phenyl)-2-phenyl-propionic...)Show InChI InChI=1S/C15H12I2O3/c16-12-7-9(8-13(17)14(12)18)6-11(15(19)20)10-4-2-1-3-5-10/h1-5,7-8,11,18H,6H2,(H,19,20) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition constant for HCV NS3 protease substrate binding site |
J Med Chem 47: 2486-98 (2004)
Article DOI: 10.1021/jm0305117
BindingDB Entry DOI: 10.7270/Q2FF3RSP |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50145815
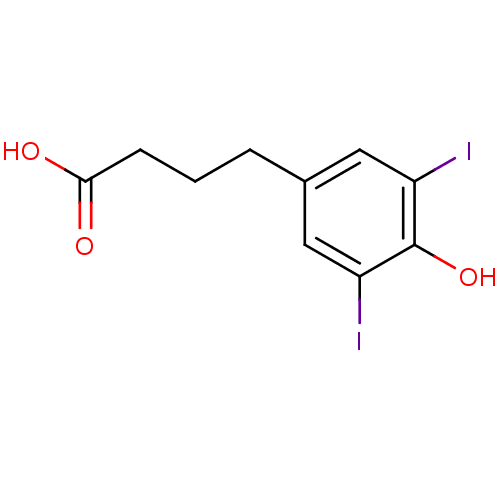
(4-(4-Hydroxy-3,5-diiodo-phenyl)-butyric acid | CHE...)Show InChI InChI=1S/C10H10I2O3/c11-7-4-6(2-1-3-9(13)14)5-8(12)10(7)15/h4-5,15H,1-3H2,(H,13,14) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition constant for HCV NS3 protease substrate binding site |
J Med Chem 47: 2486-98 (2004)
Article DOI: 10.1021/jm0305117
BindingDB Entry DOI: 10.7270/Q2FF3RSP |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50145808
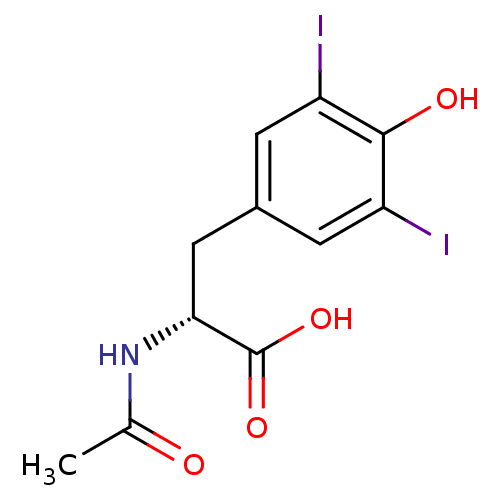
((R)-2-Acetylamino-3-(4-hydroxy-3,5-diiodo-phenyl)-...)Show InChI InChI=1S/C11H11I2NO4/c1-5(15)14-9(11(17)18)4-6-2-7(12)10(16)8(13)3-6/h2-3,9,16H,4H2,1H3,(H,14,15)(H,17,18)/t9-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition constant for HCV NS3 protease substrate binding site |
J Med Chem 47: 2486-98 (2004)
Article DOI: 10.1021/jm0305117
BindingDB Entry DOI: 10.7270/Q2FF3RSP |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50145825
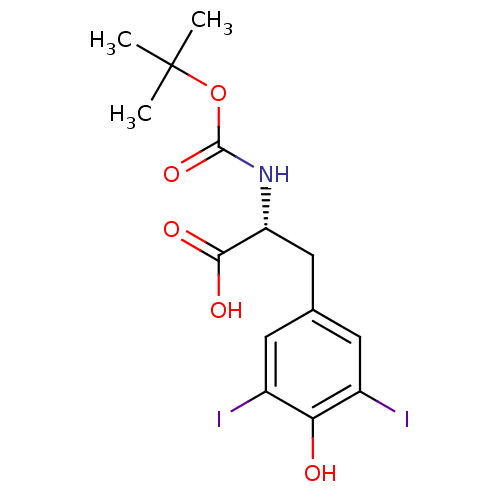
((R)-2-tert-Butoxycarbonylamino-3-(4-hydroxy-3,5-di...)Show SMILES CC(C)(C)OC(=O)N[C@H](Cc1cc(I)c(O)c(I)c1)C(O)=O Show InChI InChI=1S/C14H17I2NO5/c1-14(2,3)22-13(21)17-10(12(19)20)6-7-4-8(15)11(18)9(16)5-7/h4-5,10,18H,6H2,1-3H3,(H,17,21)(H,19,20)/t10-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition constant for HCV NS3 protease substrate binding site |
J Med Chem 47: 2486-98 (2004)
Article DOI: 10.1021/jm0305117
BindingDB Entry DOI: 10.7270/Q2FF3RSP |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50145833

((S)-2-tert-Butoxycarbonylamino-3-(4-hydroxy-3,5-di...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1cc(I)c(O)c(I)c1)C(O)=O Show InChI InChI=1S/C14H17I2NO5/c1-14(2,3)22-13(21)17-10(12(19)20)6-7-4-8(15)11(18)9(16)5-7/h4-5,10,18H,6H2,1-3H3,(H,17,21)(H,19,20)/t10-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition constant for HCV NS3 protease substrate binding site |
J Med Chem 47: 2486-98 (2004)
Article DOI: 10.1021/jm0305117
BindingDB Entry DOI: 10.7270/Q2FF3RSP |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50145835

(4-Hydroxy-3,5-diiodo-benzoic acid | CHEMBL83650)Show InChI InChI=1S/C7H4I2O3/c8-4-1-3(7(11)12)2-5(9)6(4)10/h1-2,10H,(H,11,12) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 6.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition constant for HCV NS3 protease substrate binding site |
J Med Chem 47: 2486-98 (2004)
Article DOI: 10.1021/jm0305117
BindingDB Entry DOI: 10.7270/Q2FF3RSP |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50145827
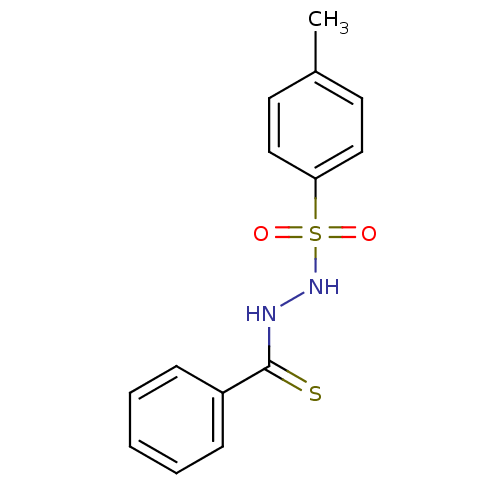
