 Found 2672 hits with Last Name = 'mincione' and Initial = 'f'
Found 2672 hits with Last Name = 'mincione' and Initial = 'f' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50094456
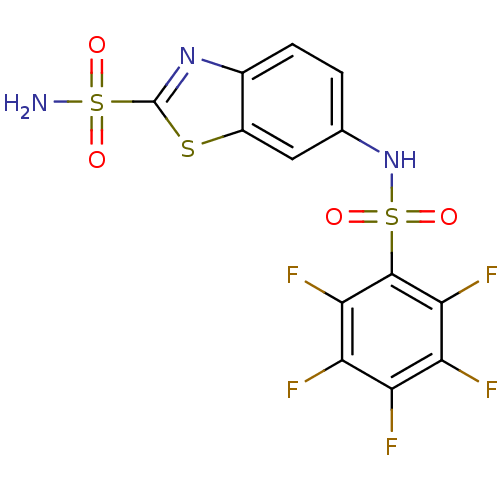
(6-Pentafluorobenzenesulfonylamino-benzothiazole-2-...)Show SMILES NS(=O)(=O)c1nc2ccc(NS(=O)(=O)c3c(F)c(F)c(F)c(F)c3F)cc2s1 Show InChI InChI=1S/C13H6F5N3O4S3/c14-7-8(15)10(17)12(11(18)9(7)16)28(24,25)21-4-1-2-5-6(3-4)26-13(20-5)27(19,22)23/h1-3,21H,(H2,19,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase II (CA2) |
J Med Chem 43: 4542-51 (2000)
BindingDB Entry DOI: 10.7270/Q2BV7HBP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50094393

(4-Methyl-5-pentafluorobenzenesulfonylimino-4,5-dih...)Show SMILES Cn1nc(sc1=NS(=O)(=O)c1c(F)c(F)c(F)c(F)c1F)S(N)(=O)=O Show InChI InChI=1S/C9H5F5N4O4S3/c1-18-8(23-9(16-18)24(15,19)20)17-25(21,22)7-5(13)3(11)2(10)4(12)6(7)14/h1H3,(H2,15,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase II (CA2) |
J Med Chem 43: 4542-51 (2000)
BindingDB Entry DOI: 10.7270/Q2BV7HBP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50094441
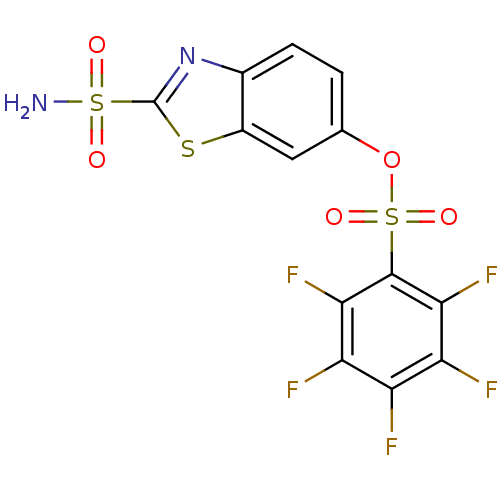
(2,3,4,5,6-Pentafluoro-benzenesulfonic acid 2-sulfa...)Show SMILES NS(=O)(=O)c1nc2ccc(OS(=O)(=O)c3c(F)c(F)c(F)c(F)c3F)cc2s1 Show InChI InChI=1S/C13H5F5N2O5S3/c14-7-8(15)10(17)12(11(18)9(7)16)28(23,24)25-4-1-2-5-6(3-4)26-13(20-5)27(19,21)22/h1-3H,(H2,19,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase II (CA2) |
J Med Chem 43: 4542-51 (2000)
BindingDB Entry DOI: 10.7270/Q2BV7HBP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50094347
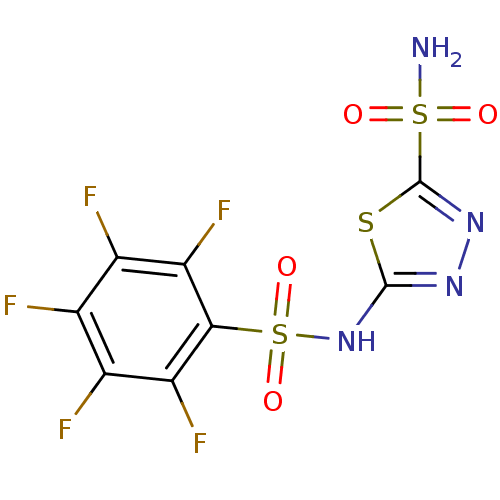
(5-(perfluorophenylsulfonamido)-1,3,4-thiadiazole-2...)Show SMILES NS(=O)(=O)c1nnc(NS(=O)(=O)c2c(F)c(F)c(F)c(F)c2F)s1 Show InChI InChI=1S/C8H3F5N4O4S3/c9-1-2(10)4(12)6(5(13)3(1)11)24(20,21)17-7-15-16-8(22-7)23(14,18)19/h(H,15,17)(H2,14,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase II (CA2) |
J Med Chem 43: 4542-51 (2000)
BindingDB Entry DOI: 10.7270/Q2BV7HBP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50097289
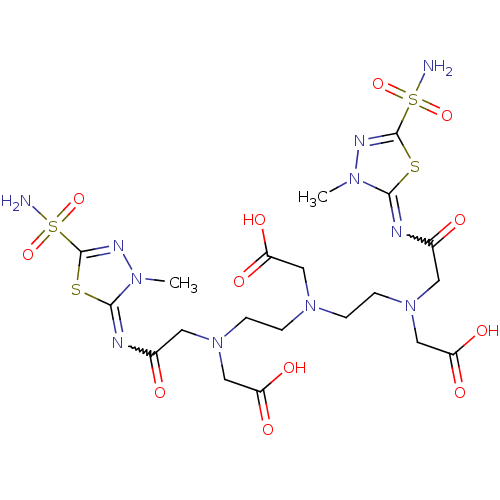
(((2-{Carboxymethyl-[2-(carboxymethyl-{[3-methyl-5-...)Show SMILES Cn1nc(sc1=NC(=O)CN(CCN(CCN(CC(O)=O)CC(=O)N=c1sc(nn1C)S(N)(=O)=O)CC(O)=O)CC(O)=O)S(N)(=O)=O |w:24.24,6.7| Show InChI InChI=1S/C20H31N11O12S4/c1-27-17(44-19(25-27)46(21,40)41)23-12(32)7-30(10-15(36)37)5-3-29(9-14(34)35)4-6-31(11-16(38)39)8-13(33)24-18-28(2)26-20(45-18)47(22,42)43/h3-11H2,1-2H3,(H,34,35)(H,36,37)(H,38,39)(H2,21,40,41)(H2,22,42,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50219176
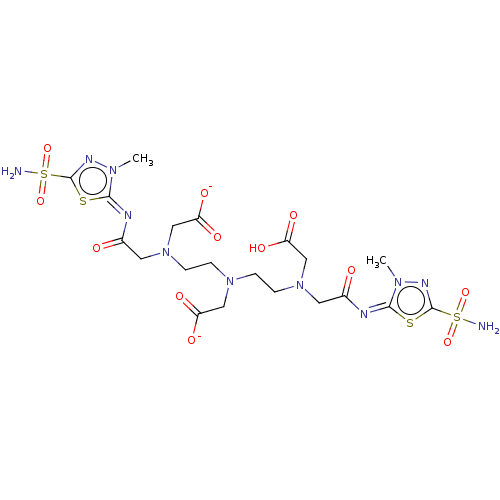
(CHEMBL2111218)Show SMILES [Zn++].Cn1nc(s\c1=N\C(=O)CN(CCN(CCN(CC([O-])=O)CC(=O)\N=c1/sc(nn1C)S(N)(=O)=O)CC([O-])=O)CC(O)=O)S(N)(=O)=O Show InChI InChI=1S/C20H31N11O12S4/c1-27-17(44-19(25-27)46(21,40)41)23-12(32)7-30(10-15(36)37)5-3-29(9-14(34)35)4-6-31(11-16(38)39)8-13(33)24-18-28(2)26-20(45-18)47(22,42)43/h3-11H2,1-2H3,(H,34,35)(H,36,37)(H,38,39)(H2,21,40,41)(H2,22,42,43)/p-2/b23-17-,24-18+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against Opioid receptor delta 1 of (endomorphin 2) in mouse vas deferens was determined |
Bioorg Med Chem Lett 11: 575-82 (2001)
BindingDB Entry DOI: 10.7270/Q2KW5GJQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50094324
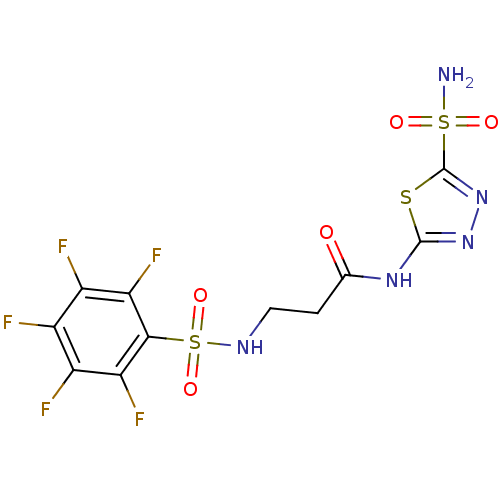
(3-Pentafluorobenzenesulfonylamino-N-(5-sulfamoyl-[...)Show SMILES NS(=O)(=O)c1nnc(NC(=O)CCNS(=O)(=O)c2c(F)c(F)c(F)c(F)c2F)s1 Show InChI InChI=1S/C11H8F5N5O5S3/c12-4-5(13)7(15)9(8(16)6(4)14)29(25,26)18-2-1-3(22)19-10-20-21-11(27-10)28(17,23)24/h18H,1-2H2,(H2,17,23,24)(H,19,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase II (CA2) |
J Med Chem 43: 4542-51 (2000)
BindingDB Entry DOI: 10.7270/Q2BV7HBP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50097303
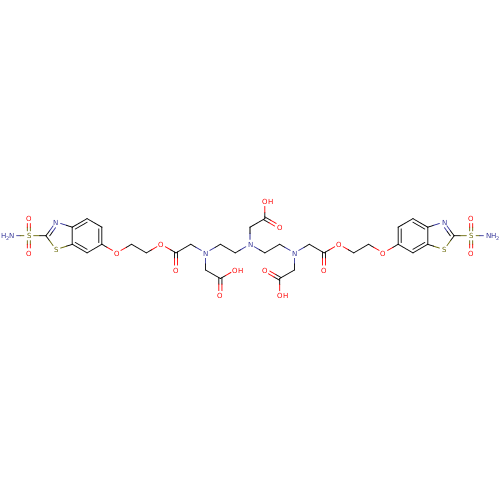
(CHEMBL346691 | {{2-[Carboxymethyl-(2-{carboxymethy...)Show SMILES NS(=O)(=O)c1nc2ccc(OCCOC(=O)CN(CCN(CCN(CC(O)=O)CC(=O)OCCOc3ccc4nc(sc4c3)S(N)(=O)=O)CC(O)=O)CC(O)=O)cc2s1 Show InChI InChI=1S/C32H39N7O16S4/c33-58(48,49)31-35-22-3-1-20(13-24(22)56-31)52-9-11-54-29(46)18-38(16-27(42)43)7-5-37(15-26(40)41)6-8-39(17-28(44)45)19-30(47)55-12-10-53-21-2-4-23-25(14-21)57-32(36-23)59(34,50)51/h1-4,13-14H,5-12,15-19H2,(H,40,41)(H,42,43)(H,44,45)(H2,33,48,49)(H2,34,50,51) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibition of cloned isozyme, human carbonic anhydrase II |
Bioorg Med Chem Lett 11: 575-82 (2001)
BindingDB Entry DOI: 10.7270/Q2KW5GJQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50097275
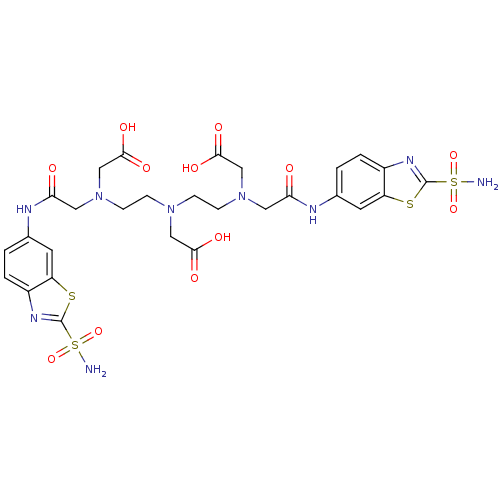
(CHEMBL286586 | {{2-[Carboxymethyl-(2-{carboxymethy...)Show SMILES NS(=O)(=O)c1nc2ccc(NC(=O)CN(CCN(CCN(CC(O)=O)CC(=O)Nc3ccc4nc(sc4c3)S(N)(=O)=O)CC(O)=O)CC(O)=O)cc2s1 Show InChI InChI=1S/C28H33N9O12S4/c29-52(46,47)27-33-18-3-1-16(9-20(18)50-27)31-22(38)11-36(14-25(42)43)7-5-35(13-24(40)41)6-8-37(15-26(44)45)12-23(39)32-17-2-4-19-21(10-17)51-28(34-19)53(30,48)49/h1-4,9-10H,5-8,11-15H2,(H,31,38)(H,32,39)(H,40,41)(H,42,43)(H,44,45)(H2,29,46,47)(H2,30,48,49) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibition of cloned isozyme, human carbonic anhydrase II |
Bioorg Med Chem Lett 11: 575-82 (2001)
BindingDB Entry DOI: 10.7270/Q2KW5GJQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50292100

