 Found 226 hits with Last Name = 'menard' and Initial = 'c'
Found 226 hits with Last Name = 'menard' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50466891
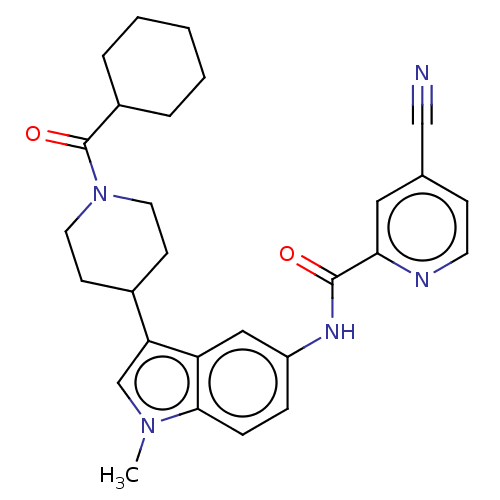
(CHEMBL4281109)Show SMILES Cn1cc(C2CCN(CC2)C(=O)C2CCCCC2)c2cc(NC(=O)c3cc(ccn3)C#N)ccc12 Show InChI InChI=1S/C28H31N5O2/c1-32-18-24(20-10-13-33(14-11-20)28(35)21-5-3-2-4-6-21)23-16-22(7-8-26(23)32)31-27(34)25-15-19(17-29)9-12-30-25/h7-9,12,15-16,18,20-21H,2-6,10-11,13-14H2,1H3,(H,31,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... |
J Med Chem 61: 10415-10439 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00392
BindingDB Entry DOI: 10.7270/Q27P922T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50466897
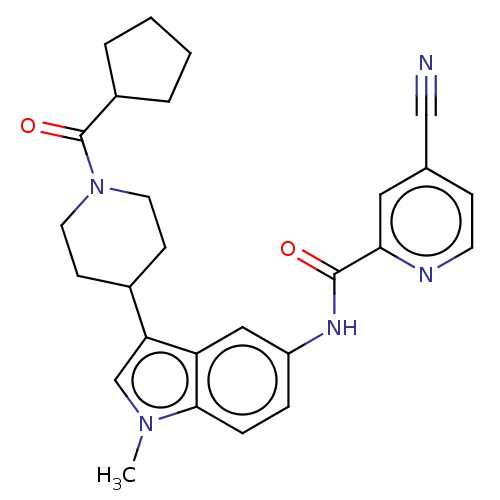
(CHEMBL4287715)Show SMILES Cn1cc(C2CCN(CC2)C(=O)C2CCCC2)c2cc(NC(=O)c3cc(ccn3)C#N)ccc12 Show InChI InChI=1S/C27H29N5O2/c1-31-17-23(19-9-12-32(13-10-19)27(34)20-4-2-3-5-20)22-15-21(6-7-25(22)31)30-26(33)24-14-18(16-28)8-11-29-24/h6-8,11,14-15,17,19-20H,2-5,9-10,12-13H2,1H3,(H,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... |
J Med Chem 61: 10415-10439 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00392
BindingDB Entry DOI: 10.7270/Q27P922T |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50466893
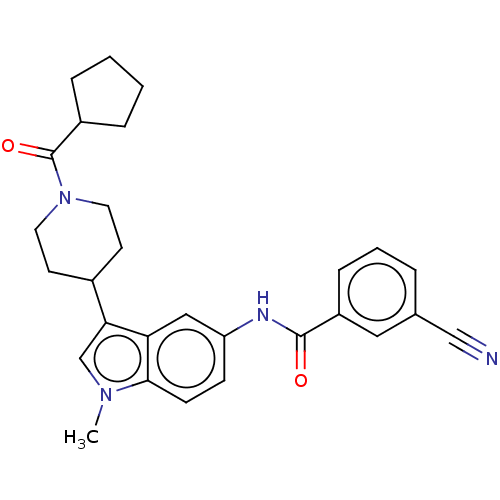
(CHEMBL4291727)Show SMILES Cn1cc(C2CCN(CC2)C(=O)C2CCCC2)c2cc(NC(=O)c3cccc(c3)C#N)ccc12 Show InChI InChI=1S/C28H30N4O2/c1-31-18-25(20-11-13-32(14-12-20)28(34)21-6-2-3-7-21)24-16-23(9-10-26(24)31)30-27(33)22-8-4-5-19(15-22)17-29/h4-5,8-10,15-16,18,20-21H,2-3,6-7,11-14H2,1H3,(H,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... |
J Med Chem 61: 10415-10439 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00392
BindingDB Entry DOI: 10.7270/Q27P922T |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50365344
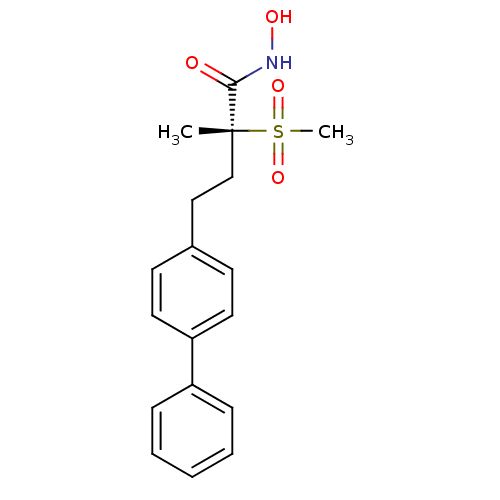
