

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrolipoyl dehydrogenase (Mycobacterium tuberculosis) | BDBM119876 (SL932 | US9073941, 503) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Medical College | Assay Description IC50 values were determined with serial dilutions (from 100 to 0.1 uM) of inhibitors by a spectrophotometric assay with DTNB, lipoamide, and NADH or ... | Biochemistry 52: 9375-84 (2013) Article DOI: 10.1021/bi401077f BindingDB Entry DOI: 10.7270/Q29K48X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prephenate dehydrogenase (Mycobacterium tuberculosis H37Rv) | BDBM119876 (SL932 | US9073941, 503) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Medical College | Assay Description IC50 values were determined with serial dilutions (from 100 to 0.1 uM) of inhibitors by a spectrophotometric assay with DTNB, lipoamide, and NADH or ... | Biochemistry 52: 9375-84 (2013) Article DOI: 10.1021/bi401077f BindingDB Entry DOI: 10.7270/Q29K48X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrolipoyl dehydrogenase (Mycobacterium tuberculosis) | BDBM119877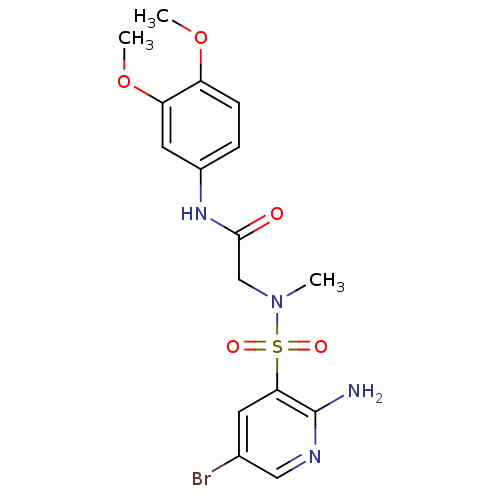 (SL809 | US9073941, 502) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Medical College | Assay Description IC50 values were determined with serial dilutions (from 100 to 0.1 uM) of inhibitors by a spectrophotometric assay with DTNB, lipoamide, and NADH or ... | Biochemistry 52: 9375-84 (2013) Article DOI: 10.1021/bi401077f BindingDB Entry DOI: 10.7270/Q29K48X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrolipoyl dehydrogenase (Mycobacterium tuberculosis) | BDBM119878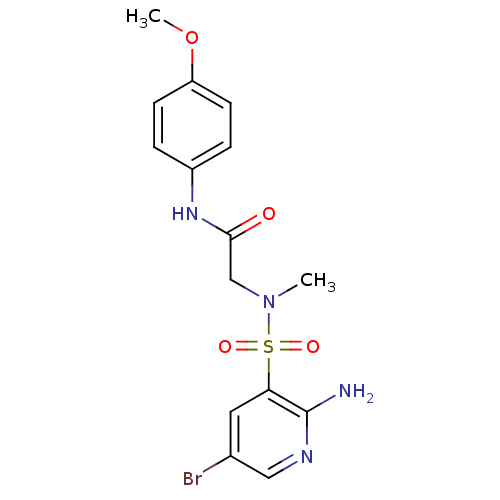 (SL827 | US9073941, 500) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Medical College | Assay Description IC50 values were determined with serial dilutions (from 100 to 0.1 uM) of inhibitors by a spectrophotometric assay with DTNB, lipoamide, and NADH or ... | Biochemistry 52: 9375-84 (2013) Article DOI: 10.1021/bi401077f BindingDB Entry DOI: 10.7270/Q29K48X6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrolipoyl dehydrogenase (Mycobacterium tuberculosis) | BDBM119880 (SL418) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Medical College | Assay Description IC50 values were determined with serial dilutions (from 100 to 0.1 uM) of inhibitors by a spectrophotometric assay with DTNB, lipoamide, and NADH or ... | Biochemistry 52: 9375-84 (2013) Article DOI: 10.1021/bi401077f BindingDB Entry