 Found 2467 hits with Last Name = 'mcdonald' and Initial = 'a'
Found 2467 hits with Last Name = 'mcdonald' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM20458
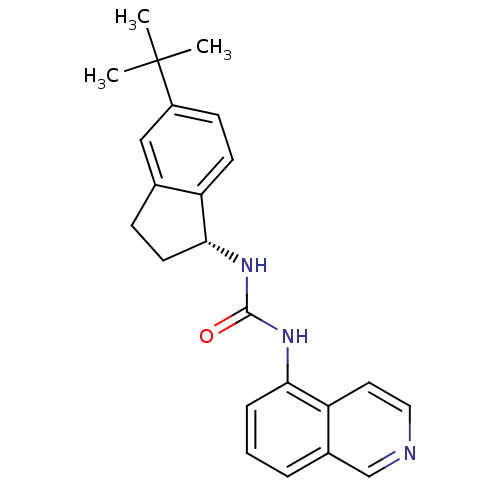
(1-[(1R)-5-tert-butyl-2,3-dihydro-1H-inden-1-yl]-3-...)Show SMILES CC(C)(C)c1ccc2[C@@H](CCc2c1)NC(=O)Nc1cccc2cnccc12 |r| Show InChI InChI=1S/C23H25N3O/c1-23(2,3)17-8-9-18-15(13-17)7-10-21(18)26-22(27)25-20-6-4-5-16-14-24-12-11-19(16)20/h4-6,8-9,11-14,21H,7,10H2,1-3H3,(H2,25,26,27)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | -46.5 | n/a | n/a | 5 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories
| Assay Description
The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate... |
J Pharmacol Exp Ther 323: 285-93 (2007)
Article DOI: 10.1124/jpet.107.124305
BindingDB Entry DOI: 10.7270/Q28K77B0 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM20464
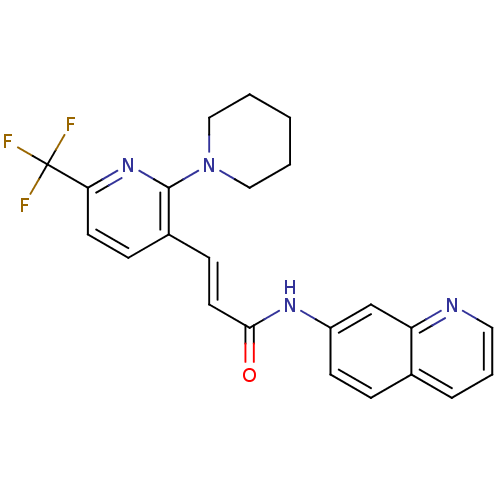
((2E)-3-[2-(piperidin-1-yl)-6-(trifluoromethyl)pyri...)Show SMILES FC(F)(F)c1ccc(\C=C\C(=O)Nc2ccc3cccnc3c2)c(n1)N1CCCCC1 Show InChI InChI=1S/C23H21F3N4O/c24-23(25,26)20-10-7-17(22(29-20)30-13-2-1-3-14-30)8-11-21(31)28-18-9-6-16-5-4-12-27-19(16)15-18/h4-12,15H,1-3,13-14H2,(H,28,31)/b11-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 27 | -43.2 | n/a | n/a | 34 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories
| Assay Description
The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate... |
J Pharmacol Exp Ther 323: 285-93 (2007)
Article DOI: 10.1124/jpet.107.124305
BindingDB Entry DOI: 10.7270/Q28K77B0 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50246766
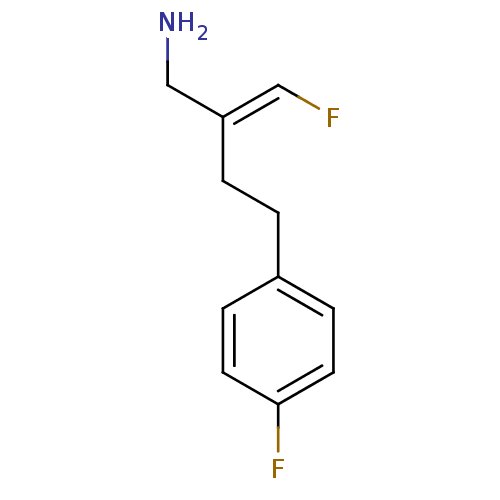
(CHEMBL489079 | Mofegiline | US9302986, Mofegiline)Show InChI InChI=1S/C11H13F2N/c12-7-10(8-14)2-1-9-3-5-11(13)6-4-9/h3-7H,1-2,8,14H2/b10-7+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MAOB expressed in Pichia pastoris by kinetic assay |
J Med Chem 51: 8019-26 (2008)
Article DOI: 10.1021/jm8011867
BindingDB Entry DOI: 10.7270/Q2542NGF |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM20334
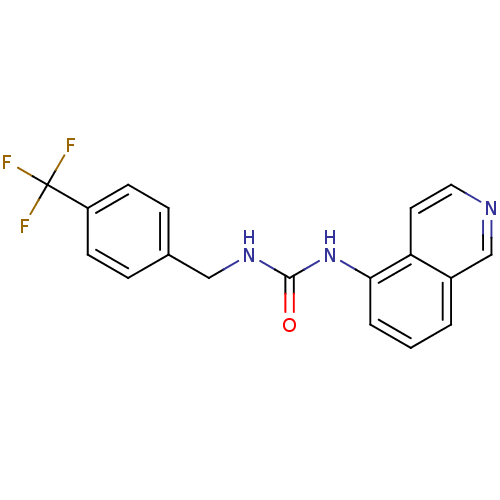
(1-Isoquinolin-5-yl-3-(4-trifluoromethyl-benzyl)-ur...)Show InChI InChI=1S/C18H14F3N3O/c19-18(20,21)14-6-4-12(5-7-14)10-23-17(25)24-16-3-1-2-13-11-22-9-8-15(13)16/h1-9,11H,10H2,(H2,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 47 | -41.8 | n/a | n/a | 11 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories
| Assay Description
The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate... |
J Pharmacol Exp Ther 323: 285-93 (2007)
Article DOI: 10.1124/jpet.107.124305
BindingDB Entry DOI: 10.7270/Q28K77B0 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM20465
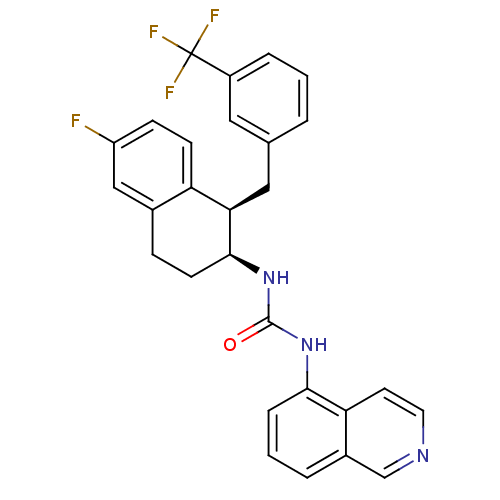
(1-[(1R,2S)-6-fluoro-1-{[3-(trifluoromethyl)phenyl]...)Show SMILES Fc1ccc2[C@@H](Cc3cccc(c3)C(F)(F)F)[C@H](CCc2c1)NC(=O)Nc1cccc2cnccc12 |r| Show InChI InChI=1S/C28H23F4N3O/c29-21-8-9-22-18(15-21)7-10-26(24(22)14-17-3-1-5-20(13-17)28(30,31)32)35-27(36)34-25-6-2-4-19-16-33-12-11-23(19)25/h1-6,8-9,11-13,15-16,24,26H,7,10,14H2,(H2,34,35,36)/t24-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 58 | -41.3 | n/a | n/a | 46 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories
| Assay Description
The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate... |
J Pharmacol Exp Ther 323: 285-93 (2007)
Article DOI: 10.1124/jpet.107.124305
BindingDB Entry DOI: 10.7270/Q28K77B0 |
More data for this
Ligand-Target Pair | |
p-hydroxybenzoate hydroxylase
(Pseudomonas fluorescens) | BDBM50038194
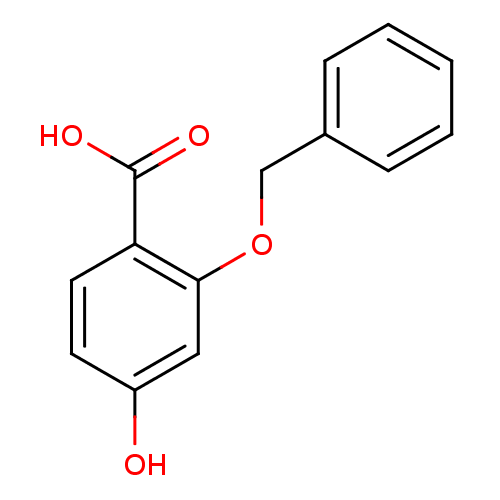
(2-Benzyloxy-4-hydroxy-benzoic acid | CHEMBL130259)Show InChI InChI=1S/C14H12O4/c15-11-6-7-12(14(16)17)13(8-11)18-9-10-4-2-1-3-5-10/h1-8,15H,9H2,(H,16,17) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against p-hydroxybenzoate hydroxylase (PHBH) from Pseudomonas fluorescence competing with p-hydroxy benzoic acid |
J Med Chem 37: 4076-8 (1995)
BindingDB Entry DOI: 10.7270/Q2959GKZ |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM20285
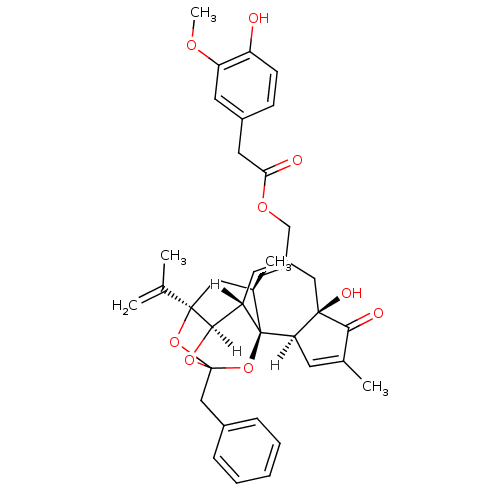
(Resiniferatoxin | [(1R,2R,6R,10S,11R,15R,17R)-13-b...)Show SMILES [H][C@]12OC3(Cc4ccccc4)O[C@]1(C[C@@H](C)[C@]1(O3)[C@]3([H])C=C(C)C(=O)[C@@]3(O)CC(COC(=O)Cc3ccc(O)c(OC)c3)=C[C@@]21[H])C(C)=C |c:47,t:23,TLB:11:3:12.14.13:44,THB:4:3:12.14.13:44| Show InChI InChI=1S/C37H40O9/c1-21(2)35-17-23(4)37-27(33(35)44-36(45-35,46-37)19-24-9-7-6-8-10-24)14-26(18-34(41)30(37)13-22(3)32(34)40)20-43-31(39)16-25-11-12-28(38)29(15-25)42-5/h6-15,23,27,30,33,38,41H,1,16-20H2,2-5H3/t23-,27+,30-,33-,34-,35-,36?,37-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 65 | -41.0 | n/a | n/a | 24 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories
| Assay Description
The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate... |
J Pharmacol Exp Ther 323: 285-93 (2007)
Article DOI: 10.1124/jpet.107.124305
BindingDB Entry DOI: 10.7270/Q28K77B0 |
More data for this
Ligand-Target Pair | |
p-hydroxybenzoate hydroxylase
(Pseudomonas fluorescens) | BDBM50038194

