

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50200120 (CHEMBL260091 | CHIR-090 | US10875832, Compound ChI...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Westf£lischen Wilhelms-Universit£t M£nster Curated by ChEMBL | Assay Description Inhibition of Escherichia coli LpxC using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetylglucosamine as substrate incubated for 30 mins prior to enzyme add... | Bioorg Med Chem 22: 1016-28 (2014) Article DOI: 10.1016/j.bmc.2013.12.057 BindingDB Entry DOI: 10.7270/Q2X92F7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50495314 (CHEMBL3103559) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Westf£lischen Wilhelms-Universit£t M£nster Curated by ChEMBL | Assay Description Inhibition of Escherichia coli LpxC using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetylglucosamine as substrate incubated for 30 mins prior to enzyme add... | Bioorg Med Chem 22: 1016-28 (2014) Article DOI: 10.1016/j.bmc.2013.12.057 BindingDB Entry DOI: 10.7270/Q2X92F7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50495317 (CHEMBL3103561) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Westf£lischen Wilhelms-Universit£t M£nster Curated by ChEMBL | Assay Description Inhibition of Escherichia coli LpxC using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetylglucosamine as substrate incubated for 30 mins prior to enzyme add... | Bioorg Med Chem 22: 1016-28 (2014) Article DOI: 10.1016/j.bmc.2013.12.057 BindingDB Entry DOI: 10.7270/Q2X92F7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50495323 (CHEMBL3103548) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 358 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Westf£lischen Wilhelms-Universit£t M£nster Curated by ChEMBL | Assay Description Inhibition of Escherichia coli LpxC using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetylglucosamine as substrate incubated for 30 mins prior to enzyme add... | Bioorg Med Chem 22: 1016-28 (2014) Article DOI: 10.1016/j.bmc.2013.12.057 BindingDB Entry DOI: 10.7270/Q2X92F7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50495312 (CHEMBL3103550) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Westf£lischen Wilhelms-Universit£t M£nster Curated by ChEMBL | Assay Description Inhibition of Escherichia coli LpxC using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetylglucosamine as substrate incubated for 30 mins prior to enzyme add... | Bioorg Med Chem 22: 1016-28 (2014) Article DOI: 10.1016/j.bmc.2013.12.057 BindingDB Entry DOI: 10.7270/Q2X92F7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50495316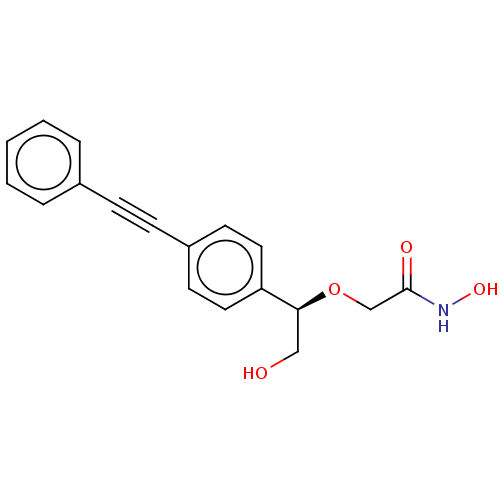 (CHEMBL3103560) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Westf£lischen Wilhelms-Universit£t M£nster Curated by ChEMBL | Assay Description Inhibition of Escherichia coli LpxC using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetylglucosamine as substrate incubated for 30 mins prior to enzyme add... | Bioorg Med Chem 22: 1016-28 (2014) Article DOI: 10.1016/j.bmc.2013.12.057 BindingDB Entry DOI: 10.7270/Q2X92F7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50495311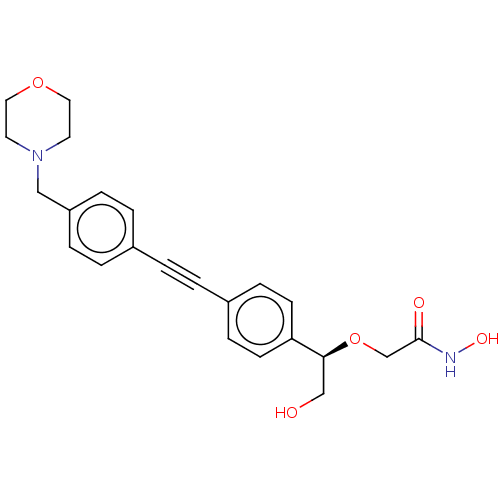 (CHEMBL3103562) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Westf£lischen Wilhelms-Universit£t M£nster Curated by ChEMBL | Assay Description Inhibition of Escherichia coli LpxC using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetylglucosamine as substrate incubated for 30 mins prior to enzyme add... | Bioorg Med Chem 22: 1016-28 (2014) Article DOI: 10.1016/j.bmc.2013.12.057 BindingDB Entry DOI: 10.7270/Q2X92F7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50523959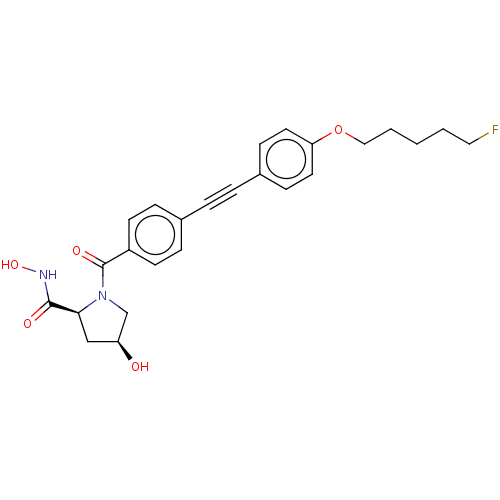 (CHEMBL4584968) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg Curated by ChEMBL | Assay Description Inhibition of human MMP13 using (7-methoxycoumarin-4-yl)acetyl-Pro-Leu-Gly-Leu-(3-(2,4-dinitrophenyl)-L-2,3-diamino-propionyl)Ala-Arg-NH2 as substrat... | Bioorg Med Chem 27: 1997-2018 (2019) Article DOI: 10.1016/j.bmc.2019.03.056 BindingDB Entry DOI: 10.7270/Q2VH5S8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50523962 (CHEMBL4527411) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg Curated by ChEMBL | Assay Description Inhibition of human MMP13 using (7-methoxycoumarin-4-yl)acetyl-Pro-Leu-Gly-Leu-(3-(2,4-dinitrophenyl)-L-2,3-diamino-propionyl)Ala-Arg-NH2 as substrat... | Bioorg Med Chem 27: 1997-2018 (2019) Article DOI: 10.1016/j.bmc.2019.03.056 BindingDB Entry DOI: 10.7270/Q2VH5S8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50523962 (CHEMBL4527411) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg Curated by ChEMBL | Assay Description Inhibition of human MMP2 using (7-methoxycoumarin-4-yl)acetyl-Pro-Leu-Gly-Leu-(3-(2,4-dinitrophenyl)-L-2,3-diamino-propionyl)Ala-Arg-NH2 as substrate... | Bioorg Med Chem 27: 1997-2018 (2019) Article DOI: 10.1016/j.bmc.2019.03.056 BindingDB Entry DOI: 10.7270/Q2VH5S8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM8465 ((2R)-N-hydroxy-2-[(4-methoxybenzene)(pyridin-3-ylm...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg Curated by ChEMBL | Assay Description Inhibition of human MMP2 using (7-methoxycoumarin-4-yl)acetyl-Pro-Leu-Gly-Leu-(3-(2,4-dinitrophenyl)-L-2,3-diamino-propionyl)Ala-Arg-NH2 as substrate... | Bioorg Med Chem 27: 1997-2018 (2019) Article DOI: 10.1016/j.bmc.2019.03.056 BindingDB Entry DOI: 10.7270/Q2VH5S8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50523959 (CHEMBL4584968) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg Curated by ChEMBL | Assay Description Inhibition of human MMP2 using (7-methoxycoumarin-4-yl)acetyl-Pro-Leu-Gly-Leu-(3-(2,4-dinitrophenyl)-L-2,3-diamino-propionyl)Ala-Arg-NH2 as substrate... | Bioorg Med Chem 27: 1997-2018 (2019) Article