DOI: 10.7270/Q29K48X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prephenate dehydrogenase (Mycobacterium tuberculosis H37Rv) | BDBM119878 (SL827 | US9073941, 500) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Medical College | Assay Description IC50 values were determined with serial dilutions (from 100 to 0.1 uM) of inhibitors by a spectrophotometric assay with DTNB, lipoamide, and NADH or ... | Biochemistry 52: 9375-84 (2013) Article DOI: 10.1021/bi401077f BindingDB Entry DOI: 10.7270/Q29K48X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prephenate dehydrogenase (Mycobacterium tuberculosis H37Rv) | BDBM119877 (SL809 | US9073941, 502) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Medical College | Assay Description IC50 values were determined with serial dilutions (from 100 to 0.1 uM) of inhibitors by a spectrophotometric assay with DTNB, lipoamide, and NADH or ... | Biochemistry 52: 9375-84 (2013) Article DOI: 10.1021/bi401077f BindingDB Entry DOI: 10.7270/Q29K48X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrolipoyl dehydrogenase (Mycobacterium tuberculosis) | BDBM119879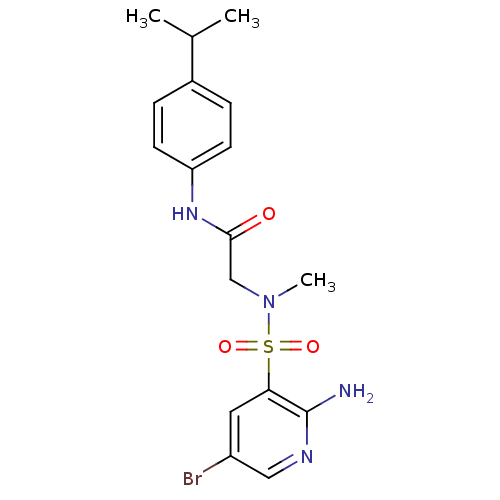 (SL917 | US9073941, 505) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 233 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Medical College | Assay Description IC50 values were determined with serial dilutions (from 100 to 0.1 uM) of inhibitors by a spectrophotometric assay with DTNB, lipoamide, and NADH or ... | Biochemistry 52: 9375-84 (2013) Article DOI: 10.1021/bi401077f BindingDB Entry DOI: 10.7270/Q29K48X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prephenate dehydrogenase (Mycobacterium tuberculosis H37Rv) | BDBM119879 (SL917 | US9073941, 505) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 289 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Medical College | Assay Description IC50 values were determined with serial dilutions (from 100 to 0.1 uM) of inhibitors by a spectrophotometric assay with DTNB, lipoamide, and NADH or ... | Biochemistry 52: 9375-84 (2013) Article DOI: 10.1021/bi401077f BindingDB Entry DOI: 10.7270/Q29K48X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prephenate dehydrogenase (Mycobacterium tuberculosis H37Rv) | BDBM119880 (SL418) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 333 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Medical College | Assay Description IC50 values were determined with serial dilutions (from 