(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human norepinephrine transporter |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50466892
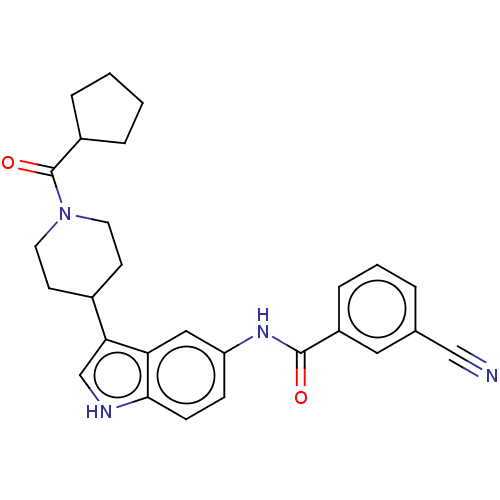
(CHEMBL4283871)Show SMILES O=C(Nc1ccc2[nH]cc(C3CCN(CC3)C(=O)C3CCCC3)c2c1)c1cccc(c1)C#N Show InChI InChI=1S/C27H28N4O2/c28-16-18-4-3-7-21(14-18)26(32)30-22-8-9-25-23(15-22)24(17-29-25)19-10-12-31(13-11-19)27(33)20-5-1-2-6-20/h3-4,7-9,14-15,17,19-20,29H,1-2,5-6,10-13H2,(H,30,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... |
J Med Chem 61: 10415-10439 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00392
BindingDB Entry DOI: 10.7270/Q27P922T |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP12 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-20
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP20 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-24
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP24 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-25
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP25 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-26
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP26 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP8 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP1 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP3 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP7 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP9 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-16
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP16 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-15
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP15 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Stromelysin-2
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP10 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human TACE |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP14 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50466913

