 Found 3860 hits with Last Name = 'miller' and Initial = 'k'
Found 3860 hits with Last Name = 'miller' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nicotinic acetylcholine receptor
(RAT) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 687-95 (2002)
Article DOI: 10.1124/jpet.302.2.687
BindingDB Entry DOI: 10.7270/Q21R6P3D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50125155
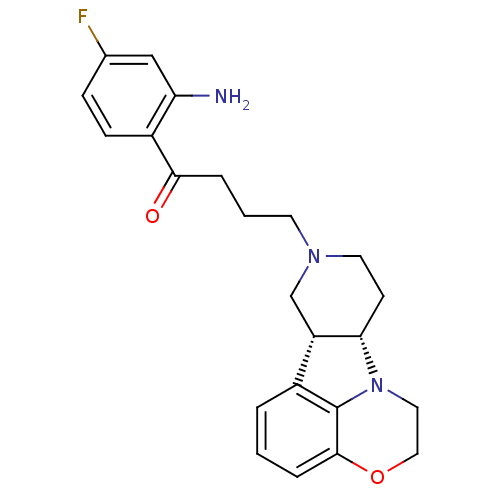
(1-(2-Amino-4-fluoro-phenyl)-4-(6bR,10aS)-1,2,6b,9,...)Show SMILES Nc1cc(F)ccc1C(=O)CCCN1CC[C@H]2[C@@H](C1)c1cccc3OCCN2c13 Show InChI InChI=1S/C23H26FN3O2/c24-15-6-7-17(19(25)13-15)21(28)4-2-9-26-10-8-20-18(14-26)16-3-1-5-22-23(16)27(20)11-12-29-22/h1,3,5-7,13,18,20H,2,4,8-12,14,25H2/t18-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity towards 5-hydroxytryptamine 2A receptor using [125I]-DOI as radioligand. |
Bioorg Med Chem Lett 13: 767-70 (2003)
BindingDB Entry DOI: 10.7270/Q25H7FNW |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50217586
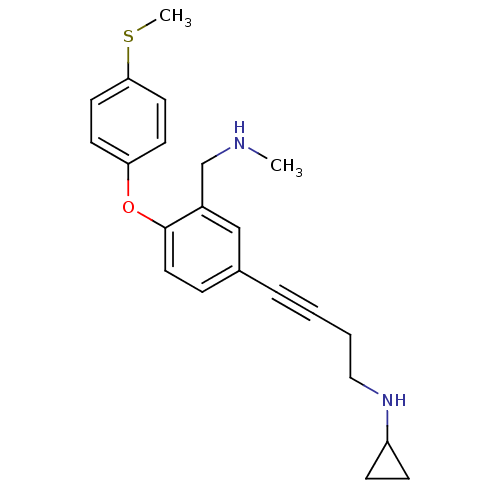
(CHEMBL442080 | N-(4-(3-((methylamino)methyl)-4-(4-...)Show InChI InChI=1S/C22H26N2OS/c1-23-16-18-15-17(5-3-4-14-24-19-7-8-19)6-13-22(18)25-20-9-11-21(26-2)12-10-20/h6,9-13,15,19,23-24H,4,7-8,14,16H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to rat SERT |
Bioorg Med Chem Lett 17: 4799-803 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.061
BindingDB Entry DOI: 10.7270/Q2F18ZFF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50125161

(1-(2-Amino-4-fluoro-phenyl)-4-(6bR,10aS)-1,2,6b,9,...)Show SMILES Nc1cc(F)ccc1C(=O)CCCN1CC[C@H]2[C@@H](C1)c1cccc3SCCN2c13 Show InChI InChI=1S/C23H26FN3OS/c24-15-6-7-17(19(25)13-15)21(28)4-2-9-26-10-8-20-18(14-26)16-3-1-5-22-23(16)27(20)11-12-29-22/h1,3,5-7,13,18,20H,2,4,8-12,14,25H2/t18-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity towards 5-hydroxytryptamine 2A receptor using [125I]-DOI as radioligand. |
Bioorg Med Chem Lett 13: 767-70 (2003)
BindingDB Entry DOI: 10.7270/Q25H7FNW |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50217584

