

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Hepacivirus C) | BDBM50485494 (CHEMBL2063089) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease D168V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485491 (CHEMBL2063088) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease D168V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485492 (Grazoprevir | Grazoprevir monohydrate | MK-5172 | ...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease D168V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50486093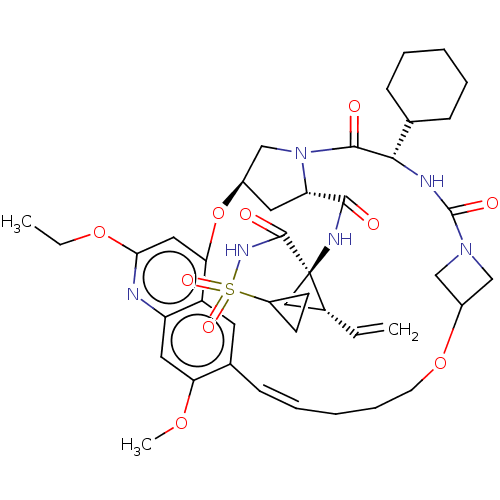 (CHEMBL2203889) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b BK NS3/4A protease A156T mutant expressed in Escherichia coli incubated for 30 mins by time-resolved fluorescence assay | Bioorg Med Chem Lett 22: 7207-13 (2012) Article DOI: 10.1016/j.bmcl.2012.09.061 BindingDB Entry DOI: 10.7270/Q28D0041 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50153088 (CHEMBL365416 | N-((3S,6R)-3-tert-Butyl-2,5-dioxo-1...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibitory effect against E. coli peptide deformylase (PDF) by AAP assay | J Med Chem 47: 4941-9 (2004) Article DOI: 10.1021/jm049592c BindingDB Entry DOI: 10.7270/Q2WD401S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50153085 (CHEMBL364836 | N-((3S,6R)-3-tert-Butyl-2,5-dioxo-1...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibitory effect against E. coli peptide deformylase (PDF) by AAP assay | J Med Chem 47: 4941-9 (2004) Article DOI: 10.1021/jm049592c BindingDB Entry DOI: 10.7270/Q2WD401S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50153083 (CHEMBL441502 | N-((3S,6R)-3-tert-Butyl-2,5-dioxo-1...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibitory effect against E. coli peptide deformylase (PDF) by AAP assay | J Med Chem 47: 4941-9 (2004) Article DOI: 10.1021/jm049592c BindingDB Entry DOI: 10.7270/Q2WD401S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50486108 (CHEMBL2203884) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b BK NS3/4A protease A156T mutant expressed in Escherichia coli incubated for 30 mins by time-resolved fluorescence assay | Bioorg Med Chem Lett 22: 7207-13 (2012) Article DOI: 10.1016/j.bmcl.2012.09.061 BindingDB Entry DOI: 10.7270/Q28D0041 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50131892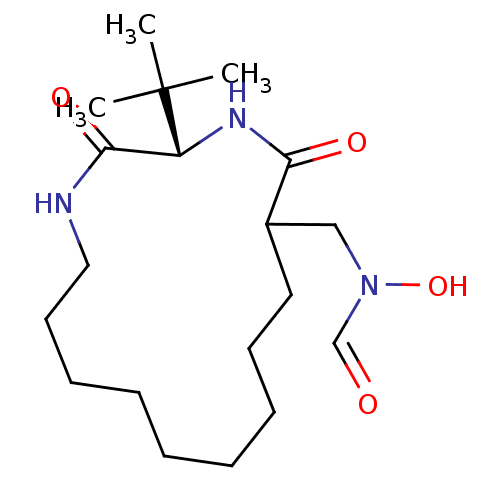 (CHEMBL421252 | N-(3-tert-Butyl-2,5-dioxo-1,4diaza-...