(CHEMBL4289304)Show SMILES CCn1cc(C2CCN(CC2)C(=O)C2CCCC2)c2cc(NC(=O)c3cc(ccn3)C#N)ccc12 Show InChI InChI=1S/C28H31N5O2/c1-2-32-18-24(20-10-13-33(14-11-20)28(35)21-5-3-4-6-21)23-16-22(7-8-26(23)32)31-27(34)25-15-19(17-29)9-12-30-25/h7-9,12,15-16,18,20-21H,2-6,10-11,13-14H2,1H3,(H,31,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... |
J Med Chem 61: 10415-10439 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00392
BindingDB Entry DOI: 10.7270/Q27P922T |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50232534
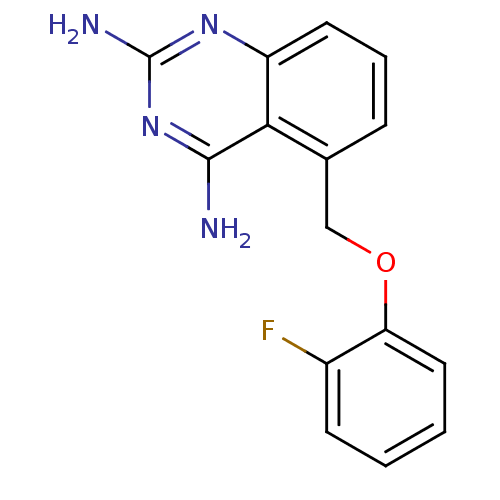
(5-((2-fluorophenoxy)methyl)quinazoline-2,4-diamine...)Show InChI InChI=1S/C15H13FN4O/c16-10-5-1-2-7-12(10)21-8-9-4-3-6-11-13(9)14(17)20-15(18)19-11/h1-7H,8H2,(H4,17,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50232526
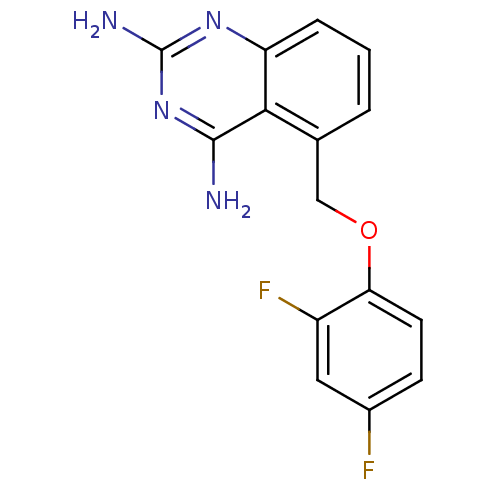
(5-((2,4-difluorophenoxy)methyl)quinazoline-2,4-dia...)Show InChI InChI=1S/C15H12F2N4O/c16-9-4-5-12(10(17)6-9)22-7-8-2-1-3-11-13(8)14(18)21-15(19)20-11/h1-6H,7H2,(H4,18,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50232538
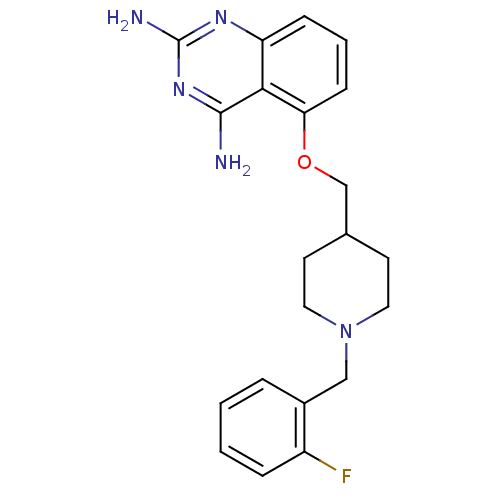
(5-((1-(2-fluorobenzyl)piperidin-4-yl)methoxy)quina...)Show InChI InChI=1S/C21H24FN5O/c22-16-5-2-1-4-15(16)12-27-10-8-14(9-11-27)13-28-18-7-3-6-17-19(18)20(23)26-21(24)25-17/h1-7,14H,8-13H2,(H4,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50237216
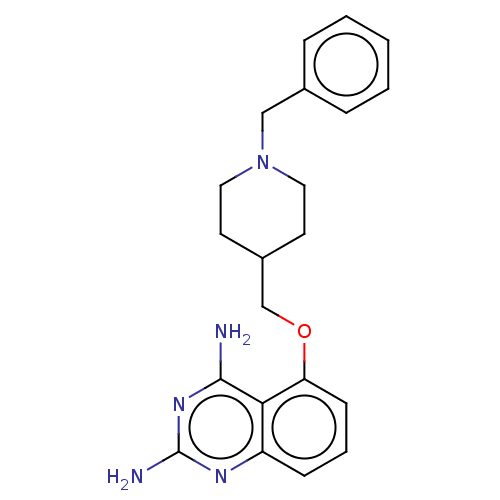
(CHEMBL4080254)Show InChI InChI=1S/C21H25N5O/c22-20-19-17(24-21(23)25-20)7-4-8-18(19)27-14-16-9-11-26(12-10-16)13-15-5-2-1-3-6-15/h1-8,16H,9-14H2,(H4,22,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50237201
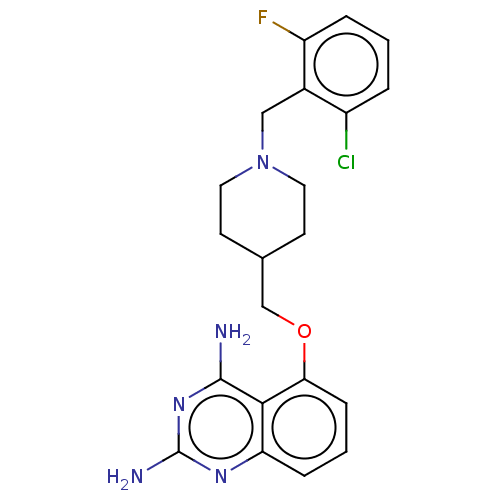
(CHEMBL4082618)Show SMILES Nc1nc(N)c2c(OCC3CCN(Cc4c(F)cccc4Cl)CC3)cccc2n1 Show InChI InChI=1S/C21H23ClFN5O/c22-15-3-1-4-16(23)14(15)11-28-9-7-13(8-10-28)12-29-18-6-2-5-17-19(18)20(24)27-21(25)26-17/h1-6,13H,7-12H2,(H4,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50232589