(CHEMBL284354 | {{2-[2-(2-{Carboxymethyl-[(2-sulfam...)Show SMILES NS(=O)(=O)c1nc2ccc(NC(=O)CN(CCOCCOCCN(CC(O)=O)CC(=O)Nc3ccc4nc(sc4c3)S(N)(=O)=O)CC(O)=O)cc2s1 Show InChI InChI=1S/C28H34N8O12S4/c29-51(43,44)27-33-19-3-1-17(11-21(19)49-27)31-23(37)13-35(15-25(39)40)5-7-47-9-10-48-8-6-36(16-26(41)42)14-24(38)32-18-2-4-20-22(12-18)50-28(34-20)52(30,45)46/h1-4,11-12H,5-10,13-16H2,(H,31,37)(H,32,38)(H,39,40)(H,41,42)(H2,29,43,44)(H2,30,45,46) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50094346
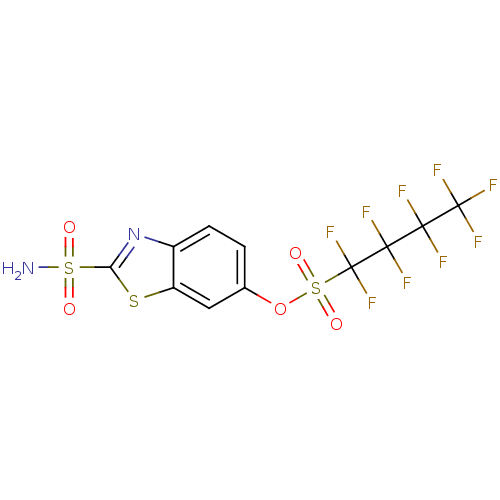
(1,1,2,2,3,3,4,4,4-Nonafluoro-butane-1-sulfonic aci...)Show SMILES NS(=O)(=O)c1nc2ccc(OS(=O)(=O)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F)cc2s1 Show InChI InChI=1S/C11H5F9N2O5S3/c12-8(13,10(16,17)18)9(14,15)11(19,20)30(25,26)27-4-1-2-5-6(3-4)28-7(22-5)29(21,23)24/h1-3H,(H2,21,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase II (CA2) |
J Med Chem 43: 4542-51 (2000)
BindingDB Entry DOI: 10.7270/Q2BV7HBP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50094403
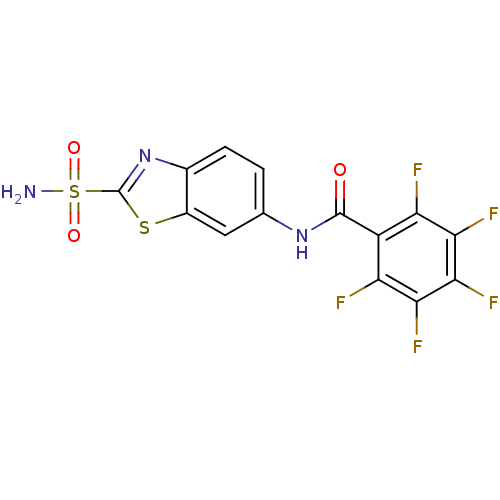
(2,3,4,5,6-Pentafluoro-N-(2-sulfamoyl-benzothiazol-...)Show SMILES NS(=O)(=O)c1nc2ccc(NC(=O)c3c(F)c(F)c(F)c(F)c3F)cc2s1 Show InChI InChI=1S/C14H6F5N3O3S2/c15-8-7(9(16)11(18)12(19)10(8)17)13(23)21-4-1-2-5-6(3-4)26-14(22-5)27(20,24)25/h1-3H,(H,21,23)(H2,20,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase II (CA2) |
J Med Chem 43: 4542-51 (2000)
BindingDB Entry DOI: 10.7270/Q2BV7HBP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50097275