DOI: 10.1016/j.bmc.2019.03.056 BindingDB Entry DOI: 10.7270/Q2VH5S8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50397360 (CHEMBL2170177 | US10188756, Compound CN110) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University of Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC8 (unknown origin) using acetyl-Gly-Ala-(N-acetyl-Lys)-amino-4-methylcoumarin as substrate preincubated for 15 mins fol... | J Med Chem 59: 2423-35 (2016) Article DOI: 10.1021/acs.jmedchem.5b01478 BindingDB Entry DOI: 10.7270/Q28054HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM8465 ((2R)-N-hydroxy-2-[(4-methoxybenzene)(pyridin-3-ylm...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg Curated by ChEMBL | Assay Description Inhibition of human MMP9 using (7-methoxycoumarin-4-yl)acetyl-Pro-Leu-Gly-Leu-(3-(2,4-dinitrophenyl)-L-2,3-diamino-propionyl)Ala-Arg-NH2 as substrate... | Bioorg Med Chem 27: 1997-2018 (2019) Article DOI: 10.1016/j.bmc.2019.03.056 BindingDB Entry DOI: 10.7270/Q2VH5S8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50523951 (CHEMBL4437091) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg Curated by ChEMBL | Assay Description Inhibition of human MMP13 using (7-methoxycoumarin-4-yl)acetyl-Pro-Leu-Gly-Leu-(3-(2,4-dinitrophenyl)-L-2,3-diamino-propionyl)Ala-Arg-NH2 as substrat... | Bioorg Med Chem 27: 1997-2018 (2019) Article DOI: 10.1016/j.bmc.2019.03.056 BindingDB Entry DOI: 10.7270/Q2VH5S8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil collagenase (Homo sapiens (Human)) | BDBM8465 ((2R)-N-hydroxy-2-[(4-methoxybenzene)(pyridin-3-ylm...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg Curated by ChEMBL | Assay Description Inhibition of human MMP8 using (7-methoxycoumarin-4-yl)acetyl-Pro-Leu-Gly-Leu-(3-(2,4-dinitrophenyl)-L-2,3-diamino-propionyl)Ala-Arg-NH2 as substrate... | Bioorg Med Chem 27: 1997-2018 (2019) Article DOI: 10.1016/j.bmc.2019.03.056 BindingDB Entry DOI: 10.7270/Q2VH5S8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil collagenase (Homo sapiens (Human)) | BDBM50523962 (CHEMBL4527411) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg Curated by ChEMBL | Assay Description Inhibition of human MMP8 using (7-methoxycoumarin-4-yl)acetyl-Pro-Leu-Gly-Leu-(3-(2,4-dinitrophenyl)-L-2,3-diamino-propionyl)Ala-Arg-NH2 as substrate... | Bioorg Med Chem 27: 1997-2018 (2019) Article DOI: 10.1016/j.bmc.2019.03.056 BindingDB Entry DOI: 10.7270/Q2VH5S8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50380399 (CHEMBL2018302 | Tubastatin A | US10227295, Compoun...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Albert-Ludwigs-University Freiburg Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC6 using RHKKAc peptide as substrate | J Med Chem 59: 1545-55 (2016) Article DOI: 10.1021/acs.jmedchem.5b01493 BindingDB Entry DOI: 10.7270/Q2X350BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM8465 ((2R)-N-hydroxy-2-[(4-methoxybenzene)(pyridin-3-ylm...