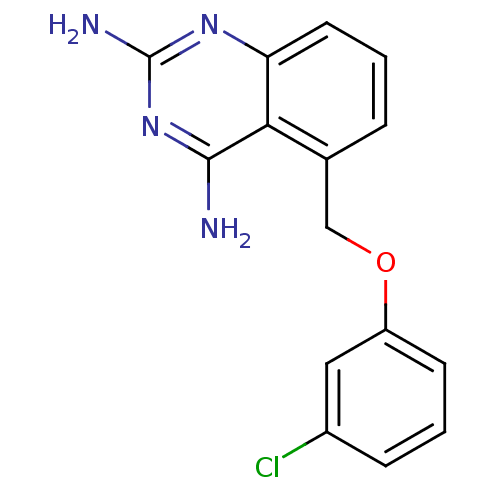
(5-((3-chlorophenoxy)methyl)quinazoline-2,4-diamine...)Show InChI InChI=1S/C15H13ClN4O/c16-10-4-2-5-11(7-10)21-8-9-3-1-6-12-13(9)14(17)20-15(18)19-12/h1-7H,8H2,(H4,17,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50237200
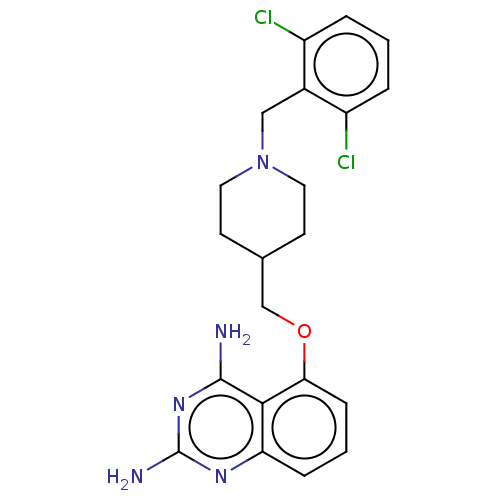
(CHEMBL4072132)Show SMILES Nc1nc(N)c2c(OCC3CCN(Cc4c(Cl)cccc4Cl)CC3)cccc2n1 Show InChI InChI=1S/C21H23Cl2N5O/c22-15-3-1-4-16(23)14(15)11-28-9-7-13(8-10-28)12-29-18-6-2-5-17-19(18)20(24)27-21(25)26-17/h1-6,13H,7-12H2,(H4,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0690 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM36530
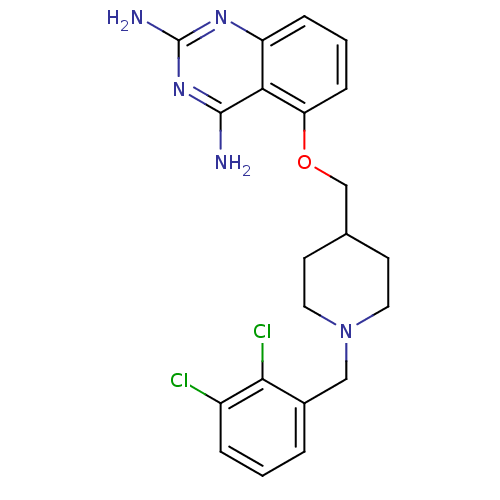
(D157493)Show SMILES Nc1nc(N)c2c(OCC3CCN(Cc4cccc(Cl)c4Cl)CC3)cccc2n1 Show InChI InChI=1S/C21H23Cl2N5O/c22-15-4-1-3-14(19(15)23)11-28-9-7-13(8-10-28)12-29-17-6-2-5-16-18(17)20(24)27-21(25)26-16/h1-6,13H,7-12H2,(H4,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards 5-HT3 receptor in rat was evaluated |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50237199
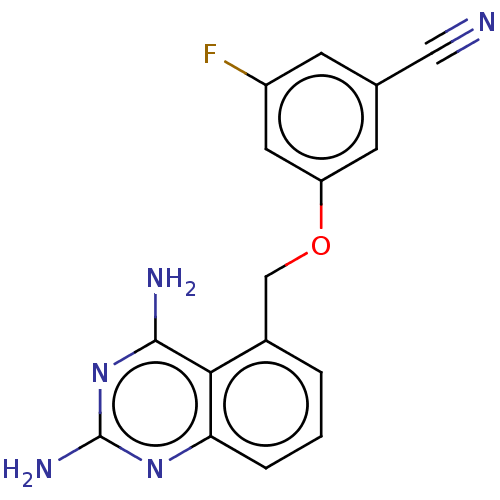
(CHEMBL4077061)Show InChI InChI=1S/C16H12FN5O/c17-11-4-9(7-18)5-12(6-11)23-8-10-2-1-3-13-14(10)15(19)22-16(20)21-13/h1-6H,8H2,(H4,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro reversal of vecuronium-induced block in isolated guinea pig hemi-diaphragm. |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50237203
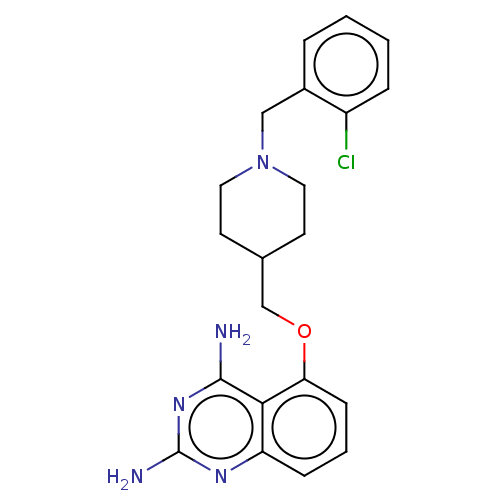
(CHEMBL250072)Show SMILES Nc1nc(N)c2c(OCC3CCN(Cc4ccccc4Cl)CC3)cccc2n1 Show InChI InChI=1S/C21H24ClN5O/c22-16-5-2-1-4-15(16)12-27-10-8-14(9-11-27)13-28-18-7-3-6-17-19(18)20(23)26-21(24)25-17/h1-7,14H,8-13H2,(H4,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50237205
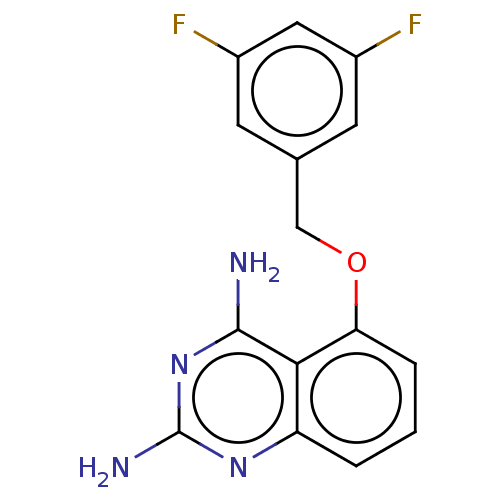
(CHEMBL398675)Show InChI InChI=1S/C15H12F2N4O/c16-9-4-8(5-10(17)6-9)7-22-12-3-1-2-11-13(12)14(18)21-15(19)20-11/h1-6H,7H2,(H4,18,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50237209