((5-(4-(4-isopropylpiperazin-1-yl)butyl)-2-(4-(meth...)Show SMILES CNCc1cc(CCCCN2CCN(CC2)C(C)C)ccc1Oc1ccc(SC)cc1 Show InChI InChI=1S/C26H39N3OS/c1-21(2)29-17-15-28(16-18-29)14-6-5-7-22-8-13-26(23(19-22)20-27-3)30-24-9-11-25(31-4)12-10-24/h8-13,19,21,27H,5-7,14-18,20H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to rat SERT |
Bioorg Med Chem Lett 17: 4799-803 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.061
BindingDB Entry DOI: 10.7270/Q2F18ZFF |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50217575
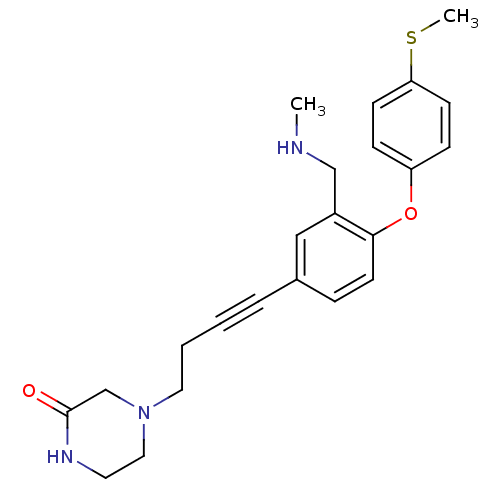
(4-(4-(3-((methylamino)methyl)-4-(4-(methylthio)phe...)Show SMILES CNCc1cc(ccc1Oc1ccc(SC)cc1)C#CCCN1CCNC(=O)C1 Show InChI InChI=1S/C23H27N3O2S/c1-24-16-19-15-18(5-3-4-13-26-14-12-25-23(27)17-26)6-11-22(19)28-20-7-9-21(29-2)10-8-20/h6-11,15,24H,4,12-14,16-17H2,1-2H3,(H,25,27) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to rat SERT |
Bioorg Med Chem Lett 17: 4799-803 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.061
BindingDB Entry DOI: 10.7270/Q2F18ZFF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50371662
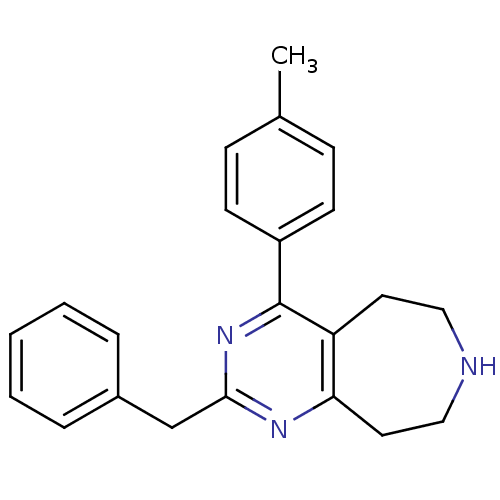
(CHEMBL269974)Show InChI InChI=1S/C22H23N3/c1-16-7-9-18(10-8-16)22-19-11-13-23-14-12-20(19)24-21(25-22)15-17-5-3-2-4-6-17/h2-10,23H,11-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human recombinant 5HT2A receptor expressed in mouse NIH3T3 cells |
Bioorg Med Chem Lett 18: 2103-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.090
BindingDB Entry DOI: 10.7270/Q2571CW5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50125173
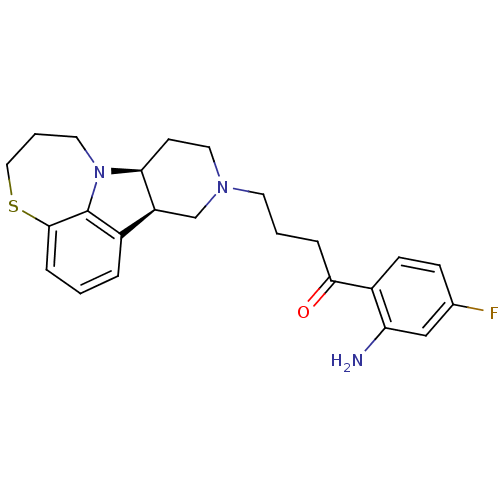
(1-(2-Amino-4-fluoro-phenyl)-4-(7bS,11aR)-6,7,8,9,1...)Show SMILES Nc1cc(F)ccc1C(=O)CCCN1CC[C@H]2[C@@H](C1)c1cccc3SCCCN2c13 Show InChI InChI=1S/C24H28FN3OS/c25-16-7-8-18(20(26)14-16)22(29)5-2-10-27-12-9-21-19(15-27)17-4-1-6-23-24(17)28(21)11-3-13-30-23/h1,4,6-8,14,19,21H,2-3,5,9-13,15,26H2/t19-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity towards 5-hydroxytryptamine 2A receptor using [125I]-DOI as radioligand. |
Bioorg Med Chem Lett 13: 767-70 (2003)
BindingDB Entry DOI: 10.7270/Q25H7FNW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50125154

(1-(2-Amino-4-fluoro-phenyl)-4-(7aS,11aR)-5,6,8,9,1...)Show SMILES Nc1cc(F)ccc1C(=O)CCCN1CC[C@H]2[C@@H](C1)c1cccc3CCCN2c13 Show InChI InChI=1S/C24H28FN3O/c25-17-8-9-19(21(26)14-17)23(29)7-3-11-27-13-10-22-20(15-27)18-6-1-4-16-5-2-12-28(22)24(16)18/h1,4,6,8-9,14,20,22H,2-3,5,7,10-13,15,26H2/t20-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity towards 5-hydroxytryptamine 2A receptor using [125I]-DOI as radioligand. |
Bioorg Med Chem Lett 13: 767-70 (2003)
BindingDB Entry DOI: 10.7270/Q25H7FNW |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50371305
