 Found 109 hits with Last Name = 'nicke' and Initial = 'a'
Found 109 hits with Last Name = 'nicke' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Casein kinase II subunit alpha'/beta
(Homo sapiens (Human)) | BDBM50530315

(CHEMBL4520531)Show InChI InChI=1S/C20H14N2O3S/c23-18-10-15(7-8-16(18)19(24)25)21-20-22-17(11-26-20)14-6-5-12-3-1-2-4-13(12)9-14/h1-11,23H,(H,21,22)(H,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t zu K£ln
Curated by ChEMBL
| Assay Description
ATP competitive inhibition of recombinant human CK2alpha2 (1 to 335 residues)/CK2beta2 (1 to 193 residues) expressed in Escherichia coli BL21(DE3) us... |
J Med Chem 63: 7766-7772 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00587
BindingDB Entry DOI: 10.7270/Q24M9834 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Casein kinase II subunit alpha'/beta
(Homo sapiens (Human)) | BDBM50540033

(CHEMBL4635554)Show InChI InChI=1S/C16H11ClN2O3S/c17-10-3-1-9(2-4-10)13-8-23-16(19-13)18-11-5-6-12(15(21)22)14(20)7-11/h1-8,20H,(H,18,19)(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 206 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t zu K£ln
Curated by ChEMBL
| Assay Description
ATP competitive inhibition of recombinant human CK2alpha2 (1 to 335 residues)/CK2beta2 (1 to 193 residues) expressed in Escherichia coli BL21(DE3) us... |
J Med Chem 63: 7766-7772 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00587
BindingDB Entry DOI: 10.7270/Q24M9834 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Casein kinase II subunit alpha'/beta
(Homo sapiens (Human)) | BDBM50540034

(CHEMBL4647986)Show InChI InChI=1S/C15H11N3O2S/c19-14(20)10-4-3-5-11(8-10)17-15-18-13(9-21-15)12-6-1-2-7-16-12/h1-9H,(H,17,18)(H,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 1.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t zu K£ln
Curated by ChEMBL
| Assay Description
ATP competitive inhibition of recombinant human CK2alpha2 (1 to 335 residues)/CK2beta2 (1 to 193 residues) expressed in Escherichia coli BL21(DE3) us... |
J Med Chem 63: 7766-7772 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00587
BindingDB Entry DOI: 10.7270/Q24M9834 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-3/beta-2
(Rattus norvegicus (Rat)) | BDBM50140093

(CHEMBL406720 | GCCSHPACAANNQDYC*)Show SMILES C[C@H](NC(=O)[C@H](CS)NC(=O)[C@H](C)NC(=O)[C@H]1CCCN1C(=O)[C@H](CC1=NCNC1)NC(=O)[C@H](CO)NC(=O)[C@H](CS)NC(=O)[C@H](CS)NC(=O)CN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CS)C(N)=O |t:26| Show InChI InChI=1S/C60H91N21O22S4/c1-25(68-56(99)39(22-106)79-49(92)26(2)69-59(102)41-4-3-11-81(41)60(103)35(13-28-18-66-24-67-28)76-55(98)36(19-82)77-58(101)40(23-107)80-57(100)38(21-105)70-45(87)17-61)48(91)72-32(14-43(63)85)53(96)74-33(15-44(64)86)52(95)71-30(9-10-42(62)84)50(93)75-34(16-46(88)89)54(97)73-31(12-27-5-7-29(83)8-6-27)51(94)78-37(20-104)47(65)90/h5-8,25-26,30-41,66,82-83,104-107H,3-4,9-24,61H2,1-2H3,(H2,62,84)(H2,63,85)(H2,64,86)(H2,65,90)(H,68,99)(H,69,102)(H,70,87)(H,71,95)(H,72,91)(H,73,97)(H,74,96)(H,75,93)(H,76,98)(H,77,101)(H,78,94)(H,79,92)(H,80,100)(H,88,89)/t25-,26-,30-,31-,32-,33-,34-,35-,36-,37-,38-,39-,40-,41+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of rat Nicotinic acetylcholine receptor alpha3-beta2 expressed in Xenopus Oocytes |
J Med Chem 47: 1234-41 (2004)
Article DOI: 10.1021/jm031010o
BindingDB Entry DOI: 10.7270/Q2RN378M |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit delta
(Rattus norvegicus) | BDBM50547502