(2-Benzyloxy-4-hydroxy-benzoic acid | CHEMBL130259)Show InChI InChI=1S/C14H12O4/c15-11-6-7-12(14(16)17)13(8-11)18-9-10-4-2-1-3-5-10/h1-8,15H,9H2,(H,16,17) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against p-hydroxybenzoate hydroxylase (PHBH) from Pseudomonas fluorescence competing with NADPH |
J Med Chem 37: 4076-8 (1995)
BindingDB Entry DOI: 10.7270/Q2959GKZ |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM20466
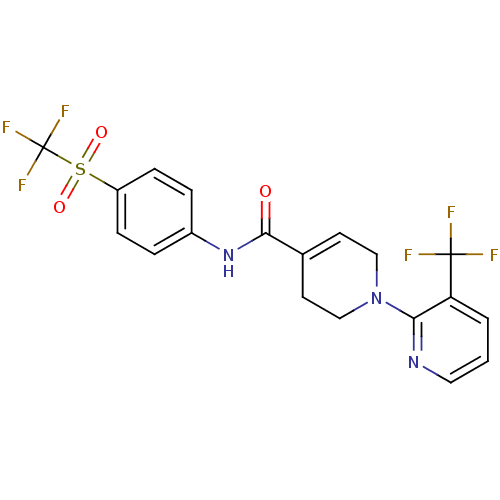
(A-784168 | CHEMBL482834 | N-[4-(trifluoromethane)s...)Show SMILES FC(F)(F)c1cccnc1N1CCC(=CC1)C(=O)Nc1ccc(cc1)S(=O)(=O)C(F)(F)F |c:14| Show InChI InChI=1S/C19H15F6N3O3S/c20-18(21,22)15-2-1-9-26-16(15)28-10-7-12(8-11-28)17(29)27-13-3-5-14(6-4-13)32(30,31)19(23,24)25/h1-7,9H,8,10-11H2,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 71 | -40.8 | n/a | n/a | 74 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories
| Assay Description
The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate... |
J Pharmacol Exp Ther 323: 285-93 (2007)
Article DOI: 10.1124/jpet.107.124305
BindingDB Entry DOI: 10.7270/Q28K77B0 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM20467
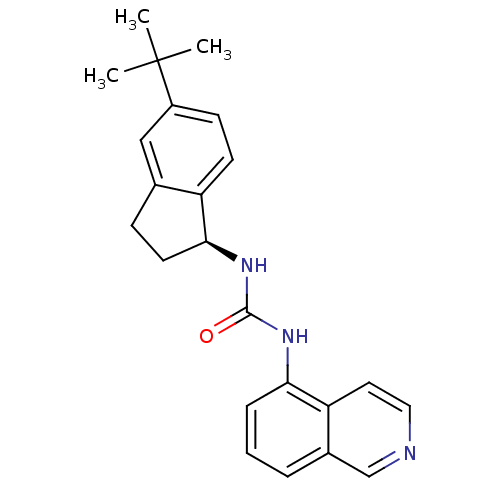
(3-[(1S)-5-tert-butyl-2,3-dihydro-1H-inden-1-yl]-1-...)Show SMILES CC(C)(C)c1ccc2[C@H](CCc2c1)NC(=O)Nc1cccc2cnccc12 |r| Show InChI InChI=1S/C23H25N3O/c1-23(2,3)17-8-9-18-15(13-17)7-10-21(18)26-22(27)25-20-6-4-5-16-14-24-12-11-19(16)20/h4-6,8-9,11-14,21H,7,10H2,1-3H3,(H2,25,26,27)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 112 | -39.7 | n/a | n/a | 34 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories
| Assay Description
The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate... |
J Pharmacol Exp Ther 323: 285-93 (2007)
Article DOI: 10.1124/jpet.107.124305
BindingDB Entry DOI: 10.7270/Q28K77B0 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM20459
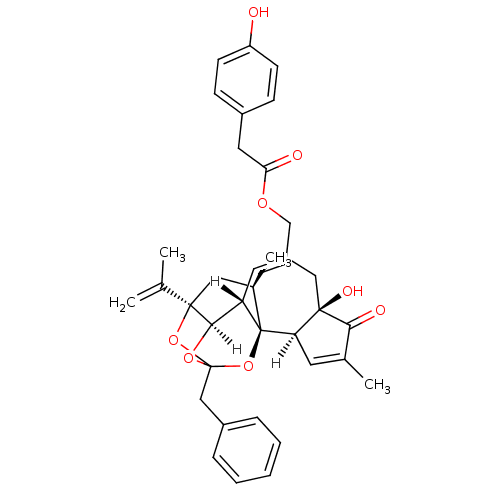
(Tinyatoxin | [(1R,2R,6R,10S,11R,15R,17R)-13-benzyl...)Show SMILES [H][C@]12OC3(Cc4ccccc4)O[C@]1(C[C@@H](C)[C@]1(O3)[C@]3([H])C=C(C)C(=O)[C@@]3(O)CC(COC(=O)Cc3ccc(O)cc3)=C[C@@]21[H])C(C)=C |c:45,t:23,TLB:11:3:12.14.13:42,THB:4:3:12.14.13:42| Show InChI InChI=1S/C36H38O8/c1-21(2)34-17-23(4)36-28(32(34)42-35(43-34,44-36)19-25-8-6-5-7-9-25)15-26(18-33(40)29(36)14-22(3)31(33)39)20-41-30(38)16-24-10-12-27(37)13-11-24/h5-15,23,28-29,32,37,40H,1,16-20H2,2-4H3/t23-,28+,29-,32-,33-,34-,35?,36-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 589 | -35.6 | n/a | n/a | 129 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories
| Assay Description
The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate... |
J Pharmacol Exp Ther 323: 285-93 (2007)
Article DOI: 10.1124/jpet.107.124305
BindingDB Entry DOI: 10.7270/Q28K77B0 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM20468
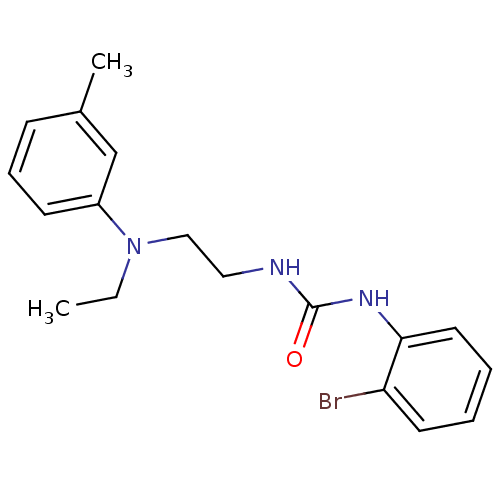
(3-(2-bromophenyl)-1-{2-[ethyl(3-methylphenyl)amino...)Show InChI InChI=1S/C18H22BrN3O/c1-3-22(15-8-6-7-14(2)13-15)12-11-20-18(23)21-17-10-5-4-9-16(17)19/h4-10,13H,3,11-12H2,1-2H3,(H2,20,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 603 | -35.5 | n/a | n/a | 95 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories
| Assay Description
The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate... |
J Pharmacol Exp Ther 323: 285-93 (2007)
Article DOI: 10.1124/jpet.107.124305
BindingDB Entry DOI: 10.7270/Q28K77B0 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50246766