(CHEMBL311209 | N-[(4-methylbenzene)sulfonamido]ben...)Show InChI InChI=1S/C14H14N2O2S2/c1-11-7-9-13(10-8-11)20(17,18)16-15-14(19)12-5-3-2-4-6-12/h2-10,16H,1H3,(H,15,19) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition constant for HCV NS3 protease substrate binding site |
J Med Chem 47: 2486-98 (2004)
Article DOI: 10.1021/jm0305117
BindingDB Entry DOI: 10.7270/Q2FF3RSP |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50380310
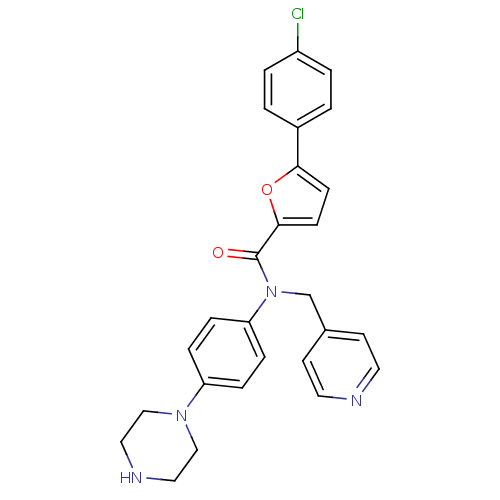
(CHEMBL2017619)Show SMILES Clc1ccc(cc1)-c1ccc(o1)C(=O)N(Cc1ccncc1)c1ccc(cc1)N1CCNCC1 Show InChI InChI=1S/C27H25ClN4O2/c28-22-3-1-21(2-4-22)25-9-10-26(34-25)27(33)32(19-20-11-13-29-14-12-20)24-7-5-23(6-8-24)31-17-15-30-16-18-31/h1-14,30H,15-19H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MK2 using Acam peptide as substrate incubated for 30 mins prior to substrate addition measured after 10 mins using 100 uM ATP by DELFIA... |
ACS Med Chem Lett 2: 632-637 (2011)
Article DOI: 10.1021/ml200113y
BindingDB Entry DOI: 10.7270/Q2J967CS |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50380309
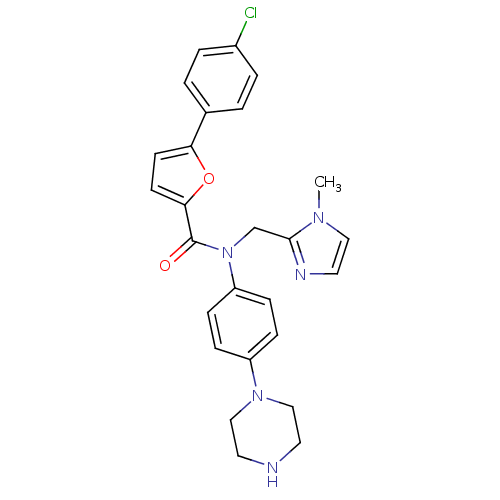
(CHEMBL2017463)Show SMILES Cn1ccnc1CN(C(=O)c1ccc(o1)-c1ccc(Cl)cc1)c1ccc(cc1)N1CCNCC1 Show InChI InChI=1S/C26H26ClN5O2/c1-30-15-14-29-25(30)18-32(22-8-6-21(7-9-22)31-16-12-28-13-17-31)26(33)24-11-10-23(34-24)19-2-4-20(27)5-3-19/h2-11,14-15,28H,12-13,16-18H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MK2 using Acam peptide as substrate incubated for 30 mins prior to substrate addition measured after 10 mins using 100 uM ATP by DELFIA... |
ACS Med Chem Lett 2: 632-637 (2011)
Article DOI: 10.1021/ml200113y
BindingDB Entry DOI: 10.7270/Q2J967CS |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50362106
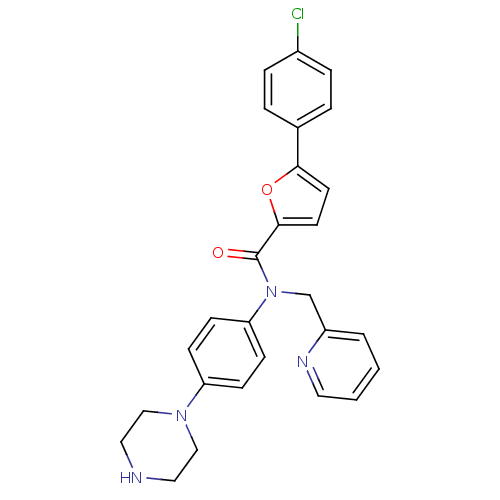
(CHEMBL1938681)Show SMILES Clc1ccc(cc1)-c1ccc(o1)C(=O)N(Cc1ccccn1)c1ccc(cc1)N1CCNCC1 Show InChI InChI=1S/C27H25ClN4O2/c28-21-6-4-20(5-7-21)25-12-13-26(34-25)27(33)32(19-22-3-1-2-14-30-22)24-10-8-23(9-11-24)31-17-15-29-16-18-31/h1-14,29H,15-19H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MK2 using Acam peptide as substrate incubated for 30 mins prior to substrate addition measured after 10 mins using 100 uM ATP by DELFIA... |
ACS Med Chem Lett 2: 632-637 (2011)
Article DOI: 10.1021/ml200113y
BindingDB Entry DOI: 10.7270/Q2J967CS |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50380305
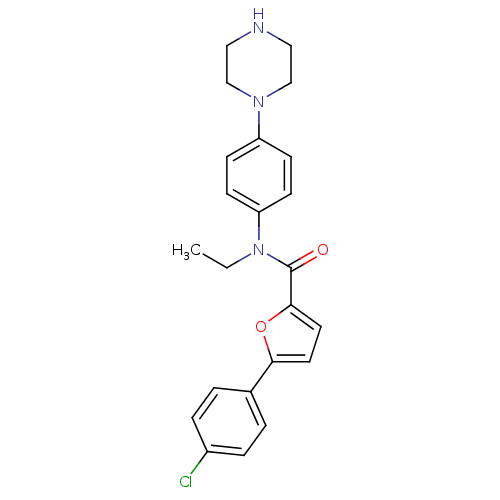
(CHEMBL2017459)Show SMILES CCN(C(=O)c1ccc(o1)-c1ccc(Cl)cc1)c1ccc(cc1)N1CCNCC1 Show InChI InChI=1S/C23H24ClN3O2/c1-2-27(20-9-7-19(8-10-20)26-15-13-25-14-16-26)23(28)22-12-11-21(29-22)17-3-5-18(24)6-4-17/h3-12,25H,2,13-16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MK2 using Acam peptide as substrate incubated for 30 mins prior to substrate addition measured after 10 mins using 100 uM ATP by DELFIA... |
ACS Med Chem Lett 2: 632-637 (2011)
Article DOI: 10.1021/ml200113y
BindingDB Entry DOI: 10.7270/Q2J967CS |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50380308