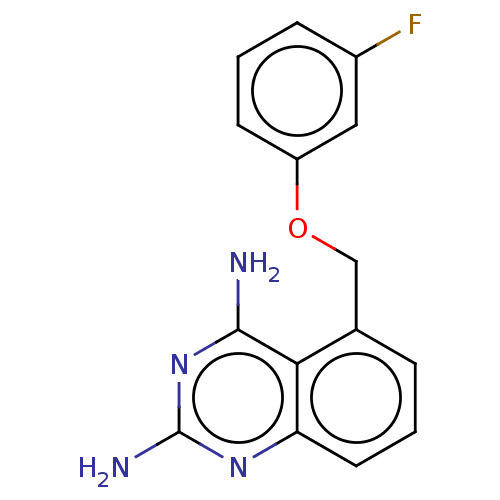
(CHEMBL4062544)Show InChI InChI=1S/C15H13FN4O/c16-10-4-2-5-11(7-10)21-8-9-3-1-6-12-13(9)14(17)20-15(18)19-12/h1-7H,8H2,(H4,17,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50237211
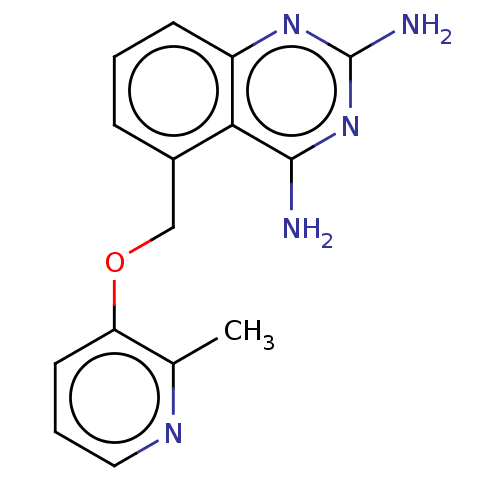
(CHEMBL4061457)Show InChI InChI=1S/C15H15N5O/c1-9-12(6-3-7-18-9)21-8-10-4-2-5-11-13(10)14(16)20-15(17)19-11/h2-7H,8H2,1H3,(H4,16,17,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50237210
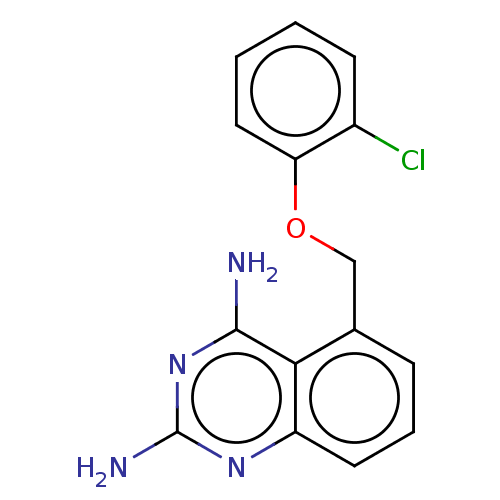
(CHEMBL399673)Show InChI InChI=1S/C15H13ClN4O/c16-10-5-1-2-7-12(10)21-8-9-4-3-6-11-13(9)14(17)20-15(18)19-11/h1-7H,8H2,(H4,17,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM36534
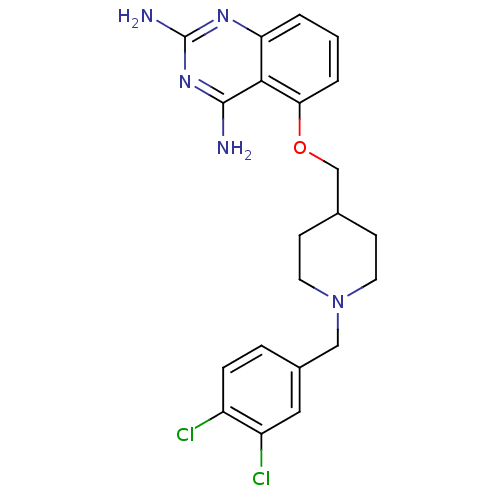
(D156095)Show SMILES Nc1nc(N)c2c(OCC3CCN(Cc4ccc(Cl)c(Cl)c4)CC3)cccc2n1 Show InChI InChI=1S/C21H23Cl2N5O/c22-15-5-4-14(10-16(15)23)11-28-8-6-13(7-9-28)12-29-18-3-1-2-17-19(18)20(24)27-21(25)26-17/h1-5,10,13H,6-9,11-12H2,(H4,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50395921
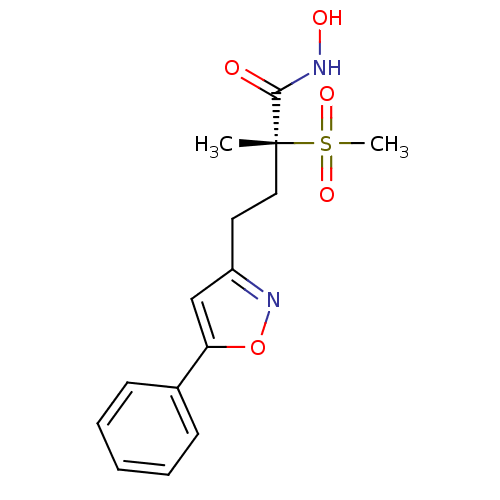
(CHEMBL2164511)Show SMILES C[C@@](CCc1cc(on1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C15H18N2O5S/c1-15(14(18)16-19,23(2,20)21)9-8-12-10-13(22-17-12)11-6-4-3-5-7-11/h3-7,10,19H,8-9H2,1-2H3,(H,16,18)/t15-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.511 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 6832-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.058
BindingDB Entry DOI: 10.7270/Q2GF0VMV |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50485077
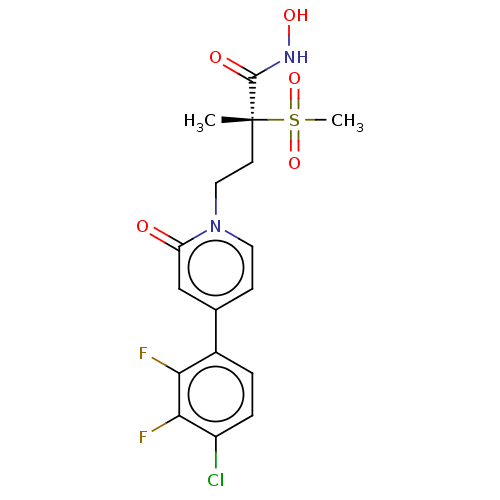
(CHEMBL2023517)Show SMILES C[C@@](CCn1ccc(cc1=O)-c1ccc(Cl)c(F)c1F)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C17H17ClF2N2O5S/c1-17(16(24)21-25,28(2,26)27)6-8-22-7-5-10(9-13(22)23)11-3-4-12(18)15(20)14(11)19/h3-5,7,9,25H,6,8H2,1-2H3,(H,21,24)/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC expressed in Escherichia coli using UDP-3-O-N-acetylglucosamine substrate measured after 30 mins by mass sp... |