(CHEMBL489079 | Mofegiline | US9302986, Mofegiline)Show InChI InChI=1S/C11H13F2N/c12-7-10(8-14)2-1-9-3-5-11(13)6-4-9/h3-7H,1-2,8,14H2/b10-7+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MAOA expressed in Pichia pastoris by kinetic assay |
J Med Chem 51: 8019-26 (2008)
Article DOI: 10.1021/jm8011867
BindingDB Entry DOI: 10.7270/Q2542NGF |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM20284
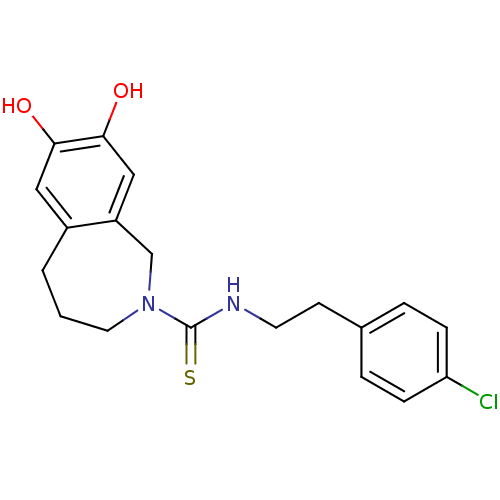
(CHEMBL391997 | CPZ | Capsazepine | N-[2-(4-chlorop...)Show InChI InChI=1S/C19H21ClN2O2S/c20-16-5-3-13(4-6-16)7-8-21-19(25)22-9-1-2-14-10-17(23)18(24)11-15(14)12-22/h3-6,10-11,23-24H,1-2,7-9,12H2,(H,21,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.29E+3 | -33.6 | n/a | n/a | 282 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories
| Assay Description
The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate... |
J Pharmacol Exp Ther 323: 285-93 (2007)
Article DOI: 10.1124/jpet.107.124305
BindingDB Entry DOI: 10.7270/Q28K77B0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM20460
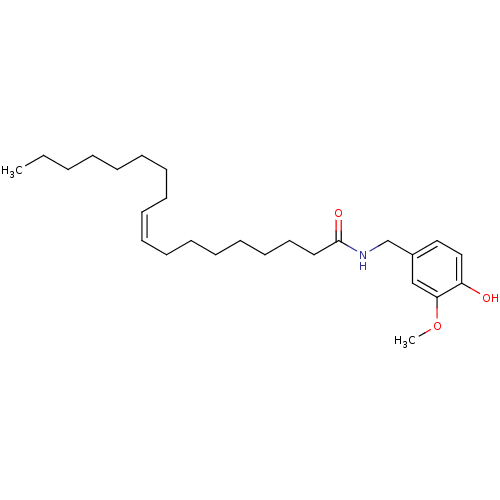
((9Z)-N-[(4-hydroxy-3-methoxyphenyl)methyl]octadec-...)Show InChI InChI=1S/C26H43NO3/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-26(29)27-22-23-19-20-24(28)25(21-23)30-2/h10-11,19-21,28H,3-9,12-18,22H2,1-2H3,(H,27,29)/b11-10- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.59E+3 | -33.1 | n/a | n/a | 132 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories
| Assay Description
The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate... |
J Pharmacol Exp Ther 323: 285-93 (2007)
Article DOI: 10.1124/jpet.107.124305
BindingDB Entry DOI: 10.7270/Q28K77B0 |
More data for this
Ligand-Target Pair | |
Membrane primary amine oxidase
(Homo sapiens (Human)) | BDBM50246766