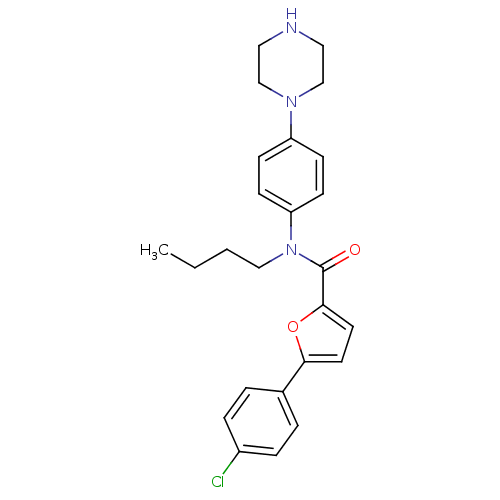
(CHEMBL2017462)Show SMILES CCCCN(C(=O)c1ccc(o1)-c1ccc(Cl)cc1)c1ccc(cc1)N1CCNCC1 Show InChI InChI=1S/C25H28ClN3O2/c1-2-3-16-29(22-10-8-21(9-11-22)28-17-14-27-15-18-28)25(30)24-13-12-23(31-24)19-4-6-20(26)7-5-19/h4-13,27H,2-3,14-18H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MK2 using Acam peptide as substrate incubated for 30 mins prior to substrate addition measured after 10 mins using 100 uM ATP by DELFIA... |
ACS Med Chem Lett 2: 632-637 (2011)
Article DOI: 10.1021/ml200113y
BindingDB Entry DOI: 10.7270/Q2J967CS |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50380304
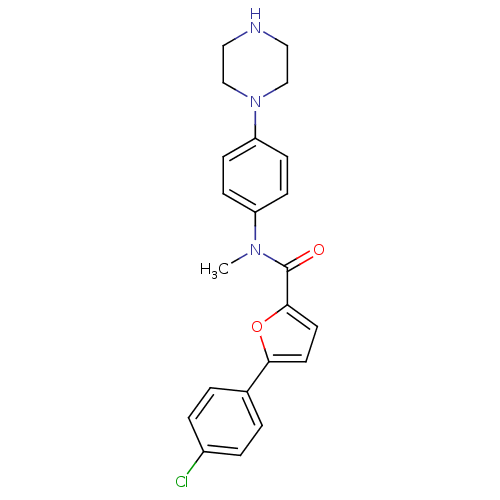
(CHEMBL2017458)Show SMILES CN(C(=O)c1ccc(o1)-c1ccc(Cl)cc1)c1ccc(cc1)N1CCNCC1 Show InChI InChI=1S/C22H22ClN3O2/c1-25(18-6-8-19(9-7-18)26-14-12-24-13-15-26)22(27)21-11-10-20(28-21)16-2-4-17(23)5-3-16/h2-11,24H,12-15H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MK2 using Acam peptide as substrate incubated for 30 mins prior to substrate addition measured after 10 mins using 100 uM ATP by DELFIA... |
ACS Med Chem Lett 2: 632-637 (2011)
Article DOI: 10.1021/ml200113y
BindingDB Entry DOI: 10.7270/Q2J967CS |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50380306
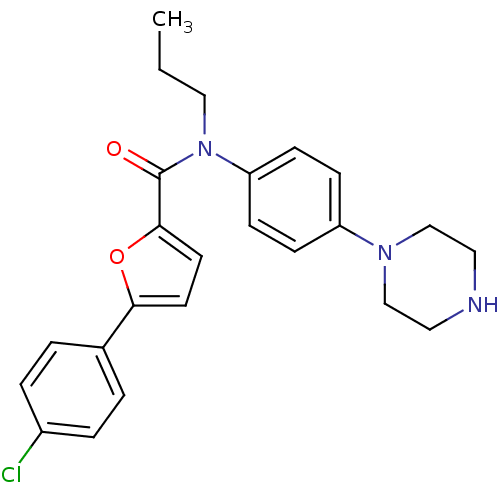
(CHEMBL2017460)Show SMILES CCCN(C(=O)c1ccc(o1)-c1ccc(Cl)cc1)c1ccc(cc1)N1CCNCC1 Show InChI InChI=1S/C24H26ClN3O2/c1-2-15-28(21-9-7-20(8-10-21)27-16-13-26-14-17-27)24(29)23-12-11-22(30-23)18-3-5-19(25)6-4-18/h3-12,26H,2,13-17H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MK2 using Acam peptide as substrate incubated for 30 mins prior to substrate addition measured after 10 mins using 100 uM ATP by DELFIA... |
ACS Med Chem Lett 2: 632-637 (2011)
Article DOI: 10.1021/ml200113y
BindingDB Entry DOI: 10.7270/Q2J967CS |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50380307
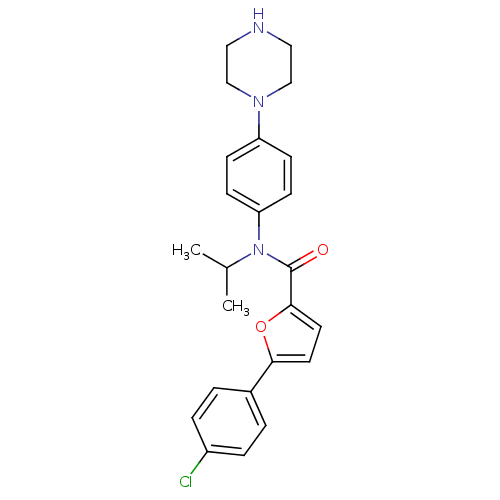
(CHEMBL2017461)Show SMILES CC(C)N(C(=O)c1ccc(o1)-c1ccc(Cl)cc1)c1ccc(cc1)N1CCNCC1 Show InChI InChI=1S/C24H26ClN3O2/c1-17(2)28(21-9-7-20(8-10-21)27-15-13-26-14-16-27)24(29)23-12-11-22(30-23)18-3-5-19(25)6-4-18/h3-12,17,26H,13-16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MK2 using Acam peptide as substrate incubated for 30 mins prior to substrate addition measured after 10 mins using 100 uM ATP by DELFIA... |
ACS Med Chem Lett 2: 632-637 (2011)
Article DOI: 10.1021/ml200113y
BindingDB Entry DOI: 10.7270/Q2J967CS |
More data for this
Ligand-Target Pair | |
Transcription factor 4
(Homo sapiens) | BDBM50556644
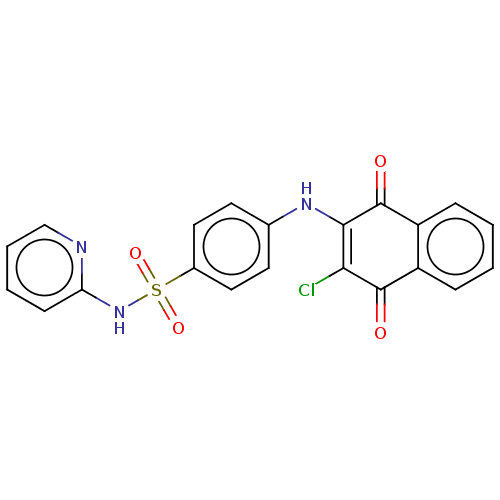
(CHEMBL1214274 | NSC-45382)Show SMILES ClC1=C(Nc2ccc(cc2)S(=O)(=O)Nc2ccccn2)C(=O)c2ccccc2C1=O |c:1| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00228
BindingDB Entry DOI: 10.7270/Q2GQ72Q3 |
More data for this
Ligand-Target Pair | |
Catenin beta-1
(Homo sapiens (Human)) | BDBM50589223
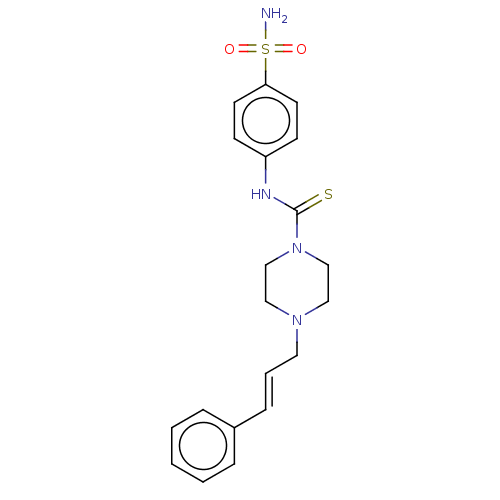