(CHEMBL4793300)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H]1CSSC[C@@H]3NC(=O)[C@H](CO)NC(=O)[C@H](Cc4ccccc4)NC(=O)[C@H](Cc4c[nH]cn4)NC(=O)[C@H](CCCCN)NC(=O)CNC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)CNC(=O)[C@H](CC(N)=O)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](C)NC3=O)C(=O)N1)NC(=O)[C@H](C)NC2=O |r| | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.184 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rat alpha1beta1epsilondelta expressed in Xenopus laevis oocytes assessed as inhibition of acetylcholine-induced channel current respons... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00957
BindingDB Entry DOI: 10.7270/Q27S7SCT |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3/beta-2
(Rattus norvegicus (Rat)) | BDBM50140086

(CHEMBL415207 | GGGCCSHPACAANNQDYC*)Show SMILES C[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CS)NC(=O)[C@H](C)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CO)NC(=O)[C@H](CS)NC(=O)[C@H](CS)NC(=O)CNC(=O)CNC(=O)CN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CS)C(N)=O Show InChI InChI=1S/C67H100N24O25S4/c1-28(54(103)77-29(2)55(104)82-36(15-47(70)95)60(109)84-37(16-48(71)96)59(108)81-34(10-11-46(69)94)57(106)85-38(17-52(100)101)61(110)83-35(13-31-6-8-33(93)9-7-31)58(107)88-41(23-117)53(72)102)78-63(112)43(25-119)89-56(105)30(3)79-66(115)45-5-4-12-91(45)67(116)39(14-32-19-73-27-76-32)86-62(111)40(22-92)87-65(114)44(26-120)90-64(113)42(24-118)80-51(99)21-75-50(98)20-74-49(97)18-68/h6-9,19,27-30,34-45,92-93,117-120H,4-5,10-18,20-26,68H2,1-3H3,(H2,69,94)(H2,70,95)(H2,71,96)(H2,72,102)(H,73,76)(H,74,97)(H,75,98)(H,77,103)(H,78,112)(H,79,115)(H,80,99)(H,81,108)(H,82,104)(H,83,110)(H,84,109)(H,85,106)(H,86,111)(H,87,114)(H,88,107)(H,89,105)(H,90,113)(H,100,101)/t28-,29-,30-,34-,35-,36-,37-,38-,39-,40-,41-,42-,43-,44-,45+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of rat Nicotinic acetylcholine receptor alpha3-beta2 expressed in Xenopus Oocytes |
J Med Chem 47: 1234-41 (2004)
Article DOI: 10.1021/jm031010o
BindingDB Entry DOI: 10.7270/Q2RN378M |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3/beta-2
(Rattus norvegicus (Rat)) | BDBM50140092
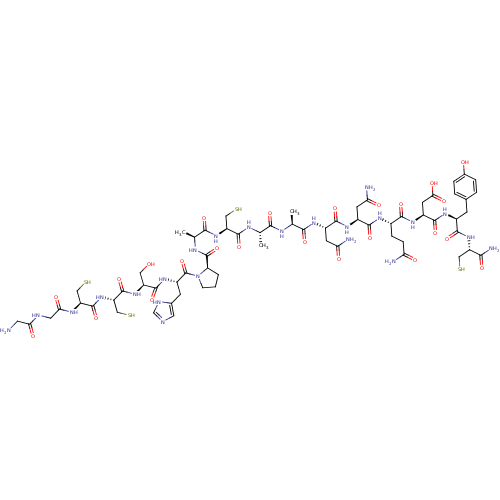
(CHEMBL437662 | GGCCSHPACAANNQDYC*)Show SMILES C[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CS)NC(=O)[C@H](C)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CO)NC(=O)[C@H](CS)NC(=O)[C@H](CS)NC(=O)CNC(=O)CN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CS)C(N)=O Show InChI InChI=1S/C65H97N23O24S4/c1-27(75-61(108)42(24-115)86-54(101)29(3)76-64(111)44-5-4-12-88(44)65(112)38(14-31-19-71-26-73-31)83-60(107)39(21-89)84-63(110)43(25-116)87-62(109)41(23-114)77-49(95)20-72-48(94)18-66)52(99)74-28(2)53(100)79-35(15-46(68)92)58(105)81-36(16-47(69)93)57(104)78-33(10-11-45(67)91)55(102)82-37(17-50(96)97)59(106)80-34(13-30-6-8-32(90)9-7-30)56(103)85-40(22-113)51(70)98/h6-9,19,26-29,33-44,89-90,113-116H,4-5,10-18,20-25,66H2,1-3H3,(H2,67,91)(H2,68,92)(H2,69,93)(H2,70,98)(H,71,73)(H,72,94)(H,74,99)(H,75,108)(H,76,111)(H,77,95)(H,78,104)(H,79,100)(H,80,106)(H,81,105)(H,82,102)(H,83,107)(H,84,110)(H,85,103)(H,86,101)(H,87,109)(H,96,97)/t27-,28-,29-,33-,34-,35-,36-,37-,38-,39-,40-,41-,42-,43-,44+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of rat Nicotinic acetylcholine receptor alpha3-beta2 expressed in Xenopus Oocytes |
J Med Chem 47: 1234-41 (2004)
Article DOI: 10.1021/jm031010o
BindingDB Entry DOI: 10.7270/Q2RN378M |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3/beta-2
(Rattus norvegicus (Rat)) | BDBM50140092