100 to 0.1 uM) of inhibitors by a spectrophotometric assay with DTNB, lipoamide, and NADH or ... | Biochemistry 52: 9375-84 (2013) Article DOI: 10.1021/bi401077f BindingDB Entry DOI: 10.7270/Q29K48X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50010993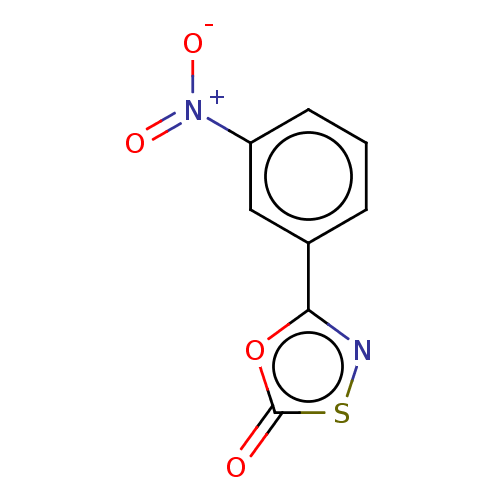 (CHEMBL3265177) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Medical College Curated by ChEMBL | Assay Description Inhibition of immunoproteasome-20S subunit beta5i in human PBMC assessed as substrate hydrolysis using suc-LLVY-AMC as substrate measured for 120 min... | ACS Med Chem Lett 5: 405-10 (2014) Article DOI: 10.1021/ml400531d BindingDB Entry DOI: 10.7270/Q2PZ5BCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50010988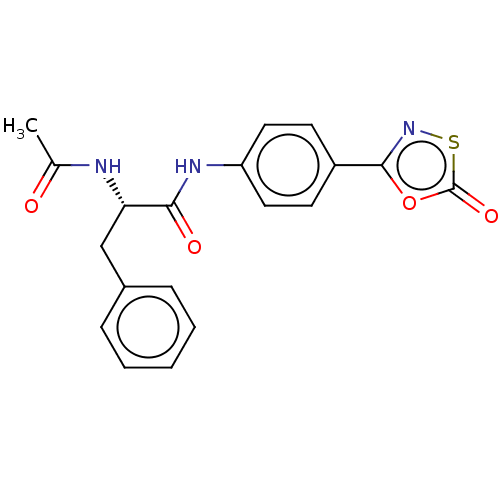 (CHEMBL3259882) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Medical College Curated by ChEMBL | Assay Description Inhibition of immunoproteasome-20S subunit beta5i in human PBMC assessed as substrate hydrolysis using suc-LLVY-AMC as substrate measured for 120 min... | ACS Med Chem Lett 5: 405-10 (2014) Article DOI: 10.1021/ml400531d BindingDB Entry DOI: 10.7270/Q2PZ5BCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50010986 (CHEMBL2336253) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Medical College Curated by ChEMBL | Assay Description Inhibition of immunoproteasome-20S subunit beta5i in human PBMC assessed as substrate hydrolysis using suc-LLVY-AMC as substrate measured for 120 min... | ACS Med Chem Lett 5: 405-10 (2014) Article DOI: 10.1021/ml400531d BindingDB Entry DOI: 10.7270/Q2PZ5BCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50010997 (CHEMBL3265182) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Medical College Curated by ChEMBL | Assay Description Inhibition of immunoproteasome-20S subunit beta5i in human PBMC assessed as substrate hydrolysis using suc-LLVY-AMC as substrate measured for 120 min... | ACS Med Chem Lett 5: 405-10 (2014) Article DOI: 10.1021/ml400531d BindingDB Entry DOI: 10.7270/Q2PZ5BCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50010995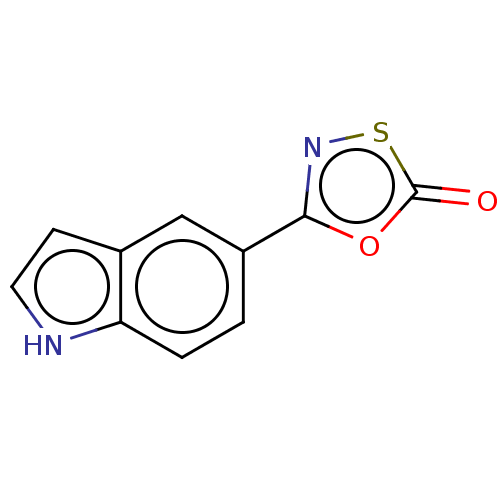 (CHEMBL3265180) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Medical College Curated by ChEMBL | Assay Description Inhibition of immunoproteasome-20S subunit beta5i in human PBMC assessed as substrate hydrolysis using suc-LLVY-AMC as substrate measured for 120 min... | ACS Med Chem Lett 5: 405-10 (2014) Article DOI: 