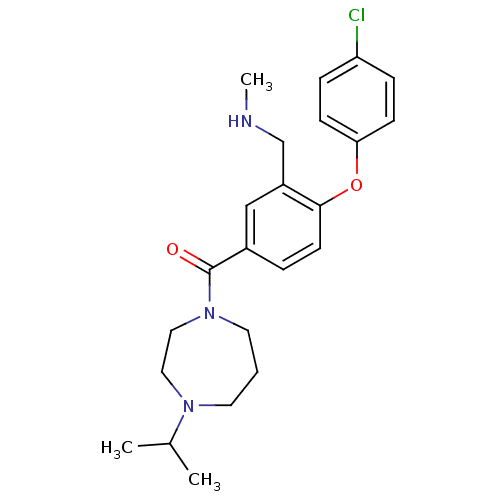
(CHEMBL272077)Show SMILES CNCc1cc(ccc1Oc1ccc(Cl)cc1)C(=O)N1CCCN(CC1)C(C)C Show InChI InChI=1S/C23H30ClN3O2/c1-17(2)26-11-4-12-27(14-13-26)23(28)18-5-10-22(19(15-18)16-25-3)29-21-8-6-20(24)7-9-21/h5-10,15,17,25H,4,11-14,16H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50217593
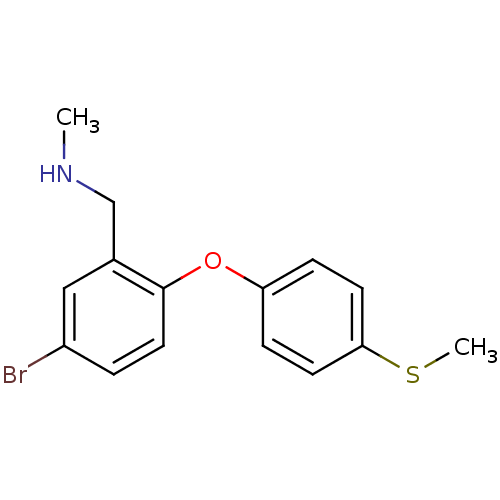
((5-bromo-2-(4-(methylthio)phenoxy)phenyl)-N-methyl...)Show InChI InChI=1S/C15H16BrNOS/c1-17-10-11-9-12(16)3-8-15(11)18-13-4-6-14(19-2)7-5-13/h3-9,17H,10H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to rat SERT |
Bioorg Med Chem Lett 17: 4799-803 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.061
BindingDB Entry DOI: 10.7270/Q2F18ZFF |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50217592
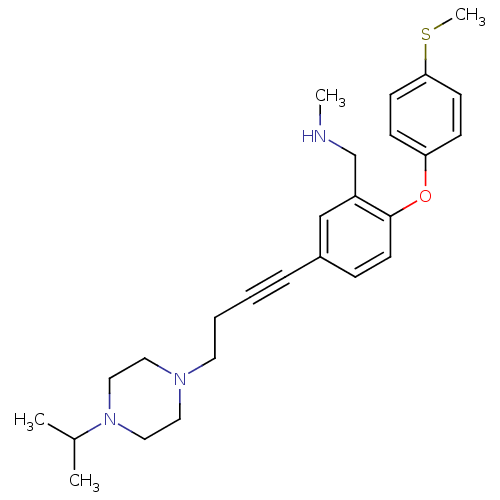
((5-(4-(4-isopropylpiperazin-1-yl)but-1-ynyl)-2-(4-...)Show SMILES CNCc1cc(ccc1Oc1ccc(SC)cc1)C#CCCN1CCN(CC1)C(C)C Show InChI InChI=1S/C26H35N3OS/c1-21(2)29-17-15-28(16-18-29)14-6-5-7-22-8-13-26(23(19-22)20-27-3)30-24-9-11-25(31-4)12-10-24/h8-13,19,21,27H,6,14-18,20H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to rat SERT |
Bioorg Med Chem Lett 17: 4799-803 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.061
BindingDB Entry DOI: 10.7270/Q2F18ZFF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50200636
(1-(4-(4-(piperidin-1-ylmethyl)phenyl)but-3-ynyl)pi...)Show InChI InChI=1S/C21H30N2/c1-4-14-22(15-5-1)16-8-3-9-20-10-12-21(13-11-20)19-23-17-6-2-7-18-23/h10-13H,1-2,4-8,14-19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.513 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells |
Eur J Med Chem 44: 4098-106 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.049
BindingDB Entry DOI: 10.7270/Q2VH5Q3G |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50414797
(CHEMBL582977)Show InChI InChI=1S/C22H31N3O/c23-22(26)21-10-15-25(16-11-21)18-20-9-6-8-19(17-20)7-2-5-14-24-12-3-1-4-13-24/h6,8-9,17,21H,1,3-5,10-16,18H2,(H2,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells |
Eur J Med Chem 44: 4098-106 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.049
BindingDB Entry DOI: 10.7270/Q2VH5Q3G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50125174
(1-(2-Amino-phenyl)-4-(7bS,11aR)-6,7,8,9,11,11a-hex...)Show SMILES Nc1ccccc1C(=O)CCCN1CC[C@H]2[C@@H](C1)c1cccc3SCCCN2c13 Show InChI InChI=1S/C24H29N3OS/c25-20-8-2-1-6-18(20)22(28)9-4-12-26-14-11-21-19(16-26)17-7-3-10-23-24(17)27(21)13-5-15-29-23/h1-3,6-8,10,19,21H,4-5,9,11-16,25H2/t19-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity towards 5-hydroxytryptamine 2A receptor using [125I]-DOI as radioligand. |
Bioorg Med Chem Lett 13: 767-70 (2003)
BindingDB Entry DOI: 10.7270/Q25H7FNW |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50217579