(CHEMBL5185515)Show SMILES NS(=O)(=O)c1ccc(NC(=S)N2CCN(C\C=C\c3ccccc3)CC2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00228
BindingDB Entry DOI: 10.7270/Q2GQ72Q3 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50380298
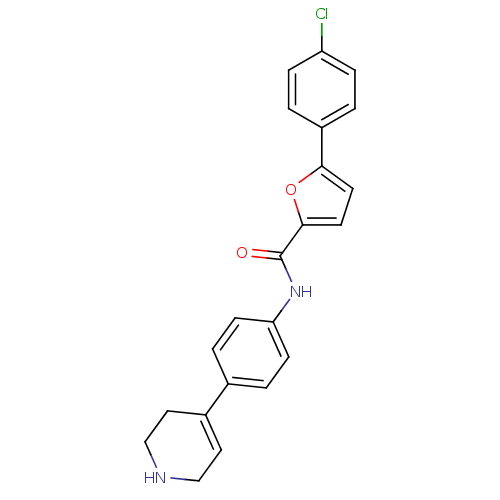
(CHEMBL2017452)Show SMILES Clc1ccc(cc1)-c1ccc(o1)C(=O)Nc1ccc(cc1)C1=CCNCC1 |t:24| Show InChI InChI=1S/C22H19ClN2O2/c23-18-5-1-17(2-6-18)20-9-10-21(27-20)22(26)25-19-7-3-15(4-8-19)16-11-13-24-14-12-16/h1-11,24H,12-14H2,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MK2 using Acam peptide as substrate incubated for 30 mins prior to substrate addition measured after 10 mins using 100 uM ATP by DELFIA... |
ACS Med Chem Lett 2: 632-637 (2011)
Article DOI: 10.1021/ml200113y
BindingDB Entry DOI: 10.7270/Q2J967CS |
More data for this
Ligand-Target Pair | |
Transcription factor 4
(Homo sapiens) | BDBM50589224

(CHEMBL5173483) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00228
BindingDB Entry DOI: 10.7270/Q2GQ72Q3 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50362169
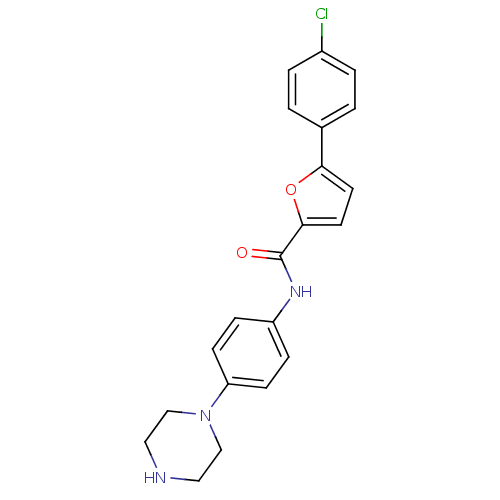
(CHEMBL1938680)Show SMILES Clc1ccc(cc1)-c1ccc(o1)C(=O)Nc1ccc(cc1)N1CCNCC1 Show InChI InChI=1S/C21H20ClN3O2/c22-16-3-1-15(2-4-16)19-9-10-20(27-19)21(26)24-17-5-7-18(8-6-17)25-13-11-23-12-14-25/h1-10,23H,11-14H2,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MK2 using Acam peptide as substrate incubated for 30 mins prior to substrate addition measured after 10 mins using 2 uM ATP by DELFIA a... |
ACS Med Chem Lett 2: 632-637 (2011)
Article DOI: 10.1021/ml200113y
BindingDB Entry DOI: 10.7270/Q2J967CS |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50362169

(CHEMBL1938680)Show SMILES Clc1ccc(cc1)-c1ccc(o1)C(=O)Nc1ccc(cc1)N1CCNCC1 Show InChI InChI=1S/C21H20ClN3O2/c22-16-3-1-15(2-4-16)19-9-10-20(27-19)21(26)24-17-5-7-18(8-6-17)25-13-11-23-12-14-25/h1-10,23H,11-14H2,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MK2 using Acam peptide as substrate incubated for 30 mins prior to substrate addition measured after 10 mins using 100 uM ATP by DELFIA... |
ACS Med Chem Lett 2: 632-637 (2011)
Article DOI: 10.1021/ml200113y
BindingDB Entry DOI: 10.7270/Q2J967CS |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50380291
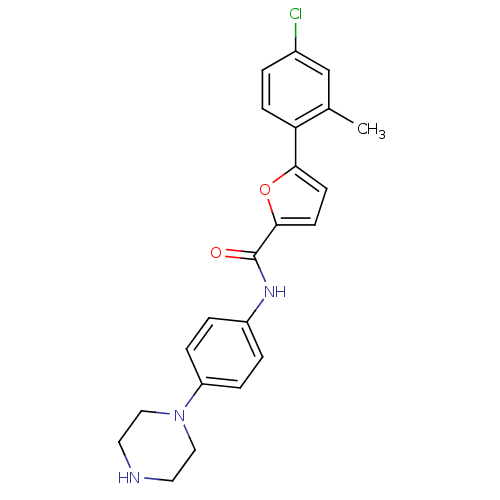
(CHEMBL2017445)Show SMILES Cc1cc(Cl)ccc1-c1ccc(o1)C(=O)Nc1ccc(cc1)N1CCNCC1 Show InChI InChI=1S/C22H22ClN3O2/c1-15-14-16(23)2-7-19(15)20-8-9-21(28-20)22(27)25-17-3-5-18(6-4-17)26-12-10-24-11-13-26/h2-9,14,24H,10-13H2,1H3,(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MK2 using Acam peptide as substrate incubated for 30 mins prior to substrate addition measured after 10 mins using 100 uM ATP by DELFIA... |
ACS Med Chem Lett 2: 632-637 (2011)
Article DOI: 10.1021/ml200113y
BindingDB Entry DOI: 10.7270/Q2J967CS |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM51932
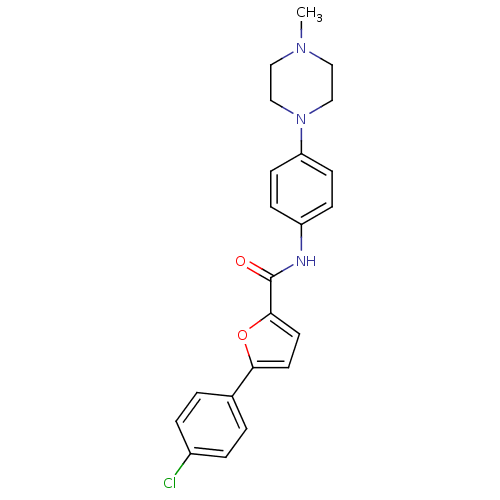
