

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neprilysin (Homo sapiens (Human)) | BDBM155343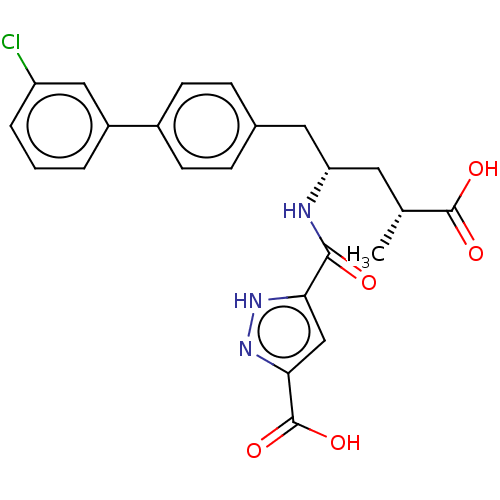 (US9006249, Example 49-2 | US9603819, Example 49-2) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The activity of a compound according to the present invention can be assessed by the following in vitro & in vivo methods and/or by the following in ... | US Patent US9603819 (2017) BindingDB Entry DOI: 10.7270/Q20V8FVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM155343 (US9006249, Example 49-2 | US9603819, Example 49-2) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Novartis AG US Patent | Assay Description See A fluorescence lifetime-based assay for protease inhibitor profiling on human kallikrein 7 Doering K, Meder G, Hinnenberger M, Woelcke J, Mayr L ... | US Patent US9006249 (2015) BindingDB Entry DOI: 10.7270/Q2NV9H0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM309469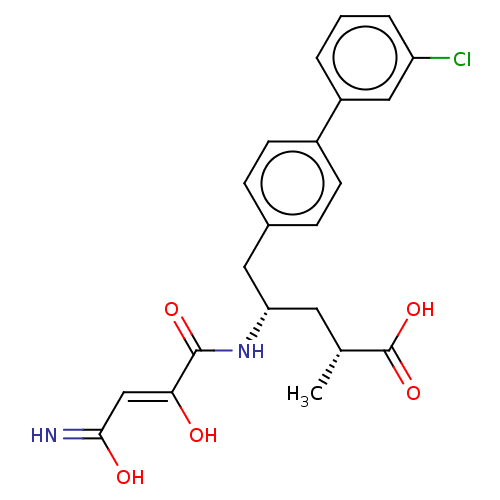 ((2R,4S)-5-(3′-Chloro-biphenyl-4-yl)-4-[(3-hy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The activity of a compound according to the present invention can be assessed by the following in vitro & in vivo methods and/or by the following in ... | US Patent US9603819 (2017) BindingDB Entry DOI: 10.7270/Q20V8FVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM153121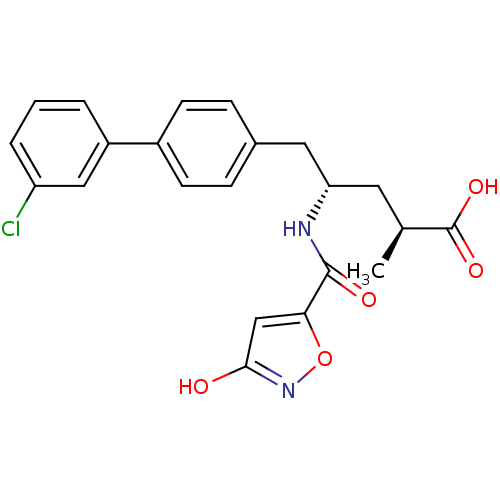 (US8993631, 29-2 | US9006249, Example 49-1) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Novartis AG US Patent | Assay Description See A fluorescence lifetime-based assay for protease inhibitor profiling on human kallikrein 7 Doering K, Meder G, Hinnenberger M, Woelcke J, Mayr L ... | US Patent US9006249 (2015) BindingDB Entry DOI: 10.7270/Q2NV9H0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM155344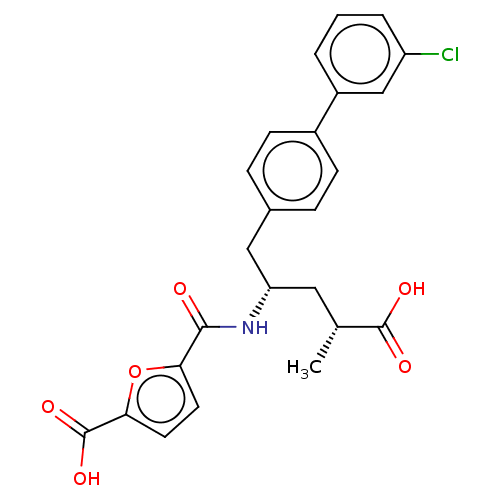 (US9006249, Example 49-3 | US9603819, Example 49-3) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Novartis AG US Patent | Assay Description See A fluorescence lifetime-based assay for protease inhibitor profiling on human kallikrein 7 Doering K, Meder G, Hinnenberger M, Woelcke J, Mayr L ... | US Patent US9006249 (2015) BindingDB Entry DOI: 10.7270/Q2NV9H0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM153128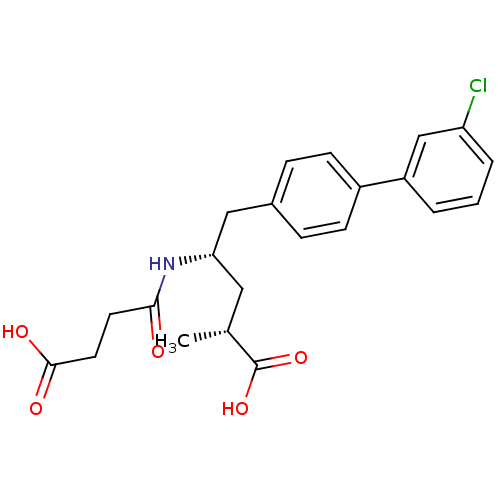 (US8993631, 36) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte... | ACS Med Chem Lett 11: 188-194 (2020) Article DOI: 10.1021/acsmedchemlett.9b00578 BindingDB Entry DOI: 10.7270/Q2C82DK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM155344 (US9006249, Example 49-3 | US9603819, Example 49-3) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The activity of