(CHEMBL437662 | GGCCSHPACAANNQDYC*)Show SMILES C[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CS)NC(=O)[C@H](C)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CO)NC(=O)[C@H](CS)NC(=O)[C@H](CS)NC(=O)CNC(=O)CN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CS)C(N)=O Show InChI InChI=1S/C65H97N23O24S4/c1-27(75-61(108)42(24-115)86-54(101)29(3)76-64(111)44-5-4-12-88(44)65(112)38(14-31-19-71-26-73-31)83-60(107)39(21-89)84-63(110)43(25-116)87-62(109)41(23-114)77-49(95)20-72-48(94)18-66)52(99)74-28(2)53(100)79-35(15-46(68)92)58(105)81-36(16-47(69)93)57(104)78-33(10-11-45(67)91)55(102)82-37(17-50(96)97)59(106)80-34(13-30-6-8-32(90)9-7-30)56(103)85-40(22-113)51(70)98/h6-9,19,26-29,33-44,89-90,113-116H,4-5,10-18,20-25,66H2,1-3H3,(H2,67,91)(H2,68,92)(H2,69,93)(H2,70,98)(H,71,73)(H,72,94)(H,74,99)(H,75,108)(H,76,111)(H,77,95)(H,78,104)(H,79,100)(H,80,106)(H,81,105)(H,82,102)(H,83,107)(H,84,110)(H,85,103)(H,86,101)(H,87,109)(H,96,97)/t27-,28-,29-,33-,34-,35-,36-,37-,38-,39-,40-,41-,42-,43-,44+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of rat Nicotinic acetylcholine receptor alpha3-beta2 expressed in Xenopus Oocytes |
J Med Chem 47: 1234-41 (2004)
Article DOI: 10.1021/jm031010o
BindingDB Entry DOI: 10.7270/Q2RN378M |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3/beta-2
(Rattus norvegicus (Rat)) | BDBM50140087
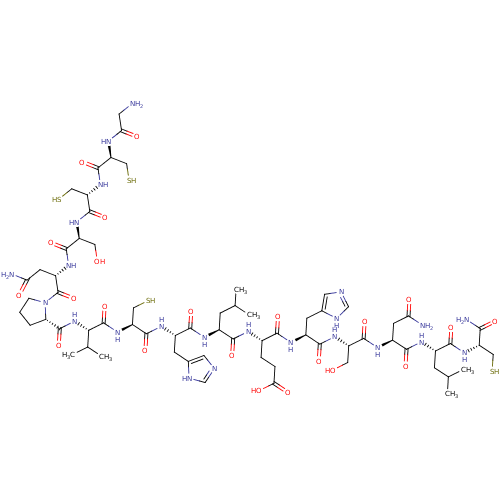
(CHEMBL265198 | GCCSNPVCHLEHSNLC*)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CS)NC(=O)[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CS)NC(=O)[C@H](CS)NC(=O)CN)C(C)C)C(=O)N[C@@H](CS)C(N)=O Show InChI InChI=1S/C67H107N23O22S4/c1-29(2)12-35(55(100)77-34(9-10-51(96)97)54(99)80-38(15-33-20-73-28-75-33)58(103)84-41(21-91)60(105)82-39(16-48(69)93)59(104)79-36(13-30(3)4)56(101)86-43(23-113)53(71)98)78-57(102)37(14-32-19-72-27-74-32)81-63(108)46(26-116)88-66(111)52(31(5)6)89-65(110)47-8-7-11-90(47)67(112)40(17-49(70)94)83-61(106)42(22-92)85-64(109)45(25-115)87-62(107)44(24-114)76-50(95)18-68/h19-20,27-31,34-47,52,91-92,113-116H,7-18,21-26,68H2,1-6H3,(H2,69,93)(H2,70,94)(H2,71,98)(H,72,74)(H,73,75)(H,76,95)(H,77,100)(H,78,102)(H,79,104)(H,80,99)(H,81,108)(H,82,105)(H,83,106)(H,84,103)(H,85,109)(H,86,101)(H,87,107)(H,88,111)(H,89,110)(H,96,97)/t34-,35-,36-,37-,38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,52-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of rat Nicotinic acetylcholine receptor alpha3-beta2 expressed in Xenopus Oocytes |
J Med Chem 47: 1234-41 (2004)
Article DOI: 10.1021/jm031010o
BindingDB Entry DOI: 10.7270/Q2RN378M |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3/beta-2
(Rattus norvegicus (Rat)) | BDBM50140088