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Binding affinity against Co(II)-substituted E. coli peptide deformylase was determined | J Med Chem 46: 3771-4 (2003) Article DOI: 10.1021/jm034113f BindingDB Entry DOI: 10.7270/Q2765DQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50486111 (CHEMBL2203888) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b BK NS3/4A protease A156T mutant expressed in Escherichia coli incubated for 30 mins by time-resolved fluorescence assay | Bioorg Med Chem Lett 22: 7207-13 (2012) Article DOI: 10.1016/j.bmcl.2012.09.061 BindingDB Entry DOI: 10.7270/Q28D0041 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50486101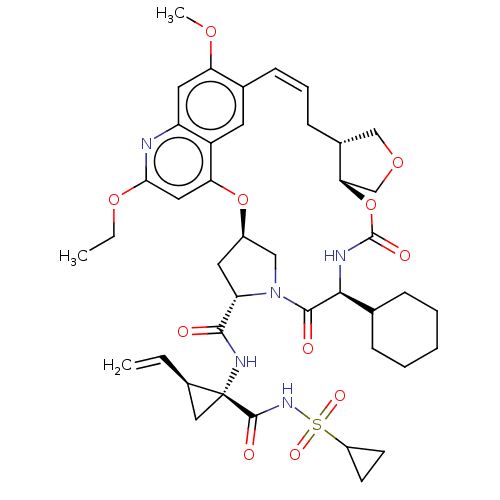 (CHEMBL2203879) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b BK NS3/4A protease A156T mutant expressed in Escherichia coli incubated for 30 mins by time-resolved fluorescence assay | Bioorg Med Chem Lett 22: 7207-13 (2012) Article DOI: 10.1016/j.bmcl.2012.09.061 BindingDB Entry DOI: 10.7270/Q28D0041 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50034711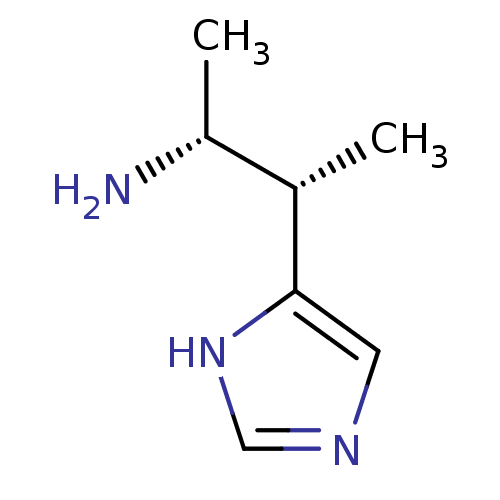 ((1R,2S)-2-(1H-Imidazol-4-yl)-1-methyl-propylamine ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding afinity against Histamine H3 receptor | J Med Chem 38: 1593-9 (1995) BindingDB Entry DOI: 10.7270/Q2BK1BC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50486103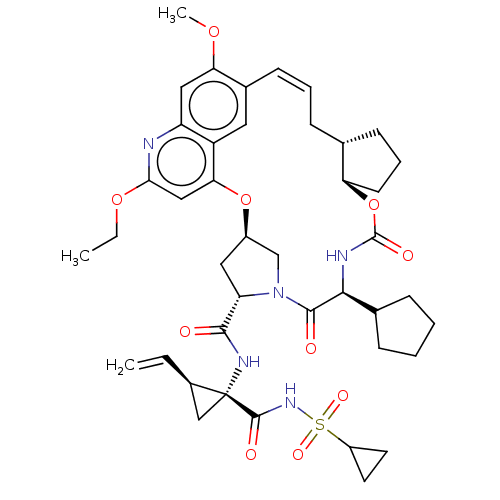 (CHEMBL2203874) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b BK NS3/4A protease A156T mutant expressed in Escherichia coli incubated for 30 mins by time-resolved fluorescence assay | Bioorg Med Chem Lett 22: 7207-13 (2012) Article DOI: 10.1016/j.bmcl.2012.09.061 BindingDB Entry DOI: 10.7270/Q28D0041 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19622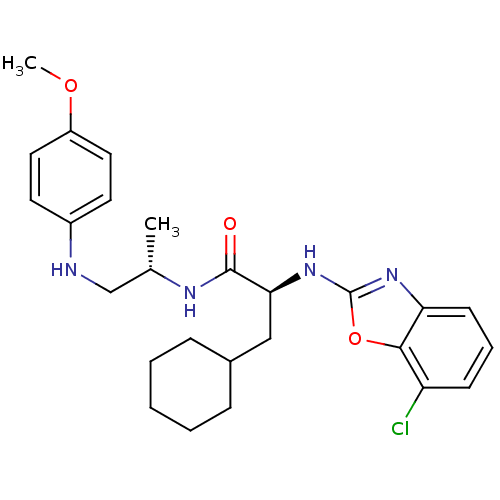 ((2S)-2-[(7-chloro-1,3-benzoxazol-2-yl)amino]-3-cyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | -53.4 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