a compound according to the present invention can be assessed by the following in vitro & in vivo methods and/or by the following in ... | US Patent US9603819 (2017) BindingDB Entry DOI: 10.7270/Q20V8FVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM130359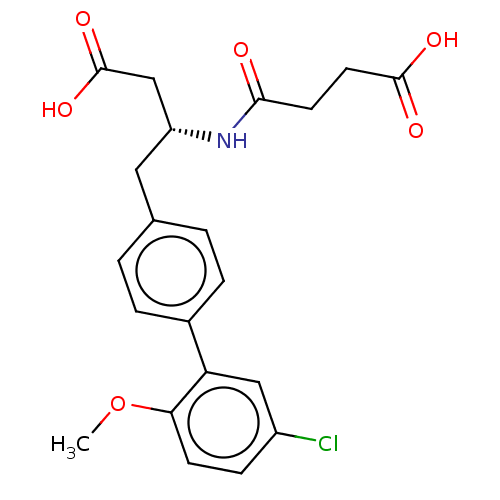 (US8822534, Example 5-39 | US8993631, 5-8) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte... | ACS Med Chem Lett 11: 188-194 (2020) Article DOI: 10.1021/acsmedchemlett.9b00578 BindingDB Entry DOI: 10.7270/Q2C82DK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50509659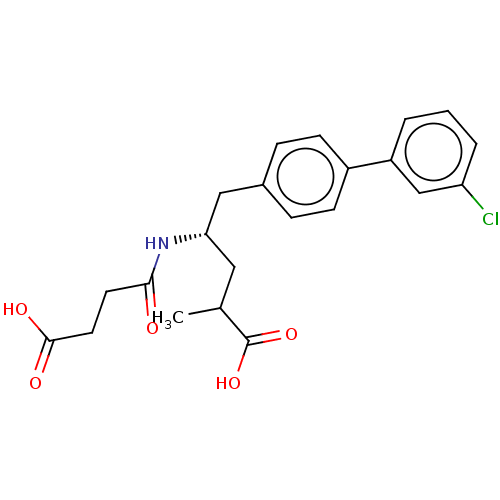 (CHEMBL4443138) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte... | ACS Med Chem Lett 11: 188-194 (2020) Article DOI: 10.1021/acsmedchemlett.9b00578 BindingDB Entry DOI: 10.7270/Q2C82DK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase WNK1 [166-489] (Homo sapiens (Human)) | BDBM203827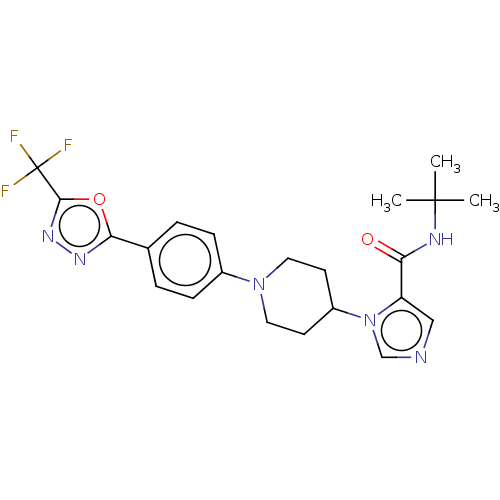 (N-(tert-butyl)-1-(1-(5-(5-(trifluoromethyl)-1,3,4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.3 | 25 |
Novartis Institutes | Assay Description Each well of the MBP-coated ScintiPlates held 100 ul of a solution containing 20 mM HEPES pH 7.3, 5 mM MnCl2 (WNK1 and WNK4) or 3 mM MnCl2 (WNK2 and ... | Nat Chem Biol 12: 896-898 (2016) Article DOI: 10.1038/nchembio.2168 BindingDB Entry DOI: 10.7270/Q2ZK5FH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM153108 (US8993631, 16-5 | US9006249, Example 3-32 | US9603...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Novartis AG US Patent | Assay Description See A fluorescence lifetime-based assay for protease inhibitor profiling on human kallikrein 7 Doering K, Meder G, Hinnenberger M, Woelcke J, Mayr L ... | US Patent US9006249 (2015) BindingDB Entry DOI: 10.7270/Q2NV9H0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM153108 (US8993631, 16-5 | US9006249, Example 3-32 | US9603...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The activity of a compound according to the present invention can be assessed by the following in vitro & in vivo methods and/or by the following in ... | US Patent US9603819 (2017) BindingDB Entry DOI: 10.7270/Q20V8FVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM130365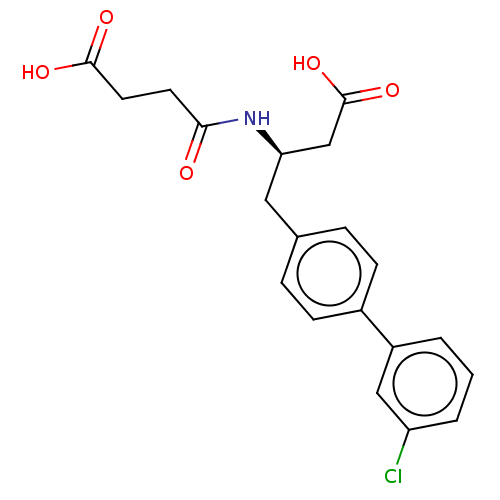 (US8822534, Example 11-1 | US8822534, Example 12-1 ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte... | ACS Med Chem Lett 11: 188-194 (2020) Article DOI: 10.1021/acsmedchemlett.9b00578 BindingDB Entry DOI: 10.7270/Q2C82DK4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50509652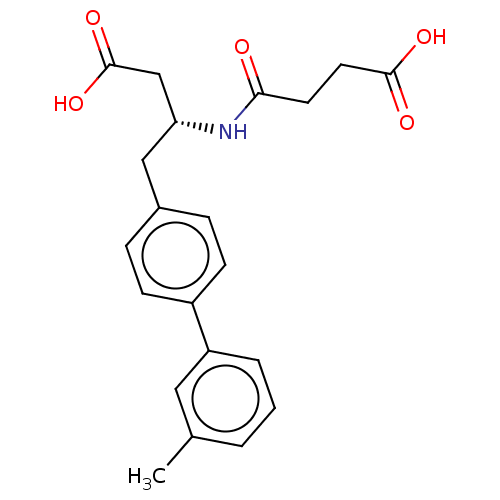 (CHEMBL4557208) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte... | ACS