 Found 221 hits with Last Name = 'naglich' and Initial = 'j'
Found 221 hits with Last Name = 'naglich' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50071844
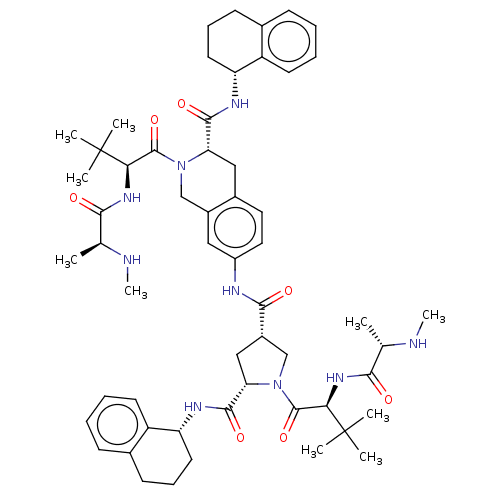
(CHEMBL3410875)Show SMILES CN[C@@H](C)C(=O)N[C@H](C(=O)N1C[C@H](C[C@H]1C(=O)N[C@@H]1CCCc2ccccc12)C(=O)Nc1ccc2C[C@H](N(Cc2c1)C(=O)[C@@H](NC(=O)[C@H](C)NC)C(C)(C)C)C(=O)N[C@@H]1CCCc2ccccc12)C(C)(C)C |r| Show InChI InChI=1S/C56H77N9O7/c1-32(57-9)48(66)62-46(55(3,4)5)53(71)64-30-37-27-39(26-25-36(37)28-44(64)51(69)60-42-23-15-19-34-17-11-13-21-40(34)42)59-50(68)38-29-45(52(70)61-43-24-16-20-35-18-12-14-22-41(35)43)65(31-38)54(72)47(56(6,7)8)63-49(67)33(2)58-10/h11-14,17-18,21-22,25-27,32-33,38,42-47,57-58H,15-16,19-20,23-24,28-31H2,1-10H3,(H,59,68)(H,60,69)(H,61,70)(H,62,66)(H,63,67)/t32-,33-,38-,42+,43+,44-,45-,46+,47+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research
Curated by ChEMBL
| Assay Description
Binding affinity to human N-terminal His-tagged cIAP1 BIR2-3 C202A/C213G mutant after 60 mins by HTRF assay |
J Med Chem 58: 1556-62 (2015)
Article DOI: 10.1021/jm501482t
BindingDB Entry DOI: 10.7270/Q2N29ZM8 |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50078359
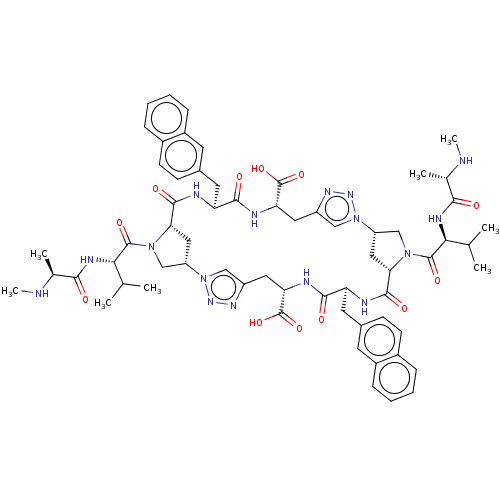
(CHEMBL3414729)Show SMILES [H][C@@]12CN(C(=O)[C@@H](NC(=O)[C@H](C)NC)C(C)C)[C@@]([H])(C1)C(=O)N[C@@H](Cc1ccc3ccccc3c1)C(=O)N[C@@H](Cc1cn(nn1)[C@]1([H])CN(C(=O)[C@@H](NC(=O)[C@H](C)NC)C(C)C)[C@@]([H])(C1)C(=O)N[C@@H](Cc1ccc3ccccc3c1)C(=O)N[C@@H](Cc1cn2nn1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C64H80N16O12/c1-33(2)53(71-55(81)35(5)65-7)61(87)77-31-45-27-51(77)59(85)67-47(23-37-17-19-39-13-9-11-15-41(39)21-37)57(83)69-50(64(91)92)26-44-30-80(76-74-44)46-28-52(78(32-46)62(88)54(34(3)4)72-56(82)36(6)66-8)60(86)68-48(24-38-18-20-40-14-10-12-16-42(40)22-38)58(84)70-49(63(89)90)25-43-29-79(45)75-73-43/h9-22,29-30,33-36,45-54,65-66H,23-28,31-32H2,1-8H3,(H,67,85)(H,68,86)(H,69,83)(H,70,84)(H,71,81)(H,72,82)(H,89,90)(H,91,92)/t35-,36-,45-,46-,47-,48-,49-,50-,51-,52-,53-,54-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ensemble Therapeutics Corp
Curated by ChEMBL
| Assay Description
Inhibition of cIAP1 BIR2-3 (154 to 352 residues) (unknown origin) fluoresceinated dimeric SMAC peptide based fluorescence polarization assay |
J Med Chem 58: 2855-61 (2015)
Article DOI: 10.1021/jm501892g
BindingDB Entry DOI: 10.7270/Q2RF5WQ0 |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 3
(Homo sapiens (Human)) | BDBM50112346
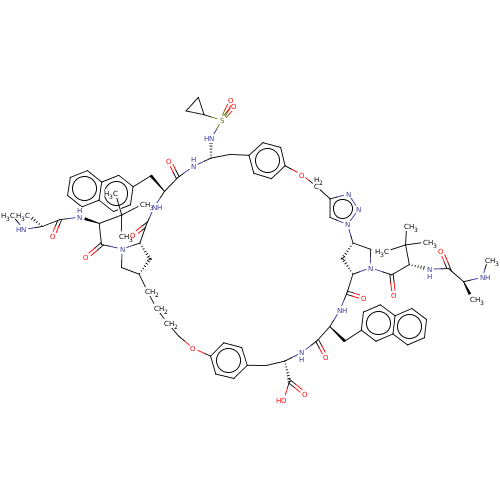
(CHEMBL3609325)Show SMILES [H][C@@]12CN(C(=O)[C@@H](NC(=O)[C@H](C)NC)C(C)(C)C)[C@@]([H])(C1)C(=O)N[C@@H](Cc1ccc3ccccc3c1)C(=O)N[C@@H](Cc1ccc(OCc3cn(nn3)[C@]3([H])CN(C(=O)[C@@H](NC(=O)[C@H](C)NC)C(C)(C)C)[C@@]([H])(C3)C(=O)N[C@@H](Cc3ccc4ccccc4c3)C(=O)N[C@@H](Cc3ccc(OCCCC2)cc3)C(O)=O)cc1)NS(=O)(=O)C1CC1 |r| Show InChI InChI=1S/C83H106N14O14S/c1-49(84-9)73(98)90-71(82(3,4)5)79(104)95-45-55-17-15-16-36-110-62-30-24-51(25-31-62)39-67(81(106)107)88-75(100)65(40-53-22-28-56-18-11-13-20-58(56)37-53)86-78(103)69-44-61(47-96(69)80(105)72(83(6,7)8)91-74(99)50(2)85-10)97-46-60(92-94-97)48-111-63-32-26-52(27-33-63)43-70(93-112(108,109)64-34-35-64)89-76(101)66(87-77(102)68(95)42-55)41-54-23-29-57-19-12-14-21-59(57)38-54/h11-14,18-33,37-38,46,49-50,55,61,64-72,84-85,93H,15-17,34-36,39-45,47-48H2,1-10H3,(H,86,103)(H,87,102)(H,88,100)(H,89,101)(H,90,98)(H,91,99)(H,106,107)/t49-,50-,55-,61-,65-,66-,67-,68-,69-,70+,71+,72+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research
Curated by ChEMBL
| Assay Description
Inhibition of cIAP BIR2-3 domain (unknown origin) |
ACS Med Chem Lett 6: 770-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00091
BindingDB Entry DOI: 10.7270/Q29P33D2 |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50071850
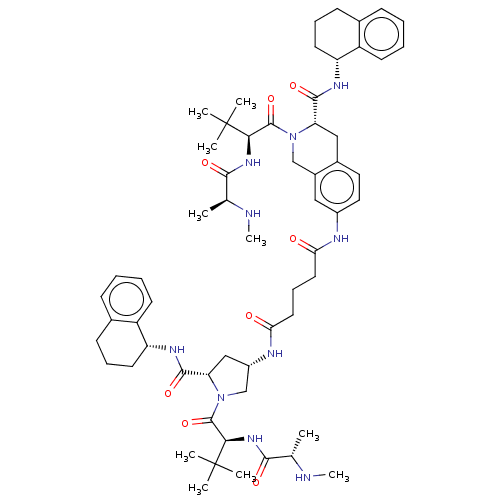
(CHEMBL3410808 | US9783573, Example 3)Show SMILES CN[C@@H](C)C(=O)N[C@H](C(=O)N1C[C@H](C[C@H]1C(=O)N[C@@H]1CCCc2ccccc12)NC(=O)CCCC(=O)Nc1ccc2C[C@H](N(Cc2c1)C(=O)[C@@H](NC(=O)[C@H](C)NC)C(C)(C)C)C(=O)N[C@@H]1CCCc2ccccc12)C(C)(C)C |r| Show InChI InChI=1S/C60H84N10O8/c1-35(61-9)53(73)67-51(59(3,4)5)57(77)69-33-40-30-41(29-28-39(40)31-47(69)55(75)65-45-24-15-20-37-18-11-13-22-43(37)45)63-49(71)26-17-27-50(72)64-42-32-48(56(76)66-46-25-16-21-38-19-12-14-23-44(38)46)70(34-42)58(78)52(60(6,7)8)68-54(74)36(2)62-10/h11-14,18-19,22-23,28-30,35-36,42,45-48,51-52,61-62H,15-17,20-21,24-27,31-34H2,1-10H3,(H,63,71)(H,64,72)(H,65,75)(H,66,76)(H,67,73)(H,68,74)/t35-,36-,42-,45+,46+,47-,48-,51+,52+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research
Curated by ChEMBL
| Assay Description
Binding affinity to human N-terminal His-tagged cIAP1 BIR2-3 C202A/C213G mutant after 60 mins by HTRF assay |
J Med Chem 58: 1556-62 (2015)
Article DOI: 10.1021/jm501482t
BindingDB Entry DOI: 10.7270/Q2N29ZM8 |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50071846

(CHEMBL3410877)Show SMILES CN[C@@H](C)C(=O)N[C@H](C(=O)N1C[C@H](C[C@H]1C(=O)N[C@@H]1CCCc2ccccc12)Nc1ccc2C[C@H](N(Cc2c1)C(=O)[C@@H](NC(=O)[C@H](C)NC)C(C)(C)C)C(=O)N[C@@H]1CCCc2ccccc12)C(C)(C)C |r| Show InChI InChI=1S/C55H77N9O6/c1-32(56-9)48(65)61-46(54(3,4)5)52(69)63-30-37-27-38(26-25-36(37)28-44(63)50(67)59-42-23-15-19-34-17-11-13-21-40(34)42)58-39-29-45(51(68)60-43-24-16-20-35-18-12-14-22-41(35)43)64(31-39)53(70)47(55(6,7)8)62-49(66)33(2)57-10/h11-14,17-18,21-22,25-27,32-33,39,42-47,56-58H,15-16,19-20,23-24,28-31H2,1-10H3,(H,59,67)(H,60,68)(H,61,65)(H,62,66)/t32-,33-,39-,42+,43+,44-,45-,46+,47+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research
Curated by ChEMBL
| Assay Description
Binding affinity to human N-terminal His-tagged cIAP1 BIR2-3 C202A/C213G mutant after 60 mins by HTRF assay |
J Med Chem 58: 1556-62 (2015)
Article DOI: 10.1021/jm501482t
BindingDB Entry DOI: 10.7270/Q2N29ZM8 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase XIAP
(Homo sapiens (Human)) | BDBM50112346