GNF | Assay Description The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... | Bioorg Med Chem Lett 16: 1975-80 (2006) Article DOI: 10.1016/j.bmcl.2005.12.095 BindingDB Entry DOI: 10.7270/Q2PZ573M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50486092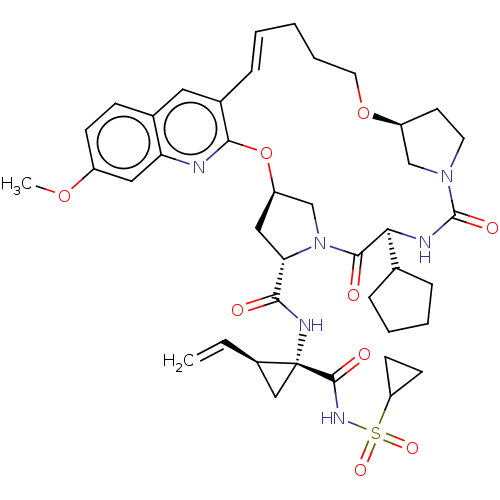 (CHEMBL2203892) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b BK NS3/4A protease D168V mutant expressed in Escherichia coli incubated for 30 mins by time-resolved fluorescence assay | Bioorg Med Chem Lett 22: 7207-13 (2012) Article DOI: 10.1016/j.bmcl.2012.09.061 BindingDB Entry DOI: 10.7270/Q28D0041 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM22904 ((2R)-1-(1H-imidazol-5-yl)propan-2-amine | (R)-alph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding afinity against Histamine H3 receptor | J Med Chem 38: 1593-9 (1995) BindingDB Entry DOI: 10.7270/Q2BK1BC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM22904 ((2R)-1-(1H-imidazol-5-yl)propan-2-amine | (R)-alph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound towards Histamine H3 receptor was determined in guinea pig brain tissue using [3H]- N alpha-methylhistamine radioligand | Bioorg Med Chem Lett 8: 243-8 (1999) BindingDB Entry DOI: 10.7270/Q2ST7P0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50486091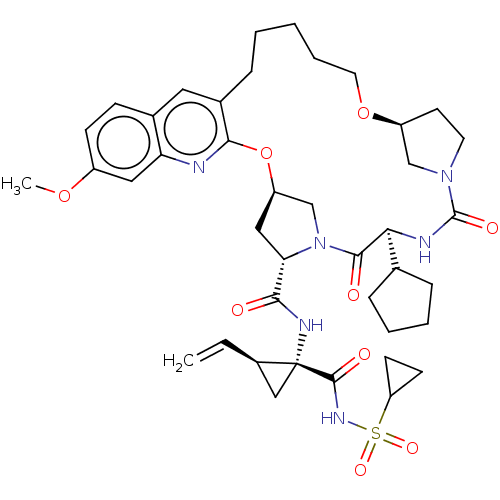 (CHEMBL2203893) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b BK NS3/4A protease A156T mutant expressed in Escherichia coli incubated for 30 mins by time-resolved fluorescence assay | Bioorg Med Chem Lett 22: 7207-13 (2012) Article DOI: 10.1016/j.bmcl.2012.09.061 BindingDB Entry DOI: 10.7270/Q28D0041 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19610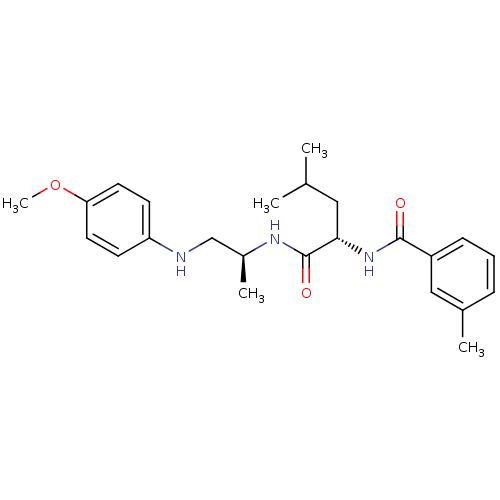 ((2S)-N-[(2S)-1-[(4-methoxyphenyl)amino]propan-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... | Bioorg Med Chem Lett 16: 1486-90 (2006) Article DOI: 10.1016/j.bmcl.2005.12.056 BindingDB Entry DOI: 10.7270/Q2TQ5ZTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50486110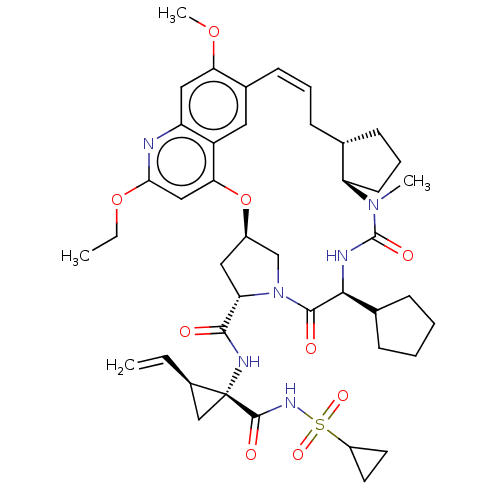 (CHEMBL2203883) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b BK NS3/4A protease A156T mutant