(CHEMBL286586 | {{2-[Carboxymethyl-(2-{carboxymethy...)Show SMILES NS(=O)(=O)c1nc2ccc(NC(=O)CN(CCN(CCN(CC(O)=O)CC(=O)Nc3ccc4nc(sc4c3)S(N)(=O)=O)CC(O)=O)CC(O)=O)cc2s1 Show InChI InChI=1S/C28H33N9O12S4/c29-52(46,47)27-33-18-3-1-16(9-20(18)50-27)31-22(38)11-36(14-25(42)43)7-5-35(13-24(40)41)6-8-37(15-26(44)45)12-23(39)32-17-2-4-19-21(10-17)51-28(34-19)53(30,48)49/h1-4,9-10H,5-8,11-15H2,(H,31,38)(H,32,39)(H,40,41)(H,42,43)(H,44,45)(H2,29,46,47)(H2,30,48,49) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50219174
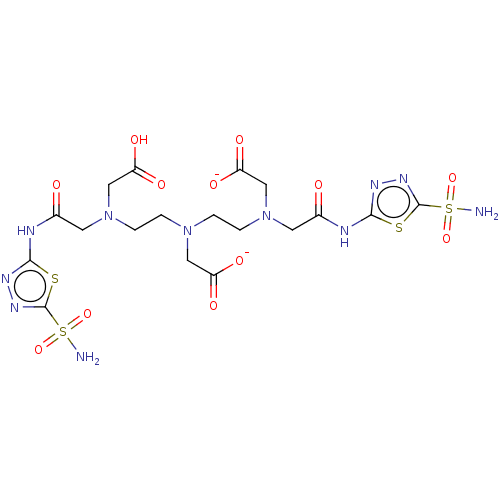
(CHEMBL2112274)Show SMILES [Zn++].NS(=O)(=O)c1nnc(NC(=O)CN(CCN(CCN(CC([O-])=O)CC(=O)Nc2nnc(s2)S(N)(=O)=O)CC([O-])=O)CC(O)=O)s1 Show InChI InChI=1S/C18H27N11O12S4/c19-44(38,39)17-25-23-15(42-17)21-10(30)5-28(8-13(34)35)3-1-27(7-12(32)33)2-4-29(9-14(36)37)6-11(31)22-16-24-26-18(43-16)45(20,40)41/h1-9H2,(H,32,33)(H,34,35)(H,36,37)(H2,19,38,39)(H2,20,40,41)(H,21,23,30)(H,22,24,31)/p-2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibition of hepatitis c virus Non structural protein 3 protease/Non structural protein 4A protease |
Bioorg Med Chem Lett 11: 575-82 (2001)
BindingDB Entry DOI: 10.7270/Q2KW5GJQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50094452
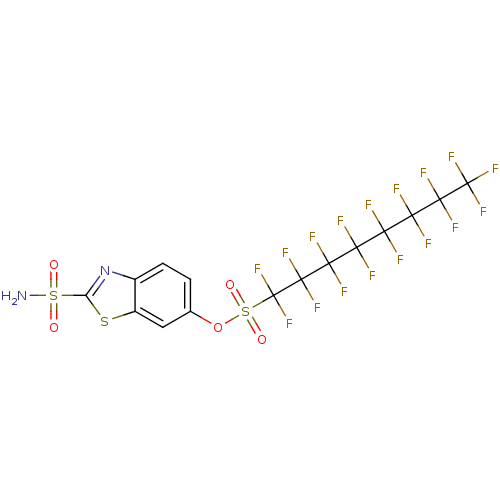
(1,1,2,2,3,3,4,4,5,5,6,6,7,7,8,8,8-Heptadecafluoro-...)Show SMILES NS(=O)(=O)c1nc2ccc(OS(=O)(=O)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F)cc2s1 Show InChI InChI=1S/C15H5F17N2O5S3/c16-8(17,10(20,21)12(24,25)14(28,29)30)9(18,19)11(22,23)13(26,27)15(31,32)42(37,38)39-4-1-2-5-6(3-4)40-7(34-5)41(33,35)36/h1-3H,(H2,33,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase II (CA2) |
J Med Chem 43: 4542-51 (2000)
BindingDB Entry DOI: 10.7270/Q2BV7HBP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50094323
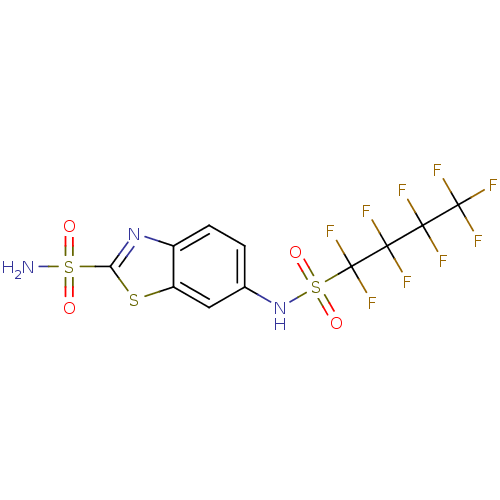
(6-(Nonafluorobutane-1-sulfonylamino)-benzothiazole...)Show SMILES NS(=O)(=O)c1nc2ccc(NS(=O)(=O)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F)cc2s1 Show InChI InChI=1S/C11H6F9N3O4S3/c12-8(13,10(16,17)18)9(14,15)11(19,20)30(26,27)23-4-1-2-5-6(3-4)28-7(22-5)29(21,24)25/h1-3,23H,(H2,21,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase II (CA2) |
J Med Chem 43: 4542-51 (2000)
BindingDB Entry DOI: 10.7270/Q2BV7HBP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50097307
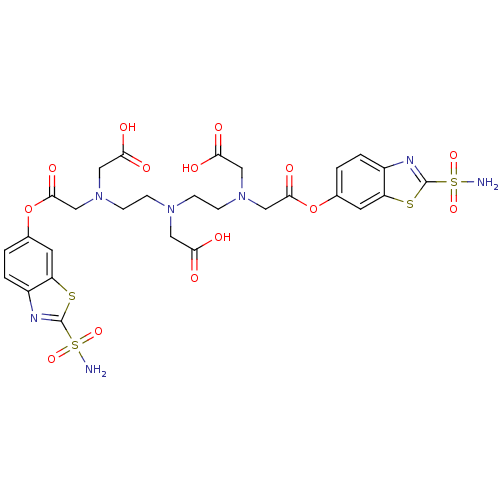
(CHEMBL408856 | [[2-(Carboxymethyl-{2-[carboxymethy...)Show SMILES NS(=O)(=O)c1nc2ccc(OC(=O)CN(CCN(CCN(CC(O)=O)CC(=O)Oc3ccc4nc(sc4c3)S(N)(=O)=O)CC(O)=O)CC(O)=O)cc2s1 Show InChI InChI=1S/C28H31N7O14S4/c29-52(44,45)27-31-18-3-1-16(9-20(18)50-27)48-25(42)14-34(12-23(38)39)7-5-33(11-22(36)37)6-8-35(13-24(40)41)15-26(43)49-17-2-4-19-21(10-17)51-28(32-19)53(30,46)47/h1-4,9-10H,5-8,11-15H2,(H,36,37)(H,38,39)(H,40,41)(H2,29,44,45)(H2,30,46,47) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50292154
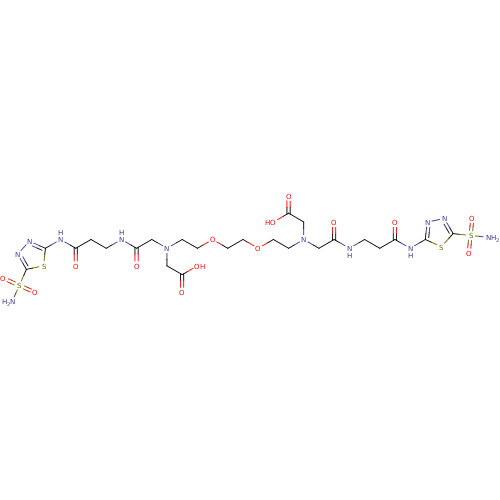
(((2-{2-[2-(Carboxymethyl-{[2-(5-sulfamoyl-[1,3,4]t...)Show SMILES NS(=O)(=O)c1nnc(NC(=O)CCNC(=O)CN(CCOCCOCCN(CC(O)=O)CC(=O)NCCC(=O)Nc2nnc(s2)S(N)(=O)=O)CC(O)=O)s1 Show InChI InChI=1S/C24H38N12O14S4/c25-53(45,46)23-33-31-21(51-23)29-15(37)1-3-27-17(39)11-35(13-19(41)42)5-7-49-9-10-50-8-6-36(14-20(43)44)12-18(40)28-4-2-16(38)30-22-32-34-24(52-22)54(26,47)48/h1-14H2,(H,27,39)(H,28,40)(H,41,42)(H,43,44)(H2,25,45,46)(H2,26,47,48)(H,29,31,37)(H,30,32,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50094326
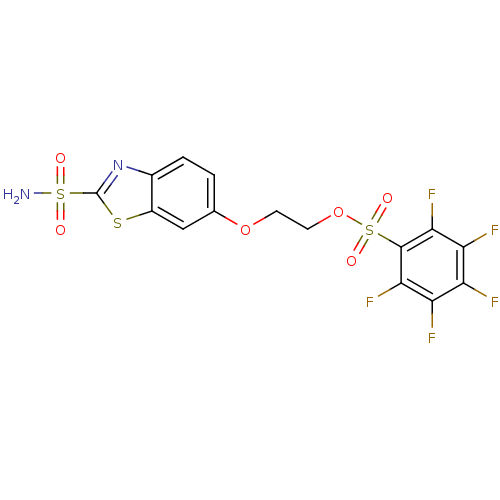
(2,3,4,5,6-Pentafluoro-benzenesulfonic acid 2-(2-su...)Show SMILES NS(=O)(=O)c1nc2ccc(OCCOS(=O)(=O)c3c(F)c(F)c(F)c(F)c3F)cc2s1 Show InChI InChI=1S/C15H9F5N2O6S3/c16-9-10(17)12(19)14(13(20)11(9)18)31(25,26)28-4-3-27-6-1-2-7-8(5-6)29-15(22-7)30(21,23)24/h1-2,5H,3-4H2,(H2,21,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase II (CA2) |
J Med Chem 43: 4542-51 (2000)
BindingDB Entry DOI: 10.7270/Q2BV7HBP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50292077
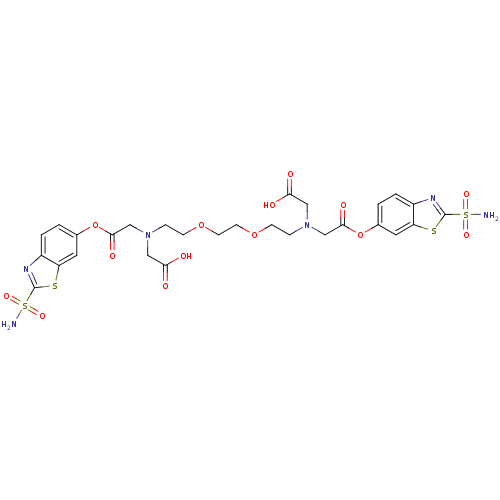
(CHEMBL33478 | [[2-(2-{2-[Carboxymethyl-(2-sulfamoy...)Show SMILES NS(=O)(=O)c1nc2ccc(OC(=O)CN(CCOCCOCCN(CC(O)=O)CC(=O)Oc3ccc4nc(sc4c3)S(N)(=O)=O)CC(O)=O)cc2s1 Show InChI InChI=1S/C28H32N6O14S4/c29-51(41,42)27-31-19-3-1-17(11-21(19)49-27)47-25(39)15-33(13-23(35)36)5-7-45-9-10-46-8-6-34(14-24(37)38)16-26(40)48-18-2-4-20-22(12-18)50-28(32-20)52(30,43)44/h1-4,11-12H,5-10,13-16H2,(H,35,36)(H,37,38)(H2,29,41,42)(H2,30,43,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50097307