((5-(4-(4-fluoropiperidin-1-yl)but-1-ynyl)-2-(4-(me...)Show InChI InChI=1S/C24H29FN2OS/c1-26-18-20-17-19(5-3-4-14-27-15-12-21(25)13-16-27)6-11-24(20)28-22-7-9-23(29-2)10-8-22/h6-11,17,21,26H,4,12-16,18H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to rat SERT |
Bioorg Med Chem Lett 17: 4799-803 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.061
BindingDB Entry DOI: 10.7270/Q2F18ZFF |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.600 | -52.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Vertex Pharmaceuticals (Europe) Limited
| Assay Description
The kinase activity was determined by incubation of enzyme and its substrate, and test compound, in the presence ATP/[gamma-32P] ATP. After incubatio... |
Nat Med 10: 262-7 (2004)
Article DOI: 10.1038/nm1003
BindingDB Entry DOI: 10.7270/Q25M63ZF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50125170

(1-(2-Amino-4-fluoro-phenyl)-4-(7bS,11aR)-4,5,6,7,8...)Show SMILES Nc1cc(F)ccc1C(=O)CCCN1CC[C@H]2[C@@H](C1)c1cccc3CCCCN2c13 Show InChI InChI=1S/C25H30FN3O/c26-18-9-10-20(22(27)15-18)24(30)8-4-12-28-14-11-23-21(16-28)19-7-3-6-17-5-1-2-13-29(23)25(17)19/h3,6-7,9-10,15,21,23H,1-2,4-5,8,11-14,16,27H2/t21-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity towards 5-hydroxytryptamine 2A receptor using [125I]-DOI as radioligand. |
Bioorg Med Chem Lett 13: 767-70 (2003)
BindingDB Entry DOI: 10.7270/Q25H7FNW |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50159110