Med Chem Lett 11: 188-194 (2020) Article DOI: 10.1021/acsmedchemlett.9b00578 BindingDB Entry DOI: 10.7270/Q2C82DK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50344356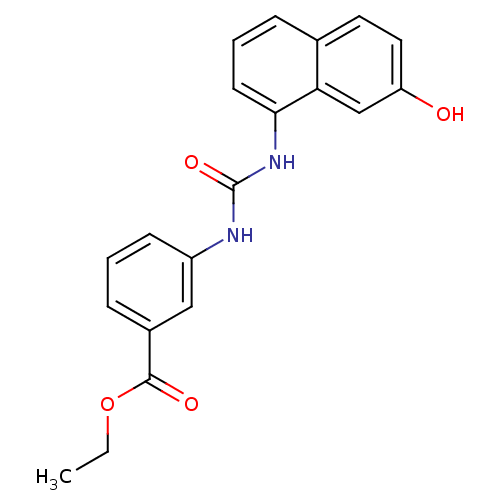 (CHEMBL1779676 | ethyl 3-(3-(7-hydroxynaphthalen-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Yakuhin, Ltd Curated by ChEMBL | Assay Description Antagonist activity at rat TRPV1 expressed in CHO cells co-expressing aequorin and CRE-luciferase reporter gene assessed as inhibition of capsaicin-i... | Bioorg Med Chem Lett 21: 3354-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.013 BindingDB Entry DOI: 10.7270/Q2NG4QXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50344356 (CHEMBL1779676 | ethyl 3-(3-(7-hydroxynaphthalen-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Yakuhin, Ltd Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells co-expressing aequorin and CRE-luciferase reporter gene assessed as inhibition of capsaicin... | Bioorg Med Chem Lett 21: 3354-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.013 BindingDB Entry DOI: 10.7270/Q2NG4QXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50344367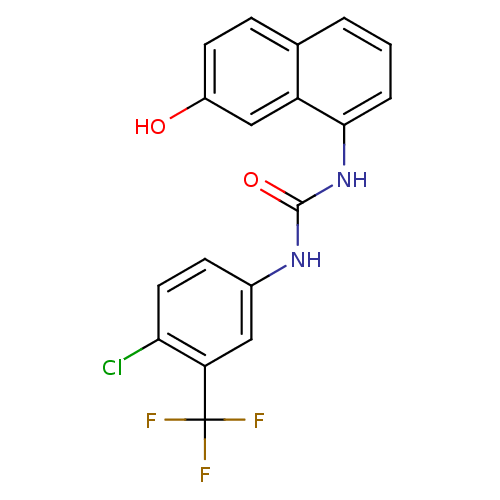 (1-(4-chloro-3-(trifluoromethyl)phenyl)-3-(7-hydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Yakuhin, Ltd Curated by ChEMBL | Assay Description Antagonist activity at rat TRPV1 expressed in CHO cells co-expressing aequorin and CRE-luciferase reporter gene assessed as inhibition of capsaicin-i... | Bioorg Med Chem Lett 21: 3354-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.013 BindingDB Entry DOI: 10.7270/Q2NG4QXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50509666 (CHEMBL4442804) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte... | ACS Med Chem Lett 11: 188-194 (2020) Article DOI: 10.1021/acsmedchemlett.9b00578 BindingDB Entry DOI: 10.7270/Q2C82DK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50344378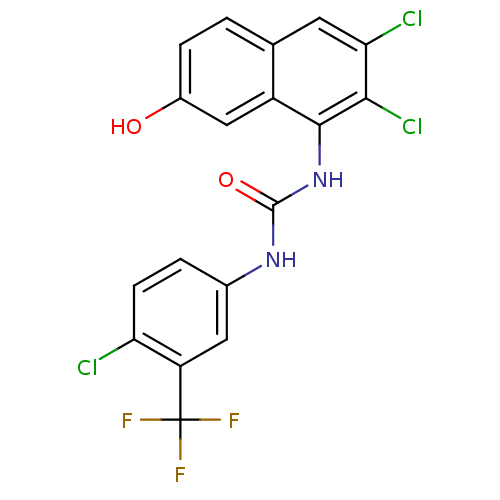 (1-(4-chloro-3-(trifluoromethyl)phenyl)-3-(2,3-dich...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Yakuhin, Ltd Curated by ChEMBL | Assay Description Antagonist activity at rat TRPV1 expressed in CHO cells co-expressing aequorin and CRE-luciferase reporter gene assessed as inhibition of capsaicin-i... | Bioorg Med Chem Lett 21: 3354-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.013 BindingDB Entry DOI: 10.7270/Q2NG4QXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50344367 (1-(4-chloro-3-(trifluoromethyl)phenyl)-3-(7-hydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Yakuhin, Ltd Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells co-expressing aequorin and CRE-luciferase