(CHEMBL408856 | [[2-(Carboxymethyl-{2-[carboxymethy...)Show SMILES NS(=O)(=O)c1nc2ccc(OC(=O)CN(CCN(CCN(CC(O)=O)CC(=O)Oc3ccc4nc(sc4c3)S(N)(=O)=O)CC(O)=O)CC(O)=O)cc2s1 Show InChI InChI=1S/C28H31N7O14S4/c29-52(44,45)27-31-18-3-1-16(9-20(18)50-27)48-25(42)14-34(12-23(38)39)7-5-33(11-22(36)37)6-8-35(13-24(40)41)15-26(43)49-17-2-4-19-21(10-17)51-28(32-19)53(30,46)47/h1-4,9-10H,5-8,11-15H2,(H,36,37)(H,38,39)(H,40,41)(H2,29,44,45)(H2,30,46,47) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibition of cloned isozyme, human carbonic anhydrase II |
Bioorg Med Chem Lett 11: 575-82 (2001)
BindingDB Entry DOI: 10.7270/Q2KW5GJQ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50094366
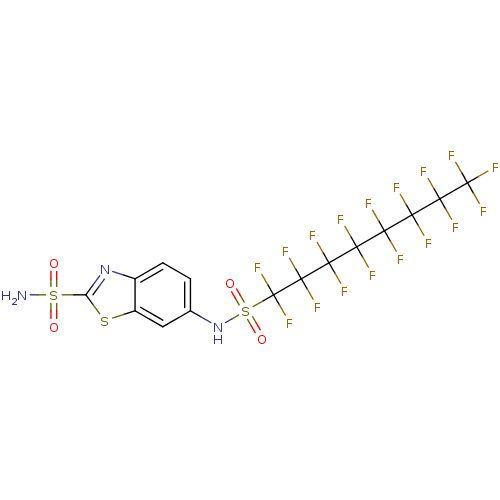
(6-(Heptadecafluorooctane-1-sulfonylamino)-benzothi...)Show SMILES NS(=O)(=O)c1nc2ccc(NS(=O)(=O)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F)cc2s1 Show InChI InChI=1S/C15H6F17N3O4S3/c16-8(17,10(20,21)12(24,25)14(28,29)30)9(18,19)11(22,23)13(26,27)15(31,32)42(38,39)35-4-1-2-5-6(3-4)40-7(34-5)41(33,36)37/h1-3,35H,(H2,33,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase II (CA2) |
J Med Chem 43: 4542-51 (2000)
BindingDB Entry DOI: 10.7270/Q2BV7HBP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50292022
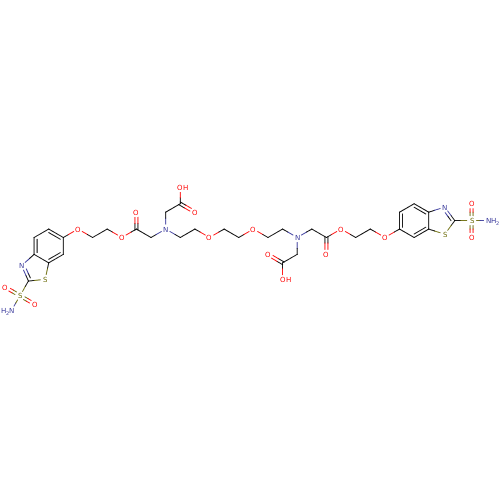
(CHEMBL35186 | {{2-[2-(2-{Carboxymethyl-[2-(2-sulfa...)Show SMILES NS(=O)(=O)c1nc2ccc(OCCOC(=O)CN(CCOCCOCCN(CC(O)=O)CC(=O)OCCOc3ccc4nc(sc4c3)S(N)(=O)=O)CC(O)=O)cc2s1 Show InChI InChI=1S/C32H40N6O16S4/c33-57(45,46)31-35-23-3-1-21(15-25(23)55-31)51-11-13-53-29(43)19-37(17-27(39)40)5-7-49-9-10-50-8-6-38(18-28(41)42)20-30(44)54-14-12-52-22-2-4-24-26(16-22)56-32(36-24)58(34,47)48/h1-4,15-16H,5-14,17-20H2,(H,39,40)(H,41,42)(H2,33,45,46)(H2,34,47,48) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50097288
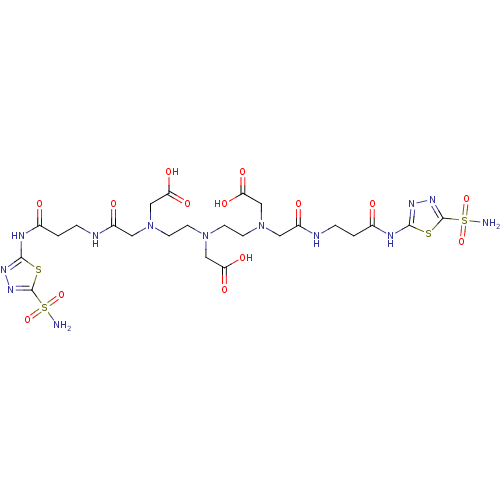
(((2-{Carboxymethyl-[2-(carboxymethyl-{[2-(5-sulfam...)Show SMILES NS(=O)(=O)c1nnc(NC(=O)CCNC(=O)CN(CCN(CCN(CC(O)=O)CC(=O)NCCC(=O)Nc2nnc(s2)S(N)(=O)=O)CC(O)=O)CC(O)=O)s1 Show InChI InChI=1S/C24H37N13O14S4/c25-54(48,49)23-33-31-21(52-23)29-14(38)1-3-27-16(40)9-36(12-19(44)45)7-5-35(11-18(42)43)6-8-37(13-20(46)47)10-17(41)28-4-2-15(39)30-22-32-34-24(53-22)55(26,50)51/h1-13H2,(H,27,40)(H,28,41)(H,42,43)(H,44,45)(H,46,47)(H2,25,48,49)(H2,26,50,51)(H,29,31,38)(H,30,32,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibition of cloned isozyme, human carbonic anhydrase II |
Bioorg Med Chem Lett 11: 575-82 (2001)
BindingDB Entry DOI: 10.7270/Q2KW5GJQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50094432