(5-(4-chlorophenyl)-N-[4-(4-methyl-1-piperazinyl)ph...)Show SMILES CN1CCN(CC1)c1ccc(NC(=O)c2ccc(o2)-c2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C22H22ClN3O2/c1-25-12-14-26(15-13-25)19-8-6-18(7-9-19)24-22(27)21-11-10-20(28-21)16-2-4-17(23)5-3-16/h2-11H,12-15H2,1H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MK2 using Acam peptide as substrate incubated for 30 mins prior to substrate addition measured after 10 mins using 100 uM ATP by DELFIA... |
ACS Med Chem Lett 2: 632-637 (2011)
Article DOI: 10.1021/ml200113y
BindingDB Entry DOI: 10.7270/Q2J967CS |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50380295
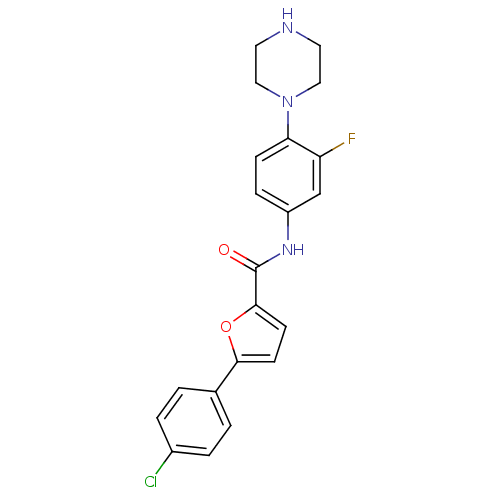
(CHEMBL2017449)Show SMILES Fc1cc(NC(=O)c2ccc(o2)-c2ccc(Cl)cc2)ccc1N1CCNCC1 Show InChI InChI=1S/C21H19ClFN3O2/c22-15-3-1-14(2-4-15)19-7-8-20(28-19)21(27)25-16-5-6-18(17(23)13-16)26-11-9-24-10-12-26/h1-8,13,24H,9-12H2,(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MK2 using Acam peptide as substrate incubated for 30 mins prior to substrate addition measured after 10 mins using 100 uM ATP by DELFIA... |
ACS Med Chem Lett 2: 632-637 (2011)
Article DOI: 10.1021/ml200113y
BindingDB Entry DOI: 10.7270/Q2J967CS |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50380294
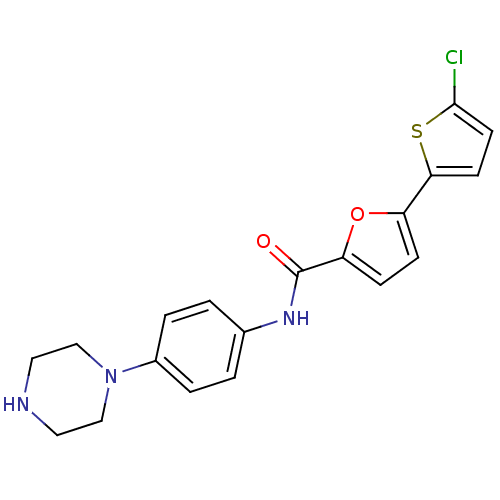
(CHEMBL2017448)Show SMILES Clc1ccc(s1)-c1ccc(o1)C(=O)Nc1ccc(cc1)N1CCNCC1 Show InChI InChI=1S/C19H18ClN3O2S/c20-18-8-7-17(26-18)15-5-6-16(25-15)19(24)22-13-1-3-14(4-2-13)23-11-9-21-10-12-23/h1-8,21H,9-12H2,(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MK2 using Acam peptide as substrate incubated for 30 mins prior to substrate addition measured after 10 mins using 100 uM ATP by DELFIA... |
ACS Med Chem Lett 2: 632-637 (2011)
Article DOI: 10.1021/ml200113y
BindingDB Entry DOI: 10.7270/Q2J967CS |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50362169

(CHEMBL1938680)Show SMILES Clc1ccc(cc1)-c1ccc(o1)C(=O)Nc1ccc(cc1)N1CCNCC1 Show InChI InChI=1S/C21H20ClN3O2/c22-16-3-1-15(2-4-16)19-9-10-20(27-19)21(26)24-17-5-7-18(8-6-17)25-13-11-23-12-14-25/h1-10,23H,11-14H2,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human IRAK4 |
ACS Med Chem Lett 2: 632-637 (2011)
Article DOI: 10.1021/ml200113y
BindingDB Entry DOI: 10.7270/Q2J967CS |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50380302
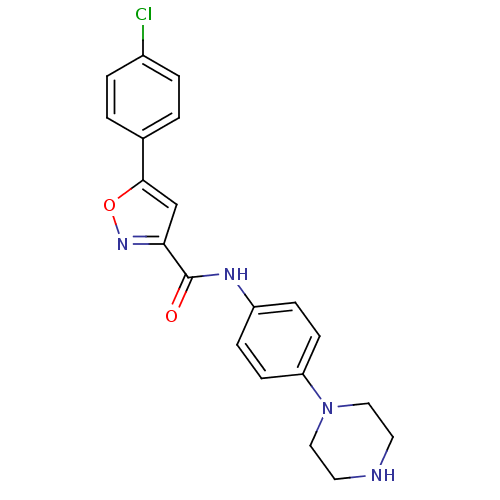
(CHEMBL2017456)Show SMILES Clc1ccc(cc1)-c1cc(no1)C(=O)Nc1ccc(cc1)N1CCNCC1 Show InChI InChI=1S/C20H19ClN4O2/c21-15-3-1-14(2-4-15)19-13-18(24-27-19)20(26)23-16-5-7-17(8-6-16)25-11-9-22-10-12-25/h1-8,13,22H,9-12H2,(H,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MK2 using Acam peptide as substrate incubated for 30 mins prior to substrate addition measured after 10 mins using 100 uM ATP by DELFIA... |
ACS Med Chem Lett 2: 632-637 (2011)
Article DOI: 10.1021/ml200113y
BindingDB Entry DOI: 10.7270/Q2J967CS |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50380290
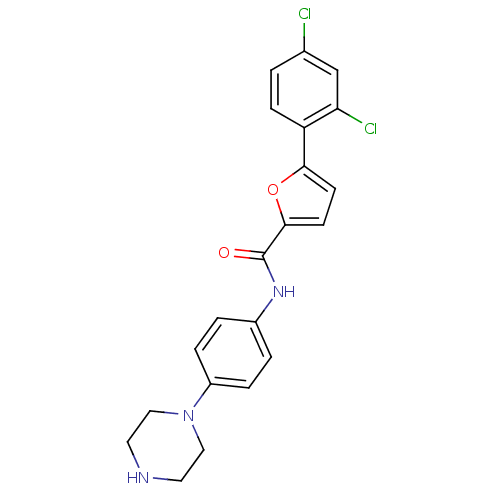