10.1021/ml400531d BindingDB Entry DOI: 10.7270/Q2PZ5BCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50011003 (CHEMBL3259879) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Medical College Curated by ChEMBL | Assay Description Inhibition of immunoproteasome-20S subunit beta5i in human PBMC assessed as substrate hydrolysis using suc-LLVY-AMC as substrate measured for 120 min... | ACS Med Chem Lett 5: 405-10 (2014) Article DOI: 10.1021/ml400531d BindingDB Entry DOI: 10.7270/Q2PZ5BCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50010999 (CHEMBL3259876) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Medical College Curated by ChEMBL | Assay Description Inhibition of immunoproteasome-20S subunit beta5i in human PBMC assessed as substrate hydrolysis using suc-LLVY-AMC as substrate measured for 120 min... | ACS Med Chem Lett 5: 405-10 (2014) Article DOI: 10.1021/ml400531d BindingDB Entry DOI: 10.7270/Q2PZ5BCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50011001 (CHEMBL3259877) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Medical College Curated by ChEMBL | Assay Description Inhibition of immunoproteasome-20S subunit beta5i in human PBMC assessed as substrate hydrolysis using suc-LLVY-AMC as substrate measured for 120 min... | ACS Med Chem Lett 5: 405-10 (2014) Article DOI: 10.1021/ml400531d BindingDB Entry DOI: 10.7270/Q2PZ5BCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50010996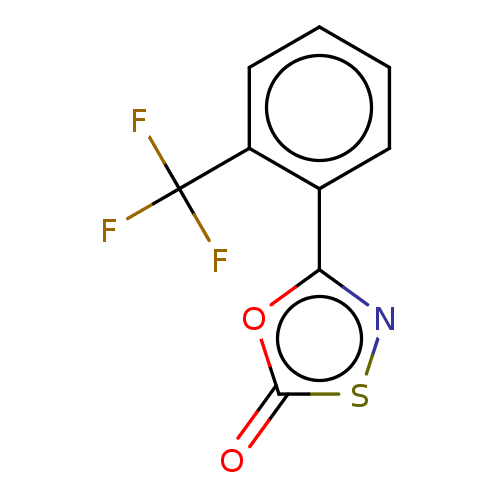 (CHEMBL3265181) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Medical College Curated by ChEMBL | Assay Description Inhibition of immunoproteasome-20S subunit beta5i in human PBMC assessed as substrate hydrolysis using suc-LLVY-AMC as substrate measured for 120 min... | ACS Med Chem Lett 5: 405-10 (2014) Article DOI: 10.1021/ml400531d BindingDB Entry DOI: 10.7270/Q2PZ5BCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50010994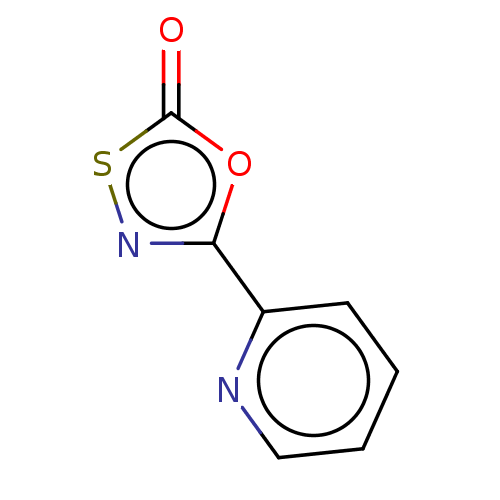 (CHEMBL3265178) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Medical College Curated by ChEMBL | Assay Description Inhibition of immunoproteasome-20S subunit beta5i in human PBMC assessed as substrate hydrolysis using suc-LLVY-AMC as substrate measured for 120 min... | ACS Med Chem Lett 5: 405-10 (2014) Article DOI: 10.1021/ml400531d BindingDB Entry DOI: 10.7270/Q2PZ5BCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interferon-induced, double-stranded RNA-activated protein kinase (Homo sapiens (Human)) | BDBM50347871 (CHEMBL1802618) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Medical College Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant PKR | Bioorg Med Chem Lett 21: 4108-14 (2011) Article DOI: 10.1016/j.bmcl.2011.04.149 BindingDB Entry DOI: 10.7270/Q2KK9C4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50010991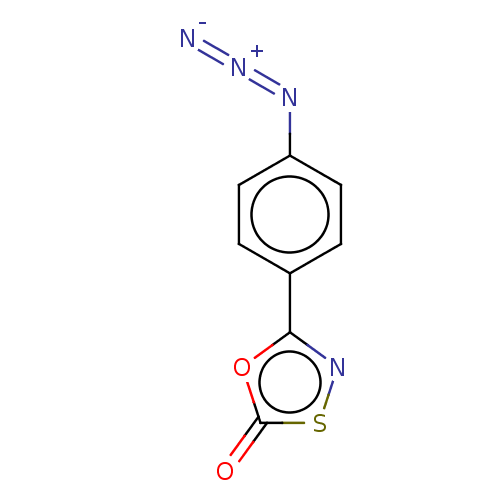 (CHEMBL3259884) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Medical College Curated by ChEMBL | Assay Description Inhibition of immunoproteasome-20S subunit beta5i in human PBMC assessed as substrate hydrolysis using suc-LLVY-AMC as substrate measured for 120 min... | ACS Med Chem Lett 5: 405-10 (2014) Article DOI: 10.1021/ml400531d BindingDB Entry DOI: 10.7270/Q2PZ5BCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50010987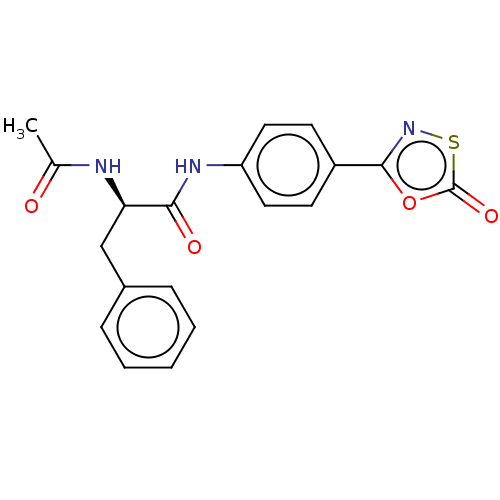 (CHEMBL3259880) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Medical College Curated by ChEMBL | Assay Description Inhibition of immunoproteasome-20S subunit beta5i in human PBMC assessed as substrate hydrolysis using suc-LLVY-AMC as substrate measured for 120 min... | ACS Med Chem Lett 5: 405-10 (2014) Article DOI: 10.1021/ml400531d BindingDB Entry DOI: 10.7270/Q2PZ5BCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50011002 (CHEMBL3259878) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Medical College Curated by ChEMBL | Assay Description Inhibition of immunoproteasome-20S subunit beta5i in human PBMC assessed as substrate hydrolysis using suc-LLVY-AMC as substrate measured for 120 min... | ACS Med Chem Lett 5: 405-10 (2014) Article DOI: 10.1021/ml400531d BindingDB Entry DOI: 10.7270/Q2PZ5BCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50332796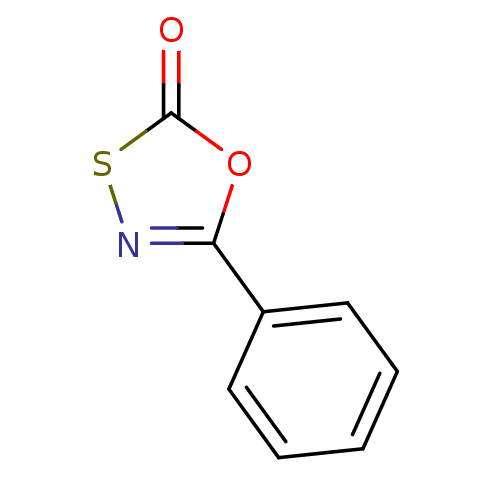 (5-phenyl-1,3,4-oxathiazol-2-one | CHEMBL1632533) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Medical College Curated by ChEMBL | Assay Description Inhibition of immunoproteasome-20S subunit beta5i in human PBMC assessed as substrate hydrolysis using suc-LLVY-AMC as substrate measured for 120 min... | ACS Med Chem Lett 5: 405-10 (2014) Article DOI: 10.1021/ml400531d BindingDB Entry DOI: 10.7270/Q2PZ5BCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50554394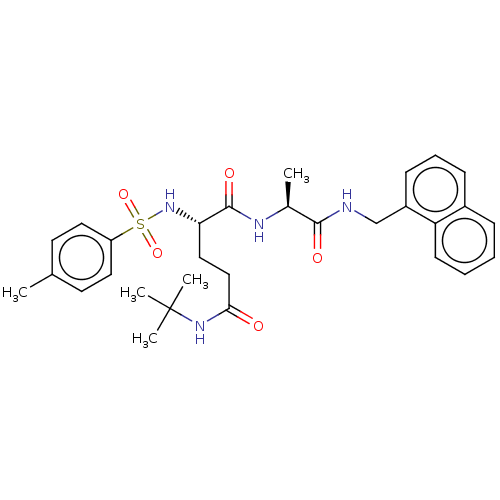 (CHEMBL4784875) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human 20S immunoproteasome beta 5 chymotrypsin-like activity | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01520 