(CHEMBL3609325)Show SMILES [H][C@@]12CN(C(=O)[C@@H](NC(=O)[C@H](C)NC)C(C)(C)C)[C@@]([H])(C1)C(=O)N[C@@H](Cc1ccc3ccccc3c1)C(=O)N[C@@H](Cc1ccc(OCc3cn(nn3)[C@]3([H])CN(C(=O)[C@@H](NC(=O)[C@H](C)NC)C(C)(C)C)[C@@]([H])(C3)C(=O)N[C@@H](Cc3ccc4ccccc4c3)C(=O)N[C@@H](Cc3ccc(OCCCC2)cc3)C(O)=O)cc1)NS(=O)(=O)C1CC1 |r| Show InChI InChI=1S/C83H106N14O14S/c1-49(84-9)73(98)90-71(82(3,4)5)79(104)95-45-55-17-15-16-36-110-62-30-24-51(25-31-62)39-67(81(106)107)88-75(100)65(40-53-22-28-56-18-11-13-20-58(56)37-53)86-78(103)69-44-61(47-96(69)80(105)72(83(6,7)8)91-74(99)50(2)85-10)97-46-60(92-94-97)48-111-63-32-26-52(27-33-63)43-70(93-112(108,109)64-34-35-64)89-76(101)66(87-77(102)68(95)42-55)41-54-23-29-57-19-12-14-21-59(57)38-54/h11-14,18-33,37-38,46,49-50,55,61,64-72,84-85,93H,15-17,34-36,39-45,47-48H2,1-10H3,(H,86,103)(H,87,102)(H,88,100)(H,89,101)(H,90,98)(H,91,99)(H,106,107)/t49-,50-,55-,61-,65-,66-,67-,68-,69-,70+,71+,72+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research
Curated by ChEMBL
| Assay Description
Inhibition of XIAP BIR3 domain (unknown origin) |
ACS Med Chem Lett 6: 770-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00091
BindingDB Entry DOI: 10.7270/Q29P33D2 |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50071847
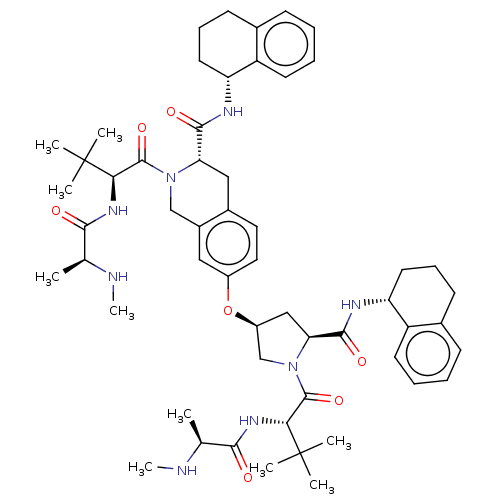
(CHEMBL3410878)Show SMILES CN[C@@H](C)C(=O)N[C@H](C(=O)N1C[C@H](C[C@H]1C(=O)N[C@@H]1CCCc2ccccc12)Oc1ccc2C[C@H](N(Cc2c1)C(=O)[C@@H](NC(=O)[C@H](C)NC)C(C)(C)C)C(=O)N[C@@H]1CCCc2ccccc12)C(C)(C)C |r| Show InChI InChI=1S/C55H76N8O7/c1-32(56-9)48(64)60-46(54(3,4)5)52(68)62-30-37-27-38(26-25-36(37)28-44(62)50(66)58-42-23-15-19-34-17-11-13-21-40(34)42)70-39-29-45(51(67)59-43-24-16-20-35-18-12-14-22-41(35)43)63(31-39)53(69)47(55(6,7)8)61-49(65)33(2)57-10/h11-14,17-18,21-22,25-27,32-33,39,42-47,56-57H,15-16,19-20,23-24,28-31H2,1-10H3,(H,58,66)(H,59,67)(H,60,64)(H,61,65)/t32-,33-,39-,42+,43+,44-,45-,46+,47+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research
Curated by ChEMBL
| Assay Description
Binding affinity to human N-terminal His-tagged cIAP1 BIR2-3 C202A/C213G mutant after 60 mins by HTRF assay |
J Med Chem 58: 1556-62 (2015)
Article DOI: 10.1021/jm501482t
BindingDB Entry DOI: 10.7270/Q2N29ZM8 |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50071820
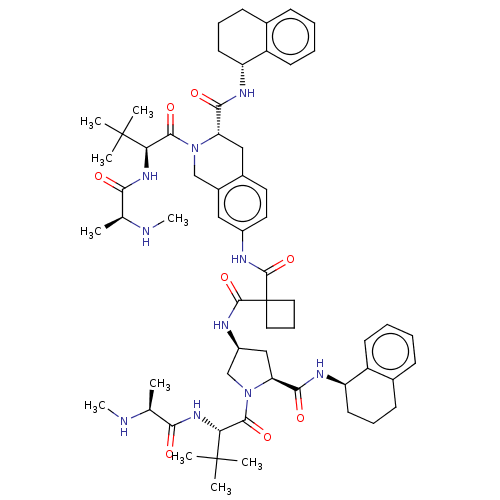
(CHEMBL3410871)Show SMILES CN[C@@H](C)C(=O)N[C@H](C(=O)N1C[C@H](C[C@H]1C(=O)N[C@@H]1CCCc2ccccc12)NC(=O)C1(CCC1)C(=O)Nc1ccc2C[C@H](N(Cc2c1)C(=O)[C@@H](NC(=O)[C@H](C)NC)C(C)(C)C)C(=O)N[C@@H]1CCCc2ccccc12)C(C)(C)C |r| Show InChI InChI=1S/C61H84N10O8/c1-35(62-9)51(72)68-49(59(3,4)5)55(76)70-33-40-30-41(27-26-39(40)31-47(70)53(74)66-45-24-15-20-37-18-11-13-22-43(37)45)64-57(78)61(28-17-29-61)58(79)65-42-32-48(54(75)67-46-25-16-21-38-19-12-14-23-44(38)46)71(34-42)56(77)50(60(6,7)8)69-52(73)36(2)63-10/h11-14,18-19,22-23,26-27,30,35-36,42,45-50,62-63H,15-17,20-21,24-25,28-29,31-34H2,1-10H3,(H,64,78)(H,65,79)(H,66,74)(H,67,75)(H,68,72)(H,69,73)/t35-,36-,42-,45+,46+,47-,48-,49+,50+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research
Curated by ChEMBL
| Assay Description
Binding affinity to human N-terminal His-tagged cIAP1 BIR2-3 C202A/C213G mutant after 60 mins by HTRF assay |
J Med Chem 58: 1556-62 (2015)
Article DOI: 10.1021/jm501482t
BindingDB Entry DOI: 10.7270/Q2N29ZM8 |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50071849
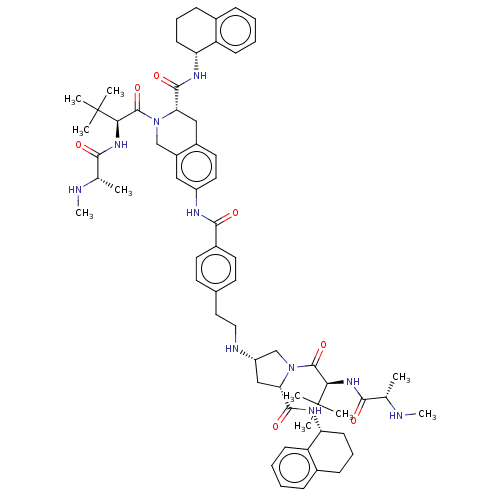
(CHEMBL3410807)Show SMILES CN[C@@H](C)C(=O)N[C@H](C(=O)N1C[C@H](C[C@H]1C(=O)N[C@@H]1CCCc2ccccc12)NCCc1ccc(cc1)C(=O)Nc1ccc2C[C@H](N(Cc2c1)C(=O)[C@@H](NC(=O)[C@H](C)NC)C(C)(C)C)C(=O)N[C@@H]1CCCc2ccccc12)C(C)(C)C |r| Show InChI InChI=1S/C64H86N10O7/c1-38(65-9)56(75)71-54(63(3,4)5)61(80)73-36-45-33-46(30-29-44(45)34-52(73)59(78)69-50-23-15-19-41-17-11-13-21-48(41)50)68-58(77)43-27-25-40(26-28-43)31-32-67-47-35-53(60(79)70-51-24-16-20-42-18-12-14-22-49(42)51)74(37-47)62(81)55(64(6,7)8)72-57(76)39(2)66-10/h11-14,17-18,21-22,25-30,33,38-39,47,50-55,65-67H,15-16,19-20,23-24,31-32,34-37H2,1-10H3,(H,68,77)(H,69,78)(H,70,79)(H,71,75)(H,72,76)/t38-,39-,47-,50+,51+,52-,53-,54+,55+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research
Curated by ChEMBL
| Assay Description
Binding affinity to human N-terminal His-tagged cIAP1 BIR2-3 C202A/C213G mutant after 60 mins by HTRF assay |
J Med Chem 58: 1556-62 (2015)
Article DOI: 10.1021/jm501482t
BindingDB Entry DOI: 10.7270/Q2N29ZM8 |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50071845
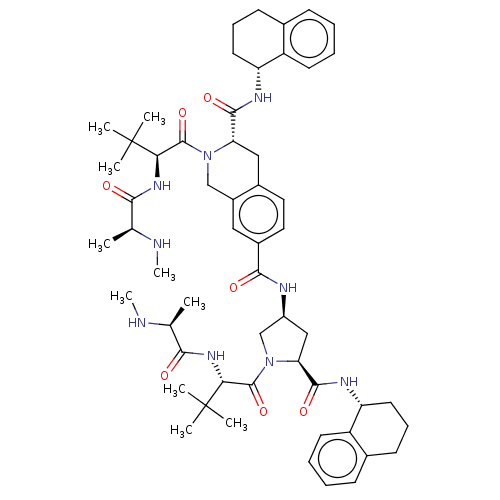
(CHEMBL3410876)Show SMILES CN[C@@H](C)C(=O)N[C@H](C(=O)N1C[C@H](C[C@H]1C(=O)N[C@@H]1CCCc2ccccc12)NC(=O)c1ccc2C[C@H](N(Cc2c1)C(=O)[C@@H](NC(=O)[C@H](C)NC)C(C)(C)C)C(=O)N[C@@H]1CCCc2ccccc12)C(C)(C)C |r| Show InChI InChI=1S/C56H77N9O7/c1-32(57-9)48(66)62-46(55(3,4)5)53(71)64-30-38-27-37(26-25-36(38)28-44(64)51(69)60-42-23-15-19-34-17-11-13-21-40(34)42)50(68)59-39-29-45(52(70)61-43-24-16-20-35-18-12-14-22-41(35)43)65(31-39)54(72)47(56(6,7)8)63-49(67)33(2)58-10/h11-14,17-18,21-22,25-27,32-33,39,42-47,57-58H,15-16,19-20,23-24,28-31H2,1-10H3,(H,59,68)(H,60,69)(H,61,70)(H,62,66)(H,63,67)/t32-,33-,39-,42+,43+,44-,45-,46+,47+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research
Curated by ChEMBL
| Assay Description
Binding affinity to human N-terminal His-tagged cIAP1 BIR2-3 C202A/C213G mutant after 60 mins by HTRF assay |
J Med Chem 58: 1556-62 (2015)
Article DOI: 10.1021/jm501482t
BindingDB Entry DOI: 10.7270/Q2N29ZM8 |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50071843