(CHEMBL2017444)Show SMILES Clc1ccc(-c2ccc(o2)C(=O)Nc2ccc(cc2)N2CCNCC2)c(Cl)c1 Show InChI InChI=1S/C21H19Cl2N3O2/c22-14-1-6-17(18(23)13-14)19-7-8-20(28-19)21(27)25-15-2-4-16(5-3-15)26-11-9-24-10-12-26/h1-8,13,24H,9-12H2,(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MK2 using Acam peptide as substrate incubated for 30 mins prior to substrate addition measured after 10 mins using 100 uM ATP by DELFIA... |
ACS Med Chem Lett 2: 632-637 (2011)
Article DOI: 10.1021/ml200113y
BindingDB Entry DOI: 10.7270/Q2J967CS |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50380288
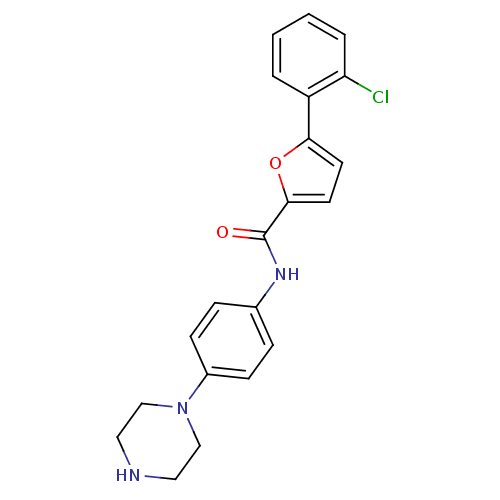
(CHEMBL2017442)Show SMILES Clc1ccccc1-c1ccc(o1)C(=O)Nc1ccc(cc1)N1CCNCC1 Show InChI InChI=1S/C21H20ClN3O2/c22-18-4-2-1-3-17(18)19-9-10-20(27-19)21(26)24-15-5-7-16(8-6-15)25-13-11-23-12-14-25/h1-10,23H,11-14H2,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MK2 using Acam peptide as substrate incubated for 30 mins prior to substrate addition measured after 10 mins using 100 uM ATP by DELFIA... |
ACS Med Chem Lett 2: 632-637 (2011)
Article DOI: 10.1021/ml200113y
BindingDB Entry DOI: 10.7270/Q2J967CS |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50380300

(CHEMBL2017454)Show SMILES Clc1ccc(cc1)-c1ccc(s1)C(=O)Nc1ccc(cc1)N1CCNCC1 Show InChI InChI=1S/C21H20ClN3OS/c22-16-3-1-15(2-4-16)19-9-10-20(27-19)21(26)24-17-5-7-18(8-6-17)25-13-11-23-12-14-25/h1-10,23H,11-14H2,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MK2 using Acam peptide as substrate incubated for 30 mins prior to substrate addition measured after 10 mins using 100 uM ATP by DELFIA... |
ACS Med Chem Lett 2: 632-637 (2011)
Article DOI: 10.1021/ml200113y
BindingDB Entry DOI: 10.7270/Q2J967CS |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50380301
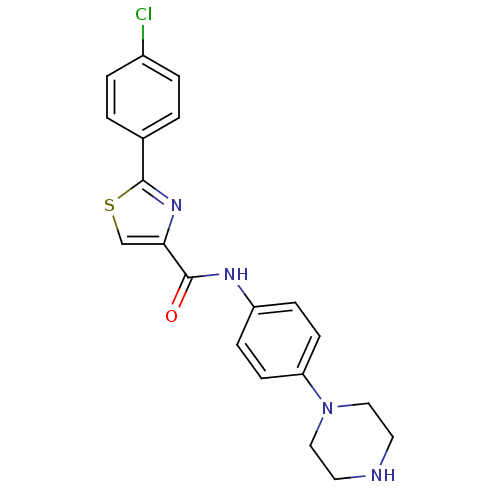
(CHEMBL2017455)Show SMILES Clc1ccc(cc1)-c1nc(cs1)C(=O)Nc1ccc(cc1)N1CCNCC1 Show InChI InChI=1S/C20H19ClN4OS/c21-15-3-1-14(2-4-15)20-24-18(13-27-20)19(26)23-16-5-7-17(8-6-16)25-11-9-22-10-12-25/h1-8,13,22H,9-12H2,(H,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MK2 using Acam peptide as substrate incubated for 30 mins prior to substrate addition measured after 10 mins using 100 uM ATP by DELFIA... |
ACS Med Chem Lett 2: 632-637 (2011)
Article DOI: 10.1021/ml200113y
BindingDB Entry DOI: 10.7270/Q2J967CS |
More data for this
Ligand-Target Pair | |
Transcription factor 4
(Homo sapiens) | BDBM50589221

(CHEMBL5192638)Show SMILES COc1cc(O)c2c3c1c1c(OC)cc(O)c4c1c(c(C[C@H](C)OC(=O)c1c(C)cc(O)cc1O)c(OC)c4=O)c3c(C[C@H](C)OC(=O)c1c(C)cc(O)cc1O)c(OC)c2=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00228
BindingDB Entry DOI: 10.7270/Q2GQ72Q3 |
More data for this
Ligand-Target Pair | |
Transcription factor 4
(Homo sapiens) | BDBM50589220

(CHEMBL3890384)Show SMILES [H][C@](C)(Cc1c(OC)c(=O)c2c(O)cc(OC)c3c4c(OC)cc(O)c5c4c(c(C[C@]([H])(C)OC(=O)c4ccccc4)c(OC)c5=O)c1c23)OC(=O)Oc1ccc(O)cc1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00228
BindingDB Entry DOI: 10.7270/Q2GQ72Q3 |
More data for this
Ligand-Target Pair | |
Transcription factor 4
(Homo sapiens) | BDBM50094484
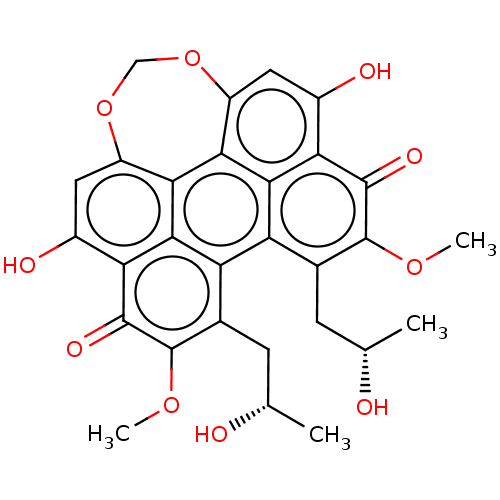