(CHEMBL451251 | GGCCSHPACAANNQDYC#)Show SMILES C[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CS)NC(=O)[C@H](C)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CO)NC(=O)[C@H](CS)NC(=O)[C@H](CS)NC(=O)CNC(=O)CN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CS)C(O)=O Show InChI InChI=1S/C65H96N22O25S4/c1-27(74-60(106)41(23-114)84-53(99)29(3)75-63(109)44-5-4-12-87(44)64(110)38(14-31-19-70-26-72-31)82-59(105)39(21-88)83-62(108)42(24-115)85-61(107)40(22-113)76-49(94)20-71-48(93)18-66)51(97)73-28(2)52(98)78-35(15-46(68)91)57(103)80-36(16-47(69)92)56(102)77-33(10-11-45(67)90)54(100)81-37(17-50(95)96)58(104)79-34(13-30-6-8-32(89)9-7-30)55(101)86-43(25-116)65(111)112/h6-9,19,26-29,33-44,88-89,113-116H,4-5,10-18,20-25,66H2,1-3H3,(H2,67,90)(H2,68,91)(H2,69,92)(H,70,72)(H,71,93)(H,73,97)(H,74,106)(H,75,109)(H,76,94)(H,77,102)(H,78,98)(H,79,104)(H,80,103)(H,81,100)(H,82,105)(H,83,108)(H,84,99)(H,85,107)(H,86,101)(H,95,96)(H,111,112)/t27-,28-,29-,33-,34-,35-,36-,37-,38-,39-,40-,41-,42-,43-,44+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of rat Nicotinic acetylcholine receptor alpha3-beta2 expressed in Xenopus Oocytes |
J Med Chem 47: 1234-41 (2004)
Article DOI: 10.1021/jm031010o
BindingDB Entry DOI: 10.7270/Q2RN378M |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3/beta-2
(Rattus norvegicus (Rat)) | BDBM50140089
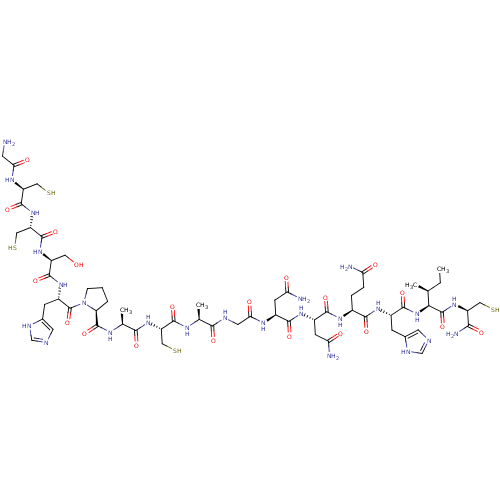
(CHEMBL437423 | GCCSHPACAGNNQHIC*)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CS)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CO)NC(=O)[C@H](CS)NC(=O)[C@H](CS)NC(=O)CN)C(=O)N[C@@H](CS)C(N)=O Show InChI InChI=1S/C61H96N24O20S4/c1-5-26(2)47(60(104)81-37(20-106)48(66)92)84-54(98)32(11-29-16-67-24-70-29)77-51(95)31(8-9-42(63)87)76-53(97)34(14-44(65)89)78-52(96)33(13-43(64)88)74-46(91)18-69-49(93)27(3)72-56(100)39(22-108)82-50(94)28(4)73-59(103)41-7-6-10-85(41)61(105)35(12-30-17-68-25-71-30)79-55(99)36(19-86)80-58(102)40(23-109)83-57(101)38(21-107)75-45(90)15-62/h16-17,24-28,31-41,47,86,106-109H,5-15,18-23,62H2,1-4H3,(H2,63,87)(H2,64,88)(H2,65,89)(H2,66,92)(H,67,70)(H,68,71)(H,69,93)(H,72,100)(H,73,103)(H,74,91)(H,75,90)(H,76,97)(H,77,95)(H,78,96)(H,79,99)(H,80,102)(H,81,104)(H,82,94)(H,83,101)(H,84,98)/t26-,27-,28-,31-,32-,33-,34-,35-,36-,37-,38-,39-,40-,41-,47-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of rat Nicotinic acetylcholine receptor alpha3-beta2 expressed in Xenopus Oocytes |
J Med Chem 47: 1234-41 (2004)
Article DOI: 10.1021/jm031010o
BindingDB Entry DOI: 10.7270/Q2RN378M |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3/beta-2
(Rattus norvegicus (Rat)) | BDBM50547502