reporter gene assessed as inhibition of capsaicin... | Bioorg Med Chem Lett 21: 3354-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.013 BindingDB Entry DOI: 10.7270/Q2NG4QXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50344367 (1-(4-chloro-3-(trifluoromethyl)phenyl)-3-(7-hydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Yakuhin, Ltd Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHOluc9aeq cells assessed as inhibition of capsaicin-stimulated response by aequorin and CRE-lucifear... | Bioorg Med Chem Lett 22: 3408-11 (2012) Article DOI: 10.1016/j.bmcl.2012.03.108 BindingDB Entry DOI: 10.7270/Q2C24XF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM50524347 (CHEMBL4535197) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of 2-((1E,3E,5E)-5-(1-(6-((((3S,5S)-1-((1-carbamoyl-1H-indol-3-yl)carbamoyl)-5-((3-chloro-2-fluorobenzyl)carbamoyl)-3-fluoropyrrolidin-3-y... | J Med Chem 62: 4656-4668 (2019) Article DOI: 10.1021/acs.jmedchem.9b00271 BindingDB Entry DOI: 10.7270/Q2CC144N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50344348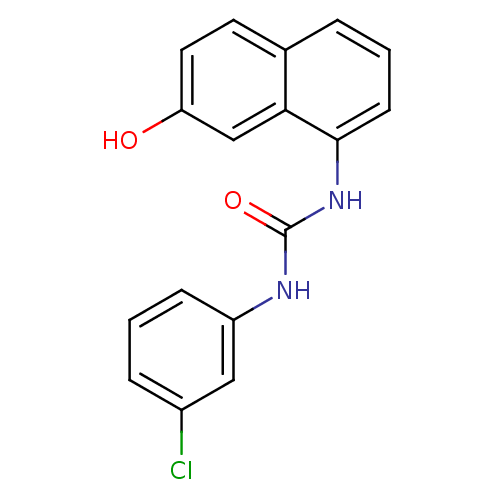 (1-(3-chlorophenyl)-3-(7-hydroxynaphthalen-1-yl)ure...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Yakuhin, Ltd Curated by ChEMBL | Assay Description Antagonist activity at rat TRPV1 expressed in CHO cells co-expressing aequorin and CRE-luciferase reporter gene assessed as inhibition of capsaicin-i... | Bioorg Med Chem Lett 21: 3354-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.013 BindingDB Entry DOI: 10.7270/Q2NG4QXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50382237 (CHEMBL2024668) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Yakuhin, Ltd Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHOluc9aeq cells assessed as inhibition of capsaicin-stimulated response by aequorin and CRE-lucifear... | Bioorg Med Chem Lett 22: 3408-11 (2012) Article DOI: 10.1016/j.bmcl.2012.03.108 BindingDB Entry DOI: 10.7270/Q2C24XF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50382237 (CHEMBL2024668) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Yakuhin, Ltd Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHOluc9aeq cells assessed as inhibition of capsaicin-stimulated response by aequorin and CRE-lucifear... | Bioorg Med Chem Lett 22: 3408-11 (2012) Article DOI: 10.1016/j.bmcl.2012.03.108 BindingDB Entry DOI: 10.7270/Q2C24XF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50344377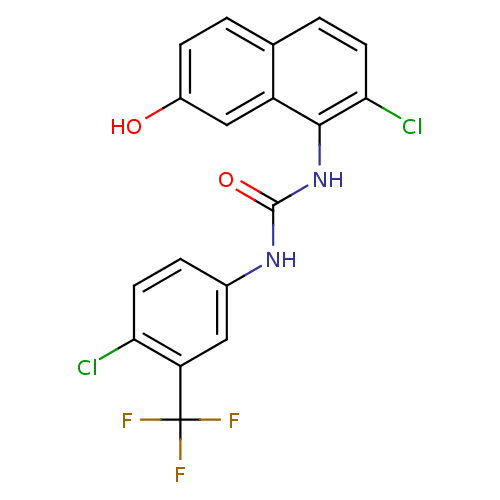 (1-(4-chloro-3-(trifluoromethyl)phenyl)-3-(2-chloro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Yakuhin, Ltd Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells co-expressing aequorin and CRE-luciferase