(CHEBI:3556 | CHEMBL2323033 | US9284299, CGP049090 ...)Show SMILES COc1c(C[C@H](C)O)c2c3c(C[C@H](C)O)c(OC)c(=O)c4c(O)cc5OCOc6cc(O)c(c2c6c5c34)c1=O |r| Show InChI InChI=1S/C27H29NO3/c29-26(27(30,23-12-6-2-7-13-23)24-14-8-3-9-15-24)31-25-17-20-28(21-18-25)19-16-22-10-4-1-5-11-22/h1-15,25,30H,16-21H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00228
BindingDB Entry DOI: 10.7270/Q2GQ72Q3 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50380303
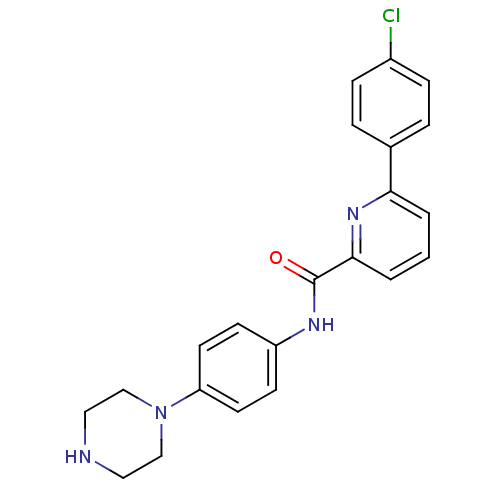
(CHEMBL2017457)Show SMILES Clc1ccc(cc1)-c1cccc(n1)C(=O)Nc1ccc(cc1)N1CCNCC1 Show InChI InChI=1S/C22H21ClN4O/c23-17-6-4-16(5-7-17)20-2-1-3-21(26-20)22(28)25-18-8-10-19(11-9-18)27-14-12-24-13-15-27/h1-11,24H,12-15H2,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MK2 using Acam peptide as substrate incubated for 30 mins prior to substrate addition measured after 10 mins using 100 uM ATP by DELFIA... |
ACS Med Chem Lett 2: 632-637 (2011)
Article DOI: 10.1021/ml200113y
BindingDB Entry DOI: 10.7270/Q2J967CS |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50380299
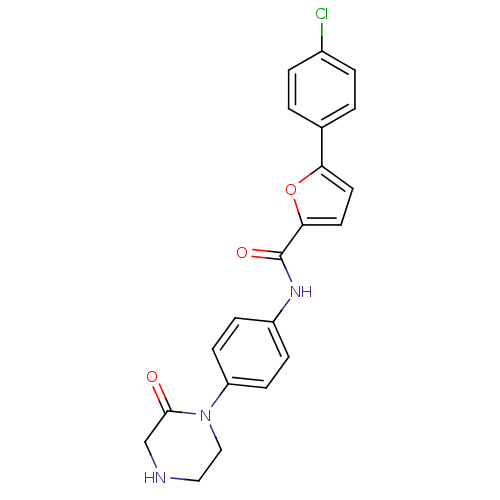
(CHEMBL2017453)Show SMILES Clc1ccc(cc1)-c1ccc(o1)C(=O)Nc1ccc(cc1)N1CCNCC1=O Show InChI InChI=1S/C21H18ClN3O3/c22-15-3-1-14(2-4-15)18-9-10-19(28-18)21(27)24-16-5-7-17(8-6-16)25-12-11-23-13-20(25)26/h1-10,23H,11-13H2,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MK2 using Acam peptide as substrate incubated for 30 mins prior to substrate addition measured after 10 mins using 100 uM ATP by DELFIA... |
ACS Med Chem Lett 2: 632-637 (2011)
Article DOI: 10.1021/ml200113y
BindingDB Entry DOI: 10.7270/Q2J967CS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50362106

(CHEMBL1938681)Show SMILES Clc1ccc(cc1)-c1ccc(o1)C(=O)N(Cc1ccccn1)c1ccc(cc1)N1CCNCC1 Show InChI InChI=1S/C27H25ClN4O2/c28-21-6-4-20(5-7-21)25-12-13-26(34-25)27(33)32(19-22-3-1-2-14-30-22)24-10-8-23(9-11-24)31-17-15-29-16-18-31/h1-14,29H,15-19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes co-incubated with tolbutamide |
ACS Med Chem Lett 2: 632-637 (2011)
Article DOI: 10.1021/ml200113y
BindingDB Entry DOI: 10.7270/Q2J967CS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50362106

(CHEMBL1938681)Show SMILES Clc1ccc(cc1)-c1ccc(o1)C(=O)N(Cc1ccccn1)c1ccc(cc1)N1CCNCC1 Show InChI InChI=1S/C27H25ClN4O2/c28-21-6-4-20(5-7-21)25-12-13-26(34-25)27(33)32(19-22-3-1-2-14-30-22)24-10-8-23(9-11-24)31-17-15-29-16-18-31/h1-14,29H,15-19H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsomes co-incubated with dextromethorphan |
ACS Med Chem Lett 2: 632-637 (2011)
Article DOI: 10.1021/ml200113y
BindingDB Entry DOI: 10.7270/Q2J967CS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50362106

(CHEMBL1938681)Show SMILES Clc1ccc(cc1)-c1ccc(o1)C(=O)N(Cc1ccccn1)c1ccc(cc1)N1CCNCC1 Show InChI InChI=1S/C27H25ClN4O2/c28-21-6-4-20(5-7-21)25-12-13-26(34-25)27(33)32(19-22-3-1-2-14-30-22)24-10-8-23(9-11-24)31-17-15-29-16-18-31/h1-14,29H,15-19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes co-incubated with testosterone |