(1-(3-(4-(piperidin-1-ylmethyl)phenoxy)propyl)piper...)Show InChI InChI=1S/C20H32N2O/c1-3-12-21(13-4-1)16-7-17-23-20-10-8-19(9-11-20)18-22-14-5-2-6-15-22/h8-11H,1-7,12-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 17: 4799-803 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.061
BindingDB Entry DOI: 10.7270/Q2F18ZFF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50371682
(CHEMBL270188)Show InChI InChI=1S/C21H20FN3/c22-17-8-6-16(7-9-17)21-18-10-12-23-13-11-19(18)24-20(25-21)14-15-4-2-1-3-5-15/h1-9,23H,10-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human recombinant 5HT2A receptor expressed in mouse NIH3T3 cells |
Bioorg Med Chem Lett 18: 2103-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.090
BindingDB Entry DOI: 10.7270/Q2571CW5 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50217590
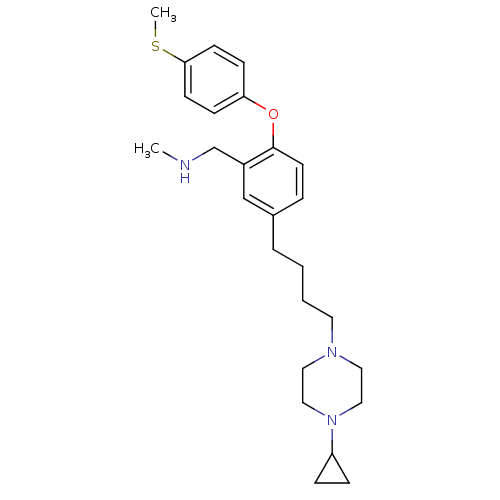
((5-(4-(4-cyclopropylpiperazin-1-yl)butyl)-2-(4-(me...)Show SMILES CNCc1cc(CCCCN2CCN(CC2)C2CC2)ccc1Oc1ccc(SC)cc1 Show InChI InChI=1S/C26H37N3OS/c1-27-20-22-19-21(6-13-26(22)30-24-9-11-25(31-2)12-10-24)5-3-4-14-28-15-17-29(18-16-28)23-7-8-23/h6,9-13,19,23,27H,3-5,7-8,14-18,20H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to rat SERT |
Bioorg Med Chem Lett 17: 4799-803 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.061
BindingDB Entry DOI: 10.7270/Q2F18ZFF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50414804
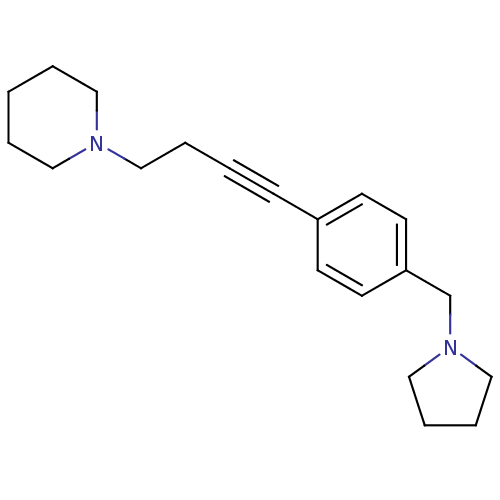
(CHEMBL574712)Show InChI InChI=1S/C20H28N2/c1-3-13-21(14-4-1)15-5-2-8-19-9-11-20(12-10-19)18-22-16-6-7-17-22/h9-12H,1,3-7,13-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.617 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells |
Eur J Med Chem 44: 4098-106 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.049
BindingDB Entry DOI: 10.7270/Q2VH5Q3G |
More data for this
Ligand-Target Pair | |
Nicotinic acetylcholine receptor
(RAT) | BDBM50054820
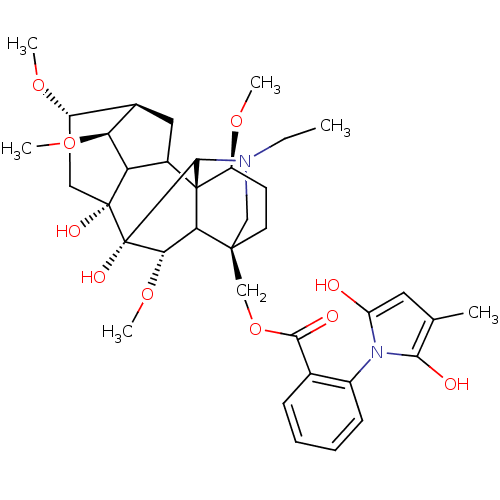
(Methyllycaconitine | [(1S,4S,5R,6S,8R,9R,13S,16S,1...)Show SMILES CCN1C[C@]2(COC(=O)c3ccccc3-n3c(O)cc(C)c3O)CC[C@H](OC)[C@@]34C5C[C@H]6[C@H](OC)C5[C@](O)(C[C@@H]6OC)[C@](O)([C@@H](OC)C23)C14 |wU:4.4,42.46,36.39,44.48,39.43,wD:28.55,32.34,25.27,31.32,TLB:28:29:32:36.38.39,1:2:47:25.23.24,25:28:42.44:4.2.3,THB:42:48:47:25.23.24,29:28:42.44:4.2.3,2:48:36.35.29:47.44,(3.83,-6.7,;5.61,-4.92,;8.1,-4.92,;7.12,-7.5,;8.22,-6.42,;8.22,-7.94,;7.82,-9.45,;8.61,-10.78,;9.94,-10.01,;8.62,-12.33,;7.29,-13.1,;7.29,-14.64,;8.62,-15.41,;9.95,-14.64,;9.95,-13.1,;11.49,-13.09,;12.9,-13.7,;13.3,-15.19,;13.93,-12.55,;13.14,-11.22,;13.91,-9.88,;11.64,-11.55,;10.86,-10.2,;6.88,-5.65,;6.87,-4.11,;8.21,-3.34,;8.2,-1.8,;6.87,-1.03,;9.54,-4.11,;10.5,-3.18,;10.3,-1.71,;11.64,-1.06,;12.67,-2.15,;14.21,-2.15,;14.96,-3.46,;11.97,-3.45,;12.65,-4.9,;13.98,-5.67,;16.6,-3.1,;16.6,-1.56,;17.69,-.47,;19.02,-1.24,;11.93,-6.28,;12.7,-7.59,;10.46,-6.53,;10.06,-8.01,;11.39,-8.78,;9.55,-5.63,;9.53,-2.57,)| Show InChI InChI=1S/C37H50N2O10/c1-7-38-17-34(18-49-32(42)20-10-8-9-11-23(20)39-26(40)14-19(2)31(39)41)13-12-25(46-4)36-22-15-21-24(45-3)16-35(43,27(22)28(21)47-5)37(44,33(36)38)30(48-6)29(34)36/h8-11,14,21-22,24-25,27-30,33,40-41,43-44H,7,12-13,15-18H2,1-6H3/t21-,22?,24+,25+,27?,28+,29?,30+,33?,34+,35-,36+,37+/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 687-95 (2002)