(CHEMBL3410873)Show SMILES CN[C@@H](C)C(=O)N[C@H](C(=O)N1C[C@H](C[C@H]1C(=O)N[C@@H]1CCCc2ccccc12)NC(=O)CNc1ccc2C[C@H](N(Cc2c1)C(=O)[C@@H](NC(=O)[C@H](C)NC)C(C)(C)C)C(=O)N[C@@H]1CCCc2ccccc12)C(C)(C)C |r| Show InChI InChI=1S/C57H80N10O7/c1-33(58-9)50(69)64-48(56(3,4)5)54(73)66-31-38-27-39(26-25-37(38)28-45(66)52(71)62-43-23-15-19-35-17-11-13-21-41(35)43)60-30-47(68)61-40-29-46(53(72)63-44-24-16-20-36-18-12-14-22-42(36)44)67(32-40)55(74)49(57(6,7)8)65-51(70)34(2)59-10/h11-14,17-18,21-22,25-27,33-34,40,43-46,48-49,58-60H,15-16,19-20,23-24,28-32H2,1-10H3,(H,61,68)(H,62,71)(H,63,72)(H,64,69)(H,65,70)/t33-,34-,40-,43+,44+,45-,46-,48+,49+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research
Curated by ChEMBL
| Assay Description
Binding affinity to human N-terminal His-tagged cIAP1 BIR2-3 C202A/C213G mutant after 60 mins by HTRF assay |
J Med Chem 58: 1556-62 (2015)
Article DOI: 10.1021/jm501482t
BindingDB Entry DOI: 10.7270/Q2N29ZM8 |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50071821
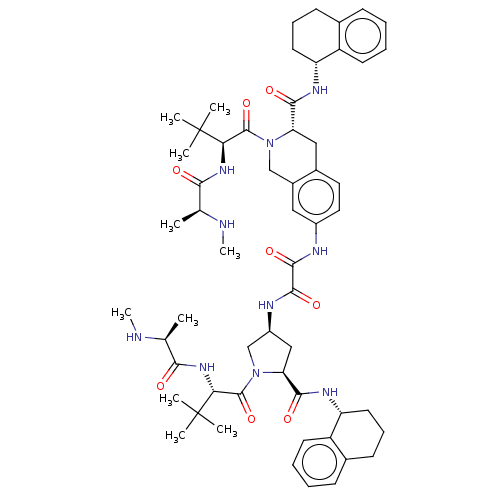
(CHEMBL3410872 | US9783573, Example 6)Show SMILES CN[C@@H](C)C(=O)N[C@H](C(=O)N1C[C@H](C[C@H]1C(=O)N[C@@H]1CCCc2ccccc12)NC(=O)C(=O)Nc1ccc2C[C@H](N(Cc2c1)C(=O)[C@@H](NC(=O)[C@H](C)NC)C(C)(C)C)C(=O)N[C@@H]1CCCc2ccccc12)C(C)(C)C |r| Show InChI InChI=1S/C57H78N10O8/c1-32(58-9)48(68)64-46(56(3,4)5)54(74)66-30-37-27-38(26-25-36(37)28-44(66)50(70)62-42-23-15-19-34-17-11-13-21-40(34)42)60-52(72)53(73)61-39-29-45(51(71)63-43-24-16-20-35-18-12-14-22-41(35)43)67(31-39)55(75)47(57(6,7)8)65-49(69)33(2)59-10/h11-14,17-18,21-22,25-27,32-33,39,42-47,58-59H,15-16,19-20,23-24,28-31H2,1-10H3,(H,60,72)(H,61,73)(H,62,70)(H,63,71)(H,64,68)(H,65,69)/t32-,33-,39-,42+,43+,44-,45-,46+,47+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research
Curated by ChEMBL
| Assay Description
Binding affinity to human N-terminal His-tagged cIAP1 BIR2-3 C202A/C213G mutant after 60 mins by HTRF assay |
J Med Chem 58: 1556-62 (2015)
Article DOI: 10.1021/jm501482t
BindingDB Entry DOI: 10.7270/Q2N29ZM8 |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50071848
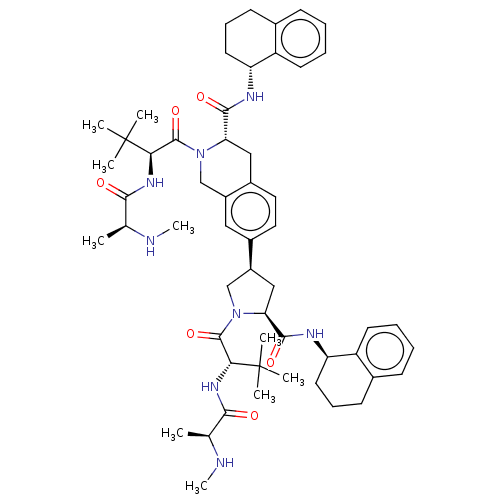
(CHEMBL3410879)Show SMILES CN[C@@H](C)C(=O)N[C@H](C(=O)N1C[C@H](C[C@H]1C(=O)N[C@@H]1CCCc2ccccc12)c1ccc2C[C@H](N(Cc2c1)C(=O)[C@@H](NC(=O)[C@H](C)NC)C(C)(C)C)C(=O)N[C@@H]1CCCc2ccccc12)C(C)(C)C |r| Show InChI InChI=1S/C55H76N8O6/c1-32(56-9)48(64)60-46(54(3,4)5)52(68)62-30-38-27-36(25-26-37(38)28-44(62)50(66)58-42-23-15-19-34-17-11-13-21-40(34)42)39-29-45(51(67)59-43-24-16-20-35-18-12-14-22-41(35)43)63(31-39)53(69)47(55(6,7)8)61-49(65)33(2)57-10/h11-14,17-18,21-22,25-27,32-33,39,42-47,56-57H,15-16,19-20,23-24,28-31H2,1-10H3,(H,58,66)(H,59,67)(H,60,64)(H,61,65)/t32-,33-,39-,42+,43+,44-,45-,46+,47+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research
Curated by ChEMBL
| Assay Description
Binding affinity to human N-terminal His-tagged cIAP1 BIR2-3 C202A/C213G mutant after 60 mins by HTRF assay |
J Med Chem 58: 1556-62 (2015)
Article DOI: 10.1021/jm501482t
BindingDB Entry DOI: 10.7270/Q2N29ZM8 |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50078360
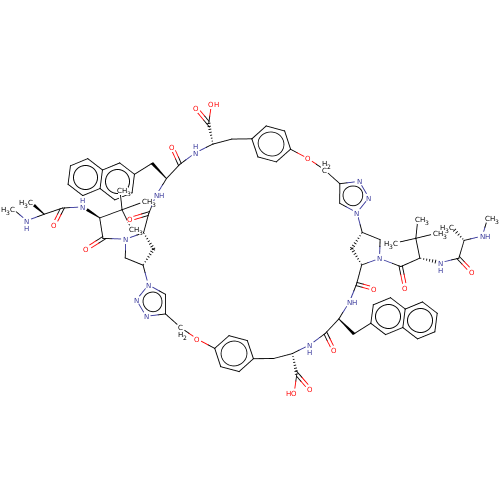
(CHEMBL3414728)Show SMILES [H][C@@]12CN(C(=O)[C@@H](NC(=O)[C@H](C)NC)C(C)(C)C)[C@@]([H])(C1)C(=O)N[C@@H](Cc1ccc3ccccc3c1)C(=O)N[C@@H](Cc1ccc(OCc3cn(nn3)[C@]3([H])CN(C(=O)[C@@H](NC(=O)[C@H](C)NC)C(C)(C)C)[C@@]([H])(C3)C(=O)N[C@@H](Cc3ccc4ccccc4c3)C(=O)N[C@@H](Cc3ccc(OCc4cn2nn4)cc3)C(O)=O)cc1)C(O)=O |r| Show InChI InChI=1S/C80H96N16O14/c1-45(81-9)69(97)87-67(79(3,4)5)75(103)93-41-57-37-65(93)73(101)83-61(35-49-19-25-51-15-11-13-17-53(51)31-49)71(99)85-63(77(105)106)33-47-23-29-60(30-24-47)110-44-56-40-96(92-90-56)58-38-66(94(42-58)76(104)68(80(6,7)8)88-70(98)46(2)82-10)74(102)84-62(36-50-20-26-52-16-12-14-18-54(52)32-50)72(100)86-64(78(107)108)34-48-21-27-59(28-22-48)109-43-55-39-95(57)91-89-55/h11-32,39-40,45-46,57-58,61-68,81-82H,33-38,41-44H2,1-10H3,(H,83,101)(H,84,102)(H,85,99)(H,86,100)(H,87,97)(H,88,98)(H,105,106)(H,107,108)/t45-,46-,57-,58-,61-,62-,63-,64-,65-,66-,67+,68+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ensemble Therapeutics Corp
Curated by ChEMBL
| Assay Description
Inhibition of cIAP1 BIR2-3 (154 to 352 residues) (unknown origin) fluoresceinated dimeric SMAC peptide based fluorescence polarization assay |
J Med Chem 58: 2855-61 (2015)
Article DOI: 10.1021/jm501892g
BindingDB Entry DOI: 10.7270/Q2RF5WQ0 |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50071863

(CHEMBL3410874)Show SMILES CN[C@@H](C)C(=O)N[C@H](C(=O)N1C[C@H](C[C@H]1C(=O)N[C@@H]1CCCc2ccccc12)NC(=O)Nc1ccc2C[C@H](N(Cc2c1)C(=O)[C@@H](NC(=O)[C@H](C)NC)C(C)(C)C)C(=O)N[C@@H]1CCCc2ccccc12)C(C)(C)C |r| Show InChI InChI=1S/C56H78N10O7/c1-32(57-9)48(67)63-46(55(3,4)5)52(71)65-30-37-27-38(26-25-36(37)28-44(65)50(69)61-42-23-15-19-34-17-11-13-21-40(34)42)59-54(73)60-39-29-45(51(70)62-43-24-16-20-35-18-12-14-22-41(35)43)66(31-39)53(72)47(56(6,7)8)64-49(68)33(2)58-10/h11-14,17-18,21-22,25-27,32-33,39,42-47,57-58H,15-16,19-20,23-24,28-31H2,1-10H3,(H,61,69)(H,62,70)(H,63,67)(H,64,68)(H2,59,60,73)/t32-,33-,39-,42+,43+,44-,45-,46+,47+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research
Curated by ChEMBL
| Assay Description
Binding affinity to human N-terminal His-tagged cIAP1 BIR2-3 C202A/C213G mutant after 60 mins by HTRF assay |
J Med Chem 58: 1556-62 (2015)
Article DOI: 10.1021/jm501482t
BindingDB Entry DOI: 10.7270/Q2N29ZM8 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50384321
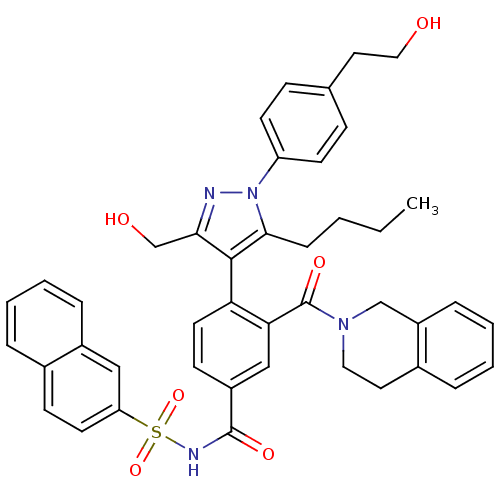
(CHEMBL2030856 | US9346795, 245)Show SMILES CCCCc1c(c(CO)nn1-c1ccc(CCO)cc1)-c1ccc(cc1C(=O)N1CCc2ccccc2C1)C(=O)NS(=O)(=O)c1ccc2ccccc2c1 |(-.54,-26.2,;-1.85,-25.39,;-1.8,-23.85,;-3.11,-23.04,;-3.07,-21.5,;-2.91,-19.96,;-4.31,-19.34,;-4.63,-17.83,;-6.1,-17.35,;-5.34,-20.48,;-4.58,-21.81,;-5.3,-23.17,;-6.84,-23.22,;-7.57,-24.57,;-6.76,-25.88,;-7.48,-27.24,;-9.02,-27.29,;-9.75,-28.65,;-5.21,-25.83,;-4.49,-24.47,;-1.57,-19.19,;-1.57,-17.66,;-.23,-16.88,;1.11,-17.66,;1.1,-19.2,;-.24,-19.97,;-.25,-21.51,;-1.59,-22.27,;1.08,-22.28,;1.08,-23.82,;2.4,-24.59,;3.74,-23.83,;5.06,-24.61,;6.4,-23.86,;6.41,-22.32,;5.08,-21.53,;3.75,-22.29,;2.42,-21.51,;2.44,-16.9,;2.45,-15.36,;3.77,-17.67,;5.1,-16.9,;4.32,-15.58,;5.86,-15.57,;6.44,-17.68,;6.43,-19.22,;7.76,-20,;9.11,-19.23,;10.45,-19.99,;11.79,-19.21,;11.77,-17.65,;10.42,-16.9,;9.1,-17.68,;7.77,-16.91,)| Show InChI InChI=1S/C43H42N4O6S/c1-2-3-12-40-41(39(28-49)44-47(40)35-17-13-29(14-18-35)22-24-48)37-20-16-33(26-38(37)43(51)46-23-21-31-9-5-7-11-34(31)27-46)42(50)45-54(52,53)36-19-15-30-8-4-6-10-32(30)25-36/h4-11,13-20,25-26,48-49H,2-3,12,21-24,27-28H2,1H3,(H,45,50) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research
Curated by ChEMBL
| Assay Description
Inhibition of biotin-labelled Bcl-xL using [FAM]-IWIAQELRRIGDEFNAYY-NH2 as substrate after 60 mins by FRET analysis |
Bioorg Med Chem Lett 22: 3951-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.106
BindingDB Entry DOI: 10.7270/Q2416Z2Z |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50384329

(CHEMBL2030864 | US9346795, 252)Show SMILES CCCCc1c(c(CO)nn1-c1ccc(Oc2cccc(Cl)c2)cc1)-c1ccc(cc1C(=O)N1CCc2ccccc2C1)C(=O)NS(=O)(=O)c1ccc2cccc(Cl)c2c1 |(-.35,-2.86,;.94,-3.7,;2.31,-3,;2.4,-1.46,;3.78,-.76,;5.2,-.17,;5.07,1.37,;6.24,2.37,;5.96,3.88,;3.58,1.72,;2.77,.41,;1.24,.4,;.46,1.73,;-1.08,1.72,;-1.84,.38,;-3.38,.36,;-4.16,1.69,;-5.7,1.67,;-6.48,2.99,;-5.73,4.34,;-4.19,4.35,;-3.43,5.69,;-3.41,3.02,;-1.05,-.96,;.48,-.94,;6.51,-.97,;7.86,-.24,;9.19,-1.04,;9.14,-2.59,;7.79,-3.32,;6.47,-2.51,;5.12,-3.24,;3.81,-2.43,;5.07,-4.78,;3.72,-5.51,;3.68,-7.04,;4.98,-7.85,;4.93,-9.38,;6.24,-10.2,;7.59,-9.48,;7.65,-7.93,;6.34,-7.13,;6.39,-5.58,;10.45,-3.39,;11.81,-2.66,;10.41,-4.93,;11.73,-5.75,;10.95,-7.08,;12.49,-7.08,;13.07,-4.99,;13.1,-3.45,;14.44,-2.69,;15.76,-3.47,;17.11,-2.71,;18.44,-3.49,;18.43,-5.05,;17.08,-5.81,;17.06,-7.35,;15.75,-5.02,;14.4,-5.78,)| Show InChI InChI=1S/C47H40Cl2N4O6S/c1-2-3-14-44-45(43(29-54)50-53(44)35-17-19-36(20-18-35)59-37-12-7-11-34(48)26-37)39-22-16-32(25-41(39)47(56)52-24-23-30-8-4-5-9-33(30)28-52)46(55)51-60(57,58)38-21-15-31-10-6-13-42(49)40(31)27-38/h4-13,15-22,25-27,54H,2-3,14,23-24,28-29H2,1H3,(H,51,55) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged Bcl2 using [FAM]-IWIAQELRRIGDEFNAYY-NH2 as substrate after 60 mins by fluorescence polarization assay |
Bioorg Med Chem Lett 22: 3951-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.106
BindingDB Entry DOI: 10.7270/Q2416Z2Z |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50384320

(CHEMBL2030855)Show SMILES CCCCc1c(c(CO)nn1-c1ccc(cc1)-c1ccccc1)-c1ccc(cc1C(=O)N1CCc2ccccc2C1)C(=O)NS(=O)(=O)c1ccc2ccccc2c1 |(46.25,-7.57,;44.94,-6.76,;44.98,-5.22,;43.67,-4.42,;43.72,-2.87,;43.88,-1.34,;42.47,-.71,;42.15,.8,;40.69,1.27,;41.44,-1.85,;42.21,-3.19,;41.49,-4.54,;39.95,-4.59,;39.22,-5.94,;40.03,-7.25,;41.58,-7.2,;42.3,-5.84,;39.31,-8.61,;37.77,-8.66,;37.04,-10.02,;37.86,-11.32,;39.4,-11.27,;40.12,-9.91,;45.22,-.57,;45.22,.97,;46.56,1.74,;47.89,.97,;47.89,-.57,;46.54,-1.34,;46.54,-2.88,;45.2,-3.64,;47.87,-3.65,;47.86,-5.19,;49.19,-5.97,;50.52,-5.21,;51.84,-5.98,;53.18,-5.23,;53.2,-3.69,;51.87,-2.9,;50.54,-3.67,;49.21,-2.88,;49.23,1.73,;49.24,3.27,;50.56,.95,;51.89,1.72,;51.11,3.05,;52.65,3.06,;53.23,.94,;53.21,-.6,;54.55,-1.38,;55.9,-.6,;57.24,-1.36,;58.57,-.58,;58.56,.97,;57.21,1.73,;55.89,.94,;54.56,1.72,)| Show InChI InChI=1S/C47H42N4O5S/c1-2-3-17-44-45(43(31-52)48-51(44)39-22-18-35(19-23-39)32-11-5-4-6-12-32)41-25-21-37(29-42(41)47(54)50-27-26-34-14-8-10-16-38(34)30-50)46(53)49-57(55,56)40-24-20-33-13-7-9-15-36(33)28-40/h4-16,18-25,28-29,52H,2-3,17,26-27,30-31H2,1H3,(H,49,53) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged Bcl2 using [FAM]-IWIAQELRRIGDEFNAYY-NH2 as substrate after 60 mins by fluorescence polarization assay |
Bioorg Med Chem Lett 22: 3951-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.106
BindingDB Entry DOI: 10.7270/Q2416Z2Z |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50384322