(CHEMBL4793300)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H]1CSSC[C@@H]3NC(=O)[C@H](CO)NC(=O)[C@H](Cc4ccccc4)NC(=O)[C@H](Cc4c[nH]cn4)NC(=O)[C@H](CCCCN)NC(=O)CNC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)CNC(=O)[C@H](CC(N)=O)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](C)NC3=O)C(=O)N1)NC(=O)[C@H](C)NC2=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rat alpha3beta2 expressed in Xenopus laevis oocytes assessed as inhibition of acetylcholine-induced channel current response at -70 mV ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00957
BindingDB Entry DOI: 10.7270/Q27S7SCT |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit gamma
(Rattus norvegicus) | BDBM50547502

(CHEMBL4793300)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H]1CSSC[C@@H]3NC(=O)[C@H](CO)NC(=O)[C@H](Cc4ccccc4)NC(=O)[C@H](Cc4c[nH]cn4)NC(=O)[C@H](CCCCN)NC(=O)CNC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)CNC(=O)[C@H](CC(N)=O)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](C)NC3=O)C(=O)N1)NC(=O)[C@H](C)NC2=O |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rat (alpha1)2betagammadelta expressed in Xenopus laevis oocytes assessed as inhibition of acetylcholine-induced channel current respons... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00957
BindingDB Entry DOI: 10.7270/Q27S7SCT |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3/beta-2
(Rattus norvegicus (Rat)) | BDBM50140094
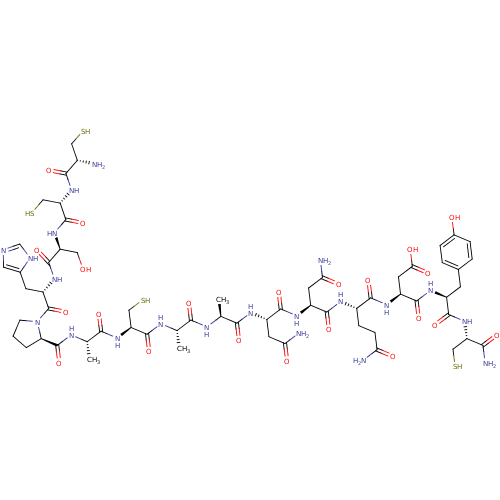
(CCSHPACAANNQDYC* | CHEMBL262475)Show SMILES C[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CS)NC(=O)[C@H](C)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CO)NC(=O)[C@H](CS)NC(=O)[C@@H](N)CS)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CS)C(N)=O Show InChI InChI=1S/C61H91N21O22S4/c1-25(70-58(101)40(22-107)80-50(93)27(3)71-60(103)42-5-4-12-82(42)61(104)37(14-29-18-67-24-68-29)77-57(100)38(19-83)78-59(102)41(23-108)81-51(94)31(62)20-105)48(91)69-26(2)49(92)73-34(15-44(64)86)55(98)75-35(16-45(65)87)54(97)72-32(10-11-43(63)85)52(95)76-36(17-46(88)89)56(99)74-33(13-28-6-8-30(84)9-7-28)53(96)79-39(21-106)47(66)90/h6-9,18,24-27,31-42,83-84,105-108H,4-5,10-17,19-23,62H2,1-3H3,(H2,63,85)(H2,64,86)(H2,65,87)(H2,66,90)(H,67,68)(H,69,91)(H,70,101)(H,71,103)(H,72,97)(H,73,92)(H,74,99)(H,75,98)(H,76,95)(H,77,100)(H,78,102)(H,79,96)(H,80,93)(H,81,94)(H,88,89)/t25-,26-,27-,31-,32-,33-,34-,35-,36-,37-,38-,39-,40-,41-,42+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.84 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of rat Nicotinic acetylcholine receptor alpha3-beta2 expressed in Xenopus Oocytes |
J Med Chem 47: 1234-41 (2004)
Article DOI: 10.1021/jm031010o
BindingDB Entry DOI: 10.7270/Q2RN378M |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit alpha/beta/delta/gamma
(Rattus norvegicus-RAT) | BDBM50547500