BindingDB Entry DOI: 10.7270/Q22R3WB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50554392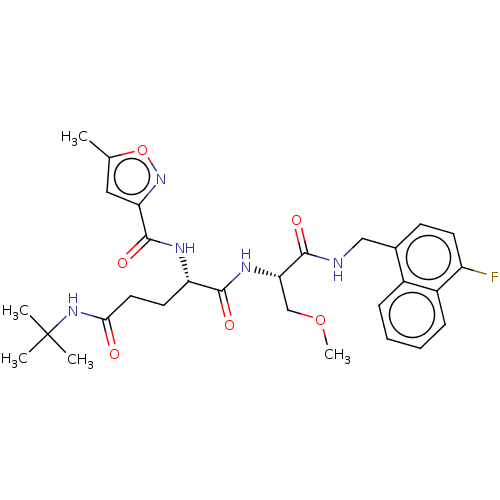 (CHEMBL4751044) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human 20S immunoproteasome beta 5 chymotrypsin-like activity | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01520 BindingDB Entry DOI: 10.7270/Q22R3WB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50554397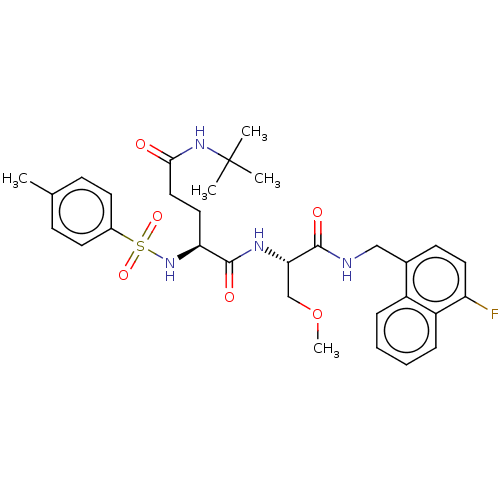 (CHEMBL4741140) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human 20S immunoproteasome beta 5 chymotrypsin-like activity | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01520 BindingDB Entry DOI: 10.7270/Q22R3WB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50554389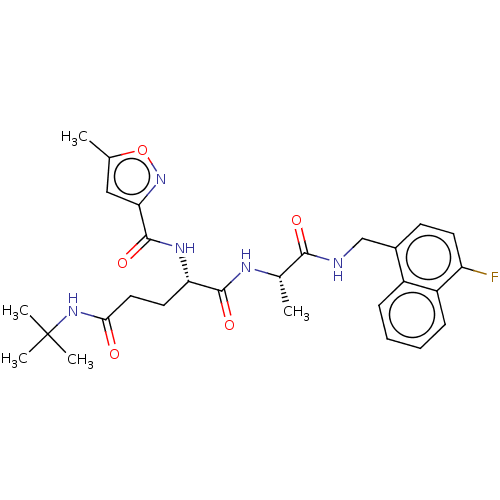 (CHEMBL4796570) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human 20S immunoproteasome beta 5 chymotrypsin-like activity | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01520 BindingDB Entry DOI: 10.7270/Q22R3WB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50554385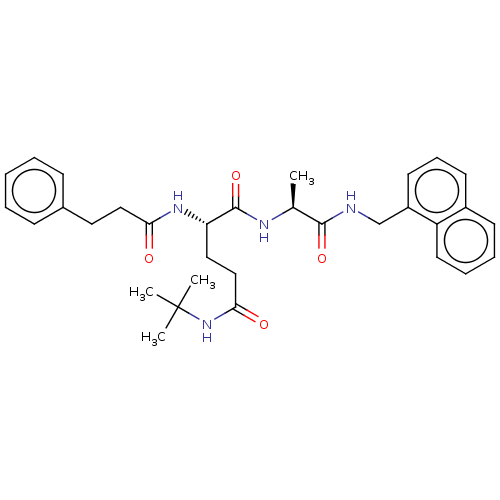 (CHEMBL4764897) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human 20S immunoproteasome beta 5 chymotrypsin-like activity | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01520 BindingDB Entry DOI: 10.7270/Q22R3WB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50554386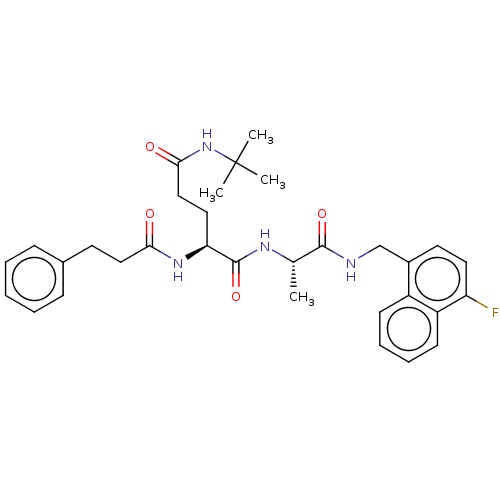 (CHEMBL4758484) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human 20S immunoproteasome beta 5 chymotrypsin-like activity | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01520 BindingDB Entry DOI: 10.7270/Q22R3WB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50554384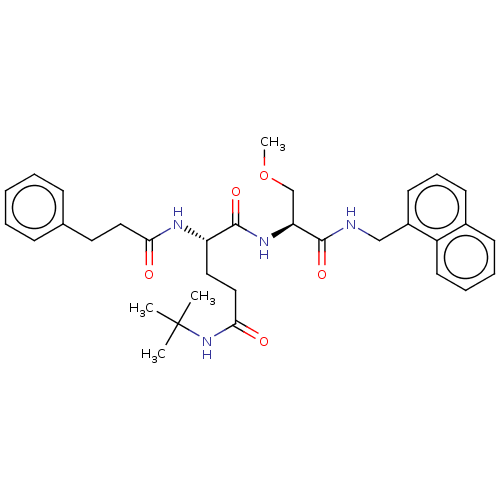 (CHEMBL4784015) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human 20S immunoproteasome beta 5 chymotrypsin-like activity | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01520 BindingDB Entry DOI: 10.7270/Q22R3WB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50554395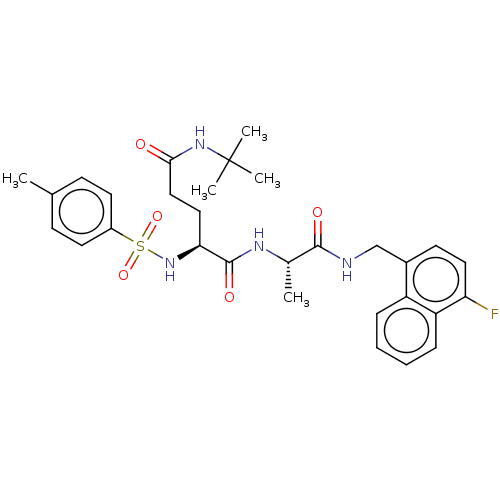 (CHEMBL4763116) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human 20S immunoproteasome beta 5 chymotrypsin-like activity | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01520 BindingDB Entry DOI: 10.7270/Q22R3WB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50554358 (CHEMBL4754892) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human 20S