(CHEMBL2030857)Show SMILES CCCCc1c(c(CO)nn1-c1ccc(CCCO)cc1)-c1ccc(cc1C(=O)N1CCc2ccccc2C1)C(=O)NS(=O)(=O)c1ccc2ccccc2c1 |(23.77,-26.25,;22.46,-25.44,;22.51,-23.9,;21.2,-23.1,;21.24,-21.55,;21.4,-20.02,;20,-19.39,;19.68,-17.88,;18.21,-17.41,;18.97,-20.53,;19.73,-21.87,;19.01,-23.22,;17.47,-23.27,;16.74,-24.62,;17.55,-25.93,;16.83,-27.29,;15.29,-27.34,;14.57,-28.7,;13.03,-28.75,;19.1,-25.88,;19.82,-24.52,;22.74,-19.25,;22.74,-17.71,;24.08,-16.94,;25.42,-17.71,;25.41,-19.25,;24.07,-20.02,;24.06,-21.56,;22.72,-22.32,;25.39,-22.33,;25.39,-23.87,;26.71,-24.65,;28.05,-23.89,;29.37,-24.66,;30.71,-23.91,;30.72,-22.37,;29.39,-21.58,;28.06,-22.35,;26.73,-21.56,;26.75,-16.95,;26.76,-15.41,;28.08,-17.73,;29.41,-16.96,;28.63,-15.63,;30.17,-15.62,;30.75,-17.74,;30.74,-19.28,;32.07,-20.06,;33.42,-19.28,;34.76,-20.04,;36.1,-19.26,;36.08,-17.71,;34.73,-16.95,;33.41,-17.74,;32.08,-16.96,)| Show InChI InChI=1S/C44H44N4O6S/c1-2-3-14-41-42(40(29-50)45-48(41)36-19-15-30(16-20-36)9-8-25-49)38-22-18-34(27-39(38)44(52)47-24-23-32-11-5-7-13-35(32)28-47)43(51)46-55(53,54)37-21-17-31-10-4-6-12-33(31)26-37/h4-7,10-13,15-22,26-27,49-50H,2-3,8-9,14,23-25,28-29H2,1H3,(H,46,51) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research
Curated by ChEMBL
| Assay Description
Inhibition of biotin-labelled Bcl-xL using [FAM]-IWIAQELRRIGDEFNAYY-NH2 as substrate after 60 mins by FRET analysis |
Bioorg Med Chem Lett 22: 3951-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.106
BindingDB Entry DOI: 10.7270/Q2416Z2Z |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50384325
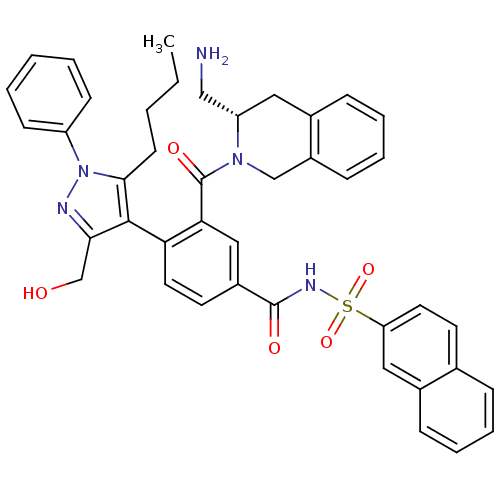
(CHEMBL2030860)Show SMILES CCCCc1c(c(CO)nn1-c1ccccc1)-c1ccc(cc1C(=O)N1Cc2ccccc2C[C@H]1CN)C(=O)NS(=O)(=O)c1ccc2ccccc2c1 |r,wU:34.39,(11.77,-38.55,;13.06,-39.38,;14.43,-38.69,;14.52,-37.15,;15.9,-36.45,;17.32,-35.86,;17.2,-34.32,;18.37,-33.32,;18.08,-31.81,;15.7,-33.96,;14.89,-35.28,;13.36,-35.29,;12.58,-33.96,;11.04,-33.97,;10.28,-35.31,;11.07,-36.64,;12.6,-36.63,;18.63,-36.66,;19.98,-35.92,;21.31,-36.73,;21.26,-38.28,;19.91,-39.01,;18.59,-38.2,;17.24,-38.93,;15.93,-38.12,;17.2,-40.47,;18.51,-41.27,;18.46,-42.82,;19.77,-43.62,;19.72,-45.16,;18.36,-45.89,;17.05,-45.07,;17.1,-43.54,;15.8,-42.73,;15.84,-41.2,;14.53,-40.39,;13.18,-41.12,;22.57,-39.08,;23.93,-38.35,;22.53,-40.62,;23.84,-41.42,;24.63,-40.1,;25.38,-41.45,;23.8,-42.97,;22.44,-43.7,;22.39,-45.24,;23.72,-46.05,;23.7,-47.6,;25.02,-48.39,;26.38,-47.63,;26.39,-46.09,;25.07,-45.3,;25.11,-43.77,)| Show InChI InChI=1S/C42H41N5O5S/c1-2-3-17-39-40(38(27-48)44-47(39)33-15-5-4-6-16-33)36-21-19-31(41(49)45-53(51,52)35-20-18-28-11-7-8-13-30(28)23-35)24-37(36)42(50)46-26-32-14-10-9-12-29(32)22-34(46)25-43/h4-16,18-21,23-24,34,48H,2-3,17,22,25-27,43H2,1H3,(H,45,49)/t34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research
Curated by ChEMBL
| Assay Description
Inhibition of biotin-labelled Bcl-xL using [FAM]-IWIAQELRRIGDEFNAYY-NH2 as substrate after 60 mins by FRET analysis |
Bioorg Med Chem Lett 22: 3951-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.106
BindingDB Entry DOI: 10.7270/Q2416Z2Z |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50078354
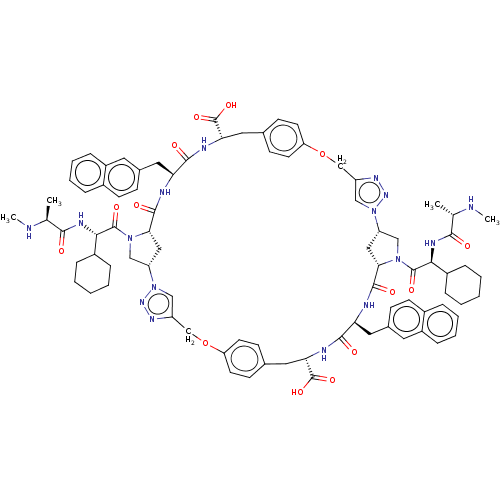
(CHEMBL3414727)Show SMILES [H][C@@]12CN(C(=O)[C@@H](NC(=O)[C@H](C)NC)C3CCCCC3)[C@@]([H])(C1)C(=O)N[C@@H](Cc1ccc3ccccc3c1)C(=O)N[C@@H](Cc1ccc(OCc3cn(nn3)[C@]3([H])CN(C(=O)[C@@H](NC(=O)[C@H](C)NC)C4CCCCC4)[C@@]([H])(C3)C(=O)N[C@@H](Cc3ccc4ccccc4c3)C(=O)N[C@@H](Cc3ccc(OCc4cn2nn4)cc3)C(O)=O)cc1)C(O)=O |r| Show InChI InChI=1S/C84H100N16O14/c1-49(85-3)75(101)91-73(57-17-7-5-8-18-57)81(107)97-45-63-41-71(97)79(105)87-67(39-53-23-29-55-15-11-13-21-59(55)35-53)77(103)89-69(83(109)110)37-51-27-33-66(34-28-51)114-48-62-44-100(96-94-62)64-42-72(98(46-64)82(108)74(58-19-9-6-10-20-58)92-76(102)50(2)86-4)80(106)88-68(40-54-24-30-56-16-12-14-22-60(56)36-54)78(104)90-70(84(111)112)38-52-25-31-65(32-26-52)113-47-61-43-99(63)95-93-61/h11-16,21-36,43-44,49-50,57-58,63-64,67-74,85-86H,5-10,17-20,37-42,45-48H2,1-4H3,(H,87,105)(H,88,106)(H,89,103)(H,90,104)(H,91,101)(H,92,102)(H,109,110)(H,111,112)/t49-,50-,63-,64-,67-,68-,69-,70-,71-,72-,73-,74-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Ensemble Therapeutics Corp
Curated by ChEMBL
| Assay Description
Inhibition of cIAP1 BIR2-3 (154 to 352 residues) (unknown origin) fluoresceinated dimeric SMAC peptide based fluorescence polarization assay |
J Med Chem 58: 2855-61 (2015)
Article DOI: 10.1021/jm501892g
BindingDB Entry DOI: 10.7270/Q2RF5WQ0 |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 3
(Homo sapiens (Human)) | BDBM50078358
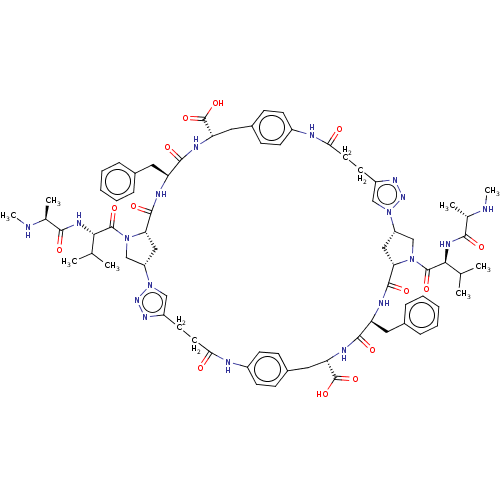
(CHEMBL3414723)Show SMILES [H][C@@]12CN(C(=O)[C@@H](NC(=O)[C@H](C)NC)C(C)C)[C@@]([H])(C1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccc(NC(=O)CCc3cn(nn3)[C@]3([H])CN(C(=O)[C@@H](NC(=O)[C@H](C)NC)C(C)C)[C@@]([H])(C3)C(=O)N[C@@H](Cc3ccccc3)C(=O)N[C@@H](Cc3ccc(NC(=O)CCc4cn2nn4)cc3)C(O)=O)cc1)C(O)=O |r| Show InChI InChI=1S/C74H94N18O14/c1-41(2)63(83-65(95)43(5)75-7)71(101)89-39-53-35-59(89)69(99)79-55(31-45-15-11-9-12-16-45)67(97)81-57(73(103)104)33-47-19-23-50(24-20-47)78-62(94)30-28-52-38-92(88-86-52)54-36-60(90(40-54)72(102)64(42(3)4)84-66(96)44(6)76-8)70(100)80-56(32-46-17-13-10-14-18-46)68(98)82-58(74(105)106)34-48-21-25-49(26-22-48)77-61(93)29-27-51-37-91(53)87-85-51/h9-26,37-38,41-44,53-60,63-64,75-76H,27-36,39-40H2,1-8H3,(H,77,93)(H,78,94)(H,79,99)(H,80,100)(H,81,97)(H,82,98)(H,83,95)(H,84,96)(H,103,104)(H,105,106)/t43-,44-,53-,54-,55-,56-,57-,58-,59-,60-,63-,64-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Ensemble Therapeutics Corp
Curated by ChEMBL
| Assay Description
Inhibition of cIAP1 BIR3 (154 to 352 residues) (unknown origin) by fluoresceinated dimeric SMAC peptide based fluorescence polarization assay |
J Med Chem 58: 2855-61 (2015)
Article DOI: 10.1021/jm501892g
BindingDB Entry DOI: 10.7270/Q2RF5WQ0 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50384324
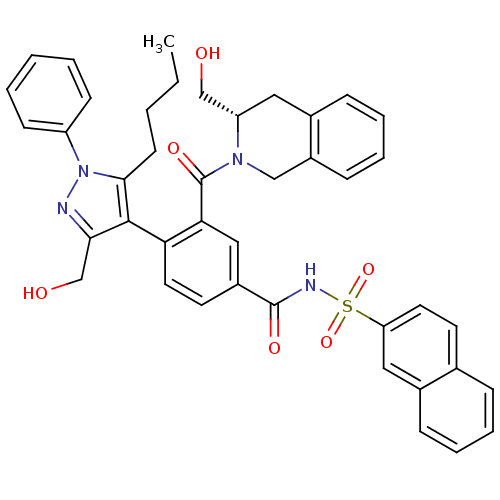
(CHEMBL2030859 | US9346795, 260)Show SMILES CCCCc1c(c(CO)nn1-c1ccccc1)-c1ccc(cc1C(=O)N1Cc2ccccc2C[C@H]1CO)C(=O)NS(=O)(=O)c1ccc2ccccc2c1 |r,wU:34.39,(-8.12,-38.1,;-6.89,-39.03,;-5.47,-38.44,;-5.28,-36.91,;-3.85,-36.31,;-2.39,-35.82,;-2.4,-34.28,;-1.16,-33.36,;-1.34,-31.83,;-3.87,-33.82,;-4.77,-35.07,;-6.3,-34.97,;-6.98,-33.59,;-8.52,-33.49,;-9.37,-34.77,;-8.68,-36.16,;-7.15,-36.25,;-1.14,-36.72,;.21,-35.98,;1.54,-36.79,;1.49,-38.33,;.14,-39.06,;-1.18,-38.25,;-2.53,-38.98,;-3.84,-38.18,;-2.57,-40.52,;-1.26,-41.33,;-1.31,-42.87,;0,-43.68,;-.05,-45.22,;-1.41,-45.94,;-2.72,-45.13,;-2.67,-43.6,;-3.97,-42.79,;-3.93,-41.25,;-5.24,-40.45,;-6.59,-41.18,;2.8,-39.14,;4.16,-38.41,;2.76,-40.68,;4.07,-41.48,;4.86,-40.16,;5.61,-41.5,;4.03,-43.02,;2.67,-43.75,;2.62,-45.29,;3.95,-46.11,;3.93,-47.65,;5.25,-48.45,;6.61,-47.69,;6.63,-46.14,;5.3,-45.36,;5.34,-43.82,)| Show InChI InChI=1S/C42H40N4O6S/c1-2-3-17-39-40(38(27-48)43-46(39)33-15-5-4-6-16-33)36-21-19-31(41(49)44-53(51,52)35-20-18-28-11-7-8-13-30(28)23-35)24-37(36)42(50)45-25-32-14-10-9-12-29(32)22-34(45)26-47/h4-16,18-21,23-24,34,47-48H,2-3,17,22,25-27H2,1H3,(H,44,49)/t34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research
Curated by ChEMBL
| Assay Description
Inhibition of biotin-labelled Bcl-xL using [FAM]-IWIAQELRRIGDEFNAYY-NH2 as substrate after 60 mins by FRET analysis |
Bioorg Med Chem Lett 22: 3951-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.106
BindingDB Entry DOI: 10.7270/Q2416Z2Z |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50384318
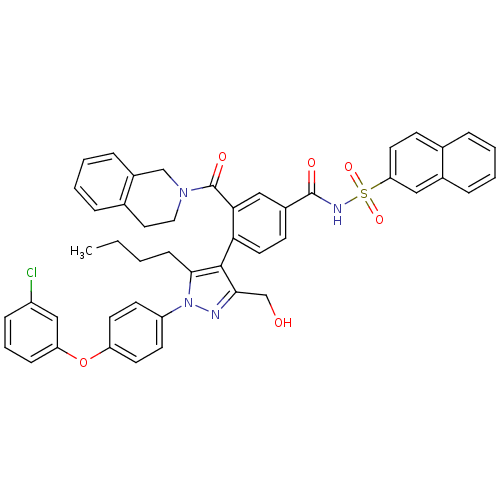
(CHEMBL2030853 | US9346795, 243)Show SMILES CCCCc1c(c(CO)nn1-c1ccc(Oc2cccc(Cl)c2)cc1)-c1ccc(cc1C(=O)N1CCc2ccccc2C1)C(=O)NS(=O)(=O)c1ccc2ccccc2c1 |(-1.41,-6.84,;-2.72,-6.03,;-2.67,-4.49,;-3.99,-3.68,;-3.94,-2.14,;-3.78,-.6,;-5.18,.02,;-5.5,1.53,;-6.97,2,;-6.22,-1.12,;-5.45,-2.46,;-6.17,-3.81,;-7.71,-3.86,;-8.44,-5.21,;-7.63,-6.52,;-8.35,-7.88,;-7.53,-9.19,;-8.27,-10.54,;-7.45,-11.85,;-5.91,-11.8,;-5.19,-10.43,;-3.65,-10.37,;-6,-9.13,;-6.08,-6.47,;-5.36,-5.11,;-2.44,.16,;-2.44,1.7,;-1.1,2.48,;.23,1.7,;.23,.16,;-1.11,-.61,;-1.12,-2.15,;-2.46,-2.91,;.21,-2.92,;.21,-4.46,;1.53,-5.23,;2.86,-4.47,;4.18,-5.25,;5.53,-4.5,;5.54,-2.96,;4.21,-2.17,;2.88,-2.93,;1.55,-2.15,;1.57,2.46,;1.58,4,;2.9,1.69,;4.23,2.45,;3.45,3.78,;4.99,3.79,;5.57,1.67,;5.56,.13,;6.89,-.65,;8.24,.13,;9.58,-.63,;10.92,.15,;10.9,1.71,;9.55,2.46,;8.23,1.68,;6.9,2.45,)| Show InChI InChI=1S/C47H41ClN4O6S/c1-2-3-15-44-45(43(30-53)49-52(44)37-18-20-38(21-19-37)58-39-14-8-13-36(48)28-39)41-23-17-34(27-42(41)47(55)51-25-24-32-10-5-7-12-35(32)29-51)46(54)50-59(56,57)40-22-16-31-9-4-6-11-33(31)26-40/h4-14,16-23,26-28,53H,2-3,15,24-25,29-30H2,1H3,(H,50,54) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged Bcl2 using [FAM]-IWIAQELRRIGDEFNAYY-NH2 as substrate after 60 mins by fluorescence polarization assay |
Bioorg Med Chem Lett 22: 3951-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.106
BindingDB Entry DOI: 10.7270/Q2416Z2Z |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50078359