(CHEMBL4759352)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc3ccccc3)NC(=O)[C@H](Cc3c[nH]cn3)NC(=O)[C@H](CCCCN)NC(=O)CNC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)CNC(=O)[C@@H](N)CC(N)=O)C(=O)N1)NC(=O)[C@H](C)NC2=O)C(N)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rat (alpha1)2betagammadelta expressed in Xenopus laevis oocytes assessed as inhibition of acetylcholine-induced channel current respons... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00957
BindingDB Entry DOI: 10.7270/Q27S7SCT |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit gamma
(Rattus norvegicus) | BDBM50547501
(CHEMBL4788244)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H]1CSSC[C@@H]3NC(=O)[C@H](CO)NC(=O)[C@H](Cc4ccccc4)NC(=O)[C@H](Cc4c[nH]cn4)NC(=O)[C@H](CCCCN)NC(=O)CNC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)CNC(=O)[C@H](CC(N)=O)NC(=O)CNC(=O)[C@H](C)NC3=O)C(=O)N1)NC(=O)[C@H](C)NC2=O |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rat (alpha1)2betagammadelta expressed in Xenopus laevis oocytes assessed as inhibition of acetylcholine-induced channel current respons... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00957
BindingDB Entry DOI: 10.7270/Q27S7SCT |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3/alpha-6/beta-2/beta-3
(Rattus norvegicus (Rat)) | BDBM50547502

(CHEMBL4793300)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H]1CSSC[C@@H]3NC(=O)[C@H](CO)NC(=O)[C@H](Cc4ccccc4)NC(=O)[C@H](Cc4c[nH]cn4)NC(=O)[C@H](CCCCN)NC(=O)CNC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)CNC(=O)[C@H](CC(N)=O)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](C)NC3=O)C(=O)N1)NC(=O)[C@H](C)NC2=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rat alpha6alpha3beta2beta3 expressed in Xenopus laevis oocytes assessed as inhibition of acetylcholine-induced channel current response... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00957
BindingDB Entry DOI: 10.7270/Q27S7SCT |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit gamma
(Rattus norvegicus) | BDBM50547503