(2,3,4,5,6-Pentafluoro-benzoic acid 2-sulfamoyl-ben...)Show SMILES NS(=O)(=O)c1nc2ccc(OC(=O)c3c(F)c(F)c(F)c(F)c3F)cc2s1 Show InChI InChI=1S/C14H5F5N2O4S2/c15-8-7(9(16)11(18)12(19)10(8)17)13(22)25-4-1-2-5-6(3-4)26-14(21-5)27(20,23)24/h1-3H,(H2,20,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase II (CA2) |
J Med Chem 43: 4542-51 (2000)
BindingDB Entry DOI: 10.7270/Q2BV7HBP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50097307

(CHEMBL408856 | [[2-(Carboxymethyl-{2-[carboxymethy...)Show SMILES NS(=O)(=O)c1nc2ccc(OC(=O)CN(CCN(CCN(CC(O)=O)CC(=O)Oc3ccc4nc(sc4c3)S(N)(=O)=O)CC(O)=O)CC(O)=O)cc2s1 Show InChI InChI=1S/C28H31N7O14S4/c29-52(44,45)27-31-18-3-1-16(9-20(18)50-27)48-25(42)14-34(12-23(38)39)7-5-33(11-22(36)37)6-8-35(13-24(40)41)15-26(43)49-17-2-4-19-21(10-17)51-28(32-19)53(30,46)47/h1-4,9-10H,5-8,11-15H2,(H,36,37)(H,38,39)(H,40,41)(H2,29,44,45)(H2,30,46,47) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50097293
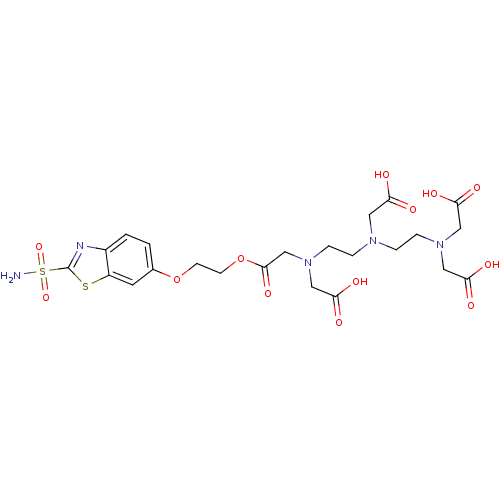
(CHEMBL34852 | {(2-{[2-(Bis-carboxymethyl-amino)-et...)Show SMILES NS(=O)(=O)c1nc2ccc(OCCOC(=O)CN(CCN(CCN(CC(O)=O)CC(O)=O)CC(O)=O)CC(O)=O)cc2s1 Show InChI InChI=1S/C23H31N5O13S2/c24-43(38,39)23-25-16-2-1-15(9-17(16)42-23)40-7-8-41-22(37)14-28(13-21(35)36)6-4-26(10-18(29)30)3-5-27(11-19(31)32)12-20(33)34/h1-2,9H,3-8,10-14H2,(H,29,30)(H,31,32)(H,33,34)(H,35,36)(H2,24,38,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50097288