(CHEMBL489079 | Mofegiline | US9302986, Mofegiline)Show InChI InChI=1S/C11H13F2N/c12-7-10(8-14)2-1-9-3-5-11(13)6-4-9/h3-7H,1-2,8,14H2/b10-7+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaxis Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human SSAO/VAP1 measuring H2O2 production by Kitz and Wilson plot analysis |
Bioorg Med Chem Lett 22: 3935-40 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.111
BindingDB Entry DOI: 10.7270/Q2SQ91D6 |
More data for this
Ligand-Target Pair | |
Membrane primary amine oxidase
(Homo sapiens (Human)) | BDBM50384084
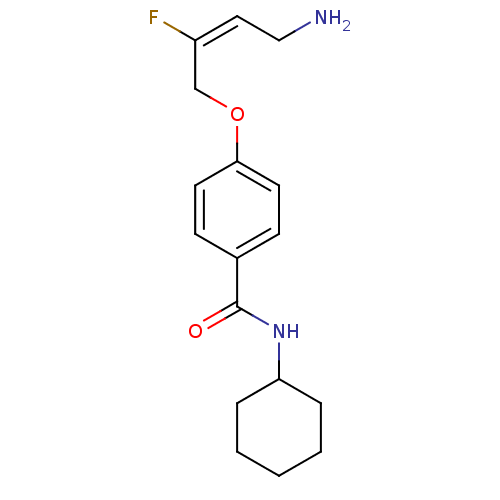
(CHEMBL2029546)Show InChI InChI=1S/C17H23FN2O2/c18-14(10-11-19)12-22-16-8-6-13(7-9-16)17(21)20-15-4-2-1-3-5-15/h6-10,15H,1-5,11-12,19H2,(H,20,21)/b14-10+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaxis Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human SSAO/VAP1 measuring H2O2 production by Kitz and Wilson plot analysis |
Bioorg Med Chem Lett 22: 3935-40 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.111
BindingDB Entry DOI: 10.7270/Q2SQ91D6 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM20462
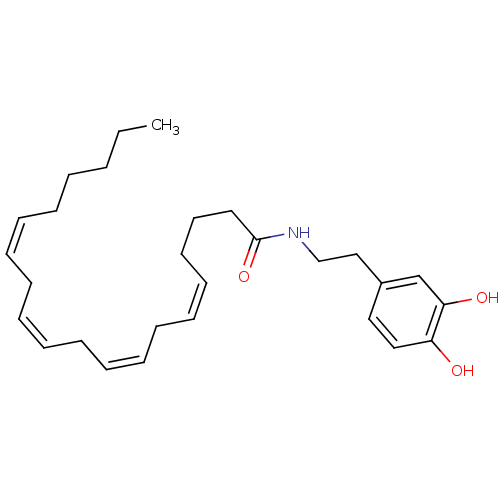
((5Z,8Z,11Z,14Z)-N-[2-(3,4-dihydroxyphenyl)ethyl]ic...)Show SMILES CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)NCCc1ccc(O)c(O)c1 Show InChI InChI=1S/C28H41NO3/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-28(32)29-23-22-25-20-21-26(30)27(31)24-25/h6-7,9-10,12-13,15-16,20-21,24,30-31H,2-5,8,11,14,17-19,22-23H2,1H3,(H,29,32)/b7-6-,10-9-,13-12-,16-15- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >6.31E+3 | >-29.7 | n/a | n/a | 1.48E+3 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories
| Assay Description
The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate... |
J Pharmacol Exp Ther 323: 285-93 (2007)
Article DOI: 10.1124/jpet.107.124305
BindingDB Entry DOI: 10.7270/Q28K77B0 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM20461
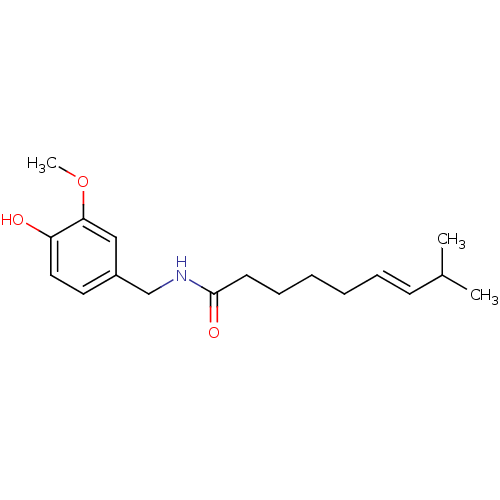
((6E)-N-[(4-hydroxy-3-methoxyphenyl)methyl]-8-methy...)Show InChI InChI=1S/C18H27NO3/c1-14(2)8-6-4-5-7-9-18(21)19-13-15-10-11-16(20)17(12-15)22-3/h6,8,10-12,14,20H,4-5,7,9,13H2,1-3H3,(H,19,21)/b8-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2.00E+4 | -26.8 | n/a | n/a | 29 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories
| Assay Description
The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate... |
J Pharmacol Exp Ther 323: 285-93 (2007)
Article DOI: 10.1124/jpet.107.124305
BindingDB Entry DOI: 10.7270/Q28K77B0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50113840
(4-Phenyl-butylamine | 4-phenylbutylamine | CHEMBL7...)Show InChI InChI=1S/C10H15N/c11-9-5-4-8-10-6-2-1-3-7-10/h1-3,6-7H,4-5,8-9,11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Inhibition of human MAOA |
J Med Chem 51: 8019-26 (2008)
Article DOI: 10.1021/jm8011867
BindingDB Entry DOI: 10.7270/Q2542NGF |
More data for this
Ligand-Target Pair | |
Glutamate receptor 1
(Rattus norvegicus (Rat)) | BDBM50010893
((R)-2-Amino-4-oxo-5-phosphono-pentanoic acid | 2-A...)Show InChI InChI=1S/C5H10NO6P/c6-4(5(8)9)1-3(7)2-13(10,11)12/h4H,1-2,6H2,(H,8,9)(H2,10,11,12)/t4-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards AMPA receptor using [3H]AMPA as radioligand; Inactive |
J Med Chem 33: 2961-3 (1990)
BindingDB Entry DOI: 10.7270/Q2BK1CXM |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 3
(Rattus norvegicus) | BDBM50004897