Article DOI: 10.1124/jpet.302.2.687
BindingDB Entry DOI: 10.7270/Q21R6P3D |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50414811
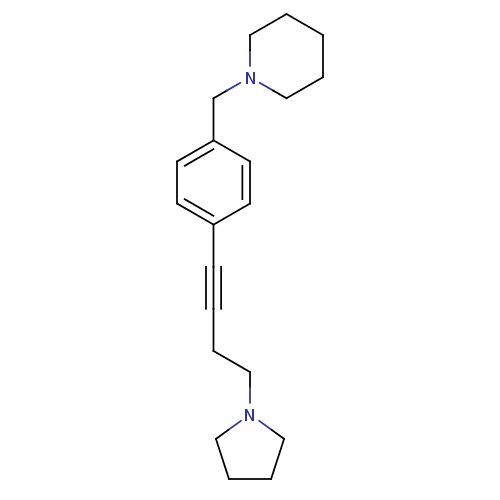
(CHEMBL574721)Show InChI InChI=1S/C20H28N2/c1-3-16-22(17-4-1)18-20-11-9-19(10-12-20)8-2-5-13-21-14-6-7-15-21/h9-12H,1,3-7,13-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.676 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells |
Eur J Med Chem 44: 4098-106 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.049
BindingDB Entry DOI: 10.7270/Q2VH5Q3G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50371669
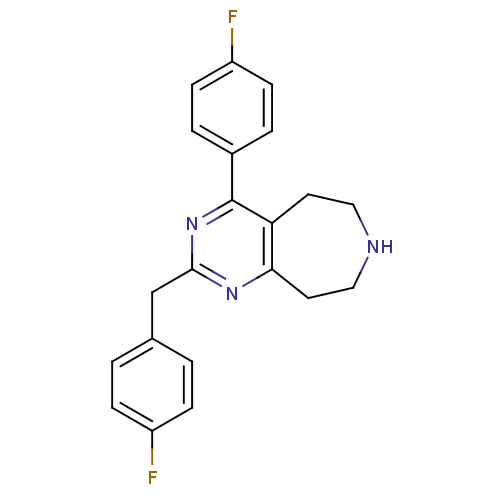
(CHEMBL402164)Show InChI InChI=1S/C21H19F2N3/c22-16-5-1-14(2-6-16)13-20-25-19-10-12-24-11-9-18(19)21(26-20)15-3-7-17(23)8-4-15/h1-8,24H,9-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human recombinant 5HT2A receptor expressed in mouse NIH3T3 cells |
Bioorg Med Chem Lett 18: 2103-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.090
BindingDB Entry DOI: 10.7270/Q2571CW5 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50217572
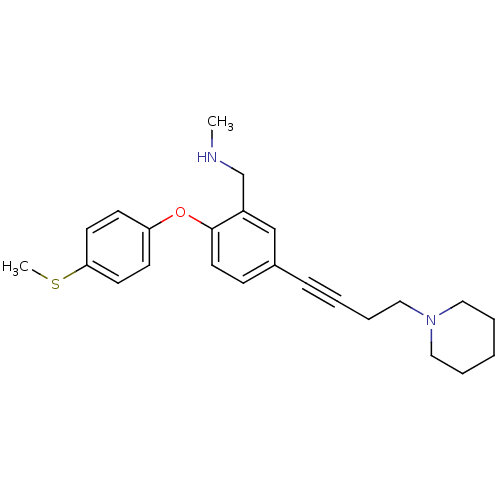
(CHEMBL393036 | N-methyl(2-(4-(methylthio)phenoxy)-...)Show InChI InChI=1S/C24H30N2OS/c1-25-19-21-18-20(8-4-7-17-26-15-5-3-6-16-26)9-14-24(21)27-22-10-12-23(28-2)13-11-22/h9-14,18,25H,3,5-7,15-17,19H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to rat SERT |
Bioorg Med Chem Lett 17: 4799-803 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.061
BindingDB Entry DOI: 10.7270/Q2F18ZFF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50217589
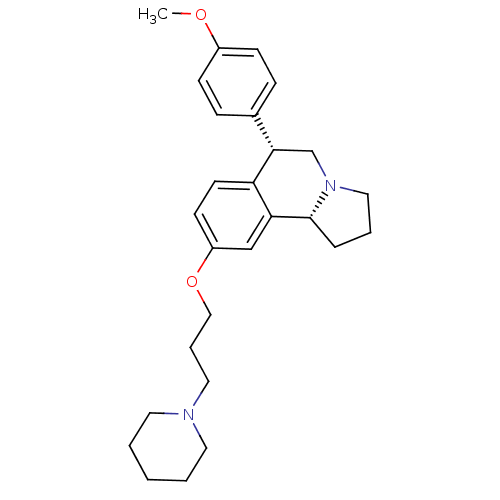
((6S,10bR)-6-(4-methoxyphenyl)-9-(3-(piperidin-1-yl...)Show SMILES COc1ccc(cc1)[C@@H]1CN2CCC[C@@H]2c2cc(OCCCN3CCCCC3)ccc12 Show InChI InChI=1S/C27H36N2O2/c1-30-22-10-8-21(9-11-22)26-20-29-17-5-7-27(29)25-19-23(12-13-24(25)26)31-18-6-16-28-14-3-2-4-15-28/h8-13,19,26-27H,2-7,14-18,20H2,1H3/t26-,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 17: 4799-803 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.061
BindingDB Entry DOI: 10.7270/Q2F18ZFF |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50217565
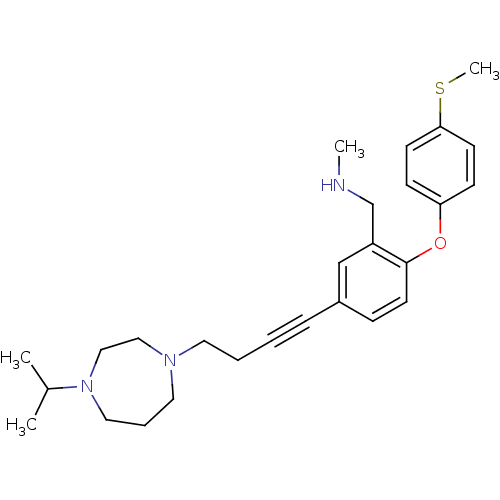
((5-(4-(4-isopropyl-1,4-diazepan-1-yl)but-1-ynyl)-2...)Show SMILES CNCc1cc(ccc1Oc1ccc(SC)cc1)C#CCCN1CCCN(CC1)C(C)C Show InChI InChI=1S/C27H37N3OS/c1-22(2)30-17-7-16-29(18-19-30)15-6-5-8-23-9-14-27(24(20-23)21-28-3)31-25-10-12-26(32-4)13-11-25/h9-14,20,22,28H,6-7,15-19,21H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to rat SERT |
Bioorg Med Chem Lett 17: 4799-803 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.061
BindingDB Entry DOI: 10.7270/Q2F18ZFF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50371294
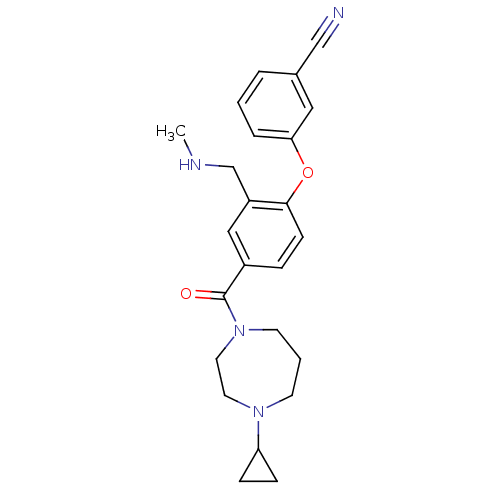
(CHEMBL257208)Show SMILES CNCc1cc(ccc1Oc1cccc(c1)C#N)C(=O)N1CCCN(CC1)C1CC1 Show InChI InChI=1S/C24H28N4O2/c1-26-17-20-15-19(6-9-23(20)30-22-5-2-4-18(14-22)16-25)24(29)28-11-3-10-27(12-13-28)21-7-8-21/h2,4-6,9,14-15,21,26H,3,7-8,10-13,17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50217572