(((2-{Carboxymethyl-[2-(carboxymethyl-{[2-(5-sulfam...)Show SMILES NS(=O)(=O)c1nnc(NC(=O)CCNC(=O)CN(CCN(CCN(CC(O)=O)CC(=O)NCCC(=O)Nc2nnc(s2)S(N)(=O)=O)CC(O)=O)CC(O)=O)s1 Show InChI InChI=1S/C24H37N13O14S4/c25-54(48,49)23-33-31-21(52-23)29-14(38)1-3-27-16(40)9-36(12-19(44)45)7-5-35(11-18(42)43)6-8-37(13-20(46)47)10-17(41)28-4-2-15(39)30-22-32-34-24(53-22)55(26,50)51/h1-13H2,(H,27,40)(H,28,41)(H,42,43)(H,44,45)(H,46,47)(H2,25,48,49)(H2,26,50,51)(H,29,31,38)(H,30,32,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50094439

(1,1,2,2,3,3,4,4,5,5,6,6,7,7,8,8,8-Heptadecafluoro-...)Show SMILES NS(=O)(=O)c1nc2ccc(OCCOS(=O)(=O)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F)cc2s1 Show InChI InChI=1S/C17H9F17N2O6S3/c18-10(19,12(22,23)14(26,27)16(30,31)32)11(20,21)13(24,25)15(28,29)17(33,34)45(39,40)42-4-3-41-6-1-2-7-8(5-6)43-9(36-7)44(35,37)38/h1-2,5H,3-4H2,(H2,35,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase II (CA2) |
J Med Chem 43: 4542-51 (2000)
BindingDB Entry DOI: 10.7270/Q2BV7HBP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50080684
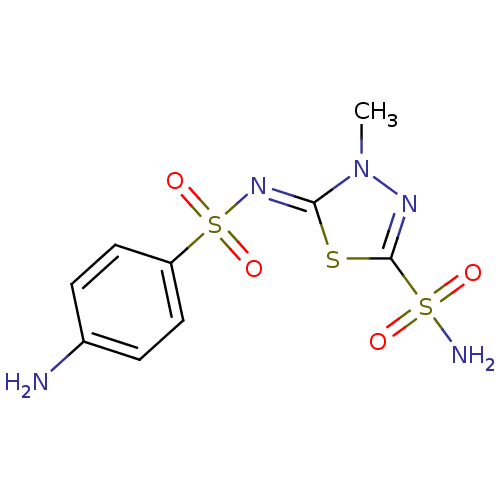
(5-(4-aminophenylsulfonylimino)-4-methyl-4,5-dihydr...)Show SMILES Cn1nc(s\c1=N/S(=O)(=O)c1ccc(N)cc1)S(N)(=O)=O Show InChI InChI=1S/C9H11N5O4S3/c1-14-8(19-9(12-14)20(11,15)16)13-21(17,18)7-4-2-6(10)3-5-7/h2-5H,10H2,1H3,(H2,11,15,16)/b13-8- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant carbonic anhydrase II (CA2) |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50097293

(CHEMBL34852 | {(2-{[2-(Bis-carboxymethyl-amino)-et...)Show SMILES NS(=O)(=O)c1nc2ccc(OCCOC(=O)CN(CCN(CCN(CC(O)=O)CC(O)=O)CC(O)=O)CC(O)=O)cc2s1 Show InChI InChI=1S/C23H31N5O13S2/c24-43(38,39)23-25-16-2-1-15(9-17(16)42-23)40-7-8-41-22(37)14-28(13-21(35)36)6-4-26(10-18(29)30)3-5-27(11-19(31)32)12-20(33)34/h1-2,9H,3-8,10-14H2,(H,29,30)(H,31,32)(H,33,34)(H,35,36)(H2,24,38,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibition of cloned isozyme, human carbonic anhydrase II |
Bioorg Med Chem Lett 11: 575-82 (2001)
BindingDB Entry DOI: 10.7270/Q2KW5GJQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50144828

(5-(2,3,5,6-Tetrafluoro-benzenesulfonylamino)-[1,3,...)Show SMILES NS(=O)(=O)c1nnc(NS(=O)(=O)c2c(F)c(F)cc(F)c2F)s1 Show InChI InChI=1S/C8H4F4N4O4S3/c9-2-1-3(10)5(12)6(4(2)11)23(19,20)16-7-14-15-8(21-7)22(13,17)18/h1H,(H,14,16)(H2,13,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human carbonic anhydrase II (hCAII) |
J Med Chem 47: 2796-804 (2004)
Article DOI: 10.1021/jm031116j
BindingDB Entry DOI: 10.7270/Q26974BC |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50292023
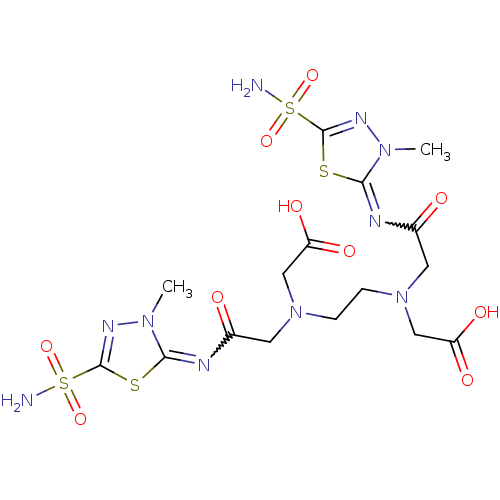
(CHEMBL1795060 | CHEMBL35495 | {(2-{Carboxymethyl-[...)Show SMILES Cn1nc(sc1=NC(=O)CN(CCN(CC(O)=O)CC(=O)N=c1sc(nn1C)S(N)(=O)=O)CC(O)=O)S(N)(=O)=O |w:21.21,6.7| Show InChI InChI=1S/C16H24N10O10S4/c1-23-13(37-15(21-23)39(17,33)34)19-9(27)5-25(7-11(29)30)3-4-26(8-12(31)32)6-10(28)20-14-24(2)22-16(38-14)40(18,35)36/h3-8H2,1-2H3,(H,29,30)(H,31,32)(H2,17,33,34)(H2,18,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50094395

(1,1,2,2,3,3,4,4,4-Nonafluoro-butane-1-sulfonic aci...)Show SMILES NS(=O)(=O)c1nc2ccc(OCCOS(=O)(=O)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F)cc2s1 Show InChI InChI=1S/C13H9F9N2O6S3/c14-10(15,12(18,19)20)11(16,17)13(21,22)33(27,28)30-4-3-29-6-1-2-7-8(5-6)31-9(24-7)32(23,25)26/h1-2,5H,3-4H2,(H2,23,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase II (CA2) |
J Med Chem 43: 4542-51 (2000)
BindingDB Entry DOI: 10.7270/Q2BV7HBP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50094430