immunoproteasome beta 5 chymotrypsin-like activity | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01520 BindingDB Entry DOI: 10.7270/Q22R3WB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50554357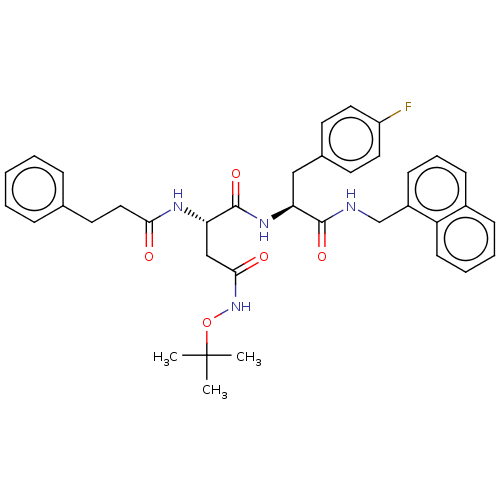 (CHEMBL4516988) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human 20S immunoproteasome beta 5 chymotrypsin-like activity | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01520 BindingDB Entry DOI: 10.7270/Q22R3WB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50554388 (CHEMBL4759896) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human 20S immunoproteasome beta 5 chymotrypsin-like activity | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01520 BindingDB Entry DOI: 10.7270/Q22R3WB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50554359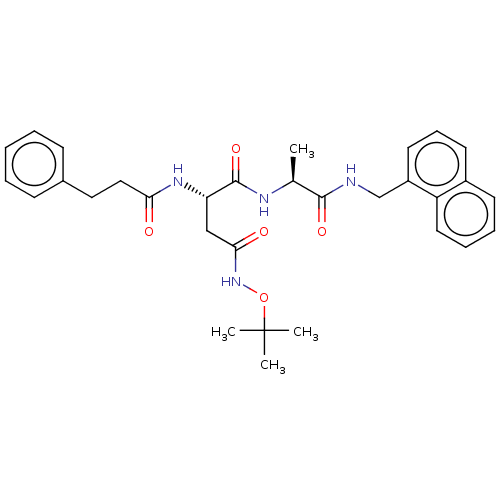 (CHEMBL4758527) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human 20S immunoproteasome beta 5 chymotrypsin-like activity | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01520 BindingDB Entry DOI: 10.7270/Q22R3WB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50326249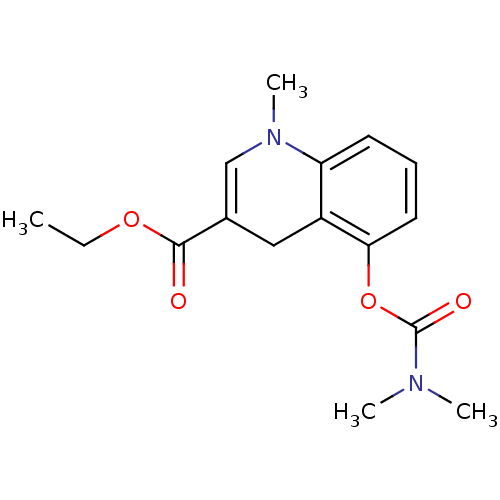 (CHEMBL1243360 | ethyl 5-(dimethylcarbamoyloxy)-1-m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of human AChE by Ellmans test | J Med Chem 53: 6490-505 (2010) Article DOI: 10.1021/jm100573q BindingDB Entry DOI: 10.7270/Q23R0T3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50554383 (CHEMBL4782251) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human 20S immunoproteasome beta 5 chymotrypsin-like activity | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01520 BindingDB Entry DOI: 10.7270/Q22R3WB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50326250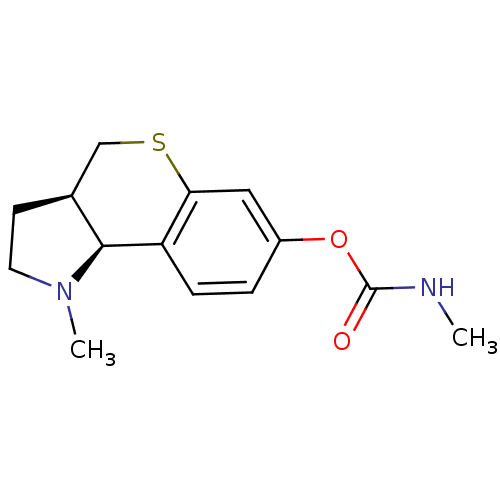 ((3aR,9bS)-1-methyl-1,2,3,3a,4,9b-hexahydrothiochro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.11 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of human AChE by Ellmans test | J Med Chem 53: 6490-505 (2010) Article DOI: 10.1021/jm100573q BindingDB Entry DOI: 10.7270/Q23R0T3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10961 ((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of human AChE by Ellmans test | J Med Chem 53: 6490-505 (2010) Article DOI: 10.1021/jm100573q BindingDB Entry DOI: 10.7270/Q23R0T3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50554393 (CHEMBL4764263) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human 