(CHEMBL3414729)Show SMILES [H][C@@]12CN(C(=O)[C@@H](NC(=O)[C@H](C)NC)C(C)C)[C@@]([H])(C1)C(=O)N[C@@H](Cc1ccc3ccccc3c1)C(=O)N[C@@H](Cc1cn(nn1)[C@]1([H])CN(C(=O)[C@@H](NC(=O)[C@H](C)NC)C(C)C)[C@@]([H])(C1)C(=O)N[C@@H](Cc1ccc3ccccc3c1)C(=O)N[C@@H](Cc1cn2nn1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C64H80N16O12/c1-33(2)53(71-55(81)35(5)65-7)61(87)77-31-45-27-51(77)59(85)67-47(23-37-17-19-39-13-9-11-15-41(39)21-37)57(83)69-50(64(91)92)26-44-30-80(76-74-44)46-28-52(78(32-46)62(88)54(34(3)4)72-56(82)36(6)66-8)60(86)68-48(24-38-18-20-40-14-10-12-16-42(40)22-38)58(84)70-49(63(89)90)25-43-29-79(45)75-73-43/h9-22,29-30,33-36,45-54,65-66H,23-28,31-32H2,1-8H3,(H,67,85)(H,68,86)(H,69,83)(H,70,84)(H,71,81)(H,72,82)(H,89,90)(H,91,92)/t35-,36-,45-,46-,47-,48-,49-,50-,51-,52-,53-,54-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Ensemble Therapeutics Corp
Curated by ChEMBL
| Assay Description
Inhibition of XIAP BIR2-3 (125 to 356 residues) C202A/C213G mutant (unknown origin) fluoresceinated dimeric SMAC peptide based fluorescence polarizat... |
J Med Chem 58: 2855-61 (2015)
Article DOI: 10.1021/jm501892g
BindingDB Entry DOI: 10.7270/Q2RF5WQ0 |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50384358
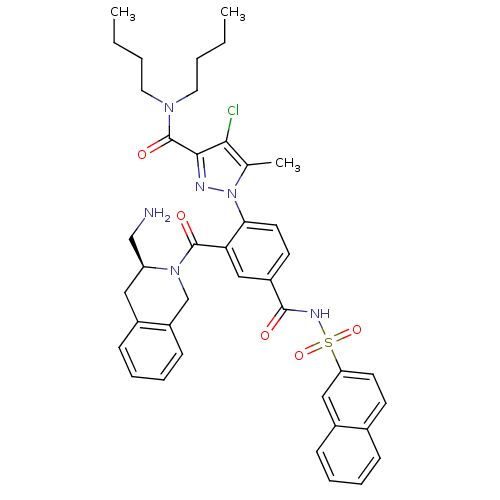
(CHEMBL2031027 | US9346795, 92)Show SMILES CCCCN(CCCC)C(=O)c1nn(c(C)c1Cl)-c1ccc(cc1C(=O)N1Cc2ccccc2C[C@H]1CN)C(=O)NS(=O)(=O)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C41H45ClN6O5S/c1-4-6-20-46(21-7-5-2)41(51)38-37(42)27(3)48(44-38)36-19-17-31(39(49)45-54(52,53)34-18-16-28-12-8-9-14-30(28)23-34)24-35(36)40(50)47-26-32-15-11-10-13-29(32)22-33(47)25-43/h8-19,23-24,33H,4-7,20-22,25-26,43H2,1-3H3,(H,45,49)/t33-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research
Curated by ChEMBL
| Assay Description
Inhibition in GST-tagged Bcl2 using [FAM]-IWIAQELRRIGDEFNAYY-NH2 as substrate after 60 mins by fluorescence polarization assay |
Bioorg Med Chem Lett 22: 3946-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.103
BindingDB Entry DOI: 10.7270/Q20866BM |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50384340
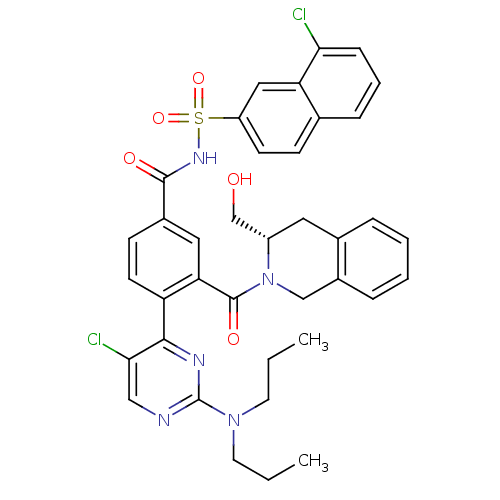
(CHEMBL2031009 | US9346795, 328)Show SMILES CCCN(CCC)c1ncc(Cl)c(n1)-c1ccc(cc1C(=O)N1Cc2ccccc2C[C@H]1CO)C(=O)NS(=O)(=O)c1ccc2cccc(Cl)c2c1 |r| Show InChI InChI=1S/C38H37Cl2N5O5S/c1-3-16-44(17-4-2)38-41-21-34(40)35(42-38)30-15-13-26(19-32(30)37(48)45-22-27-9-6-5-8-25(27)18-28(45)23-46)36(47)43-51(49,50)29-14-12-24-10-7-11-33(39)31(24)20-29/h5-15,19-21,28,46H,3-4,16-18,22-23H2,1-2H3,(H,43,47)/t28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research
Curated by ChEMBL
| Assay Description
Inhibition of biotin-labelled Bcl-xL using [FAM]-IWIAQELRRIGDEFNAYY-NH2 as substrate after 60 mins by FRET analysis |
Bioorg Med Chem Lett 22: 3951-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.106
BindingDB Entry DOI: 10.7270/Q2416Z2Z |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50384340

(CHEMBL2031009 | US9346795, 328)Show SMILES CCCN(CCC)c1ncc(Cl)c(n1)-c1ccc(cc1C(=O)N1Cc2ccccc2C[C@H]1CO)C(=O)NS(=O)(=O)c1ccc2cccc(Cl)c2c1 |r| Show InChI InChI=1S/C38H37Cl2N5O5S/c1-3-16-44(17-4-2)38-41-21-34(40)35(42-38)30-15-13-26(19-32(30)37(48)45-22-27-9-6-5-8-25(27)18-28(45)23-46)36(47)43-51(49,50)29-14-12-24-10-7-11-33(39)31(24)20-29/h5-15,19-21,28,46H,3-4,16-18,22-23H2,1-2H3,(H,43,47)/t28-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged Bcl2 using [FAM]-IWIAQELRRIGDEFNAYY-NH2 as substrate after 60 mins by fluorescence polarization assay |
Bioorg Med Chem Lett 22: 3951-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.106
BindingDB Entry DOI: 10.7270/Q2416Z2Z |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50030280
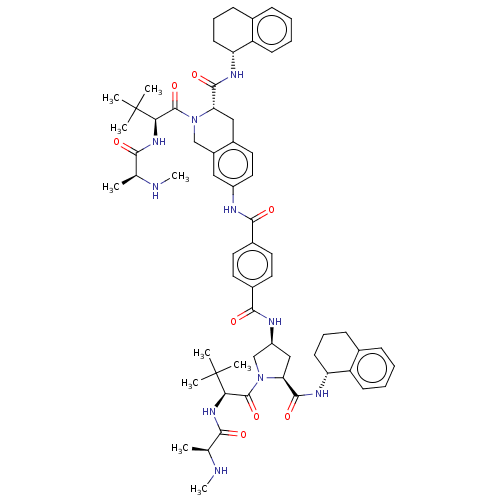
(CHEMBL3339214 | US9783573, Example 10)Show SMILES CN[C@@H](C)C(=O)N[C@H](C(=O)N1C[C@H](C[C@H]1C(=O)N[C@@H]1CCCc2ccccc12)NC(=O)c1ccc(cc1)C(=O)Nc1ccc2C[C@H](N(Cc2c1)C(=O)[C@@H](NC(=O)[C@H](C)NC)C(C)(C)C)C(=O)N[C@@H]1CCCc2ccccc12)C(C)(C)C |r| Show InChI InChI=1S/C63H82N10O8/c1-36(64-9)54(74)70-52(62(3,4)5)60(80)72-34-43-31-44(30-29-42(43)32-50(72)58(78)68-48-23-15-19-38-17-11-13-21-46(38)48)66-56(76)40-25-27-41(28-26-40)57(77)67-45-33-51(59(79)69-49-24-16-20-39-18-12-14-22-47(39)49)73(35-45)61(81)53(63(6,7)8)71-55(75)37(2)65-10/h11-14,17-18,21-22,25-31,36-37,45,48-53,64-65H,15-16,19-20,23-24,32-35H2,1-10H3,(H,66,76)(H,67,77)(H,68,78)(H,69,79)(H,70,74)(H,71,75)/t36-,37-,45-,48+,49+,50-,51-,52+,53+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research
Curated by ChEMBL
| Assay Description
Binding affinity to human N-terminal His-tagged cIAP1 BIR2-3 C202A/C213G mutant after 60 mins by HTRF assay |
J Med Chem 58: 1556-62 (2015)
Article DOI: 10.1021/jm501482t
BindingDB Entry DOI: 10.7270/Q2N29ZM8 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50384358