((APV)2-Amino-5-phosphono-pentanoic acid | (R)-2-Am...)Show InChI InChI=1S/C5H12NO5P/c6-4(5(7)8)2-1-3-12(9,10)11/h4H,1-3,6H2,(H,7,8)(H2,9,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards Ionotropic glutamate receptor kainate using [3H]-kainic acid as radioligand; Inactive |
J Med Chem 33: 2961-3 (1990)
BindingDB Entry DOI: 10.7270/Q2BK1CXM |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 3
(Rattus norvegicus) | BDBM50002360

((CPP)4-(3-Phosphono-propyl)-piperazine-2-carboxyli...)Show InChI InChI=1S/C8H17N2O5P/c11-8(12)7-6-10(4-2-9-7)3-1-5-16(13,14)15/h7,9H,1-6H2,(H,11,12)(H2,13,14,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards Ionotropic glutamate receptor kainate using [3H]-kainic acid as radioligand; Inactive |
J Med Chem 33: 2961-3 (1990)
BindingDB Entry DOI: 10.7270/Q2BK1CXM |
More data for this
Ligand-Target Pair | |
Glutamate receptor 1
(Rattus norvegicus (Rat)) | BDBM50004927

(4-Phosphonomethyl-piperidine-2-carboxylic acid | 4...)Show InChI InChI=1S/C7H14NO5P/c9-7(10)6-3-5(1-2-8-6)4-14(11,12)13/h5-6,8H,1-4H2,(H,9,10)(H2,11,12,13)/t5-,6+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards AMPA receptor using [3H]AMPA as radioligand; Inactive |
J Med Chem 33: 2961-3 (1990)
BindingDB Entry DOI: 10.7270/Q2BK1CXM |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 3
(Rattus norvegicus) | BDBM50004927

(4-Phosphonomethyl-piperidine-2-carboxylic acid | 4...)Show InChI InChI=1S/C7H14NO5P/c9-7(10)6-3-5(1-2-8-6)4-14(11,12)13/h5-6,8H,1-4H2,(H,9,10)(H2,11,12,13)/t5-,6+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards Ionotropic glutamate receptor kainate using [3H]-kainic acid as radioligand; Inactive |
J Med Chem 33: 2961-3 (1990)
BindingDB Entry DOI: 10.7270/Q2BK1CXM |
More data for this
Ligand-Target Pair | |
Glutamate receptor 1
(Rattus norvegicus (Rat)) | BDBM50002363
((APH)2-Amino-7-phosphono-heptanoic acid | 2-Amino-...)Show InChI InChI=1S/C7H16NO5P/c8-6(7(9)10)4-2-1-3-5-14(11,12)13/h6H,1-5,8H2,(H,9,10)(H2,11,12,13) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards AMPA receptor using [3H]AMPA as radioligand; Inactive |
J Med Chem 33: 2961-3 (1990)
BindingDB Entry DOI: 10.7270/Q2BK1CXM |
More data for this
Ligand-Target Pair | |
Glutamate receptor 1
(Rattus norvegicus (Rat)) | BDBM50004897

((APV)2-Amino-5-phosphono-pentanoic acid | (R)-2-Am...)Show InChI InChI=1S/C5H12NO5P/c6-4(5(7)8)2-1-3-12(9,10)11/h4H,1-3,6H2,(H,7,8)(H2,9,10,11) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards AMPA receptor using [3H]AMPA as radioligand; Inactive |
J Med Chem 33: 2961-3 (1990)
BindingDB Entry DOI: 10.7270/Q2BK1CXM |
More data for this
Ligand-Target Pair | |
Glutamate receptor 1
(Rattus norvegicus (Rat)) | BDBM50002360

((CPP)4-(3-Phosphono-propyl)-piperazine-2-carboxyli...)Show InChI InChI=1S/C8H17N2O5P/c11-8(12)7-6-10(4-2-9-7)3-1-5-16(13,14)15/h7,9H,1-6H2,(H,11,12)(H2,13,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards AMPA receptor using [3H]AMPA as radioligand; Inactive |
J Med Chem 33: 2961-3 (1990)
BindingDB Entry DOI: 10.7270/Q2BK1CXM |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 3
(Rattus norvegicus) | BDBM50002363

((APH)2-Amino-7-phosphono-heptanoic acid | 2-Amino-...)Show InChI InChI=1S/C7H16NO5P/c8-6(7(9)10)4-2-1-3-5-14(11,12)13/h6H,1-5,8H2,(H,9,10)(H2,11,12,13) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards Ionotropic glutamate receptor kainate using [3H]-kainic acid as radioligand; Inactive |
J Med Chem 33: 2961-3 (1990)
BindingDB Entry DOI: 10.7270/Q2BK1CXM |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 3
(Rattus norvegicus) | BDBM50010893