(CHEMBL393036 | N-methyl(2-(4-(methylthio)phenoxy)-...)Show InChI InChI=1S/C24H30N2OS/c1-25-19-21-18-20(8-4-7-17-26-15-5-3-6-16-26)9-14-24(21)27-22-10-12-23(28-2)13-11-22/h9-14,18,25H,3,5-7,15-17,19H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to human SERT |
Bioorg Med Chem Lett 17: 4799-803 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.061
BindingDB Entry DOI: 10.7270/Q2F18ZFF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50217589

((6S,10bR)-6-(4-methoxyphenyl)-9-(3-(piperidin-1-yl...)Show SMILES COc1ccc(cc1)[C@@H]1CN2CCC[C@@H]2c2cc(OCCCN3CCCCC3)ccc12 Show InChI InChI=1S/C27H36N2O2/c1-30-22-10-8-21(9-11-22)26-20-29-17-5-7-27(29)25-19-23(12-13-24(25)26)31-18-6-16-28-14-3-2-4-15-28/h8-13,19,26-27H,2-7,14-18,20H2,1H3/t26-,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50125173

(1-(2-Amino-4-fluoro-phenyl)-4-(7bS,11aR)-6,7,8,9,1...)Show SMILES Nc1cc(F)ccc1C(=O)CCCN1CC[C@H]2[C@@H](C1)c1cccc3SCCCN2c13 Show InChI InChI=1S/C24H28FN3OS/c25-16-7-8-18(20(26)14-16)22(29)5-2-10-27-12-9-21-19(15-27)17-4-1-6-23-24(17)28(21)11-3-13-30-23/h1,4,6-8,14,19,21H,2-3,5,9-13,15,26H2/t19-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity towards DA D2 receptor using [3H]-N-methyl-spiperone as radioligand. |
Bioorg Med Chem Lett 13: 767-70 (2003)
BindingDB Entry DOI: 10.7270/Q25H7FNW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50125163

(1-(2-Amino-phenyl)-4-(7bS,11aR)-4,5,6,7,8,9,11,11a...)Show SMILES Nc1ccccc1C(=O)CCCN1CC[C@H]2[C@@H](C1)c1cccc3CCCCN2c13 Show InChI InChI=1S/C25H31N3O/c26-22-11-2-1-9-20(22)24(29)12-6-14-27-16-13-23-21(17-27)19-10-5-8-18-7-3-4-15-28(23)25(18)19/h1-2,5,8-11,21,23H,3-4,6-7,12-17,26H2/t21-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity towards 5-hydroxytryptamine 2A receptor using [125I]-DOI as radioligand. |
Bioorg Med Chem Lett 13: 767-70 (2003)
BindingDB Entry DOI: 10.7270/Q25H7FNW |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50209802
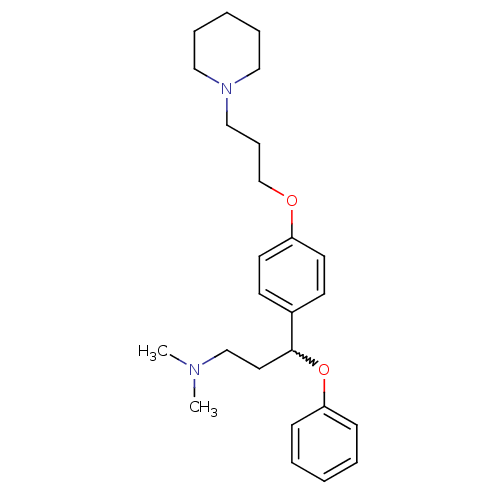
(CHEMBL438490 | N,N-dimethyl-3-phenoxy-3-(4-(3-(pip...)Show SMILES CN(C)CCC(Oc1ccccc1)c1ccc(OCCCN2CCCCC2)cc1 |w:5.5| Show InChI InChI=1S/C25H36N2O2/c1-26(2)20-16-25(29-24-10-5-3-6-11-24)22-12-14-23(15-13-22)28-21-9-19-27-17-7-4-8-18-27/h3,5-6,10-15,25H,4,7-9,16-21H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity at human histamine H3 |
Bioorg Med Chem Lett 17: 5325-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.017
BindingDB Entry DOI: 10.7270/Q24749K4 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50414798
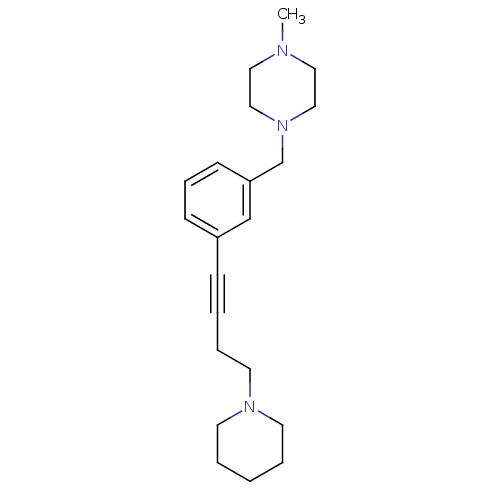
(CHEMBL575172)Show InChI InChI=1S/C21H31N3/c1-22-14-16-24(17-15-22)19-21-10-7-9-20(18-21)8-3-6-13-23-11-4-2-5-12-23/h7,9-10,18H,2,4-6,11-17,19H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.724 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells |
Eur J Med Chem 44: 4098-106 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.049
BindingDB Entry DOI: 10.7270/Q2VH5Q3G |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Displacement of [3H]BMS-725519 from human CB1 receptor expressed in CHO cells after 90 mins by scintillation counting |
Bioorg Med Chem Lett 21: 6856-60 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.016
BindingDB Entry DOI: 10.7270/Q2WS8TN3 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50414800
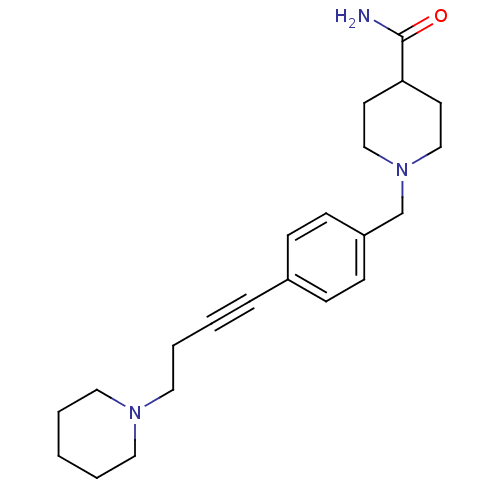
(CHEMBL583182)Show InChI InChI=1S/C22H31N3O/c23-22(26)21-11-16-25(17-12-21)18-20-9-7-19(8-10-20)6-2-5-15-24-13-3-1-4-14-24/h7-10,21H,1,3-5,11-18H2,(H2,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.759 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells |
Eur J Med Chem 44: 4098-106 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.049
BindingDB Entry DOI: 10.7270/Q2VH5Q3G |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50414792
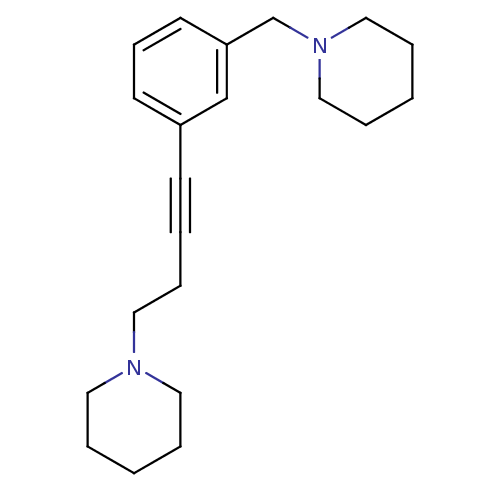
(CHEMBL573817)Show InChI InChI=1S/C21H30N2/c1-4-13-22(14-5-1)15-8-3-10-20-11-9-12-21(18-20)19-23-16-6-2-7-17-23/h9,11-12,18H,1-2,4-8,13-17,19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.776 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells |
Eur J Med Chem 44: 4098-106 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.049
BindingDB Entry DOI: 10.7270/Q2VH5Q3G |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50217576