(CHEMBL2031027 | US9346795, 92)Show SMILES CCCCN(CCCC)C(=O)c1nn(c(C)c1Cl)-c1ccc(cc1C(=O)N1Cc2ccccc2C[C@H]1CN)C(=O)NS(=O)(=O)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C41H45ClN6O5S/c1-4-6-20-46(21-7-5-2)41(51)38-37(42)27(3)48(44-38)36-19-17-31(39(49)45-54(52,53)34-18-16-28-12-8-9-14-30(28)23-34)24-35(36)40(50)47-26-32-15-11-10-13-29(32)22-33(47)25-43/h8-19,23-24,33H,4-7,20-22,25-26,43H2,1-3H3,(H,45,49)/t33-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research
Curated by ChEMBL
| Assay Description
Inhibition of Bcl-XL using fluoresceinated 18-mer Bim as substrate after 60 mins by FRET analysis |
Bioorg Med Chem Lett 22: 3946-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.103
BindingDB Entry DOI: 10.7270/Q20866BM |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 3
(Homo sapiens (Human)) | BDBM50078359

(CHEMBL3414729)Show SMILES [H][C@@]12CN(C(=O)[C@@H](NC(=O)[C@H](C)NC)C(C)C)[C@@]([H])(C1)C(=O)N[C@@H](Cc1ccc3ccccc3c1)C(=O)N[C@@H](Cc1cn(nn1)[C@]1([H])CN(C(=O)[C@@H](NC(=O)[C@H](C)NC)C(C)C)[C@@]([H])(C1)C(=O)N[C@@H](Cc1ccc3ccccc3c1)C(=O)N[C@@H](Cc1cn2nn1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C64H80N16O12/c1-33(2)53(71-55(81)35(5)65-7)61(87)77-31-45-27-51(77)59(85)67-47(23-37-17-19-39-13-9-11-15-41(39)21-37)57(83)69-50(64(91)92)26-44-30-80(76-74-44)46-28-52(78(32-46)62(88)54(34(3)4)72-56(82)36(6)66-8)60(86)68-48(24-38-18-20-40-14-10-12-16-42(40)22-38)58(84)70-49(63(89)90)25-43-29-79(45)75-73-43/h9-22,29-30,33-36,45-54,65-66H,23-28,31-32H2,1-8H3,(H,67,85)(H,68,86)(H,69,83)(H,70,84)(H,71,81)(H,72,82)(H,89,90)(H,91,92)/t35-,36-,45-,46-,47-,48-,49-,50-,51-,52-,53-,54-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Ensemble Therapeutics Corp
Curated by ChEMBL
| Assay Description
Inhibition of cIAP1 BIR3 (154 to 352 residues) (unknown origin) by fluoresceinated dimeric SMAC peptide based fluorescence polarization assay |
J Med Chem 58: 2855-61 (2015)
Article DOI: 10.1021/jm501892g
BindingDB Entry DOI: 10.7270/Q2RF5WQ0 |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 3
(Homo sapiens (Human)) | BDBM50078357
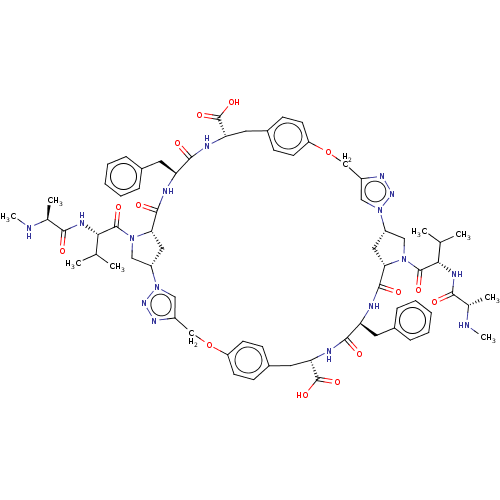
(CHEMBL3414724)Show SMILES [H][C@@]12CN(C(=O)[C@@H](NC(=O)[C@H](C)NC)C(C)C)[C@@]([H])(C1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccc(OCc3cn(nn3)[C@]3([H])CN(C(=O)[C@@H](NC(=O)[C@H](C)NC)C(C)C)[C@@]([H])(C3)C(=O)N[C@@H](Cc3ccccc3)C(=O)N[C@@H](Cc3ccc(OCc4cn2nn4)cc3)C(O)=O)cc1)C(O)=O |r| Show InChI InChI=1S/C70H88N16O14/c1-39(2)59(77-61(87)41(5)71-7)67(93)83-35-49-31-57(83)65(91)73-53(27-43-15-11-9-12-16-43)63(89)75-55(69(95)96)29-45-21-25-52(26-22-45)100-38-48-34-86(82-80-48)50-32-58(84(36-50)68(94)60(40(3)4)78-62(88)42(6)72-8)66(92)74-54(28-44-17-13-10-14-18-44)64(90)76-56(70(97)98)30-46-19-23-51(24-20-46)99-37-47-33-85(49)81-79-47/h9-26,33-34,39-42,49-50,53-60,71-72H,27-32,35-38H2,1-8H3,(H,73,91)(H,74,92)(H,75,89)(H,76,90)(H,77,87)(H,78,88)(H,95,96)(H,97,98)/t41-,42-,49-,50-,53-,54-,55-,56-,57-,58-,59-,60-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Ensemble Therapeutics Corp
Curated by ChEMBL
| Assay Description
Inhibition of cIAP1 BIR3 (154 to 352 residues) (unknown origin) by fluoresceinated dimeric SMAC peptide based fluorescence polarization assay |
J Med Chem 58: 2855-61 (2015)
Article DOI: 10.1021/jm501892g
BindingDB Entry DOI: 10.7270/Q2RF5WQ0 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50384312
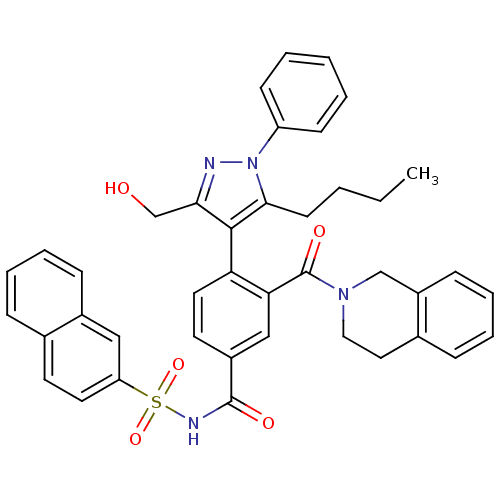
(CHEMBL2030847)Show SMILES CCCCc1c(c(CO)nn1-c1ccccc1)-c1ccc(cc1C(=O)N1CCc2ccccc2C1)C(=O)NS(=O)(=O)c1ccc2ccccc2c1 |(-.21,-22.28,;-1.52,-21.47,;-1.48,-19.93,;-2.79,-19.12,;-2.74,-17.58,;-2.58,-16.04,;-3.99,-15.42,;-4.31,-13.91,;-5.77,-13.43,;-5.02,-16.56,;-4.25,-17.89,;-4.97,-19.25,;-6.52,-19.3,;-7.24,-20.65,;-6.43,-21.96,;-4.88,-21.91,;-4.16,-20.55,;-1.24,-15.27,;-1.24,-13.74,;.1,-12.96,;1.43,-13.74,;1.43,-15.28,;.08,-16.05,;.08,-17.59,;-1.26,-18.35,;1.41,-18.36,;1.4,-19.9,;2.73,-20.67,;4.06,-19.91,;5.38,-20.69,;6.72,-19.94,;6.74,-18.4,;5.41,-17.61,;4.08,-18.37,;2.75,-17.59,;2.77,-12.98,;2.78,-11.44,;4.1,-13.75,;5.43,-12.98,;4.65,-11.66,;6.19,-11.65,;6.77,-13.76,;6.75,-15.3,;8.09,-16.08,;9.44,-15.31,;10.78,-16.07,;12.11,-15.29,;12.1,-13.73,;10.75,-12.98,;9.43,-13.76,;8.1,-12.99,)| Show InChI InChI=1S/C41H38N4O5S/c1-2-3-17-38-39(37(27-46)42-45(38)33-15-5-4-6-16-33)35-21-19-31(25-36(35)41(48)44-23-22-29-12-8-10-14-32(29)26-44)40(47)43-51(49,50)34-20-18-28-11-7-9-13-30(28)24-34/h4-16,18-21,24-25,46H,2-3,17,22-23,26-27H2,1H3,(H,43,47) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research
Curated by ChEMBL
| Assay Description
Inhibition of biotin-labelled Bcl-xL using [FAM]-IWIAQELRRIGDEFNAYY-NH2 as substrate after 60 mins by FRET analysis |
Bioorg Med Chem Lett 22: 3951-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.106
BindingDB Entry DOI: 10.7270/Q2416Z2Z |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50384324

(CHEMBL2030859 | US9346795, 260)Show SMILES CCCCc1c(c(CO)nn1-c1ccccc1)-c1ccc(cc1C(=O)N1Cc2ccccc2C[C@H]1CO)C(=O)NS(=O)(=O)c1ccc2ccccc2c1 |r,wU:34.39,(-8.12,-38.1,;-6.89,-39.03,;-5.47,-38.44,;-5.28,-36.91,;-3.85,-36.31,;-2.39,-35.82,;-2.4,-34.28,;-1.16,-33.36,;-1.34,-31.83,;-3.87,-33.82,;-4.77,-35.07,;-6.3,-34.97,;-6.98,-33.59,;-8.52,-33.49,;-9.37,-34.77,;-8.68,-36.16,;-7.15,-36.25,;-1.14,-36.72,;.21,-35.98,;1.54,-36.79,;1.49,-38.33,;.14,-39.06,;-1.18,-38.25,;-2.53,-38.98,;-3.84,-38.18,;-2.57,-40.52,;-1.26,-41.33,;-1.31,-42.87,;0,-43.68,;-.05,-45.22,;-1.41,-45.94,;-2.72,-45.13,;-2.67,-43.6,;-3.97,-42.79,;-3.93,-41.25,;-5.24,-40.45,;-6.59,-41.18,;2.8,-39.14,;4.16,-38.41,;2.76,-40.68,;4.07,-41.48,;4.86,-40.16,;5.61,-41.5,;4.03,-43.02,;2.67,-43.75,;2.62,-45.29,;3.95,-46.11,;3.93,-47.65,;5.25,-48.45,;6.61,-47.69,;6.63,-46.14,;5.3,-45.36,;5.34,-43.82,)| Show InChI InChI=1S/C42H40N4O6S/c1-2-3-17-39-40(38(27-48)43-46(39)33-15-5-4-6-16-33)36-21-19-31(41(49)44-53(51,52)35-20-18-28-11-7-8-13-30(28)23-35)24-37(36)42(50)45-25-32-14-10-9-12-29(32)22-34(45)26-47/h4-16,18-21,23-24,34,47-48H,2-3,17,22,25-27H2,1H3,(H,44,49)/t34-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged Bcl2 using [FAM]-IWIAQELRRIGDEFNAYY-NH2 as substrate after 60 mins by fluorescence polarization assay |
Bioorg Med Chem Lett 22: 3951-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.106
BindingDB Entry DOI: 10.7270/Q2416Z2Z |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50384355

(CHEMBL2031024 | US9346795, 91)Show SMILES CCCCN(CCCC)C(=O)c1nn(c(C)c1Cl)-c1ccc(cc1C(=O)N1Cc2ccccc2C[C@H]1CO)C(=O)NS(=O)(=O)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C41H44ClN5O6S/c1-4-6-20-45(21-7-5-2)41(51)38-37(42)27(3)47(43-38)36-19-17-31(39(49)44-54(52,53)34-18-16-28-12-8-9-14-30(28)23-34)24-35(36)40(50)46-25-32-15-11-10-13-29(32)22-33(46)26-48/h8-19,23-24,33,48H,4-7,20-22,25-26H2,1-3H3,(H,44,49)/t33-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research
Curated by ChEMBL
| Assay Description
Inhibition of Bcl-XL using fluoresceinated 18-mer Bim as substrate after 60 mins by FRET analysis |
Bioorg Med Chem Lett 22: 3946-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.103
BindingDB Entry DOI: 10.7270/Q20866BM |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50384320

(CHEMBL2030855)Show SMILES CCCCc1c(c(CO)nn1-c1ccc(cc1)-c1ccccc1)-c1ccc(cc1C(=O)N1CCc2ccccc2C1)C(=O)NS(=O)(=O)c1ccc2ccccc2c1 |(46.25,-7.57,;44.94,-6.76,;44.98,-5.22,;43.67,-4.42,;43.72,-2.87,;43.88,-1.34,;42.47,-.71,;42.15,.8,;40.69,1.27,;41.44,-1.85,;42.21,-3.19,;41.49,-4.54,;39.95,-4.59,;39.22,-5.94,;40.03,-7.25,;41.58,-7.2,;42.3,-5.84,;39.31,-8.61,;37.77,-8.66,;37.04,-10.02,;37.86,-11.32,;39.4,-11.27,;40.12,-9.91,;45.22,-.57,;45.22,.97,;46.56,1.74,;47.89,.97,;47.89,-.57,;46.54,-1.34,;46.54,-2.88,;45.2,-3.64,;47.87,-3.65,;47.86,-5.19,;49.19,-5.97,;50.52,-5.21,;51.84,-5.98,;53.18,-5.23,;53.2,-3.69,;51.87,-2.9,;50.54,-3.67,;49.21,-2.88,;49.23,1.73,;49.24,3.27,;50.56,.95,;51.89,1.72,;51.11,3.05,;52.65,3.06,;53.23,.94,;53.21,-.6,;54.55,-1.38,;55.9,-.6,;57.24,-1.36,;58.57,-.58,;58.56,.97,;57.21,1.73,;55.89,.94,;54.56,1.72,)| Show InChI InChI=1S/C47H42N4O5S/c1-2-3-17-44-45(43(31-52)48-51(44)39-22-18-35(19-23-39)32-11-5-4-6-12-32)41-25-21-37(29-42(41)47(54)50-27-26-34-14-8-10-16-38(34)30-50)46(53)49-57(55,56)40-24-20-33-13-7-9-15-36(33)28-40/h4-16,18-25,28-29,52H,2-3,17,26-27,30-31H2,1H3,(H,49,53) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research
Curated by ChEMBL
| Assay Description
Inhibition of biotin-labelled Bcl-xL using [FAM]-IWIAQELRRIGDEFNAYY-NH2 as substrate after 60 mins by FRET analysis |
Bioorg Med Chem Lett 22: 3951-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.106
BindingDB Entry DOI: 10.7270/Q2416Z2Z |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50384326