((R)-2-Amino-4-oxo-5-phosphono-pentanoic acid | 2-A...)Show InChI InChI=1S/C5H10NO6P/c6-4(5(8)9)1-3(7)2-13(10,11)12/h4H,1-2,6H2,(H,8,9)(H2,10,11,12)/t4-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards Ionotropic glutamate receptor kainate using [3H]-kainic acid as radioligand; Inactive |
J Med Chem 33: 2961-3 (1990)
BindingDB Entry DOI: 10.7270/Q2BK1CXM |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50232114
((R)-1-(5-tert-butyl-2,3-dihydro-1H-inden-1-yl)-3-(...)Show SMILES CC(C)(C)c1ccc2[C@@H](CCc2c1)NC(=O)Nc1cccc2[nH]ncc12 Show InChI InChI=1S/C21H24N4O/c1-21(2,3)14-8-9-15-13(11-14)7-10-18(15)24-20(26)23-17-5-4-6-19-16(17)12-22-25-19/h4-6,8-9,11-12,18H,7,10H2,1-3H3,(H,22,25)(H2,23,24,26)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 5.5 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Blockade of pH 5.5-induced activation of TRPV1 |
J Med Chem 51: 392-5 (2008)
Article DOI: 10.1021/jm701007g
BindingDB Entry DOI: 10.7270/Q218367F |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50321857

(8-{4-(4-Fluorophenyl)-5-[4-(trifluoromethyl)phenyl...)Show SMILES OC1CCc2cccc(Nc3nc(c(o3)-c3ccc(cc3)C(F)(F)F)-c3ccc(F)cc3)c2C1 Show InChI InChI=1S/C26H20F4N2O2/c27-19-11-6-16(7-12-19)23-24(17-4-9-18(10-5-17)26(28,29)30)34-25(32-23)31-22-3-1-2-15-8-13-20(33)14-21(15)22/h1-7,9-12,20,33H,8,13-14H2,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant TRPV1 receptor expressed in human 1321 cells assessed as inhibition of capsaicin-induced in intracellular ca... |
Bioorg Med Chem 18: 4821-9 (2011)
Article DOI: 10.1016/j.bmc.2010.04.099
BindingDB Entry DOI: 10.7270/Q27H1KJJ |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50228080

(2',15'-dimethyl-5,5'-dioxo-(9'R)-spiro[tetrahydrof...)Show SMILES CC(=O)S[C@@H]1CC2=CC(=O)CC[C@]2(C)[C@H]2CC[C@@]3(C)[C@@H](CC[C@@]33CCC(=O)O3)[C@H]12 |r,t:6| Show InChI InChI=1S/C24H32O4S/c1-14(25)29-19-13-15-12-16(26)4-8-22(15,2)17-5-9-23(3)18(21(17)19)6-10-24(23)11-7-20(27)28-24/h12,17-19,21H,4-11,13H2,1-3H3/t17-,18-,19+,21+,22-,23-,24+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Antagonist activity at mineralocorticoid receptor ligand binding domain expressed in african green monkey COS7 cells co-transfected with Gal4-LBD by ... |
J Nat Prod 72: 1944-8 (2009)
Article DOI: 10.1021/np9004882
BindingDB Entry DOI: 10.7270/Q2R49RQR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM128907

(US8802711, 76)Show SMILES Cc1cc2c(NC(=O)N[C@@H]3CC[C@@H](C3)c3cccc(F)c3)c(F)ccc2cn1 Show InChI InChI=1S/C22H21F2N3O/c1-13-9-19-16(12-25-13)6-8-20(24)21(19)27-22(28)26-18-7-5-15(11-18)14-3-2-4-17(23)10-14/h2-4,6,8-10,12,15,18H,5,7,11H2,1H3,(H2,26,27,28)/t15-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The functional activity of compounds at the TRPV1 receptor was determined by measurement of intracellular Ca2+ levels ([Ca2+]i) using the Fluorescenc... |
US Patent US8802711 (2014)
BindingDB Entry DOI: 10.7270/Q2QZ28NC |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50056351
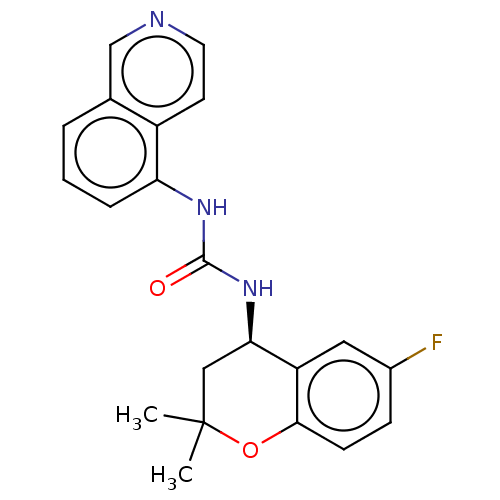
(CHEMBL3326569)Show SMILES CC1(C)C[C@@H](NC(=O)Nc2cccc3cnccc23)c2cc(F)ccc2O1 |r| Show InChI InChI=1S/C21H20FN3O2/c1-21(2)11-18(16-10-14(22)6-7-19(16)27-21)25-20(26)24-17-5-3-4-13-12-23-9-8-15(13)17/h3-10,12,18H,11H2,1-2H3,(H2,24,25,26)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity against human TRPV1 expressed in HEK293 cells assessed as capsaicin-induced calcium flux by FLIPR assay |
J Med Chem 57: 7412-24 (2014)
Article DOI: 10.1021/jm500916t
BindingDB Entry DOI: 10.7270/Q2Z3219J |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50321856

(4-{2-[7-Hydroxy-5,6,7,8-tetrahydronaphthalen-1-yla...)Show SMILES OC1CCc2cccc(Nc3nc(c(o3)-c3ccc(cc3)C(F)(F)F)-c3ccc(cc3)C#N)c2C1 Show InChI InChI=1S/C27H20F3N3O2/c28-27(29,30)20-11-8-19(9-12-20)25-24(18-6-4-16(15-31)5-7-18)33-26(35-25)32-23-3-1-2-17-10-13-21(34)14-22(17)23/h1-9,11-12,21,34H,10,13-14H2,(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant TRPV1 receptor expressed in human 1321 cells assessed as inhibition of capsaicin-induced in intracellular ca... |
Bioorg Med Chem 18: 4821-9 (2011)
Article DOI: 10.1016/j.bmc.2010.04.099
BindingDB Entry DOI: 10.7270/Q27H1KJJ |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50321853