ACS Med Chem Lett 2: 632-637 (2011)
Article DOI: 10.1021/ml200113y
BindingDB Entry DOI: 10.7270/Q2J967CS |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50380289
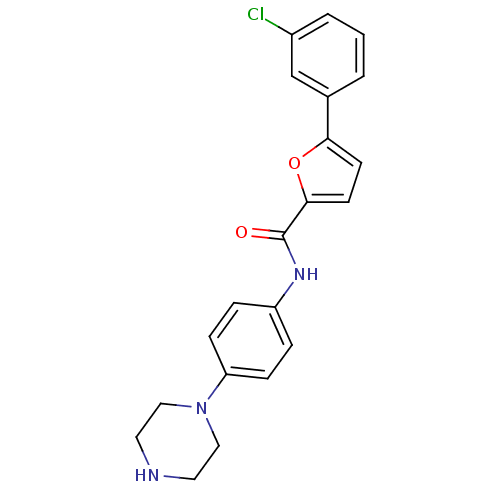
(CHEMBL2017443)Show SMILES Clc1cccc(c1)-c1ccc(o1)C(=O)Nc1ccc(cc1)N1CCNCC1 Show InChI InChI=1S/C21H20ClN3O2/c22-16-3-1-2-15(14-16)19-8-9-20(27-19)21(26)24-17-4-6-18(7-5-17)25-12-10-23-11-13-25/h1-9,14,23H,10-13H2,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MK2 using Acam peptide as substrate incubated for 30 mins prior to substrate addition measured after 10 mins using 100 uM ATP by DELFIA... |
ACS Med Chem Lett 2: 632-637 (2011)
Article DOI: 10.1021/ml200113y
BindingDB Entry DOI: 10.7270/Q2J967CS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50362106

(CHEMBL1938681)Show SMILES Clc1ccc(cc1)-c1ccc(o1)C(=O)N(Cc1ccccn1)c1ccc(cc1)N1CCNCC1 Show InChI InChI=1S/C27H25ClN4O2/c28-21-6-4-20(5-7-21)25-12-13-26(34-25)27(33)32(19-22-3-1-2-14-30-22)24-10-8-23(9-11-24)31-17-15-29-16-18-31/h1-14,29H,15-19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes using tolbutamide as substrate incubated for 30 mins prior to substrate addition |
ACS Med Chem Lett 2: 632-637 (2011)
Article DOI: 10.1021/ml200113y
BindingDB Entry DOI: 10.7270/Q2J967CS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50362106

(CHEMBL1938681)Show SMILES Clc1ccc(cc1)-c1ccc(o1)C(=O)N(Cc1ccccn1)c1ccc(cc1)N1CCNCC1 Show InChI InChI=1S/C27H25ClN4O2/c28-21-6-4-20(5-7-21)25-12-13-26(34-25)27(33)32(19-22-3-1-2-14-30-22)24-10-8-23(9-11-24)31-17-15-29-16-18-31/h1-14,29H,15-19H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate incubated for 30 mins prior to substrate addition |
ACS Med Chem Lett 2: 632-637 (2011)
Article DOI: 10.1021/ml200113y
BindingDB Entry DOI: 10.7270/Q2J967CS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50362106

(CHEMBL1938681)Show SMILES Clc1ccc(cc1)-c1ccc(o1)C(=O)N(Cc1ccccn1)c1ccc(cc1)N1CCNCC1 Show InChI InChI=1S/C27H25ClN4O2/c28-21-6-4-20(5-7-21)25-12-13-26(34-25)27(33)32(19-22-3-1-2-14-30-22)24-10-8-23(9-11-24)31-17-15-29-16-18-31/h1-14,29H,15-19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes co-incubated with testosterone |
ACS Med Chem Lett 2: 632-637 (2011)
Article DOI: 10.1021/ml200113y
BindingDB Entry DOI: 10.7270/Q2J967CS |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50380296
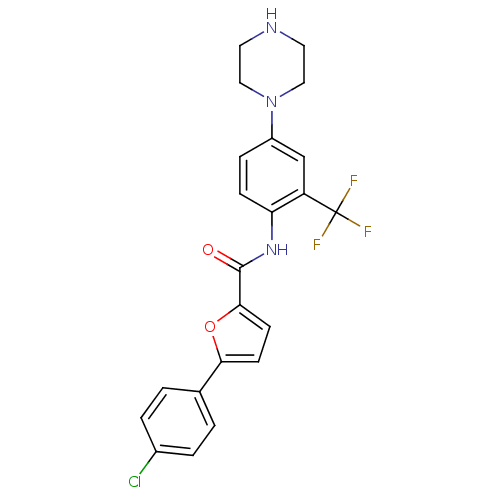
(CHEMBL2017450)Show SMILES FC(F)(F)c1cc(ccc1NC(=O)c1ccc(o1)-c1ccc(Cl)cc1)N1CCNCC1 Show InChI InChI=1S/C22H19ClF3N3O2/c23-15-3-1-14(2-4-15)19-7-8-20(31-19)21(30)28-18-6-5-16(13-17(18)22(24,25)26)29-11-9-27-10-12-29/h1-8,13,27H,9-12H2,(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MK2 using Acam peptide as substrate incubated for 30 mins prior to substrate addition measured after 10 mins using 100 uM ATP by DELFIA... |
ACS Med Chem Lett 2: 632-637 (2011)
Article DOI: 10.1021/ml200113y
BindingDB Entry DOI: 10.7270/Q2J967CS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data