expressed in Escherichia coli incubated for 30 mins by time-resolved fluorescence assay | Bioorg Med Chem Lett 22: 7207-13 (2012) Article DOI: 10.1016/j.bmcl.2012.09.061 BindingDB Entry DOI: 10.7270/Q28D0041 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50486090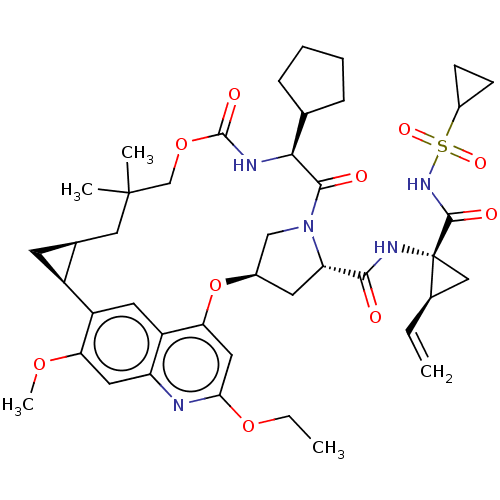 (CHEMBL2203882) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b BK NS3/4A protease D168V mutant expressed in Escherichia coli incubated for 30 mins by time-resolved fluorescence assay | Bioorg Med Chem Lett 22: 7207-13 (2012) Article DOI: 10.1016/j.bmcl.2012.09.061 BindingDB Entry DOI: 10.7270/Q28D0041 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50486105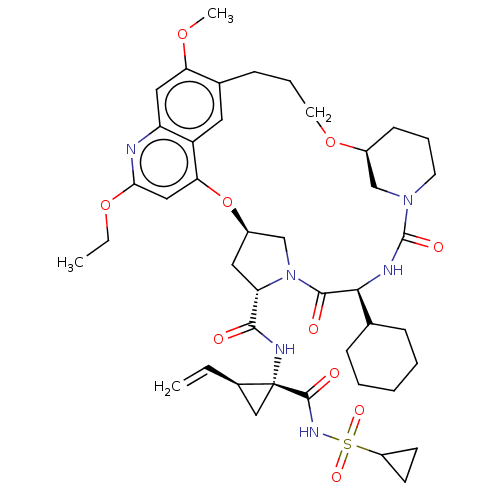 (CHEMBL2203886) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b BK NS3/4A protease A156T mutant expressed in Escherichia coli incubated for 30 mins by time-resolved fluorescence assay | Bioorg Med Chem Lett 22: 7207-13 (2012) Article DOI: 10.1016/j.bmcl.2012.09.061 BindingDB Entry DOI: 10.7270/Q28D0041 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50212688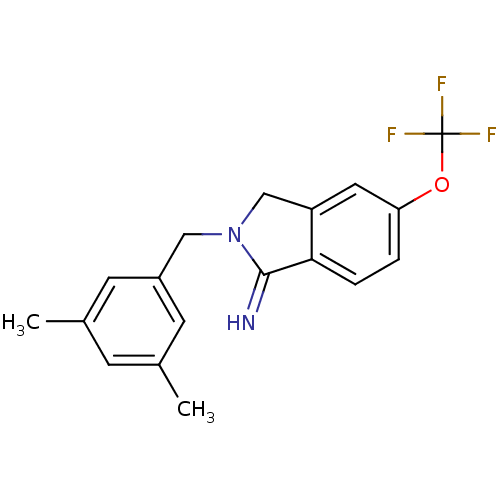 (2-(3,5-dimethyl-benzyl)-5-trifluoromethoxy-2,3-dih...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to NMDA NR2B receptor | Bioorg Med Chem Lett 17: 3997-4000 (2007) Article DOI: 10.1016/j.bmcl.2007.04.084 BindingDB Entry DOI: 10.7270/Q21J99GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50486097 (CHEMBL2203875) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b BK NS3/4A protease A156T mutant expressed in Escherichia coli incubated for 30 mins by time-resolved fluorescence assay | Bioorg Med Chem Lett 22: 7207-13 (2012) Article DOI: 10.1016/j.bmcl.2012.09.061 BindingDB Entry DOI: 10.7270/Q28D0041 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19600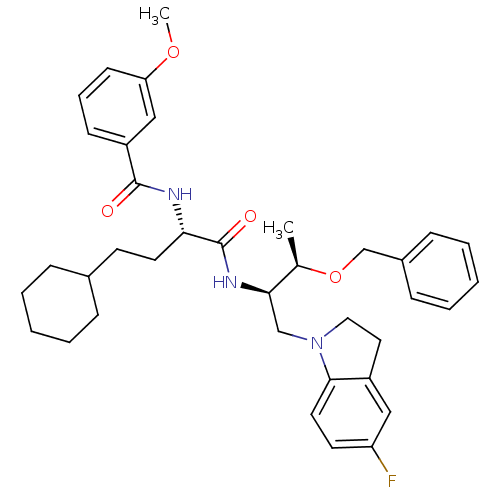 ((2S)-N-[(2R,3R)-3-(benzyloxy)-1-(5-fluoro-2,3-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... | Bioorg Med Chem Lett 16: 1486-90 (2006) Article DOI: 10.1016/j.bmcl.2005.12.056 BindingDB Entry DOI: 10.7270/Q2TQ5ZTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50069825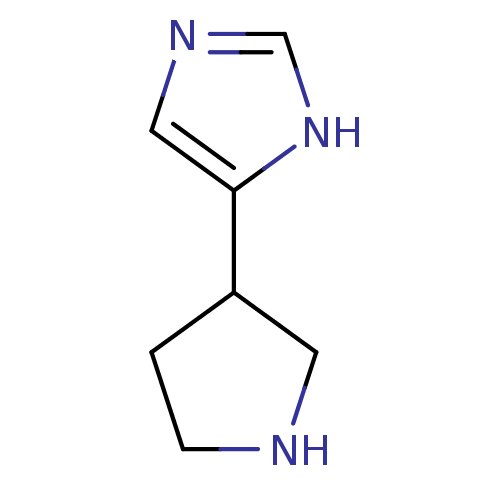 (4-Pyrrolidin-3-yl-1H-imidazole | CHEMBL79983) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-N-alpha-methylhistamine binding to guinea pig brain Histamine H3 receptor | Bioorg Med Chem Lett 8: 243-8 (1999) BindingDB Entry DOI: 10.7270/Q2ST7P0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50212685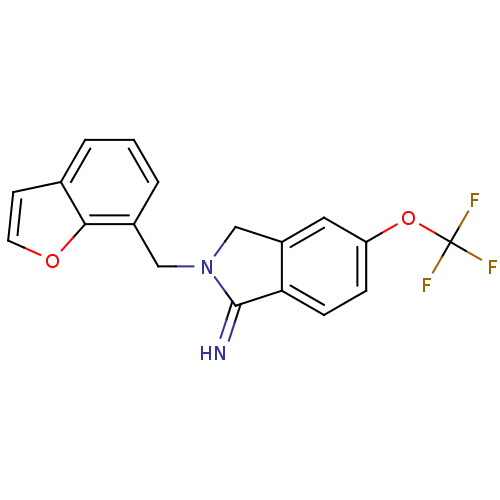 (2-benzofuran-7-ylmethyl-5-trifluoromethoxy-2,3-dih...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to NMDA NR2B receptor | Bioorg Med Chem Lett 17: 3997-4000 (2007) Article DOI: 10.1016/j.bmcl.2007.04.084 BindingDB Entry DOI: 10.7270/Q21J99GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50486107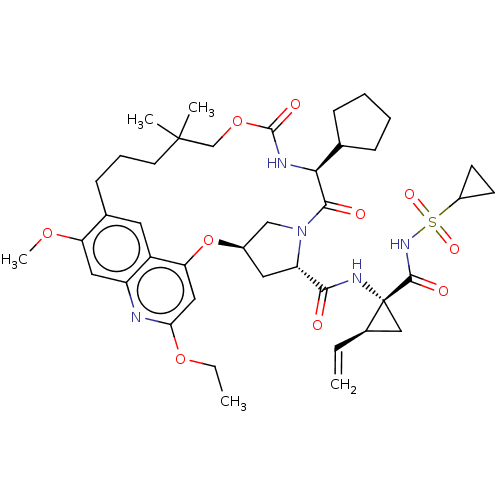 (CHEMBL2203873) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b BK NS3/4A protease D168V mutant expressed in Escherichia coli incubated for 30 mins by time-resolved fluorescence assay | Bioorg Med Chem Lett 22: 7207-13 (2012) Article DOI: 10.1016/j.bmcl.2012.09.061 BindingDB Entry DOI: 10.7270/Q28D0041 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50069816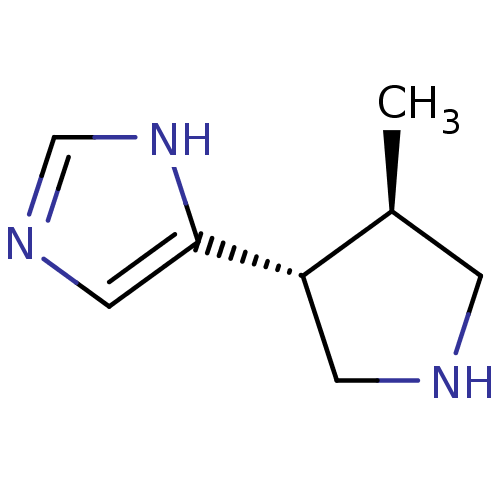 (4-((3R,4R)-4-Methyl-pyrrolidin-3-yl)-1H-imidazole ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-N-alpha-methylhistamine binding to guinea pig brain Histamine H3 receptor | Bioorg Med Chem Lett 8: 243-8 (1999) BindingDB Entry DOI: 10.7270/Q2ST7P0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50486094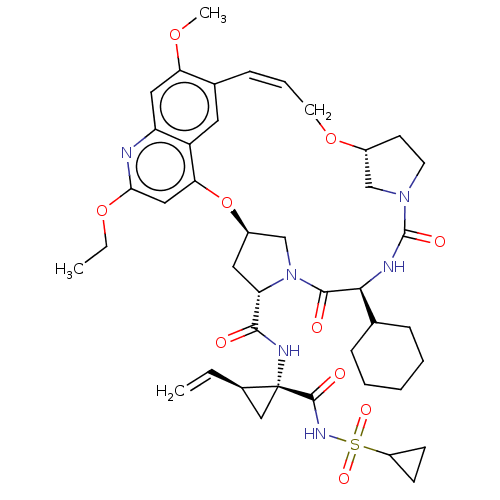 (CHEMBL2203885) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b BK NS3/4A protease A156T mutant expressed in Escherichia coli incubated for 30 mins by time-resolved fluorescence assay | Bioorg Med Chem Lett 22: 7207-13 (2012) Article DOI: 10.1016/j.bmcl.2012.09.061 BindingDB Entry DOI: 10.7270/Q28D0041 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485491 (CHEMBL2063088) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156T mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50034708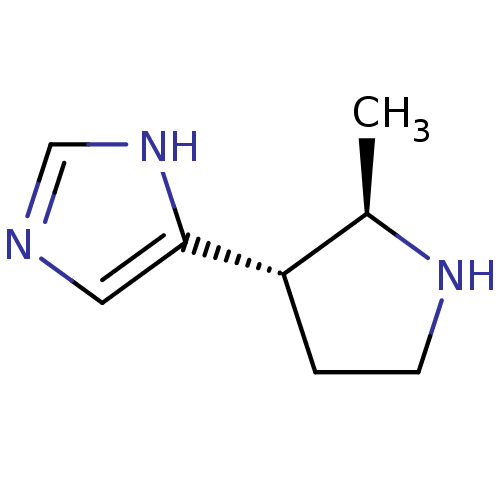 (4-((2R,3S)-2-Methyl-pyrrolidin-3-yl)-1H-imidazole ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding afinity against Histamine H3 receptor | J Med Chem 38: 1593-9 (1995) BindingDB Entry DOI: 