(CHEMBL236046 | N-methyl(2-(4-(methylthio)phenoxy)-...)Show InChI InChI=1S/C23H28N2O2S/c1-24-18-20-17-19(5-3-4-12-25-13-15-26-16-14-25)6-11-23(20)27-21-7-9-22(28-2)10-8-21/h6-11,17,24H,4,12-16,18H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to human SERT |
Bioorg Med Chem Lett 17: 4799-803 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.061
BindingDB Entry DOI: 10.7270/Q2F18ZFF |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50217576

(CHEMBL236046 | N-methyl(2-(4-(methylthio)phenoxy)-...)Show InChI InChI=1S/C23H28N2O2S/c1-24-18-20-17-19(5-3-4-12-25-13-15-26-16-14-25)6-11-23(20)27-21-7-9-22(28-2)10-8-21/h6-11,17,24H,4,12-16,18H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human SERT |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50125169
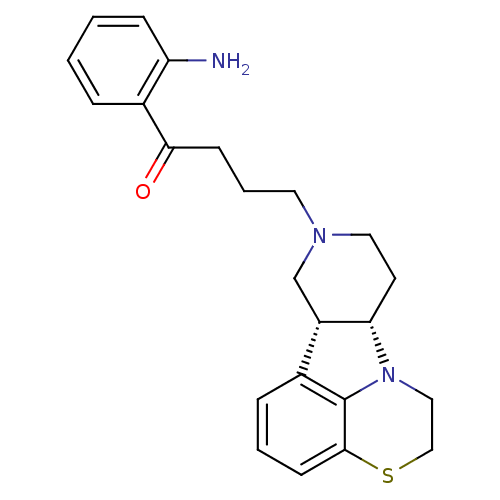
(1-(2-Amino-phenyl)-4-(6bR,10aS)-1,2,6b,9,10,10a-he...)Show SMILES Nc1ccccc1C(=O)CCCN1CC[C@H]2[C@@H](C1)c1cccc3SCCN2c13 Show InChI InChI=1S/C23H27N3OS/c24-19-7-2-1-5-17(19)21(27)8-4-11-25-12-10-20-18(15-25)16-6-3-9-22-23(16)26(20)13-14-28-22/h1-3,5-7,9,18,20H,4,8,10-15,24H2/t18-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity towards 5-hydroxytryptamine 2A receptor using [125I]-DOI as radioligand. |
Bioorg Med Chem Lett 13: 767-70 (2003)
BindingDB Entry DOI: 10.7270/Q25H7FNW |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50371289
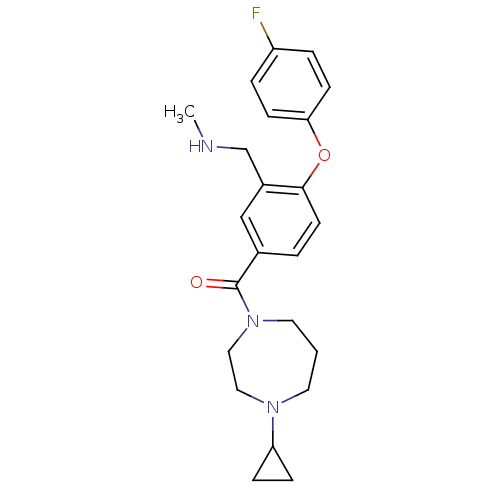
(CHEMBL258349)Show SMILES CNCc1cc(ccc1Oc1ccc(F)cc1)C(=O)N1CCCN(CC1)C1CC1 Show InChI InChI=1S/C23H28FN3O2/c1-25-16-18-15-17(3-10-22(18)29-21-8-4-19(24)5-9-21)23(28)27-12-2-11-26(13-14-27)20-6-7-20/h3-5,8-10,15,20,25H,2,6-7,11-14,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50371290
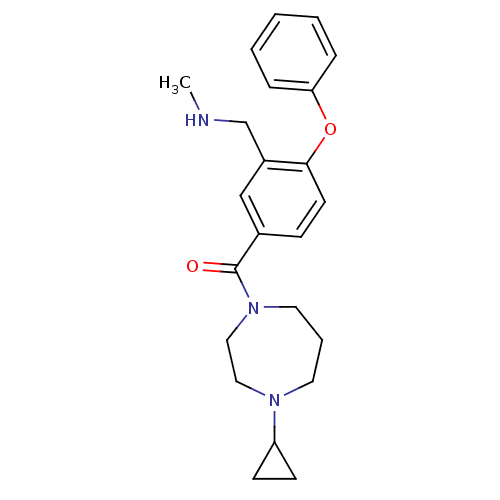
(CHEMBL401683)Show SMILES CNCc1cc(ccc1Oc1ccccc1)C(=O)N1CCCN(CC1)C1CC1 Show InChI InChI=1S/C23H29N3O2/c1-24-17-19-16-18(8-11-22(19)28-21-6-3-2-4-7-21)23(27)26-13-5-12-25(14-15-26)20-9-10-20/h2-4,6-8,11,16,20,24H,5,9-10,12-15,17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50125177
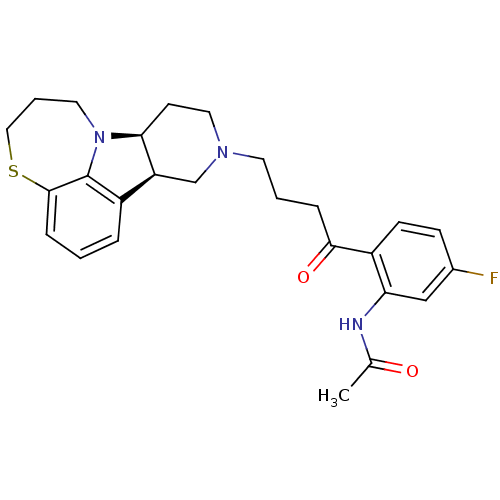
(CHEMBL162768 | N-[5-Fluoro-2-((7bS,11aR)-4-6,7,8,9...)Show SMILES CC(=O)Nc1cc(F)ccc1C(=O)CCCN1CC[C@H]2[C@@H](C1)c1cccc3SCCCN2c13 Show InChI InChI=1S/C26H30FN3O2S/c1-17(31)28-22-15-18(27)8-9-20(22)24(32)6-3-11-29-13-10-23-21(16-29)19-5-2-7-25-26(19)30(23)12-4-14-33-25/h2,5,7-9,15,21,23H,3-4,6,10-14,16H2,1H3,(H,28,31)/t21-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity towards 5-hydroxytryptamine 2A receptor using [125I]-DOI as radioligand. |
Bioorg Med Chem Lett 13: 767-70 (2003)
BindingDB Entry DOI: 10.7270/Q25H7FNW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50125151
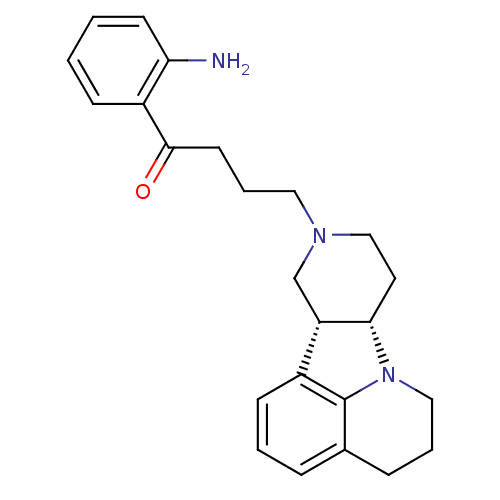
(1-(2-Amino-phenyl)-4-(7aS,11aR)-5,6,8,9,11,11a-hex...)Show SMILES Nc1ccccc1C(=O)CCCN1CC[C@H]2[C@@H](C1)c1cccc3CCCN2c13 Show InChI InChI=1S/C24H29N3O/c25-21-10-2-1-8-19(21)23(28)11-5-13-26-15-12-22-20(16-26)18-9-3-6-17-7-4-14-27(22)24(17)18/h1-3,6,8-10,20,22H,4-5,7,11-16,25H2/t20-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity towards 5-hydroxytryptamine 2A receptor using [125I]-DOI as radioligand. |
Bioorg Med Chem Lett 13: 767-70 (2003)
BindingDB Entry DOI: 10.7270/Q25H7FNW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50371681
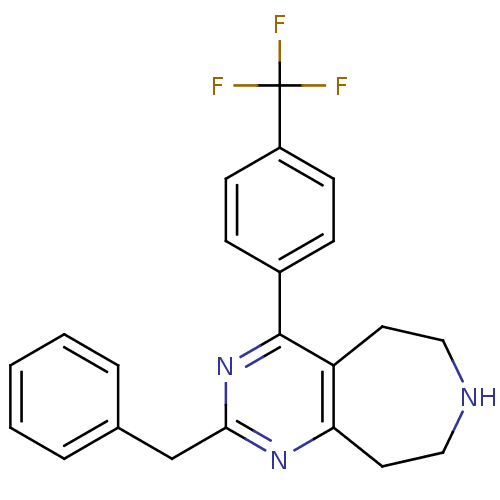
(CHEMBL271418)Show SMILES FC(F)(F)c1ccc(cc1)-c1nc(Cc2ccccc2)nc2CCNCCc12 Show InChI InChI=1S/C22H20F3N3/c23-22(24,25)17-8-6-16(7-9-17)21-18-10-12-26-13-11-19(18)27-20(28-21)14-15-4-2-1-3-5-15/h1-9,26H,10-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human recombinant 5HT2A receptor expressed in mouse NIH3T3 cells |
Bioorg Med Chem Lett 18: 2103-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.090
BindingDB Entry DOI: 10.7270/Q2571CW5 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50414813

(CHEMBL573328)Show InChI InChI=1S/C23H35N3/c1-21(2)26-18-16-25(17-19-26)20-23-11-9-22(10-12-23)8-4-7-15-24-13-5-3-6-14-24/h9-12,21H,3,5-7,13-20H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.871 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells |
Eur J Med Chem 44: 4098-106 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.049
BindingDB Entry DOI: 10.7270/Q2VH5Q3G |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50414799

(CHEMBL573815)Show InChI InChI=1S/C21H30N2O/c24-21-11-16-23(17-12-21)18-20-9-7-19(8-10-20)6-2-5-15-22-13-3-1-4-14-22/h7-10,21,24H,1,3-5,11-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.891 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells |
Eur J Med Chem 44: 4098-106 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.049
BindingDB Entry DOI: 10.7270/Q2VH5Q3G |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50217576

(CHEMBL236046 | N-methyl(2-(4-(methylthio)phenoxy)-...)Show InChI InChI=1S/C23H28N2O2S/c1-24-18-20-17-19(5-3-4-12-25-13-15-26-16-14-25)6-11-23(20)27-21-7-9-22(28-2)10-8-21/h6-11,17,24H,4,12-16,18H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to rat SERT |
Bioorg Med Chem Lett 17: 4799-803 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.061
BindingDB Entry DOI: 10.7270/Q2F18ZFF |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50217595

(CHEMBL237317 | N-methyl(2-(4-(methylthio)phenoxy)-...)Show InChI InChI=1S/C23H28N2OS2/c1-24-18-20-17-19(5-3-4-12-25-13-15-28-16-14-25)6-11-23(20)26-21-7-9-22(27-2)10-8-21/h6-11,17,24H,4,12-16,18H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to rat SERT |
Bioorg Med Chem Lett 17: 4799-803 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.061
BindingDB Entry DOI: 10.7270/Q2F18ZFF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data