(CHEMBL2030861)Show SMILES CCCCc1c(c(CO)nn1-c1ccccc1)-c1ccc(cc1C(=O)N1CCc2ccccc2C1)C(=O)NS(=O)(=O)CC[Si](C)(C)C |(33.41,-39.78,;34.7,-40.62,;36.07,-39.92,;36.15,-38.39,;37.53,-37.68,;38.96,-37.09,;38.83,-35.56,;40,-34.56,;39.72,-33.04,;37.33,-35.2,;36.53,-36.51,;35,-36.53,;34.22,-35.2,;32.68,-35.21,;31.92,-36.55,;32.71,-37.88,;34.24,-37.86,;40.27,-37.9,;41.62,-37.16,;42.94,-37.97,;42.9,-39.51,;41.54,-40.24,;40.23,-39.43,;38.87,-40.17,;37.56,-39.36,;38.83,-41.71,;37.48,-42.44,;37.43,-43.97,;38.74,-44.78,;38.69,-46.31,;39.99,-47.13,;41.35,-46.4,;41.41,-44.86,;40.1,-44.05,;40.15,-42.51,;44.21,-40.32,;45.57,-39.59,;44.17,-41.86,;45.48,-42.66,;46.27,-41.34,;47.02,-42.69,;45.43,-44.21,;46.75,-45.01,;46.72,-46.55,;48.03,-47.35,;45.37,-47.29,;46.7,-48.08,)| Show InChI InChI=1S/C36H44N4O5SSi/c1-5-6-16-33-34(32(25-41)37-40(33)29-14-8-7-9-15-29)30-18-17-27(35(42)38-46(44,45)21-22-47(2,3)4)23-31(30)36(43)39-20-19-26-12-10-11-13-28(26)24-39/h7-15,17-18,23,41H,5-6,16,19-22,24-25H2,1-4H3,(H,38,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research
Curated by ChEMBL
| Assay Description
Inhibition of biotin-labelled Bcl-xL using [FAM]-IWIAQELRRIGDEFNAYY-NH2 as substrate after 60 mins by FRET analysis |
Bioorg Med Chem Lett 22: 3951-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.106
BindingDB Entry DOI: 10.7270/Q2416Z2Z |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50384322

(CHEMBL2030857)Show SMILES CCCCc1c(c(CO)nn1-c1ccc(CCCO)cc1)-c1ccc(cc1C(=O)N1CCc2ccccc2C1)C(=O)NS(=O)(=O)c1ccc2ccccc2c1 |(23.77,-26.25,;22.46,-25.44,;22.51,-23.9,;21.2,-23.1,;21.24,-21.55,;21.4,-20.02,;20,-19.39,;19.68,-17.88,;18.21,-17.41,;18.97,-20.53,;19.73,-21.87,;19.01,-23.22,;17.47,-23.27,;16.74,-24.62,;17.55,-25.93,;16.83,-27.29,;15.29,-27.34,;14.57,-28.7,;13.03,-28.75,;19.1,-25.88,;19.82,-24.52,;22.74,-19.25,;22.74,-17.71,;24.08,-16.94,;25.42,-17.71,;25.41,-19.25,;24.07,-20.02,;24.06,-21.56,;22.72,-22.32,;25.39,-22.33,;25.39,-23.87,;26.71,-24.65,;28.05,-23.89,;29.37,-24.66,;30.71,-23.91,;30.72,-22.37,;29.39,-21.58,;28.06,-22.35,;26.73,-21.56,;26.75,-16.95,;26.76,-15.41,;28.08,-17.73,;29.41,-16.96,;28.63,-15.63,;30.17,-15.62,;30.75,-17.74,;30.74,-19.28,;32.07,-20.06,;33.42,-19.28,;34.76,-20.04,;36.1,-19.26,;36.08,-17.71,;34.73,-16.95,;33.41,-17.74,;32.08,-16.96,)| Show InChI InChI=1S/C44H44N4O6S/c1-2-3-14-41-42(40(29-50)45-48(41)36-19-15-30(16-20-36)9-8-25-49)38-22-18-34(27-39(38)44(52)47-24-23-32-11-5-7-13-35(32)28-47)43(51)46-55(53,54)37-21-17-31-10-4-6-12-33(31)26-37/h4-7,10-13,15-22,26-27,49-50H,2-3,8-9,14,23-25,28-29H2,1H3,(H,46,51) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged Bcl2 using [FAM]-IWIAQELRRIGDEFNAYY-NH2 as substrate after 60 mins by fluorescence polarization assay |
Bioorg Med Chem Lett 22: 3951-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.106
BindingDB Entry DOI: 10.7270/Q2416Z2Z |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50384339
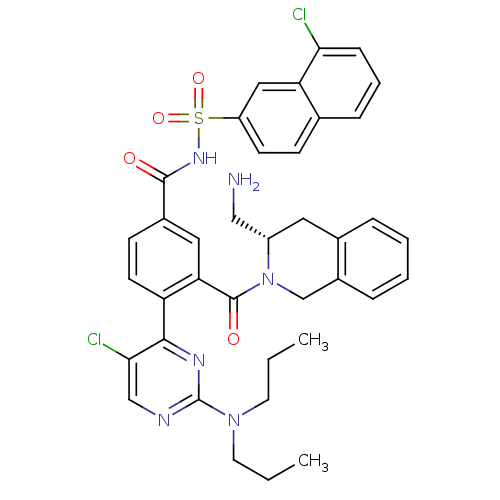
(CHEMBL2031008 | US9346795, 329)Show SMILES CCCN(CCC)c1ncc(Cl)c(n1)-c1ccc(cc1C(=O)N1Cc2ccccc2C[C@H]1CN)C(=O)NS(=O)(=O)c1ccc2cccc(Cl)c2c1 |r| Show InChI InChI=1S/C38H38Cl2N6O4S/c1-3-16-45(17-4-2)38-42-22-34(40)35(43-38)30-15-13-26(19-32(30)37(48)46-23-27-9-6-5-8-25(27)18-28(46)21-41)36(47)44-51(49,50)29-14-12-24-10-7-11-33(39)31(24)20-29/h5-15,19-20,22,28H,3-4,16-18,21,23,41H2,1-2H3,(H,44,47)/t28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research
Curated by ChEMBL
| Assay Description
Inhibition of biotin-labelled Bcl-xL using [FAM]-IWIAQELRRIGDEFNAYY-NH2 as substrate after 60 mins by FRET analysis |
Bioorg Med Chem Lett 22: 3951-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.106
BindingDB Entry DOI: 10.7270/Q2416Z2Z |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50384341
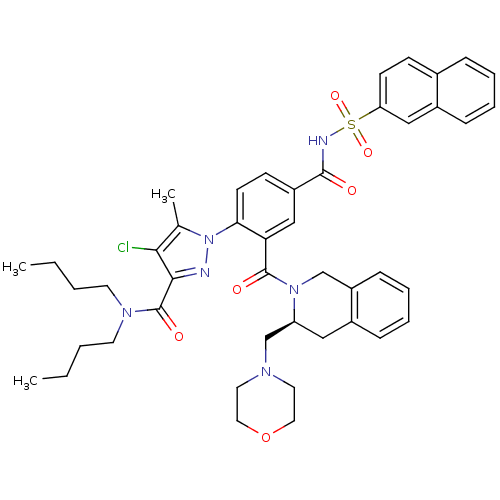
(CHEMBL2031029)Show SMILES CCCCN(CCCC)C(=O)c1nn(c(C)c1Cl)-c1ccc(cc1C(=O)N1Cc2ccccc2C[C@H]1CN1CCOCC1)C(=O)NS(=O)(=O)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C45H51ClN6O6S/c1-4-6-20-50(21-7-5-2)45(55)42-41(46)31(3)52(47-42)40-19-17-35(43(53)48-59(56,57)38-18-16-32-12-8-9-14-34(32)27-38)28-39(40)44(54)51-29-36-15-11-10-13-33(36)26-37(51)30-49-22-24-58-25-23-49/h8-19,27-28,37H,4-7,20-26,29-30H2,1-3H3,(H,48,53)/t37-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research
Curated by ChEMBL
| Assay Description
Inhibition in GST-tagged Bcl2 using [FAM]-IWIAQELRRIGDEFNAYY-NH2 as substrate after 60 mins by fluorescence polarization assay |
Bioorg Med Chem Lett 22: 3946-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.103
BindingDB Entry DOI: 10.7270/Q20866BM |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50384347
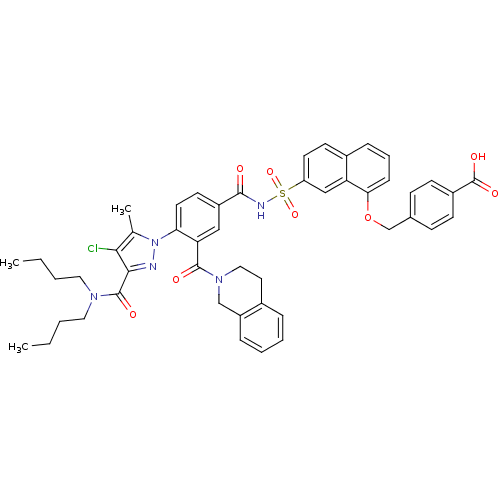
(CHEMBL2031016)Show SMILES CCCCN(CCCC)C(=O)c1nn(c(C)c1Cl)-c1ccc(cc1C(=O)N1CCc2ccccc2C1)C(=O)NS(=O)(=O)c1ccc2cccc(OCc3ccc(cc3)C(O)=O)c2c1 Show InChI InChI=1S/C48H48ClN5O8S/c1-4-6-24-52(25-7-5-2)47(57)44-43(49)31(3)54(50-44)41-22-20-36(27-40(41)46(56)53-26-23-33-11-8-9-12-37(33)29-53)45(55)51-63(60,61)38-21-19-34-13-10-14-42(39(34)28-38)62-30-32-15-17-35(18-16-32)48(58)59/h8-22,27-28H,4-7,23-26,29-30H2,1-3H3,(H,51,55)(H,58,59) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research
Curated by ChEMBL
| Assay Description
Inhibition in GST-tagged Bcl2 using [FAM]-IWIAQELRRIGDEFNAYY-NH2 as substrate after 60 mins by fluorescence polarization assay |
Bioorg Med Chem Lett 22: 3946-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.103
BindingDB Entry DOI: 10.7270/Q20866BM |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50384323
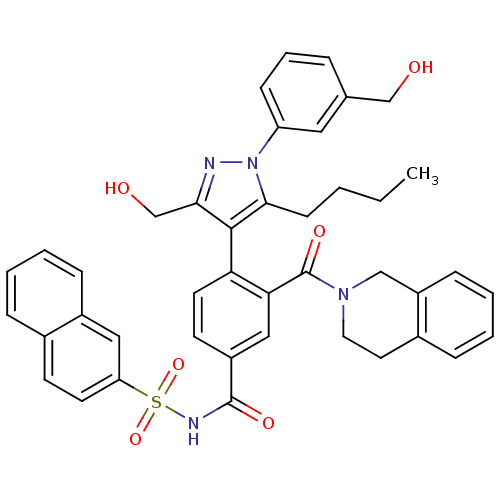
(CHEMBL2030858)Show SMILES CCCCc1c(c(CO)nn1-c1cccc(CO)c1)-c1ccc(cc1C(=O)N1CCc2ccccc2C1)C(=O)NS(=O)(=O)c1ccc2ccccc2c1 |(48.56,-26.2,;47.25,-25.39,;47.3,-23.85,;45.99,-23.04,;46.03,-21.5,;46.2,-19.96,;44.79,-19.34,;44.47,-17.83,;43,-17.35,;43.76,-20.48,;44.52,-21.82,;43.8,-23.17,;42.26,-23.22,;41.53,-24.57,;42.34,-25.88,;43.89,-25.83,;44.71,-27.13,;43.99,-28.49,;44.61,-24.47,;47.53,-19.2,;47.53,-17.66,;48.87,-16.88,;50.21,-17.66,;50.2,-19.2,;48.86,-19.97,;48.85,-21.51,;47.52,-22.27,;50.18,-22.28,;50.18,-23.82,;51.5,-24.59,;52.84,-23.83,;54.16,-24.61,;55.5,-23.86,;55.51,-22.32,;54.19,-21.53,;52.85,-22.29,;51.52,-21.51,;51.54,-16.9,;51.55,-15.36,;52.87,-17.67,;54.21,-16.91,;53.43,-15.58,;54.97,-15.57,;55.54,-17.69,;55.53,-19.23,;56.86,-20.01,;58.21,-19.23,;59.56,-19.99,;60.89,-19.21,;60.87,-17.65,;59.53,-16.9,;58.2,-17.68,;56.87,-16.91,)| Show InChI InChI=1S/C42H40N4O6S/c1-2-3-15-39-40(38(27-48)43-46(39)34-14-8-9-28(22-34)26-47)36-19-17-32(24-37(36)42(50)45-21-20-30-11-5-7-13-33(30)25-45)41(49)44-53(51,52)35-18-16-29-10-4-6-12-31(29)23-35/h4-14,16-19,22-24,47-48H,2-3,15,20-21,25-27H2,1H3,(H,44,49) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research
Curated by ChEMBL
| Assay Description
Inhibition of biotin-labelled Bcl-xL using [FAM]-IWIAQELRRIGDEFNAYY-NH2 as substrate after 60 mins by FRET analysis |
Bioorg Med Chem Lett 22: 3951-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.106
BindingDB Entry DOI: 10.7270/Q2416Z2Z |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50384329