reporter gene assessed as inhibition of capsaicin... | Bioorg Med Chem Lett 21: 3354-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.013 BindingDB Entry DOI: 10.7270/Q2NG4QXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50344345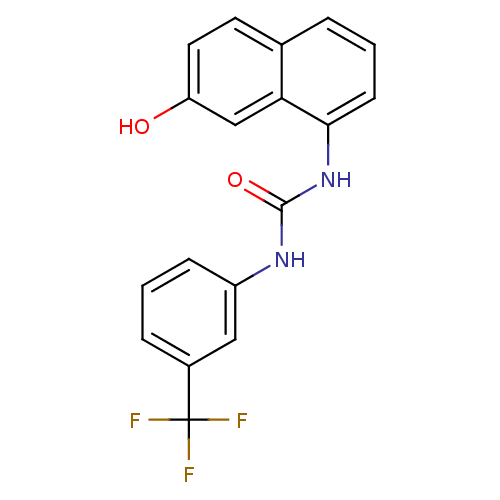 (1-(7-hydroxynaphthalen-1-yl)-3-(3-(trifluoromethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Yakuhin, Ltd Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells co-expressing aequorin and CRE-luciferase reporter gene assessed as inhibition of capsaicin... | Bioorg Med Chem Lett 21: 3354-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.013 BindingDB Entry DOI: 10.7270/Q2NG4QXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM20412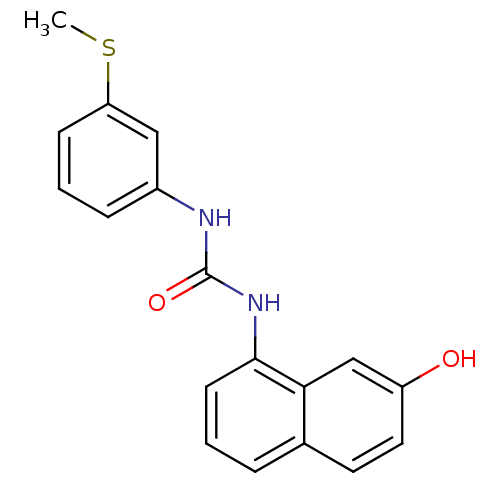 (3-(7-hydroxynaphthalen-1-yl)-1-[3-(methylsulfanyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Yakuhin, Ltd Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells co-expressing aequorin and CRE-luciferase reporter gene assessed as inhibition of capsaicin... | Bioorg Med Chem Lett 21: 3354-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.013 BindingDB Entry DOI: 10.7270/Q2NG4QXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50344377 (1-(4-chloro-3-(trifluoromethyl)phenyl)-3-(2-chloro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Yakuhin, Ltd Curated by ChEMBL | Assay Description Antagonist activity at rat TRPV1 expressed in CHO cells co-expressing aequorin and CRE-luciferase reporter gene assessed as inhibition of capsaicin-i... | Bioorg Med Chem Lett 21: 3354-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.013 BindingDB Entry DOI: 10.7270/Q2NG4QXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM130355 (US8822534, Example 5-7 | US8993631, 5-3) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte... | ACS Med Chem Lett 11: 188-194 (2020) Article DOI: 10.1021/acsmedchemlett.9b00578 BindingDB Entry DOI: 10.7270/Q2C82DK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50344345 (1-(7-hydroxynaphthalen-1-yl)-3-(3-(trifluoromethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Yakuhin, Ltd Curated by ChEMBL | Assay Description Antagonist activity at rat TRPV1 expressed in CHO cells co-expressing aequorin and CRE-luciferase reporter gene assessed as inhibition of capsaicin-i... | Bioorg Med Chem Lett 21: 3354-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.013 BindingDB Entry DOI: 10.7270/Q2NG4QXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50344363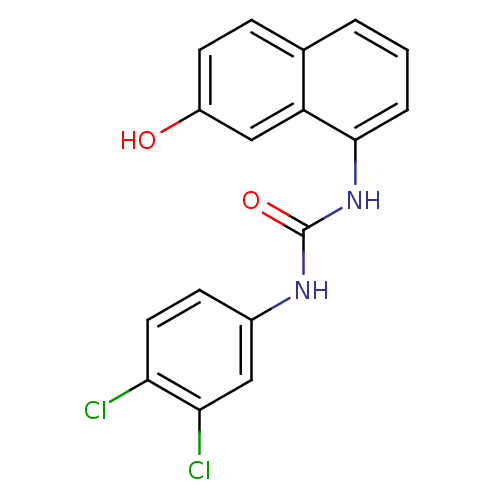 (1-(3,4-dichlorophenyl)-3-(7-hydroxynaphthalen-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Yakuhin, Ltd Curated by ChEMBL | Assay Description Antagonist