(2,3,4,5,6-Pentafluoro-benzoic acid 2-(2-sulfamoyl-...)Show SMILES NS(=O)(=O)c1nc2ccc(OCCOC(=O)c3c(F)c(F)c(F)c(F)c3F)cc2s1 Show InChI InChI=1S/C16H9F5N2O5S2/c17-10-9(11(18)13(20)14(21)12(10)19)15(24)28-4-3-27-6-1-2-7-8(5-6)29-16(23-7)30(22,25)26/h1-2,5H,3-4H2,(H2,22,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase II (CA2) |
J Med Chem 43: 4542-51 (2000)
BindingDB Entry DOI: 10.7270/Q2BV7HBP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50292007
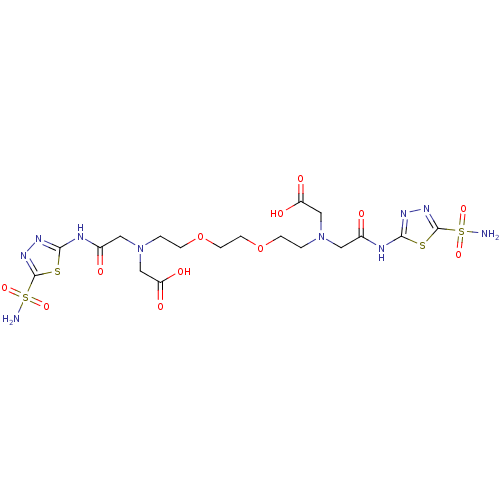
(CHEMBL1795064 | CHEMBL286828 | {{2-[2-(2-{Carboxym...)Show SMILES NS(=O)(=O)c1nnc(NC(=O)CN(CCOCCOCCN(CC(O)=O)CC(=O)Nc2nnc(s2)S(N)(=O)=O)CC(O)=O)s1 Show InChI InChI=1S/C18H28N10O12S4/c19-43(35,36)17-25-23-15(41-17)21-11(29)7-27(9-13(31)32)1-3-39-5-6-40-4-2-28(10-14(33)34)8-12(30)22-16-24-26-18(42-16)44(20,37)38/h1-10H2,(H,31,32)(H,33,34)(H2,19,35,36)(H2,20,37,38)(H,21,23,29)(H,22,24,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50097309
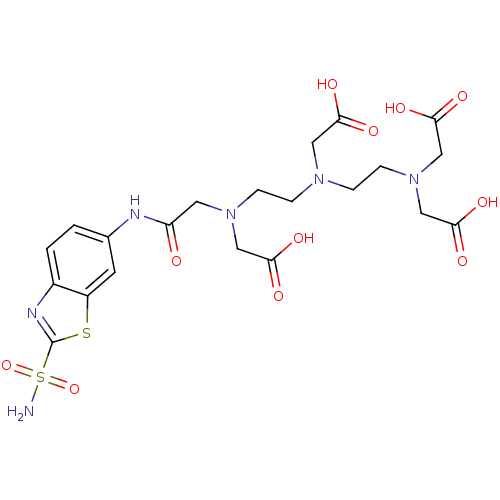
((Carboxymethyl-{2-[carboxymethyl-(2-{carboxymethyl...)Show SMILES NS(=O)(=O)c1nc2ccc(NC(=O)CN(CCN(CCN(CC(O)=O)CC(O)=O)CC(O)=O)CC(O)=O)cc2s1 Show InChI InChI=1S/C21H28N6O11S2/c22-40(37,38)21-24-14-2-1-13(7-15(14)39-21)23-16(28)8-26(10-18(31)32)5-3-25(9-17(29)30)4-6-27(11-19(33)34)12-20(35)36/h1-2,7H,3-6,8-12H2,(H,23,28)(H,29,30)(H,31,32)(H,33,34)(H,35,36)(H2,22,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50292132
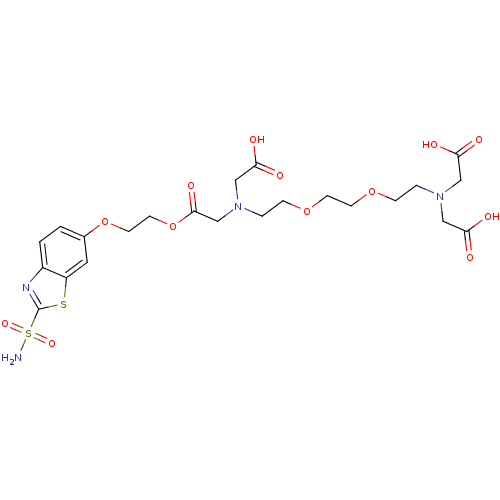
(CHEMBL286131 | {(2-{2-[2-(Bis-carboxymethyl-amino)...)Show SMILES NS(=O)(=O)c1nc2ccc(OCCOC(=O)CN(CCOCCOCCN(CC(O)=O)CC(O)=O)CC(O)=O)cc2s1 Show InChI InChI=1S/C23H32N4O13S2/c24-42(35,36)23-25-17-2-1-16(11-18(17)41-23)39-9-10-40-22(34)15-27(14-21(32)33)4-6-38-8-7-37-5-3-26(12-19(28)29)13-20(30)31/h1-2,11H,3-10,12-15H2,(H,28,29)(H,30,31)(H,32,33)(H2,24,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
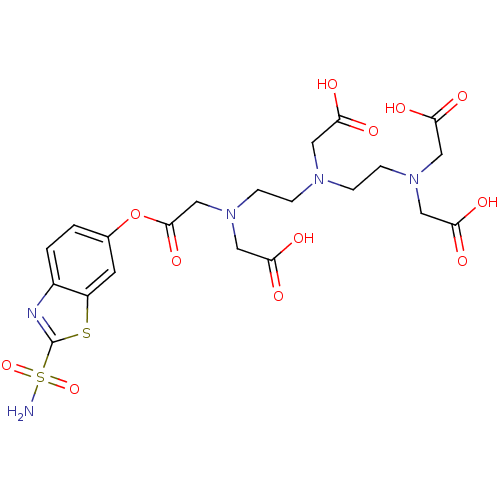
(Homo sapiens (Human)) | BDBM50097298
(CHEMBL34317 | [(2-{[2-(Bis-carboxymethyl-amino)-et...)Show SMILES NS(=O)(=O)c1nc2ccc(OC(=O)CN(CCN(CCN(CC(O)=O)CC(O)=O)CC(O)=O)CC(O)=O)cc2s1 Show InChI InChI=1S/C21H27N5O12S2/c22-40(36,37)21-23-14-2-1-13(7-15(14)39-21)38-20(35)12-26(11-19(33)34)6-4-24(8-16(27)28)3-5-25(9-17(29)30)10-18(31)32/h1-2,7H,3-6,8-12H2,(H,27,28)(H,29,30)(H,31,32)(H,33,34)(H2,22,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
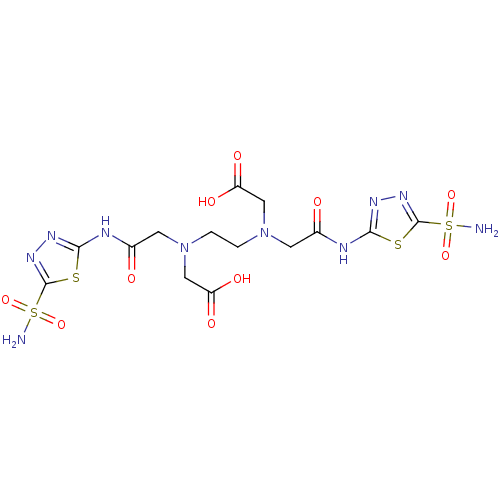
(Homo sapiens (Human)) | BDBM50228325
(CHEMBL1795059 | CHEMBL34546 | {(2-{Carboxymethyl-[...)Show SMILES NS(=O)(=O)c1nnc(NC(=O)CN(CCN(CC(O)=O)CC(=O)Nc2nnc(s2)S(N)(=O)=O)CC(O)=O)s1 Show InChI InChI=1S/C14H20N10O10S4/c15-37(31,32)13-21-19-11(35-13)17-7(25)3-23(5-9(27)28)1-2-24(6-10(29)30)4-8(26)18-12-20-22-14(36-12)38(16,33)34/h1-6H2,(H,27,28)(H,29,30)(H2,15,31,32)(H2,16,33,34)(H,17,19,25)(H,18,20,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50097309

((Carboxymethyl-{2-[carboxymethyl-(2-{carboxymethyl...)Show SMILES NS(=O)(=O)c1nc2ccc(NC(=O)CN(CCN(CCN(CC(O)=O)CC(O)=O)CC(O)=O)CC(O)=O)cc2s1 Show InChI InChI=1S/C21H28N6O11S2/c22-40(37,38)21-24-14-2-1-13(7-15(14)39-21)23-16(28)8-26(10-18(31)32)5-3-25(9-17(29)30)4-6-27(11-19(33)34)12-20(35)36/h1-2,7H,3-6,8-12H2,(H,23,28)(H,29,30)(H,31,32)(H,33,34)(H,35,36)(H2,22,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibition of cloned isozyme, human carbonic anhydrase II |
Bioorg Med Chem Lett 11: 575-82 (2001)
BindingDB Entry DOI: 10.7270/Q2KW5GJQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50292008
(CHEMBL1795065 | CHEMBL35272 | {{2-[2-(2-{Carboxyme...)Show SMILES Cn1nc(sc1=NC(=O)CN(CCOCCOCCN(CC(O)=O)CC(=O)N=c1sc(nn1C)S(N)(=O)=O)CC(O)=O)S(N)(=O)=O |w:27.27,6.7| Show InChI InChI=1S/C20H32N10O12S4/c1-27-17(43-19(25-27)45(21,37)38)23-13(31)9-29(11-15(33)34)3-5-41-7-8-42-6-4-30(12-16(35)36)10-14(32)24-18-28(2)26-20(44-18)46(22,39)40/h3-12H2,1-2H3,(H,33,34)(H,35,36)(H2,21,37,38)(H2,22,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Bos taurus (bovine)) | BDBM50080684