(8-{4-Ethyl-5-[4-(trifluoromethyl)phenyl]oxazol-2-y...)Show SMILES CCc1nc(Nc2cccc3CCC(O)Cc23)oc1-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C22H21F3N2O2/c1-2-18-20(14-6-9-15(10-7-14)22(23,24)25)29-21(26-18)27-19-5-3-4-13-8-11-16(28)12-17(13)19/h3-7,9-10,16,28H,2,8,11-12H2,1H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant TRPV1 receptor expressed in human 1321 cells assessed as inhibition of capsaicin-induced in intracellular ca... |
Bioorg Med Chem 18: 4821-9 (2011)
Article DOI: 10.1016/j.bmc.2010.04.099
BindingDB Entry DOI: 10.7270/Q27H1KJJ |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM128912

(US8802711, 81)Show SMILES Cn1c2cccc(NC(=O)N[C@@H]3CC[C@H](C3)c3ccccc3F)c2ccc1=O Show InChI InChI=1S/C22H22FN3O2/c1-26-20-8-4-7-19(17(20)11-12-21(26)27)25-22(28)24-15-10-9-14(13-15)16-5-2-3-6-18(16)23/h2-8,11-12,14-15H,9-10,13H2,1H3,(H2,24,25,28)/t14-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The functional activity of compounds at the TRPV1 receptor was determined by measurement of intracellular Ca2+ levels ([Ca2+]i) using the Fluorescenc... |
US Patent US8802711 (2014)
BindingDB Entry DOI: 10.7270/Q2QZ28NC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-9
(RAT) | BDBM82070

(CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3/t10-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
SIBIA Neurosciences Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards Nicotinic acetylcholine receptor by the displacement of [3H]-nicotine from rat cortical membranes |
J Med Chem 42: 1684-6 (1999)
Article DOI: 10.1021/jm990035d
BindingDB Entry DOI: 10.7270/Q2QC02PQ |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50338002
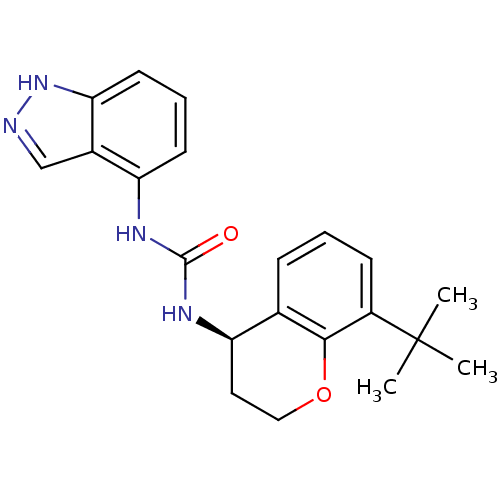
((R)-1-(8-tert-butylchroman-4-yl)-3-(1H-indazol-4-y...)Show SMILES CC(C)(C)c1cccc2[C@@H](CCOc12)NC(=O)Nc1cccc2[nH]ncc12 |r| Show InChI InChI=1S/C21H24N4O2/c1-21(2,3)15-7-4-6-13-17(10-11-27-19(13)15)24-20(26)23-16-8-5-9-18-14(16)12-22-25-18/h4-9,12,17H,10-11H2,1-3H3,(H,22,25)(H2,23,24,26)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 assessed as inhibition of calcium influx |
Bioorg Med Chem Lett 21: 1338-41 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.056
BindingDB Entry DOI: 10.7270/Q20K28W6 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50056358
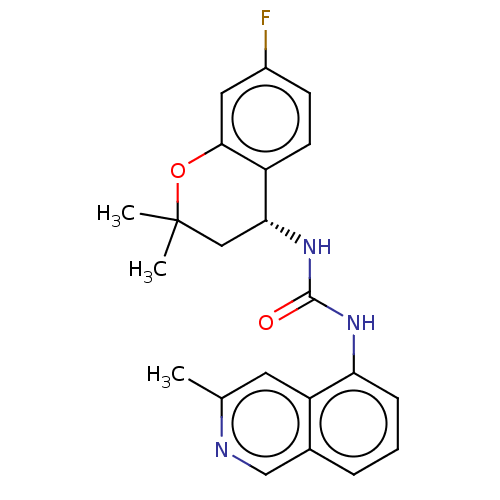
(CHEMBL3326581)Show SMILES Cc1cc2c(NC(=O)N[C@@H]3CC(C)(C)Oc4cc(F)ccc34)cccc2cn1 |r| Show InChI InChI=1S/C22H22FN3O2/c1-13-9-17-14(12-24-13)5-4-6-18(17)25-21(27)26-19-11-22(2,3)28-20-10-15(23)7-8-16(19)20/h4-10,12,19H,11H2,1-3H3,(H2,25,26,27)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity against human TRPV1 expressed in HEK293 cells assessed as capsaicin-induced calcium flux by FLIPR assay |
J Med Chem 57: 7412-24 (2014)
Article DOI: 10.1021/jm500916t
BindingDB Entry DOI: 10.7270/Q2Z3219J |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50232113
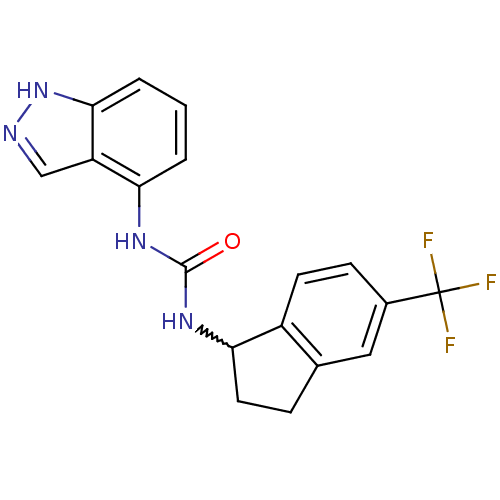
(1-(1H-indazol-4-yl)-3-(5-(trifluoromethyl)-2,3-dih...)Show SMILES FC(F)(F)c1ccc2C(CCc2c1)NC(=O)Nc1cccc2[nH]ncc12 |w:8.14| Show InChI InChI=1S/C18H15F3N4O/c19-18(20,21)11-5-6-12-10(8-11)4-7-15(12)24-17(26)23-14-2-1-3-16-13(14)9-22-25-16/h1-3,5-6,8-9,15H,4,7H2,(H,22,25)(H2,23,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Blockade of human TRPV1 receptor assessed as inhibition of capsaicin-induced calcium flux |
J Med Chem 51: 392-5 (2008)
Article DOI: 10.1021/jm701007g
BindingDB Entry DOI: 10.7270/Q218367F |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50232113