10.7270/Q2BK1BC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50486090 (CHEMBL2203882) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b BK NS3/4A protease A156T mutant expressed in Escherichia coli incubated for 30 mins by time-resolved fluorescence assay | Bioorg Med Chem Lett 22: 7207-13 (2012) Article DOI: 10.1016/j.bmcl.2012.09.061 BindingDB Entry DOI: 10.7270/Q28D0041 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50034708 (4-((2R,3S)-2-Methyl-pyrrolidin-3-yl)-1H-imidazole ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding afinity against Histamine H3 receptor | J Med Chem 38: 1593-9 (1995) BindingDB Entry DOI: 10.7270/Q2BK1BC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485494 (CHEMBL2063089) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156T mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50309343 (8-methyl-2,3,4,5,5a,6-hexahydro-[1,4]diazepino[1,7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co Curated by ChEMBL | Assay Description Displacement of [125I]DOI from human recombinant 5HT2C receptor expressed in HEK293E cells | Bioorg Med Chem Lett 20: 1128-33 (2010) Article DOI: 10.1016/j.bmcl.2009.12.014 BindingDB Entry DOI: 10.7270/Q2K074DK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50486092 (CHEMBL2203892) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b BK NS3/4A protease A156T mutant expressed in Escherichia coli incubated for 30 mins by time-resolved fluorescence assay | Bioorg Med Chem Lett 22: 7207-13 (2012) Article DOI: 10.1016/j.bmcl.2012.09.061 BindingDB Entry DOI: 10.7270/Q28D0041 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50153086 (CHEMBL188671 | N-[(1R,2S)-3-(4-Amino-butyl)-2,5-di...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibitory effect against E. coli peptide deformylase (PDF) by AAP assay | J Med Chem 47: 4941-9 (2004) Article DOI: 10.1021/jm049592c BindingDB Entry DOI: 10.7270/Q2WD401S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50069823 (4-((3S,4R)-4-Methyl-pyrrolidin-3-yl)-1H-imidazole ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-N-alpha-methylhistamine binding to guinea pig brain Histamine H3 receptor | Bioorg Med Chem Lett 8: 243-8 (1999) BindingDB Entry DOI: 10.7270/Q2ST7P0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19627 ((2S)-2-[(7-chloro-1,3-benzoxazol-2-yl)amino]-3-cyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | -50.6 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