activity at rat TRPV1 expressed in CHO cells co-expressing aequorin and CRE-luciferase reporter gene assessed as inhibition of capsaicin-i... | Bioorg Med Chem Lett 21: 3354-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.013 BindingDB Entry DOI: 10.7270/Q2NG4QXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50344372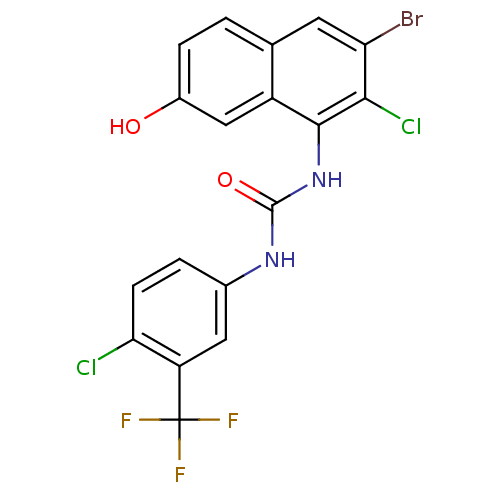 (1-(3-bromo-2-chloro-7-hydroxynaphthalen-1-yl)-3-(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Yakuhin, Ltd Curated by ChEMBL | Assay Description Antagonist activity at rat TRPV1 expressed in CHO cells co-expressing aequorin and CRE-luciferase reporter gene assessed as inhibition of capsaicin-i... | Bioorg Med Chem Lett 21: 3354-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.013 BindingDB Entry DOI: 10.7270/Q2NG4QXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50344363 (1-(3,4-dichlorophenyl)-3-(7-hydroxynaphthalen-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Yakuhin, Ltd Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells co-expressing aequorin and CRE-luciferase reporter gene assessed as inhibition of capsaicin... | Bioorg Med Chem Lett 21: 3354-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.013 BindingDB Entry DOI: 10.7270/Q2NG4QXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50344346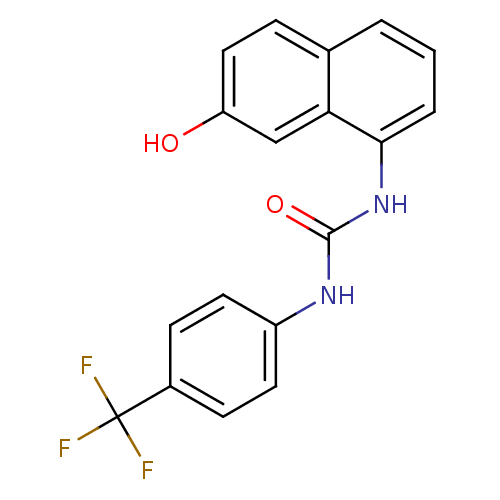 (1-(7-hydroxynaphthalen-1-yl)-3-(4-(trifluoromethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Yakuhin, Ltd Curated by ChEMBL | Assay Description Antagonist activity at rat TRPV1 expressed in CHO cells co-expressing aequorin and CRE-luciferase reporter gene assessed as inhibition of capsaicin-i... | Bioorg Med Chem Lett 21: 3354-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.013 BindingDB Entry DOI: 10.7270/Q2NG4QXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM50462108 (CHEMBL4246585) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of 2-((1E,3E,5E)-5-(1-(6-((((3S,5S)-1-((1-carbamoyl-1H-indol-3-yl)carbamoyl)-5-((3-chloro-2-fluorobenzyl)carbamoyl)-3-fluoropyrrolidin-3-y... | J Med Chem 62: 4656-4668 (2019) Article DOI: 10.1021/acs.jmedchem.9b00271 BindingDB Entry DOI: 10.7270/Q2CC144N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase WNK1 [1-491] (Homo sapiens (Human)) | BDBM203827 (N-(tert-butyl)-1-(1-(5-(5-(trifluoromethyl)-1,3,4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.3 | 25 |