20S immunoproteasome beta 5 chymotrypsin-like activity | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01520 BindingDB Entry DOI: 10.7270/Q22R3WB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50554391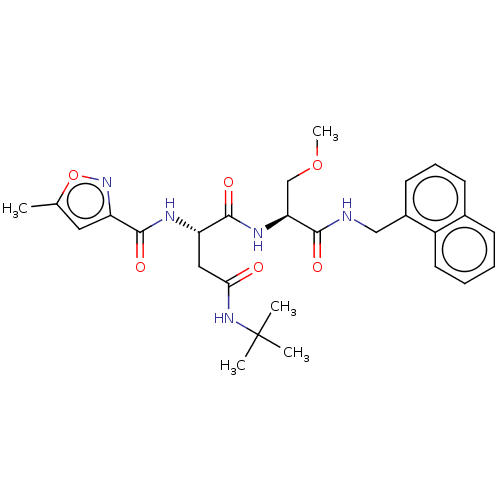 (CHEMBL4776824) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human 20S immunoproteasome beta 5 chymotrypsin-like activity | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01520 BindingDB Entry DOI: 10.7270/Q22R3WB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50554365 (CHEMBL4764833) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human 20S immunoproteasome beta 5 chymotrypsin-like activity | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01520 BindingDB Entry DOI: 10.7270/Q22R3WB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10973 ((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of human AChE by Ellmans test | J Med Chem 53: 6490-505 (2010) Article DOI: 10.1021/jm100573q BindingDB Entry DOI: 10.7270/Q23R0T3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50554388 (CHEMBL4759896) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human 20S immunoproteasome beta 5 chymotrypsin-like activity in human KARPAS-1106P cells incubated for 2 hrs by proteasome-Glo assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01520 BindingDB Entry DOI: 10.7270/Q22R3WB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50554396 (CHEMBL4764229) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human 20S immunoproteasome beta 5 chymotrypsin-like activity | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01520 BindingDB Entry DOI: 10.7270/Q22R3WB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50326251 ((3aR,9bS)-1-methyl-2,3,3a,4,5,9b-hexahydro-1H-benz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of human AChE by Ellmans test | J Med Chem 53: 6490-505 (2010) Article DOI: 10.1021/jm100573q BindingDB Entry DOI: 10.7270/Q23R0T3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM651205 (US20240043470, Compound 4-13) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | UniChem | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50554390 (CHEMBL4784421) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human 20S immunoproteasome beta 5 chymotrypsin-like activity | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01520 BindingDB Entry DOI: 10.7270/Q22R3WB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1836 total ) | Next | Last >> |