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg Curated by ChEMBL | Assay Description Inhibition of human MMP13 using (7-methoxycoumarin-4-yl)acetyl-Pro-Leu-Gly-Leu-(3-(2,4-dinitrophenyl)-L-2,3-diamino-propionyl)Ala-Arg-NH2 as substrat... | Bioorg Med Chem 27: 1997-2018 (2019) Article DOI: 10.1016/j.bmc.2019.03.056 BindingDB Entry DOI: 10.7270/Q2VH5S8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50164533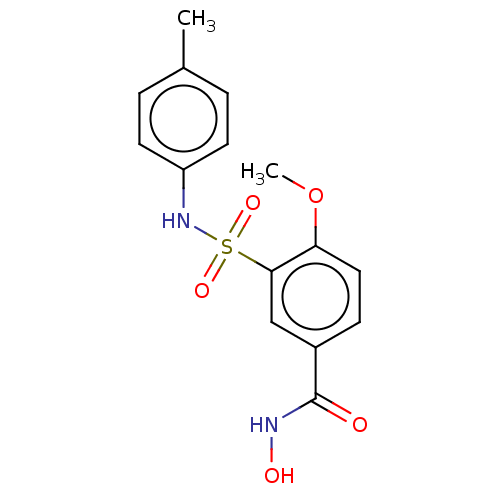 (CHEMBL3800182) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University of Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC8 using Fluor de Lys as substrate preincubated for 90 mins followed by BML-KI176 addition measured after 45 mins ... | J Med Chem 59: 2423-35 (2016) Article DOI: 10.1021/acs.jmedchem.5b01478 BindingDB Entry DOI: 10.7270/Q28054HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil collagenase (Homo sapiens (Human)) | BDBM50523959 (CHEMBL4584968) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg Curated by ChEMBL | Assay Description Inhibition of human MMP8 using (7-methoxycoumarin-4-yl)acetyl-Pro-Leu-Gly-Leu-(3-(2,4-dinitrophenyl)-L-2,3-diamino-propionyl)Ala-Arg-NH2 as substrate... | Bioorg Med Chem 27: 1997-2018 (2019) Article DOI: 10.1016/j.bmc.2019.03.056 BindingDB Entry DOI: 10.7270/Q2VH5S8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50164532 (CHEMBL3797516) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University of Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC8 using Fluor de Lys as substrate preincubated for 90 mins followed by BML-KI176 addition measured after 45 mins ... | J Med Chem 59: 2423-35 (2016) Article DOI: 10.1021/acs.jmedchem.5b01478 BindingDB Entry DOI: 10.7270/Q28054HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50164518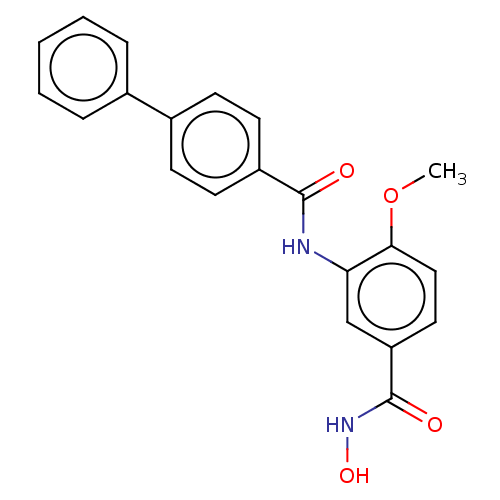 (CHEMBL3800394) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University of Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC8 using Fluor de Lys as substrate preincubated for 90 mins followed by BML-KI176 addition measured after 45 mins ... | J Med Chem 59: 2423-35 (2016) Article DOI: 10.1021/acs.jmedchem.5b01478 BindingDB Entry DOI: 10.7270/Q28054HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50246866 (CHEMBL4078458 | US11505523, Compound 22d) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University of Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of human HDAC8 (1 to 377 residues) expressed in Escherichia coli BL21(DE3) using Boc-Lys(TFA)-AMC as substrate preincubated for 5 mins fol... | J Med Chem 60: 10188-10204 (2017) Article DOI: 10.1021/acs.jmedchem.7b01447 BindingDB Entry DOI: 10.7270/Q2DZ0BQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50164532 (CHEMBL3797516) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University of Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of