GNF | Assay Description The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... | Bioorg Med Chem Lett 16: 1975-80 (2006) Article DOI: 10.1016/j.bmcl.2005.12.095 BindingDB Entry DOI: 10.7270/Q2PZ573M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50034708 (4-((2R,3S)-2-Methyl-pyrrolidin-3-yl)-1H-imidazole ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-N-alpha-methylhistamine binding to guinea pig brain Histamine H3 receptor | Bioorg Med Chem Lett 8: 243-8 (1999) BindingDB Entry DOI: 10.7270/Q2ST7P0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM103905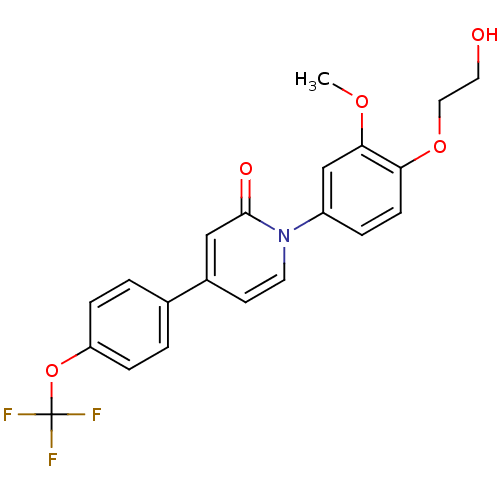 (JNK3 inhibitor 2 | US8563583, C-1) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Compounds were characterized in an in vitro binding assay to determine their Ki or ability to antagonized binding of a peptide agonist to the human m... | US Patent US8563583 (2013) BindingDB Entry DOI: 10.7270/Q22F7M2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50203311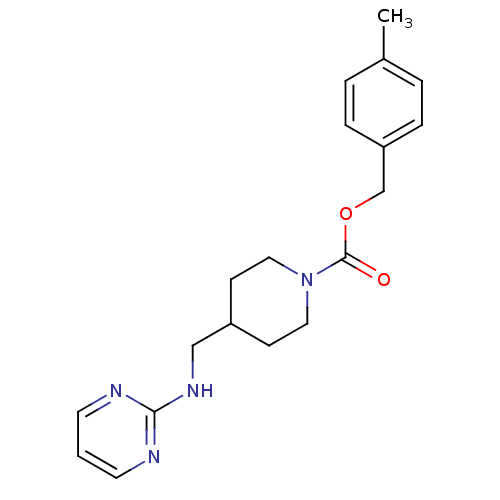 (4-methylbenzyl 4-[(2-pyrimidinylamino)methyl]-1-pi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](E)-N1-(2-methoxybenzyl)cinnamamidine from human NR2B expressed in Ltk- cells | J Med Chem 50: 807-19 (2007) Article DOI: 10.1021/jm060983w BindingDB Entry DOI: 10.7270/Q2FX7944 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50212684 (2-(2-methoxy-benzyl)-5-trifluoromethoxy-2,3-dihydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to NMDA NR2B receptor | Bioorg Med Chem Lett 17: 3997-4000 (2007) Article DOI: 10.1016/j.bmcl.2007.04.084 BindingDB Entry DOI: 10.7270/Q21J99GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50203310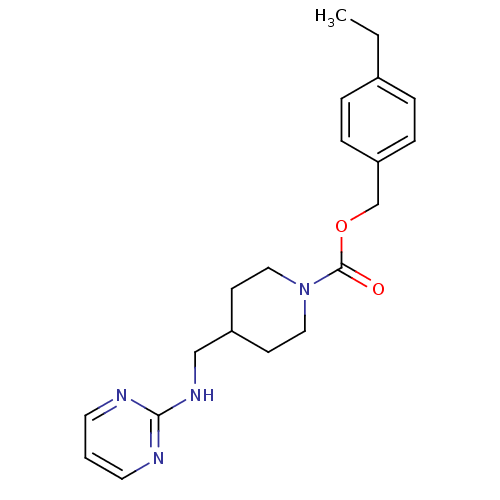 (4-ethylbenzyl 4-[(2-pyrimidinylamino)methyl]-1-pip...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](E)-N1-(2-methoxybenzyl)cinnamamidine from human NR2B expressed in Ltk- cells | J Med Chem 50: 807-19 (2007) Article DOI: 10.1021/jm060983w BindingDB Entry DOI: 10.7270/Q2FX7944 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (RAT) | BDBM22868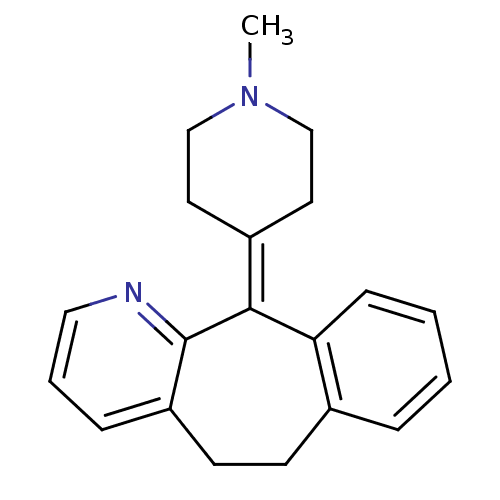 (11-(1-methylpiperidin-4-ylidene)-6,11-dihydro-5H-b...