J Med Chem 55: 1662-70 (2012)
Article DOI: 10.1021/jm2014875
BindingDB Entry DOI: 10.7270/Q2W38050 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50485061

(CHEMBL2023524)Show SMILES C[C@@](CCn1ccc(cc1=O)-c1ccc(cc1)-n1nccn1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C19H21N5O5S/c1-19(18(26)22-27,30(2,28)29)8-12-23-11-7-15(13-17(23)25)14-3-5-16(6-4-14)24-20-9-10-21-24/h3-7,9-11,13,27H,8,12H2,1-2H3,(H,22,26)/t19-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC expressed in Escherichia coli using UDP-3-O-N-acetylglucosamine substrate measured after 30 mins by mass sp... |
J Med Chem 55: 1662-70 (2012)
Article DOI: 10.1021/jm2014875
BindingDB Entry DOI: 10.7270/Q2W38050 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50395911
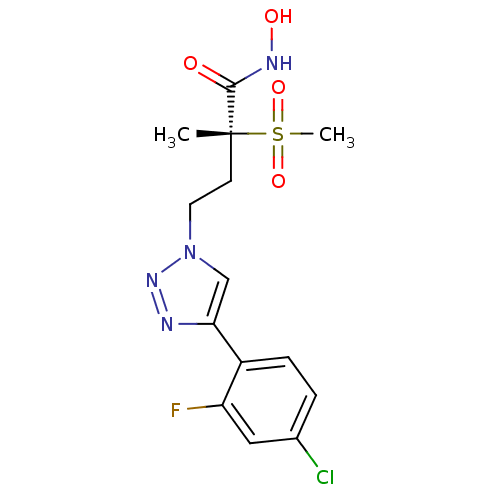
(CHEMBL2164521)Show SMILES C[C@@](CCn1cc(nn1)-c1ccc(Cl)cc1F)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C14H16ClFN4O4S/c1-14(13(21)18-22,25(2,23)24)5-6-20-8-12(17-19-20)10-4-3-9(15)7-11(10)16/h3-4,7-8,22H,5-6H2,1-2H3,(H,18,21)/t14-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.657 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 6832-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.058
BindingDB Entry DOI: 10.7270/Q2GF0VMV |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50395920
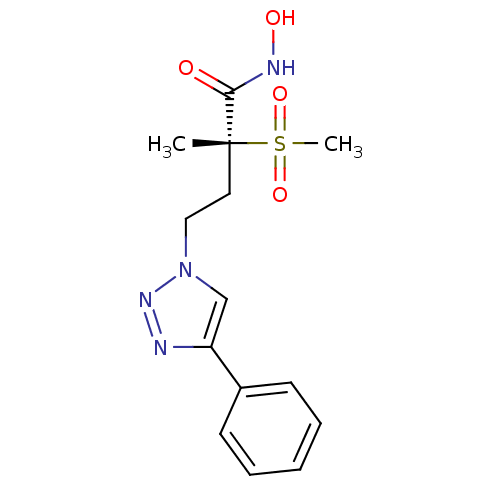
(CHEMBL2164512)Show SMILES C[C@@](CCn1cc(nn1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C14H18N4O4S/c1-14(13(19)16-20,23(2,21)22)8-9-18-10-12(15-17-18)11-6-4-3-5-7-11/h3-7,10,20H,8-9H2,1-2H3,(H,16,19)/t14-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 6832-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.058
BindingDB Entry DOI: 10.7270/Q2GF0VMV |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50395910