recombinant human full length C-terminal His-tagged HDAC8 expressed in Escherichia coli BL21(DE3) using fluor de Lys(R) as substrate af... | J Med Chem 60: 10188-10204 (2017) Article DOI: 10.1021/acs.jmedchem.7b01447 BindingDB Entry DOI: 10.7270/Q2DZ0BQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50164516 (CHEMBL3800057) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University of Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC8 using Fluor de Lys as substrate preincubated for 90 mins followed by BML-KI176 addition measured after 45 mins ... | J Med Chem 59: 2423-35 (2016) Article DOI: 10.1021/acs.jmedchem.5b01478 BindingDB Entry DOI: 10.7270/Q28054HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50164517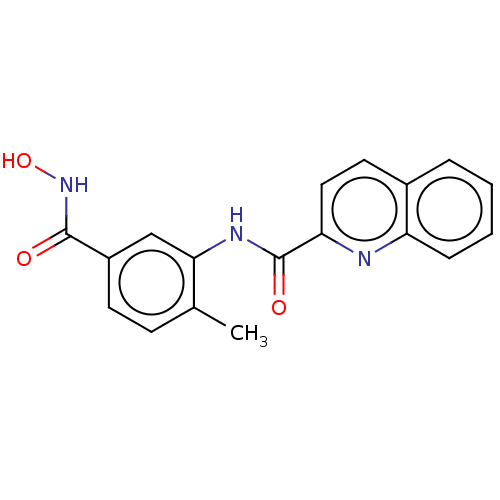 (CHEMBL3798183) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University of Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC8 using Fluor de Lys as substrate preincubated for 90 mins followed by BML-KI176 addition measured after 45 mins ... | J Med Chem 59: 2423-35 (2016) Article DOI: 10.1021/acs.jmedchem.5b01478 BindingDB Entry DOI: 10.7270/Q28054HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50523956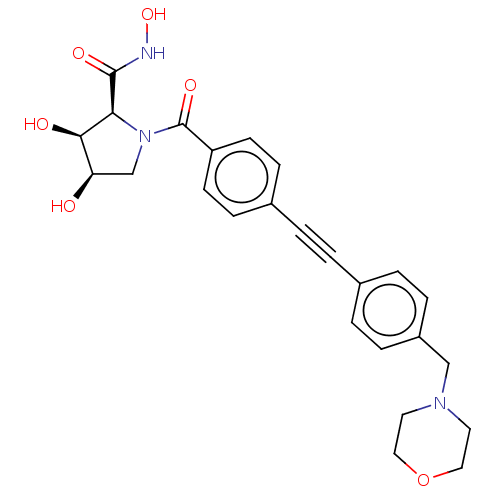 (CHEMBL4536692) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg Curated by ChEMBL | Assay Description Inhibition of human MMP2 using (7-methoxycoumarin-4-yl)acetyl-Pro-Leu-Gly-Leu-(3-(2,4-dinitrophenyl)-L-2,3-diamino-propionyl)Ala-Arg-NH2 as substrate... | Bioorg Med Chem 27: 1997-2018 (2019) Article DOI: 10.1016/j.bmc.2019.03.056 BindingDB Entry DOI: 10.7270/Q2VH5S8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50246847 (CHEMBL4089635) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University of Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of recombinant human full length C-terminal His-tagged HDAC8 expressed in Escherichia coli BL21(DE3) using fluor de Lys(R) as substrate af... | J Med Chem 60: 10188-10204 (2017) Article DOI: 10.1021/acs.jmedchem.7b01447 BindingDB Entry DOI: 10.7270/Q2DZ0BQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50246874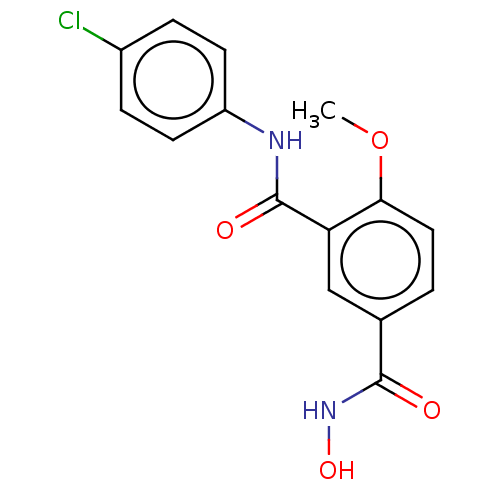 (CHEMBL4095643) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University of Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of recombinant human full length C-terminal His-tagged HDAC8 expressed in Escherichia coli BL21(DE3) using fluor de Lys(R) as substrate af... | J Med Chem 60: 10188-10204 (2017) Article DOI: 10.1021/acs.jmedchem.7b01447 BindingDB Entry DOI: 10.7270/Q2DZ0BQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50523956 (CHEMBL4536692) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg Curated by ChEMBL | Assay Description Inhibition of human MMP13 using (7-methoxycoumarin-4-yl)acetyl-Pro-Leu-Gly-Leu-(3-(2,4-dinitrophenyl)-L-2,3-diamino-propionyl)Ala-Arg-NH2 as substrat... | Bioorg