Novartis Institutes | Assay Description Each well of the MBP-coated ScintiPlates held 100 ul of a solution containing 20 mM HEPES pH 7.3, 5 mM MnCl2 (WNK1 and WNK4) or 3 mM MnCl2 (WNK2 and ... | Nat Chem Biol 12: 896-898 (2016) Article DOI: 10.1038/nchembio.2168 BindingDB Entry DOI: 10.7270/Q2ZK5FH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50344346 (1-(7-hydroxynaphthalen-1-yl)-3-(4-(trifluoromethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Yakuhin, Ltd Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells co-expressing aequorin and CRE-luciferase reporter gene assessed as inhibition of capsaicin... | Bioorg Med Chem Lett 21: 3354-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.013 BindingDB Entry DOI: 10.7270/Q2NG4QXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50509647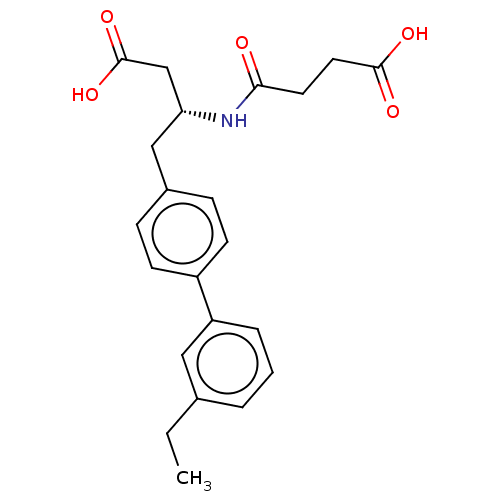 (CHEMBL4518426) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte... | ACS Med Chem Lett 11: 188-194 (2020) Article DOI: 10.1021/acsmedchemlett.9b00578 BindingDB Entry DOI: 10.7270/Q2C82DK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50344355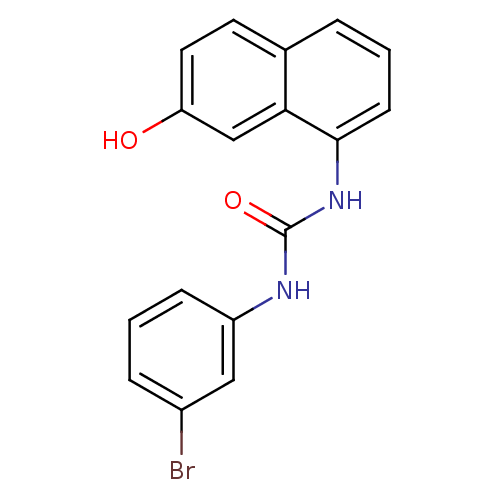 (1-(3-bromophenyl)-3-(7-hydroxynaphthalen-1-yl)urea...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Yakuhin, Ltd Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells co-expressing aequorin and CRE-luciferase reporter gene assessed as inhibition of capsaicin... | Bioorg Med Chem Lett 21: 3354-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.013 BindingDB Entry DOI: 10.7270/Q2NG4QXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50344378 (1-(4-chloro-3-(trifluoromethyl)phenyl)-3-(2,3-dich...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Yakuhin, Ltd Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells co-expressing aequorin and CRE-luciferase reporter gene assessed as inhibition of capsaicin... | Bioorg Med Chem Lett 21: 3354-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.013 BindingDB Entry DOI: 10.7270/Q2NG4QXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor B (Homo sapiens (Human)) | BDBM50540314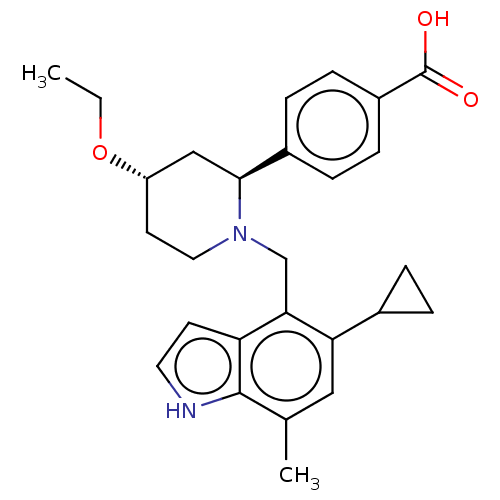 (CHEMBL4639592) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human serine protease factor B by TR-FRET based competition binding assay | J Med Chem 63: 5697-5722 (2020) Article DOI: 10.1021/acs.jmedchem.9b01870 BindingDB Entry DOI: 10.7270/Q21N84P4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50034842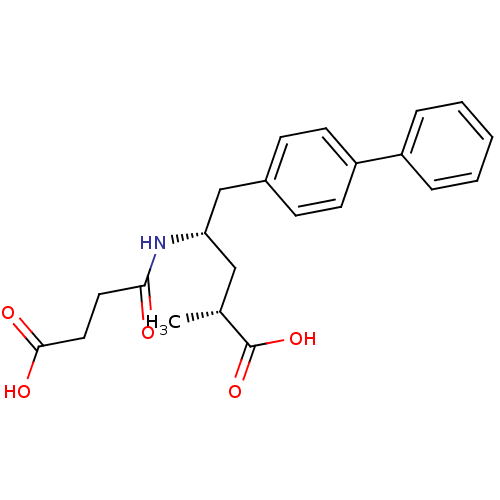 ((2R,4S)-5-Biphenyl-4-yl-4-(3-carboxy-propionylamin...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte... | ACS Med Chem Lett 11: 188-194 (2020) Article DOI: 10.1021/acsmedchemlett.9b00578 BindingDB Entry DOI: 10.7270/Q2C82DK4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase WNK1 [1-434] (Homo sapiens (Human)) | BDBM203827 (N-(tert-butyl)-1-(1-(5-(5-(trifluoromethyl)-1,3,4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.3 | 25 |