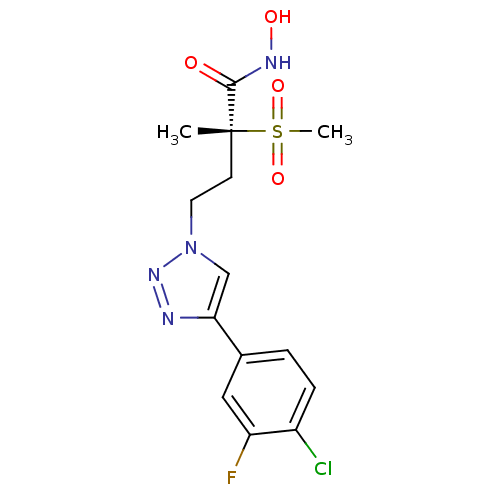
(CHEMBL2164522)Show SMILES C[C@@](CCn1cc(nn1)-c1ccc(Cl)c(F)c1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C14H16ClFN4O4S/c1-14(13(21)18-22,25(2,23)24)5-6-20-8-12(17-19-20)9-3-4-10(15)11(16)7-9/h3-4,7-8,22H,5-6H2,1-2H3,(H,18,21)/t14-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.912 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 6832-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.058
BindingDB Entry DOI: 10.7270/Q2GF0VMV |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
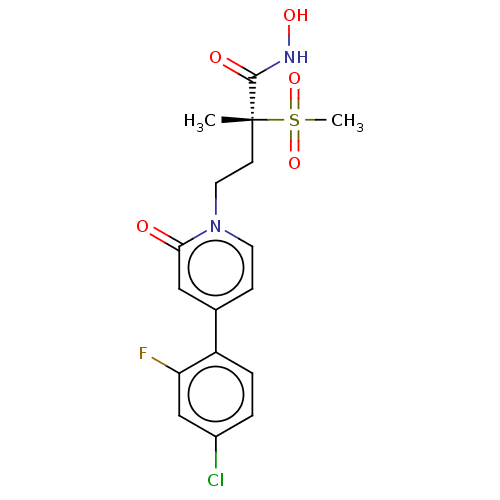
(Pseudomonas aeruginosa) | BDBM50485056
(CHEMBL2023515)Show SMILES C[C@@](CCn1ccc(cc1=O)-c1ccc(Cl)cc1F)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C17H18ClFN2O5S/c1-17(16(23)20-24,27(2,25)26)6-8-21-7-5-11(9-15(21)22)13-4-3-12(18)10-14(13)19/h3-5,7,9-10,24H,6,8H2,1-2H3,(H,20,23)/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC expressed in Escherichia coli using UDP-3-O-N-acetylglucosamine substrate measured after 30 mins by mass sp... |
J Med Chem 55: 1662-70 (2012)
Article DOI: 10.1021/jm2014875
BindingDB Entry DOI: 10.7270/Q2W38050 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50237217
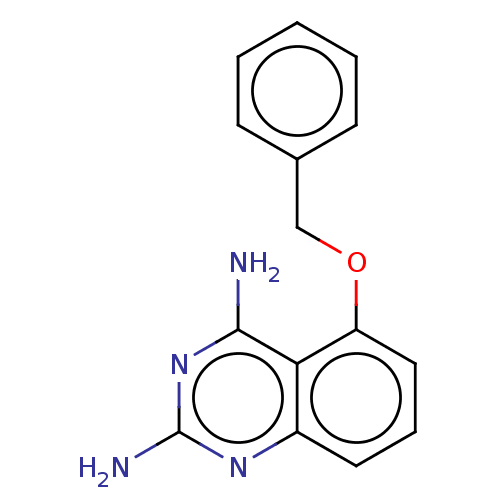
(CHEMBL342595)Show InChI InChI=1S/C15H14N4O/c16-14-13-11(18-15(17)19-14)7-4-8-12(13)20-9-10-5-2-1-3-6-10/h1-8H,9H2,(H4,16,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards rat 5-hydroxytryptamine 3 receptor was evaluated |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50485060
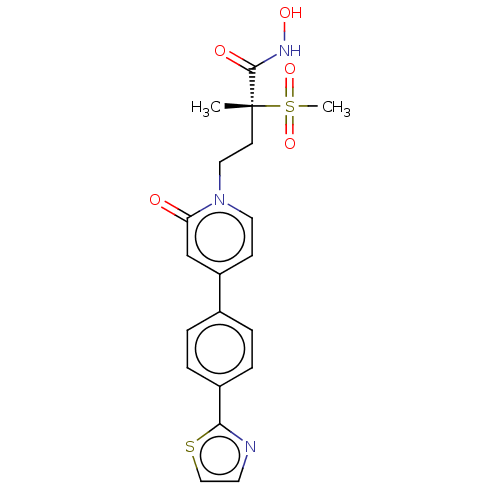
(CHEMBL2023522)Show SMILES C[C@@](CCn1ccc(cc1=O)-c1ccc(cc1)-c1nccs1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C20H21N3O5S2/c1-20(19(25)22-26,30(2,27)28)8-11-23-10-7-16(13-17(23)24)14-3-5-15(6-4-14)18-21-9-12-29-18/h3-7,9-10,12-13,26H,8,11H2,1-2H3,(H,22,25)/t20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC expressed in Escherichia coli using UDP-3-O-N-acetylglucosamine substrate measured after 30 mins by mass sp... |