(CHEMBL2030864 | US9346795, 252)Show SMILES CCCCc1c(c(CO)nn1-c1ccc(Oc2cccc(Cl)c2)cc1)-c1ccc(cc1C(=O)N1CCc2ccccc2C1)C(=O)NS(=O)(=O)c1ccc2cccc(Cl)c2c1 |(-.35,-2.86,;.94,-3.7,;2.31,-3,;2.4,-1.46,;3.78,-.76,;5.2,-.17,;5.07,1.37,;6.24,2.37,;5.96,3.88,;3.58,1.72,;2.77,.41,;1.24,.4,;.46,1.73,;-1.08,1.72,;-1.84,.38,;-3.38,.36,;-4.16,1.69,;-5.7,1.67,;-6.48,2.99,;-5.73,4.34,;-4.19,4.35,;-3.43,5.69,;-3.41,3.02,;-1.05,-.96,;.48,-.94,;6.51,-.97,;7.86,-.24,;9.19,-1.04,;9.14,-2.59,;7.79,-3.32,;6.47,-2.51,;5.12,-3.24,;3.81,-2.43,;5.07,-4.78,;3.72,-5.51,;3.68,-7.04,;4.98,-7.85,;4.93,-9.38,;6.24,-10.2,;7.59,-9.48,;7.65,-7.93,;6.34,-7.13,;6.39,-5.58,;10.45,-3.39,;11.81,-2.66,;10.41,-4.93,;11.73,-5.75,;10.95,-7.08,;12.49,-7.08,;13.07,-4.99,;13.1,-3.45,;14.44,-2.69,;15.76,-3.47,;17.11,-2.71,;18.44,-3.49,;18.43,-5.05,;17.08,-5.81,;17.06,-7.35,;15.75,-5.02,;14.4,-5.78,)| Show InChI InChI=1S/C47H40Cl2N4O6S/c1-2-3-14-44-45(43(29-54)50-53(44)35-17-19-36(20-18-35)59-37-12-7-11-34(48)26-37)39-22-16-32(25-41(39)47(56)52-24-23-30-8-4-5-9-33(30)28-52)46(55)51-60(57,58)38-21-15-31-10-6-13-42(49)40(31)27-38/h4-13,15-22,25-27,54H,2-3,14,23-24,28-29H2,1H3,(H,51,55) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research
Curated by ChEMBL
| Assay Description
Inhibition of biotin-labelled Bcl-xL using [FAM]-IWIAQELRRIGDEFNAYY-NH2 as substrate after 60 mins by FRET analysis |
Bioorg Med Chem Lett 22: 3951-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.106
BindingDB Entry DOI: 10.7270/Q2416Z2Z |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50384339

(CHEMBL2031008 | US9346795, 329)Show SMILES CCCN(CCC)c1ncc(Cl)c(n1)-c1ccc(cc1C(=O)N1Cc2ccccc2C[C@H]1CN)C(=O)NS(=O)(=O)c1ccc2cccc(Cl)c2c1 |r| Show InChI InChI=1S/C38H38Cl2N6O4S/c1-3-16-45(17-4-2)38-42-22-34(40)35(43-38)30-15-13-26(19-32(30)37(48)46-23-27-9-6-5-8-25(27)18-28(46)21-41)36(47)44-51(49,50)29-14-12-24-10-7-11-33(39)31(24)20-29/h5-15,19-20,22,28H,3-4,16-18,21,23,41H2,1-2H3,(H,44,47)/t28-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged Bcl2 using [FAM]-IWIAQELRRIGDEFNAYY-NH2 as substrate after 60 mins by fluorescence polarization assay |
Bioorg Med Chem Lett 22: 3951-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.106
BindingDB Entry DOI: 10.7270/Q2416Z2Z |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 3
(Homo sapiens (Human)) | BDBM50078360

(CHEMBL3414728)Show SMILES [H][C@@]12CN(C(=O)[C@@H](NC(=O)[C@H](C)NC)C(C)(C)C)[C@@]([H])(C1)C(=O)N[C@@H](Cc1ccc3ccccc3c1)C(=O)N[C@@H](Cc1ccc(OCc3cn(nn3)[C@]3([H])CN(C(=O)[C@@H](NC(=O)[C@H](C)NC)C(C)(C)C)[C@@]([H])(C3)C(=O)N[C@@H](Cc3ccc4ccccc4c3)C(=O)N[C@@H](Cc3ccc(OCc4cn2nn4)cc3)C(O)=O)cc1)C(O)=O |r| Show InChI InChI=1S/C80H96N16O14/c1-45(81-9)69(97)87-67(79(3,4)5)75(103)93-41-57-37-65(93)73(101)83-61(35-49-19-25-51-15-11-13-17-53(51)31-49)71(99)85-63(77(105)106)33-47-23-29-60(30-24-47)110-44-56-40-96(92-90-56)58-38-66(94(42-58)76(104)68(80(6,7)8)88-70(98)46(2)82-10)74(102)84-62(36-50-20-26-52-16-12-14-18-54(52)32-50)72(100)86-64(78(107)108)34-48-21-27-59(28-22-48)109-43-55-39-95(57)91-89-55/h11-32,39-40,45-46,57-58,61-68,81-82H,33-38,41-44H2,1-10H3,(H,83,101)(H,84,102)(H,85,99)(H,86,100)(H,87,97)(H,88,98)(H,105,106)(H,107,108)/t45-,46-,57-,58-,61-,62-,63-,64-,65-,66-,67+,68+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Ensemble Therapeutics Corp
Curated by ChEMBL
| Assay Description
Inhibition of cIAP1 BIR3 (154 to 352 residues) (unknown origin) by fluoresceinated dimeric SMAC peptide based fluorescence polarization assay |
J Med Chem 58: 2855-61 (2015)
Article DOI: 10.1021/jm501892g
BindingDB Entry DOI: 10.7270/Q2RF5WQ0 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50384341

(CHEMBL2031029)Show SMILES CCCCN(CCCC)C(=O)c1nn(c(C)c1Cl)-c1ccc(cc1C(=O)N1Cc2ccccc2C[C@H]1CN1CCOCC1)C(=O)NS(=O)(=O)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C45H51ClN6O6S/c1-4-6-20-50(21-7-5-2)45(55)42-41(46)31(3)52(47-42)40-19-17-35(43(53)48-59(56,57)38-18-16-32-12-8-9-14-34(32)27-38)28-39(40)44(54)51-29-36-15-11-10-13-33(36)26-37(51)30-49-22-24-58-25-23-49/h8-19,27-28,37H,4-7,20-26,29-30H2,1-3H3,(H,48,53)/t37-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research
Curated by ChEMBL
| Assay Description
Inhibition of Bcl-XL using fluoresceinated 18-mer Bim as substrate after 60 mins by FRET analysis |
Bioorg Med Chem Lett 22: 3946-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.103
BindingDB Entry DOI: 10.7270/Q20866BM |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50384353
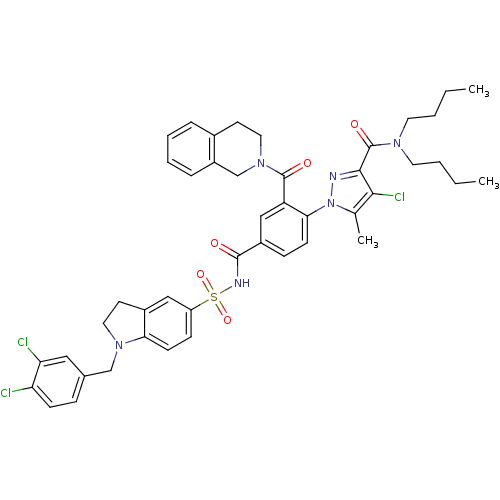
(CHEMBL2031022 | US9346795, 59)Show SMILES CCCCN(CCCC)C(=O)c1nn(c(C)c1Cl)-c1ccc(cc1C(=O)N1CCc2ccccc2C1)C(=O)NS(=O)(=O)c1ccc2N(Cc3ccc(Cl)c(Cl)c3)CCc2c1 Show InChI InChI=1S/C45H47Cl3N6O5S/c1-4-6-20-51(21-7-5-2)45(57)42-41(48)29(3)54(49-42)40-16-13-33(26-36(40)44(56)53-23-18-31-10-8-9-11-34(31)28-53)43(55)50-60(58,59)35-14-17-39-32(25-35)19-22-52(39)27-30-12-15-37(46)38(47)24-30/h8-17,24-26H,4-7,18-23,27-28H2,1-3H3,(H,50,55) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research
Curated by ChEMBL
| Assay Description
Inhibition in GST-tagged Bcl2 using [FAM]-IWIAQELRRIGDEFNAYY-NH2 as substrate after 60 mins by fluorescence polarization assay |
Bioorg Med Chem Lett 22: 3946-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.103
BindingDB Entry DOI: 10.7270/Q20866BM |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50384347

(CHEMBL2031016)Show SMILES CCCCN(CCCC)C(=O)c1nn(c(C)c1Cl)-c1ccc(cc1C(=O)N1CCc2ccccc2C1)C(=O)NS(=O)(=O)c1ccc2cccc(OCc3ccc(cc3)C(O)=O)c2c1 Show InChI InChI=1S/C48H48ClN5O8S/c1-4-6-24-52(25-7-5-2)47(57)44-43(49)31(3)54(50-44)41-22-20-36(27-40(41)46(56)53-26-23-33-11-8-9-12-37(33)29-53)45(55)51-63(60,61)38-21-19-34-13-10-14-42(39(34)28-38)62-30-32-15-17-35(18-16-32)48(58)59/h8-22,27-28H,4-7,23-26,29-30H2,1-3H3,(H,51,55)(H,58,59) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research
Curated by ChEMBL
| Assay Description
Inhibition of Bcl-XL using fluoresceinated 18-mer Bim as substrate after 60 mins by FRET analysis |
Bioorg Med Chem Lett 22: 3946-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.103
BindingDB Entry DOI: 10.7270/Q20866BM |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 3
(Homo sapiens (Human)) | BDBM50078355
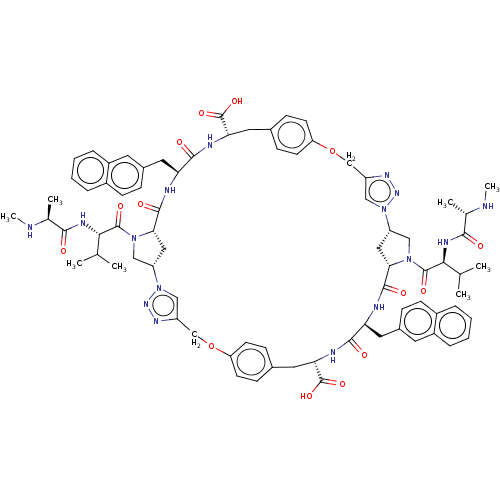
(CHEMBL3414726)Show SMILES [H][C@@]12CN(C(=O)[C@@H](NC(=O)[C@H](C)NC)C(C)C)[C@@]([H])(C1)C(=O)N[C@@H](Cc1ccc3ccccc3c1)C(=O)N[C@@H](Cc1ccc(OCc3cn(nn3)[C@]3([H])CN(C(=O)[C@@H](NC(=O)[C@H](C)NC)C(C)C)[C@@]([H])(C3)C(=O)N[C@@H](Cc3ccc4ccccc4c3)C(=O)N[C@@H](Cc3ccc(OCc4cn2nn4)cc3)C(O)=O)cc1)C(O)=O |r| Show InChI InChI=1S/C78H92N16O14/c1-43(2)67(85-69(95)45(5)79-7)75(101)91-39-57-35-65(91)73(99)81-61(33-49-17-23-51-13-9-11-15-53(51)29-49)71(97)83-63(77(103)104)31-47-21-27-60(28-22-47)108-42-56-38-94(90-88-56)58-36-66(92(40-58)76(102)68(44(3)4)86-70(96)46(6)80-8)74(100)82-62(34-50-18-24-52-14-10-12-16-54(52)30-50)72(98)84-64(78(105)106)32-48-19-25-59(26-20-48)107-41-55-37-93(57)89-87-55/h9-30,37-38,43-46,57-58,61-68,79-80H,31-36,39-42H2,1-8H3,(H,81,99)(H,82,100)(H,83,97)(H,84,98)(H,85,95)(H,86,96)(H,103,104)(H,105,106)/t45-,46-,57-,58-,61-,62-,63-,64-,65-,66-,67-,68-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Ensemble Therapeutics Corp
Curated by ChEMBL
| Assay Description
Inhibition of cIAP1 BIR3 (154 to 352 residues) (unknown origin) by fluoresceinated dimeric SMAC peptide based fluorescence polarization assay |
J Med Chem 58: 2855-61 (2015)
Article DOI: 10.1021/jm501892g
BindingDB Entry DOI: 10.7270/Q2RF5WQ0 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50384352
(CHEMBL2031021 | US9346795, 53)Show SMILES CCCCN(CCCC)C(=O)c1nn(c(C)c1Cl)-c1ccc(cc1C(=O)N1CCc2ccccc2C1)C(=O)NS(=O)(=O)c1ccc2N(CC)CCc2c1 Show InChI InChI=1S/C40H47ClN6O5S/c1-5-8-20-45(21-9-6-2)40(50)37-36(41)27(4)47(42-37)35-16-14-30(25-33(35)39(49)46-23-18-28-12-10-11-13-31(28)26-46)38(48)43-53(51,52)32-15-17-34-29(24-32)19-22-44(34)7-3/h10-17,24-25H,5-9,18-23,26H2,1-4H3,(H,43,48) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research
Curated by ChEMBL
| Assay Description
Inhibition of Bcl-XL using fluoresceinated 18-mer Bim as substrate after 60 mins by FRET analysis |
Bioorg Med Chem Lett 22: 3946-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.103
BindingDB Entry DOI: 10.7270/Q20866BM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

















