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Corporation Curated by ChEMBL | Assay Description Binding affinity to histamine H1 receptor in rat brain membranes was evaluated using [3H]-pyrilamine as radioligand | J Med Chem 34: 457-61 (1991) BindingDB Entry DOI: 10.7270/Q2BV7FK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM103907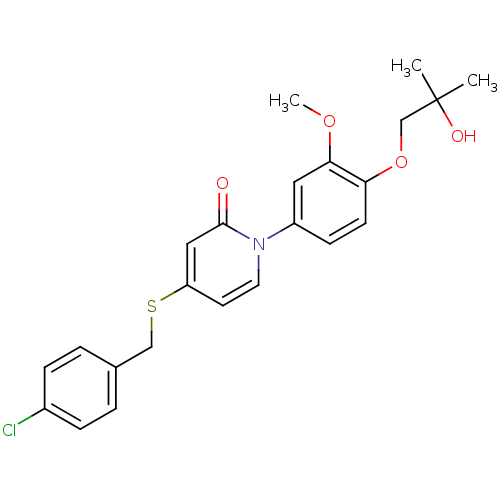 (JNK3 inhibitor 4 | US8563583, D-4) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Compounds were characterized in an in vitro binding assay to determine their Ki or ability to antagonized binding of a peptide agonist to the human m... | US Patent US8563583 (2013) BindingDB Entry DOI: 10.7270/Q22F7M2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50486106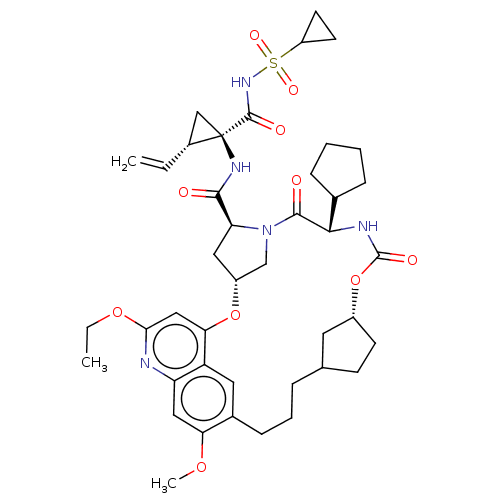 (CHEMBL2203878) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b BK NS3/4A protease A156T mutant expressed in Escherichia coli incubated for 30 mins by time-resolved fluorescence assay | Bioorg Med Chem Lett 22: 7207-13 (2012) Article DOI: 10.1016/j.bmcl.2012.09.061 BindingDB Entry DOI: 10.7270/Q28D0041 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50486095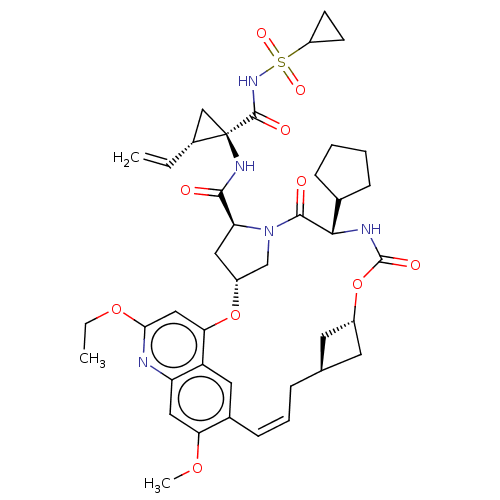 (CHEMBL2203881) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b BK NS3/4A protease A156T mutant expressed in Escherichia coli incubated for 30 mins by time-resolved fluorescence assay | Bioorg Med Chem Lett 22: 7207-13 (2012) Article DOI: 10.1016/j.bmcl.2012.09.061 BindingDB Entry DOI: 10.7270/Q28D0041 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19631 ((2S)-2-(1,3-benzoxazol-2-ylamino)-N-[(2R)-1-(benzy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5 | -49.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
GNF | Assay Description The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... | Bioorg Med Chem Lett 16: 1975-80 (2006) Article DOI: 10.1016/j.bmcl.2005.12.095 BindingDB Entry DOI: 10.7270/Q2PZ573M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 5170 total ) | Next | Last >> |