J Med Chem 55: 1662-70 (2012)
Article DOI: 10.1021/jm2014875
BindingDB Entry DOI: 10.7270/Q2W38050 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50485076
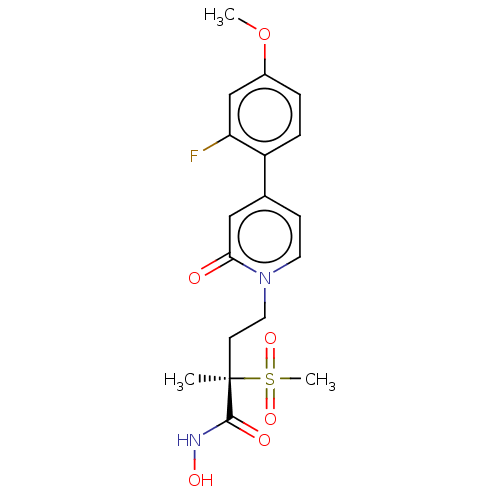
(CHEMBL2023402)Show SMILES COc1ccc(c(F)c1)-c1ccn(CC[C@](C)(C(=O)NO)S(C)(=O)=O)c(=O)c1 |r| Show InChI InChI=1S/C18H21FN2O6S/c1-18(17(23)20-24,28(3,25)26)7-9-21-8-6-12(10-16(21)22)14-5-4-13(27-2)11-15(14)19/h4-6,8,10-11,24H,7,9H2,1-3H3,(H,20,23)/t18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC expressed in Escherichia coli using UDP-3-O-N-acetylglucosamine substrate measured after 30 mins by mass sp... |
J Med Chem 55: 1662-70 (2012)
Article DOI: 10.1021/jm2014875
BindingDB Entry DOI: 10.7270/Q2W38050 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50485083
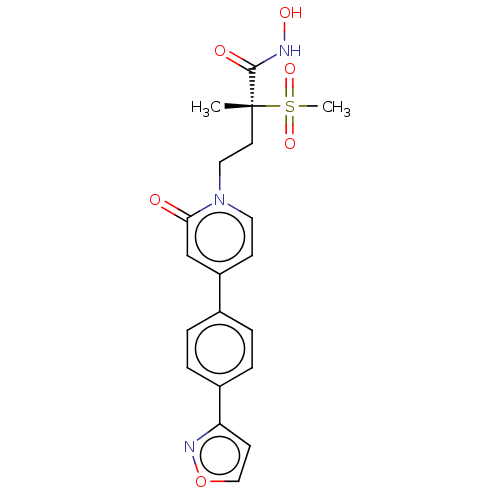
(CHEMBL2023523)Show SMILES C[C@@](CCn1ccc(cc1=O)-c1ccc(cc1)-c1ccon1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C20H21N3O6S/c1-20(19(25)21-26,30(2,27)28)9-11-23-10-7-16(13-18(23)24)14-3-5-15(6-4-14)17-8-12-29-22-17/h3-8,10,12-13,26H,9,11H2,1-2H3,(H,21,25)/t20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC expressed in Escherichia coli using UDP-3-O-N-acetylglucosamine substrate measured after 30 mins by mass sp... |
J Med Chem 55: 1662-70 (2012)
Article DOI: 10.1021/jm2014875
BindingDB Entry DOI: 10.7270/Q2W38050 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50395909

(CHEMBL2164523)Show SMILES COc1ccc(-c2cn(CC[C@](C)(C(=O)NO)S(C)(=O)=O)nn2)c(F)c1 |r| Show InChI InChI=1S/C15H19FN4O5S/c1-15(14(21)18-22,26(3,23)24)6-7-20-9-13(17-19-20)11-5-4-10(25-2)8-12(11)16/h4-5,8-9,22H,6-7H2,1-3H3,(H,18,21)/t15-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 6832-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.058
BindingDB Entry DOI: 10.7270/Q2GF0VMV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data