(1-(1H-indazol-4-yl)-3-(5-(trifluoromethyl)-2,3-dih...)Show SMILES FC(F)(F)c1ccc2C(CCc2c1)NC(=O)Nc1cccc2[nH]ncc12 |w:8.14| Show InChI InChI=1S/C18H15F3N4O/c19-18(20,21)11-5-6-12-10(8-11)4-7-15(12)24-17(26)23-14-2-1-3-16-13(14)9-22-25-16/h1-3,5-6,8-9,15H,4,7H2,(H,22,25)(H2,23,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 assessed as inhibition of calcium influx |
Bioorg Med Chem Lett 21: 1338-41 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.056
BindingDB Entry DOI: 10.7270/Q20K28W6 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50232114

((R)-1-(5-tert-butyl-2,3-dihydro-1H-inden-1-yl)-3-(...)Show SMILES CC(C)(C)c1ccc2[C@@H](CCc2c1)NC(=O)Nc1cccc2[nH]ncc12 Show InChI InChI=1S/C21H24N4O/c1-21(2,3)14-8-9-15-13(11-14)7-10-18(15)24-20(26)23-17-5-4-6-19-16(17)12-22-25-19/h4-6,8-9,11-12,18H,7,10H2,1-3H3,(H,22,25)(H2,23,24,26)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Blockade of N-arachidonoyl-dopamine-induced activation of TRPV1 |
J Med Chem 51: 392-5 (2008)
Article DOI: 10.1021/jm701007g
BindingDB Entry DOI: 10.7270/Q218367F |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Antagonist activity at glucocorticoid receptor ligand binding domain expressed in african green monkey COS7 cells co-transfected with Gal4-LBD by luc... |
J Nat Prod 72: 1944-8 (2009)
Article DOI: 10.1021/np9004882
BindingDB Entry DOI: 10.7270/Q2R49RQR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50321850
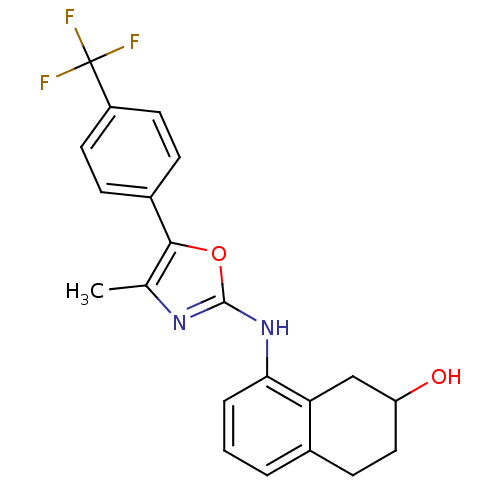
(8-{5-[3-Methyl-4-(trifluoromethyl)phenyl]oxazol-2-...)Show SMILES Cc1nc(Nc2cccc3CCC(O)Cc23)oc1-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C21H19F3N2O2/c1-12-19(14-5-8-15(9-6-14)21(22,23)24)28-20(25-12)26-18-4-2-3-13-7-10-16(27)11-17(13)18/h2-6,8-9,16,27H,7,10-11H2,1H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant TRPV1 receptor expressed in human 1321 cells assessed as inhibition of capsaicin-induced in intracellular ca... |
Bioorg Med Chem 18: 4821-9 (2011)
Article DOI: 10.1016/j.bmc.2010.04.099
BindingDB Entry DOI: 10.7270/Q27H1KJJ |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM128913

(US8802711, 82)Show SMILES Cc1cc2c(NC(=O)N[C@@H]3CC[C@H](C3)c3ccccc3F)c(F)ccc2cn1 Show InChI InChI=1S/C22H21F2N3O/c1-13-10-18-15(12-25-13)7-9-20(24)21(18)27-22(28)26-16-8-6-14(11-16)17-4-2-3-5-19(17)23/h2-5,7,9-10,12,14,16H,6,8,11H2,1H3,(H2,26,27,28)/t14-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The functional activity of compounds at the TRPV1 receptor was determined by measurement of intracellular Ca2+ levels ([Ca2+]i) using the Fluorescenc... |
US Patent US8802711 (2014)
BindingDB Entry DOI: 10.7270/Q2QZ28NC |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM128905

(US8802711, 74)Show SMILES Cc1cc2c(NC(=O)N[C@H]3CC[C@@H](C3)c3cccc(F)c3)c(F)ccc2cn1 Show InChI InChI=1S/C22H21F2N3O/c1-13-9-19-16(12-25-13)6-8-20(24)21(19)27-22(28)26-18-7-5-15(11-18)14-3-2-4-17(23)10-14/h2-4,6,8-10,12,15,18H,5,7,11H2,1H3,(H2,26,27,28)/t15-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The functional activity of compounds at the TRPV1 receptor was determined by measurement of intracellular Ca2+ levels ([Ca2+]i) using the Fluorescenc... |
US Patent US8802711 (2014)
BindingDB Entry DOI: 10.7270/Q2QZ28NC |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50319456
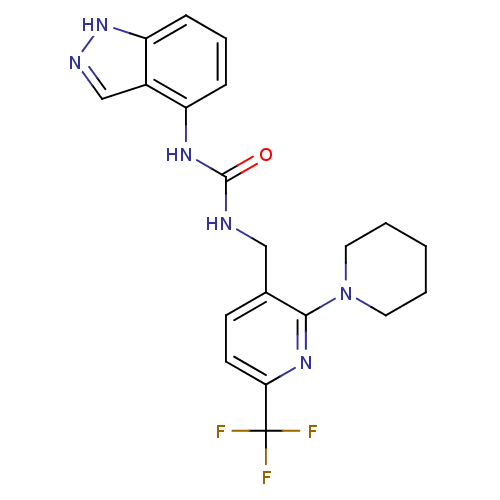
(1-(1H-indazol-4-yl)-3-((2-(piperidin-1-yl)-6-(trif...)Show SMILES FC(F)(F)c1ccc(CNC(=O)Nc2cccc3[nH]ncc23)c(n1)N1CCCCC1 Show InChI InChI=1S/C20H21F3N6O/c21-20(22,23)17-8-7-13(18(27-17)29-9-2-1-3-10-29)11-24-19(30)26-15-5-4-6-16-14(15)12-25-28-16/h4-8,12H,1-3,9-11H2,(H,25,28)(H2,24,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 |
Bioorg Med Chem Lett 20: 3291-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.047
BindingDB Entry DOI: 10.7270/Q2B27VF7 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Rattus norvegicus (rat)) | BDBM50246766

(CHEMBL489079 | Mofegiline | US9302986, Mofegiline)Show InChI InChI=1S/C11H13F2N/c12-7-10(8-14)2-1-9-3-5-11(13)6-4-9/h3-7H,1-2,8,14H2/b10-7+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Inhibition of rat membrane MAOB |
J Med Chem 51: 8019-26 (2008)
Article DOI: 10.1021/jm8011867
BindingDB Entry DOI: 10.7270/Q2542NGF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data