(5-(4-aminophenylsulfonylimino)-4-methyl-4,5-dihydr...)Show SMILES Cn1nc(s\c1=N/S(=O)(=O)c1ccc(N)cc1)S(N)(=O)=O Show InChI InChI=1S/C9H11N5O4S3/c1-14-8(19-9(12-14)20(11,15)16)13-21(17,18)7-4-2-6(10)3-5-7/h2-5H,10H2,1H3,(H2,11,15,16)/b13-8- | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine carbonic anhydrase IV (CA4), isolated from bovine lung microsomes |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50097298

(CHEMBL34317 | [(2-{[2-(Bis-carboxymethyl-amino)-et...)Show SMILES NS(=O)(=O)c1nc2ccc(OC(=O)CN(CCN(CCN(CC(O)=O)CC(O)=O)CC(O)=O)CC(O)=O)cc2s1 Show InChI InChI=1S/C21H27N5O12S2/c22-40(36,37)21-23-14-2-1-13(7-15(14)39-21)38-20(35)12-26(11-19(33)34)6-4-24(8-16(27)28)3-5-25(9-17(29)30)10-18(31)32/h1-2,7H,3-6,8-12H2,(H,27,28)(H,29,30)(H,31,32)(H,33,34)(H2,22,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibition of cloned isozyme, human carbonic anhydrase II |
Bioorg Med Chem Lett 11: 575-82 (2001)
BindingDB Entry DOI: 10.7270/Q2KW5GJQ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50094450
(6-Trifluoromethanesulfonylamino-benzothiazole-2-su...)Show SMILES NS(=O)(=O)c1nc2ccc(NS(=O)(=O)C(F)(F)F)cc2s1 Show InChI InChI=1S/C8H6F3N3O4S3/c9-8(10,11)21(17,18)14-4-1-2-5-6(3-4)19-7(13-5)20(12,15)16/h1-3,14H,(H2,12,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase II (CA2) |
J Med Chem 43: 4542-51 (2000)
BindingDB Entry DOI: 10.7270/Q2BV7HBP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50097300
((Carboxymethyl-{2-[carboxymethyl-(2-{carboxymethyl...)Show SMILES NS(=O)(=O)c1nnc(NC(=O)CN(CCN(CCN(CC(O)=O)CC(O)=O)CC(O)=O)CC(O)=O)s1 Show InChI InChI=1S/C16H25N7O11S2/c17-36(33,34)16-20-19-15(35-16)18-10(24)5-22(7-12(27)28)3-1-21(6-11(25)26)2-4-23(8-13(29)30)9-14(31)32/h1-9H2,(H,25,26)(H,27,28)(H,29,30)(H,31,32)(H2,17,33,34)(H,18,19,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50094337
(CHEMBL344156 | Trifluoro-methanesulfonic acid 2-su...)Show SMILES NS(=O)(=O)c1nc2ccc(OS(=O)(=O)C(F)(F)F)cc2s1 Show InChI InChI=1S/C8H5F3N2O5S3/c9-8(10,11)21(16,17)18-4-1-2-5-6(3-4)19-7(13-5)20(12,14)15/h1-3H,(H2,12,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase II (CA2) |
J Med Chem 43: 4542-51 (2000)
BindingDB Entry DOI: 10.7270/Q2BV7HBP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50292119
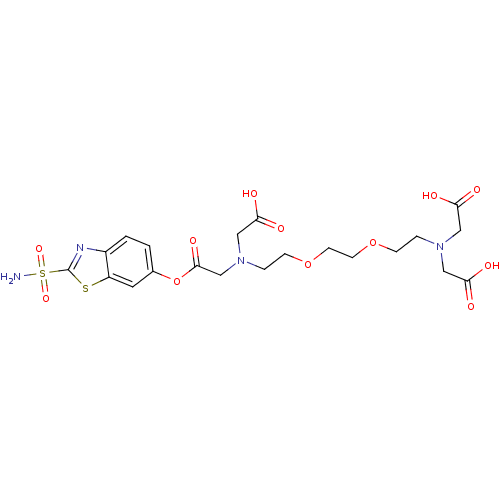
(CHEMBL284500 | [(2-{2-[2-(Bis-carboxymethyl-amino)...)Show SMILES NS(=O)(=O)c1nc2ccc(OC(=O)CN(CCOCCOCCN(CC(O)=O)CC(O)=O)CC(O)=O)cc2s1 Show InChI InChI=1S/C21H28N4O12S2/c22-39(33,34)21-23-15-2-1-14(9-16(15)38-21)37-20(32)13-25(12-19(30)31)4-6-36-8-7-35-5-3-24(10-17(26)27)11-18(28)29/h1-2,9H,3-8,10-13H2,(H,26,27)(H,28,29)(H,30,31)(H2,22,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50097300

((Carboxymethyl-{2-[carboxymethyl-(2-{carboxymethyl...)Show SMILES NS(=O)(=O)c1nnc(NC(=O)CN(CCN(CCN(CC(O)=O)CC(O)=O)CC(O)=O)CC(O)=O)s1 Show InChI InChI=1S/C16H25N7O11S2/c17-36(33,34)16-20-19-15(35-16)18-10(24)5-22(7-12(27)28)3-1-21(6-11(25)26)2-4-23(8-13(29)30)9-14(31)32/h1-9H2,(H,25,26)(H,27,28)(H,29,30)(H,31,32)(H2,17,33,34)(H,18,19,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibition of cloned isozyme, human carbonic anhydrase II |
Bioorg Med Chem Lett 11: 575-82 (2001)
BindingDB Entry DOI: 10.7270/Q2KW5GJQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50094320
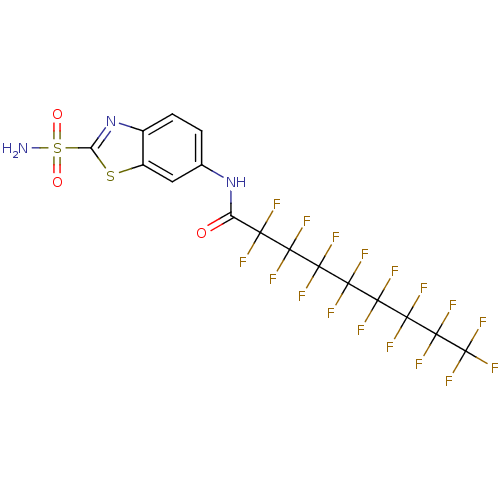
(2,2,3,3,4,4,5,5,6,6,7,7,8,8,9,9,9-Heptadecafluoro-...)Show SMILES NS(=O)(=O)c1nc2ccc(NC(=O)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F)cc2s1 Show InChI InChI=1S/C16H6F17N3O3S2/c17-9(18,7(37)35-4-1-2-5-6(3-4)40-8(36-5)41(34,38)39)10(19,20)11(21,22)12(23,24)13(25,26)14(27,28)15(29,30)16(31,32)33/h1-3H,(H,35,37)(H2,34,38,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase II (CA2) |
J Med Chem 43: 4542-51 (2000)
BindingDB Entry DOI: 10.7270/Q2BV7HBP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data