Med Chem 27: 1997-2018 (2019) Article DOI: 10.1016/j.bmc.2019.03.056 BindingDB Entry DOI: 10.7270/Q2VH5S8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University of Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC6 using Cbz-(Ac)Lys-AMC as substrate preincubated for 90 mins followed by trypsin addition measured after 20 mins... | J Med Chem 59: 2423-35 (2016) Article DOI: 10.1021/acs.jmedchem.5b01478 BindingDB Entry DOI: 10.7270/Q28054HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50399672 (CHEMBL2178342) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg Curated by ChEMBL | Assay Description Inhibition of human His-thioredoxin-tagged HDAC8 expressed in Escherichia coli BL21 (DE3) cells using Fluor de Lys (R)-HDAC8 as substrate by fluorome... | J Med Chem 61: 10000-10016 (2018) Article DOI: 10.1021/acs.jmedchem.8b01087 BindingDB Entry DOI: 10.7270/Q2VM4FT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil collagenase (Homo sapiens (Human)) | BDBM50523963 (CHEMBL4452902) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg Curated by ChEMBL | Assay Description Inhibition of human MMP8 using (7-methoxycoumarin-4-yl)acetyl-Pro-Leu-Gly-Leu-(3-(2,4-dinitrophenyl)-L-2,3-diamino-propionyl)Ala-Arg-NH2 as substrate... | Bioorg Med Chem 27: 1997-2018 (2019) Article DOI: 10.1016/j.bmc.2019.03.056 BindingDB Entry DOI: 10.7270/Q2VH5S8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Albert-Ludwigs-University Freiburg Curated by ChEMBL | Assay Description Inhibition of human HDAC6 using (Z-(Ac)Lys-AMC) as substrate after 90 mins by fluorescence analysis | J Med Chem 59: 1545-55 (2016) Article DOI: 10.1021/acs.jmedchem.5b01493 BindingDB Entry DOI: 10.7270/Q2X350BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50450625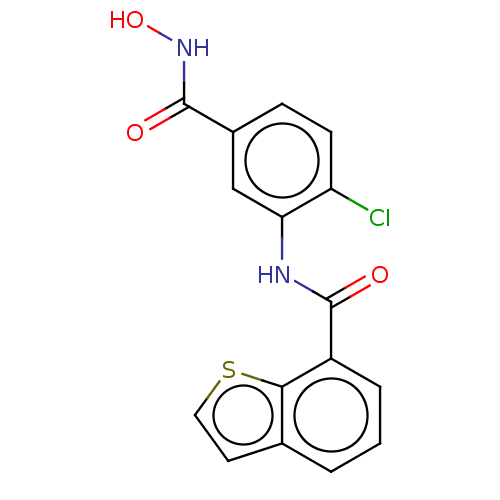 (CHEMBL4160522) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged full length human HDAC6 expressed in baculovirus infected Sf9 insect cells using Z(Ac)Lys-AMC as substrate by flu... | J Med Chem 61: 10000-10016 (2018) Article DOI: 10.1021/acs.jmedchem.8b01087 BindingDB Entry DOI: 10.7270/Q2VM4FT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50523961 (CHEMBL4441587) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg Curated by ChEMBL | Assay Description Inhibition of human MMP2 using (7-methoxycoumarin-4-yl)acetyl-Pro-Leu-Gly-Leu-(3-(2,4-dinitrophenyl)-L-2,3-diamino-propionyl)Ala-Arg-NH2 as substrate... | Bioorg Med Chem 27: 1997-2018 (2019) Article DOI: 10.1016/j.bmc.2019.03.056 BindingDB Entry DOI: 10.7270/Q2VH5S8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50523961 (CHEMBL4441587) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg Curated by ChEMBL | Assay Description Inhibition of human MMP13 using (7-methoxycoumarin-4-yl)acetyl-Pro-Leu-Gly-Leu-(3-(2,4-dinitrophenyl)-L-2,3-diamino-propionyl)Ala-Arg-NH2 as substrat... | Bioorg Med Chem 27: 1997-2018 (2019) Article DOI: 10.1016/j.bmc.2019.03.056 BindingDB Entry DOI: 10.7270/Q2VH5S8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50200120 (CHEMBL260091 | CHIR-090 | US10875832, Compound ChI...