Novartis Institutes | Assay Description Each well of the MBP-coated ScintiPlates held 100 ul of a solution containing 20 mM HEPES pH 7.3, 5 mM MnCl2 (WNK1 and WNK4) or 3 mM MnCl2 (WNK2 and ... | Nat Chem Biol 12: 896-898 (2016) Article DOI: 10.1038/nchembio.2168 BindingDB Entry DOI: 10.7270/Q2ZK5FH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50344348 (1-(3-chlorophenyl)-3-(7-hydroxynaphthalen-1-yl)ure...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Yakuhin, Ltd Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells co-expressing aequorin and CRE-luciferase reporter gene assessed as inhibition of capsaicin... | Bioorg Med Chem Lett 21: 3354-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.013 BindingDB Entry DOI: 10.7270/Q2NG4QXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM130354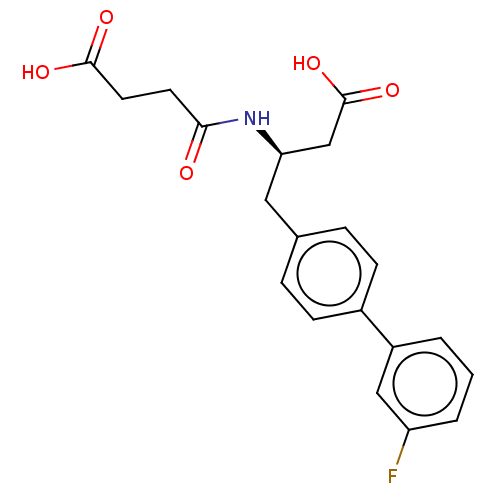 (US8822534, Example 5-4 | US8993631, 5-2) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte... | ACS Med Chem Lett 11: 188-194 (2020) Article DOI: 10.1021/acsmedchemlett.9b00578 BindingDB Entry DOI: 10.7270/Q2C82DK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50344372 (1-(3-bromo-2-chloro-7-hydroxynaphthalen-1-yl)-3-(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Yakuhin, Ltd Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells co-expressing aequorin and CRE-luciferase reporter gene assessed as inhibition of capsaicin... | Bioorg Med Chem Lett 21: 3354-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.013 BindingDB Entry DOI: 10.7270/Q2NG4QXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50509655 (CHEMBL4466321) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte... | ACS Med Chem Lett 11: 188-194 (2020) Article DOI: 10.1021/acsmedchemlett.9b00578 BindingDB Entry DOI: 10.7270/Q2C82DK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM20412 (3-(7-hydroxynaphthalen-1-yl)-1-[3-(methylsulfanyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Yakuhin, Ltd Curated by ChEMBL | Assay Description Antagonist activity at rat TRPV1 expressed in CHO cells co-expressing aequorin and CRE-luciferase reporter gene assessed as inhibition of capsaicin-i... | Bioorg Med Chem Lett 21: 3354-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.013 BindingDB Entry DOI: 10.7270/Q2NG4QXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM309463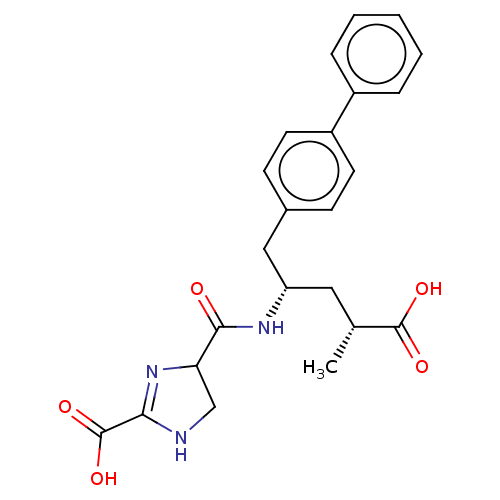 (US9603819, Example 3-60) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The activity of a compound according to the present invention can be assessed by the following in vitro & in vivo methods and/or by the following in ... | US Patent US9603819 (2017) BindingDB Entry DOI: 10.7270/Q20V8FVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 501 total ) | Next | Last >> |