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Westf£lischen Wilhelms-Universit£t M£nster Curated by ChEMBL | Assay Description Inhibition of Escherichia coli LpxC using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetylglucosamine as substrate incubated for 30 mins prior to enzyme add... | Bioorg Med Chem 22: 1016-28 (2014) Article DOI: 10.1016/j.bmc.2013.12.057 BindingDB Entry DOI: 10.7270/Q2X92F7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50148612 (CHEMBL3770095) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Albert-Ludwigs-University Freiburg Curated by ChEMBL | Assay Description Inhibition of human HDAC6 using (Z-(Ac)Lys-AMC) as substrate after 90 mins by fluorescence analysis | J Med Chem 59: 1545-55 (2016) Article DOI: 10.1021/acs.jmedchem.5b01493 BindingDB Entry DOI: 10.7270/Q2X350BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50105327 (JNJ-26481585 | Quisinostat) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg Curated by ChEMBL | Assay Description Inhibition of human His-thioredoxin-tagged HDAC8 expressed in Escherichia coli BL21 (DE3) cells using Fluor de Lys (R)-HDAC8 as substrate by fluorome... | J Med Chem 61: 10000-10016 (2018) Article DOI: 10.1021/acs.jmedchem.8b01087 BindingDB Entry DOI: 10.7270/Q2VM4FT7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 8 (Schistosoma mansoni) | BDBM50164641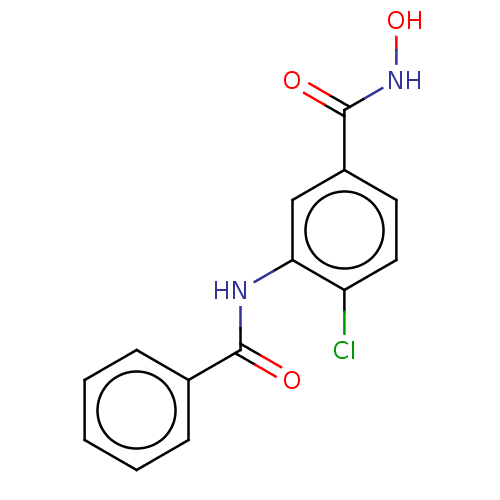 (CHEMBL3797843) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University of Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of recombinant Schistosoma mansoni HDAC8 expressed in Escherichia coli using Fluor de Lys as substrate preincubated for 90 mins followed b... | J Med Chem 59: 2423-35 (2016) Article DOI: 10.1021/acs.jmedchem.5b01478 BindingDB Entry DOI: 10.7270/Q28054HC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50246846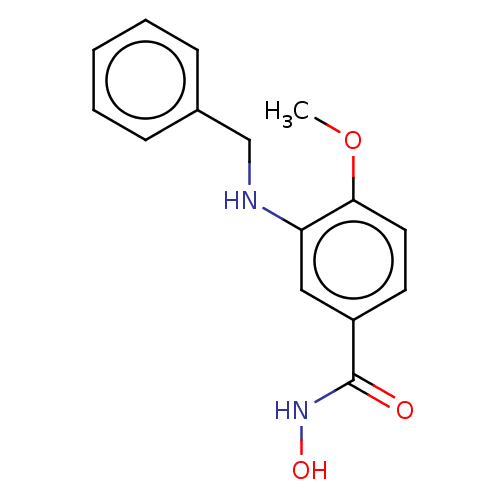 (CHEMBL4105068) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University of Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of recombinant human full length C-terminal His-tagged HDAC8 expressed in Escherichia coli BL21(DE3) using fluor de Lys(R) as substrate af... | J Med Chem 60: 10188-10204 (2017) Article DOI: 10.1021/acs.jmedchem.7b01447 BindingDB Entry DOI: 10.7270/Q2DZ0BQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50164658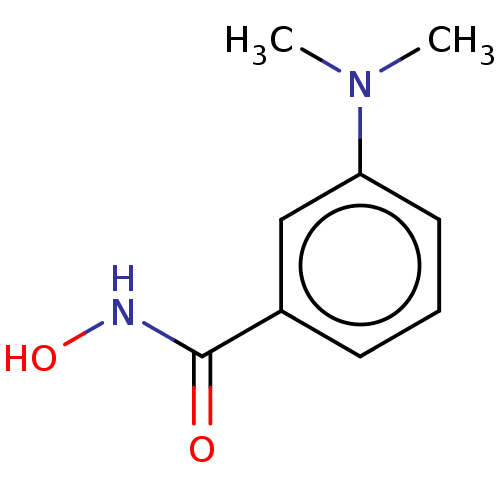 (CHEMBL3798268) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University of Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC8 using Fluor de Lys as substrate preincubated for 90 mins followed by BML-KI176 addition measured after 45 mins ... | J Med Chem 59: 2423-35 (2016) Article DOI: 10.1021/acs.jmedchem.5b01478 BindingDB Entry DOI: 10.7270/Q28054HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50246848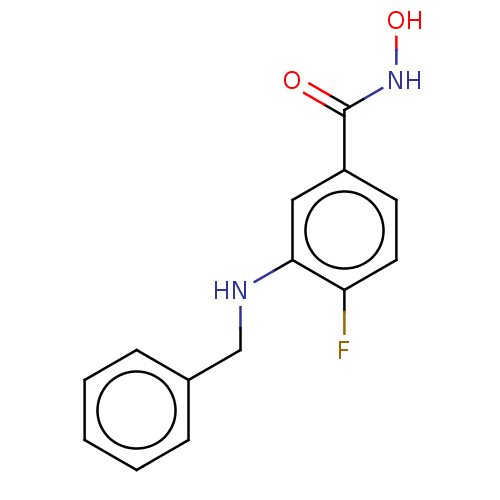 (CHEMBL4061831) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University of Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of recombinant human full length C-terminal His-tagged HDAC8 expressed in Escherichia coli BL21(DE3) using fluor de Lys(R) as substrate af... | J Med Chem 60: 10188-10204 (2017) Article DOI: 10.1021/acs.jmedchem.7b01447 BindingDB Entry DOI: 10.7270/Q2DZ0BQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Schistosoma mansoni) | BDBM50164518 (CHEMBL3800394) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University of Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of recombinant Schistosoma mansoni HDAC8 expressed in Escherichia coli using Fluor de Lys as substrate preincubated for 90 mins followed b... | J Med Chem 59: 2423-35 (2016) Article DOI: 10.1021/acs.jmedchem.5b01478 BindingDB Entry DOI: 10.7270/Q28054HC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50164519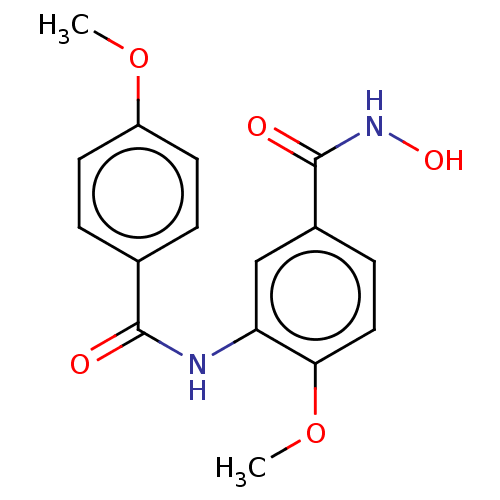 (CHEMBL3799297) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University of Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC8 using Fluor de Lys as substrate preincubated for 90 mins followed by BML-KI176 addition measured after 45 mins ... | J Med Chem 59: 2423-35 (2016) Article DOI: 10.1021/acs.jmedchem.5b01478 BindingDB Entry DOI: 10.7270/Q28054HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50397360 (CHEMBL2170177 | US10188756, Compound CN110) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg Curated by ChEMBL | Assay Description Inhibition of human His-thioredoxin-tagged HDAC8 expressed in Escherichia coli BL21 (DE3) cells using Fluor de Lys (R)-HDAC8 as substrate by fluorome... | J Med Chem 61: 10000-10016 (2018) Article DOI: 10.1021/acs.jmedchem.8b01087 BindingDB Entry DOI: 10.7270/Q2VM4FT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50397360 (CHEMBL2170177 | US10188756, Compound CN110) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University of Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC8 using Fluor de Lys as substrate preincubated for 90 mins followed by BML-KI176 addition measured after 45 mins ... | J Med Chem 59: 2423-35 (2016) Article DOI: 10.1021/acs.jmedchem.5b01478 BindingDB Entry DOI: 10.7270/Q28054HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50148617 (CHEMBL3771189) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Albert-Ludwigs-University Freiburg Curated by ChEMBL | Assay Description Inhibition of human HDAC6 using (Z-(Ac)Lys-AMC) as substrate after 90 mins by fluorescence analysis | J Med Chem 59: 1545-55 (2016) Article DOI: 10.1021/acs.jmedchem.5b01493 BindingDB Entry DOI: 10.7270/Q2X350BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 367 total ) | Next | Last >> |