(CHEMBL4753712)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H]1CSSC[C@@H]3NC(=O)[C@H](CO)NC(=O)[C@H](Cc4ccccc4)NC(=O)[C@H](Cc4c[nH]cn4)NC(=O)[C@H](CCCCN)NC(=O)CNC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)CNC(=O)[C@H](CC(N)=O)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)CNC3=O)C(=O)N1)NC(=O)[C@H](C)NC2=O |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rat (alpha1)2betagammadelta expressed in Xenopus laevis oocytes assessed as inhibition of acetylcholine-induced channel current respons... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00957
BindingDB Entry DOI: 10.7270/Q27S7SCT |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3/beta-2
(Rattus norvegicus (Rat)) | BDBM50140085
(CHEMBL266702 | GCCSLPPCAANNPDYC*)Show SMILES CC(C)C[C@@H](NC(=O)[C@@H](CO)NC(=O)[C@@H](CS)NC(=O)[C@@H](CS)NC(=O)CN)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](CS)C(=O)N[C@@H](C)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccc(S)cc1)C(=O)N[C@@H](CS)C(N)=O Show InChI InChI=1S/C65H99N19O21S5/c1-29(2)18-37(76-57(97)39(24-85)78-60(100)43(28-109)80-59(99)41(26-107)72-49(88)23-66)63(103)84-17-7-10-46(84)65(105)83-16-6-9-45(83)62(102)81-42(27-108)58(98)71-30(3)52(92)70-31(4)53(93)73-35(20-47(67)86)55(95)77-38(21-48(68)87)64(104)82-15-5-8-44(82)61(101)75-36(22-50(89)90)56(96)74-34(19-32-11-13-33(110)14-12-32)54(94)79-40(25-106)51(69)91/h11-14,29-31,34-46,85,106-110H,5-10,15-28,66H2,1-4H3,(H2,67,86)(H2,68,87)(H2,69,91)(H,70,92)(H,71,98)(H,72,88)(H,73,93)(H,74,96)(H,75,101)(H,76,97)(H,77,95)(H,78,100)(H,79,94)(H,80,99)(H,81,102)(H,89,90)/t30-,31-,34-,35-,36-,37+,38-,39+,40-,41+,42-,43+,44+,45-,46-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of rat Nicotinic acetylcholine receptor alpha3-beta2 expressed in Xenopus Oocytes |
J Med Chem 47: 1234-41 (2004)
Article DOI: 10.1021/jm031010o
BindingDB Entry DOI: 10.7270/Q2RN378M |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3/beta-2
(Rattus norvegicus (Rat)) | BDBM50140091
(CHEMBL413505 | IRDgammaCCSNPACRVNNOHVC#)Show SMILES CC[C@H](C)[C@H](N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(O)=O)C(=O)NC(CC(C(O)=O)C(O)=O)C(=O)N[C@@H](CS)C(=O)N[C@@H](CS)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N1CC(O)CC1C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CS)C(N)=O Show InChI InChI=1S/C84H137N31O30S4/c1-8-35(6)59(88)76(137)100-40(12-9-15-95-83(90)91)64(125)102-45(24-58(121)122)68(129)101-43(20-39(81(142)143)82(144)145)66(127)110-52(31-149)73(134)111-51(30-148)72(133)107-48(27-116)70(131)106-46(22-56(86)119)79(140)114-17-11-14-53(114)74(135)98-36(7)63(124)109-50(29-147)71(132)99-41(13-10-16-96-84(92)93)65(126)112-60(33(2)3)77(138)104-44(21-55(85)118)67(128)105-47(23-57(87)120)80(141)115-26-38(117)19-54(115)75(136)103-42(18-37-25-94-32-97-37)69(130)113-61(34(4)5)78(139)108-49(28-146)62(89)123/h25,32-36,38-54,59-61,116-117,146-149H,8-24,26-31,88H2,1-7H3,(H2,85,118)(H2,86,119)(H2,87,120)(H2,89,123)(H,94,97)(H,98,135)(H,99,132)(H,100,137)(H,101,129)(H,102,125)(H,103,136)(H,104,138)(H,105,128)(H,106,131)(H,107,133)(H,108,139)(H,109,124)(H,110,127)(H,111,134)(H,112,126)(H,113,130)(H,121,122)(H,142,143)(H,144,145)(H4,90,91,95)(H4,92,93,96)/t35-,36-,38?,40-,41-,42-,43?,44-,45-,46-,47-,48-,49-,50-,51-,52-,53-,54?,59-,60-,61-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of rat Nicotinic acetylcholine receptor alpha3-beta2 expressed in Xenopus Oocytes |
J Med Chem 47: 1234-41 (2004)
Article DOI: 10.1021/jm031010o
BindingDB Entry DOI: 10.7270/Q2RN378M |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3/beta-2
(Rattus norvegicus (Rat)) | BDBM50547501

(CHEMBL4788244)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H]1CSSC[C@@H]3NC(=O)[C@H](CO)NC(=O)[C@H](Cc4ccccc4)NC(=O)[C@H](Cc4c[nH]cn4)NC(=O)[C@H](CCCCN)NC(=O)CNC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)CNC(=O)[C@H](CC(N)=O)NC(=O)CNC(=O)[C@H](C)NC3=O)C(=O)N1)NC(=O)[C@H](C)NC2=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rat alpha3beta2 expressed in Xenopus laevis oocytes assessed as inhibition of acetylcholine-induced channel current response at -70 mV ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00957
BindingDB Entry DOI: 10.7270/Q27S7SCT |
More data for this
Ligand-Target Pair | |
G2/mitotic-specific cyclin-B1
(Rattus norvegicus) | BDBM50140090
(CHEMBL217150 | RDPCCSNPVCTVHNPQIC*)Show SMILES CC(C)C[C@@H](NC(=O)[C@@H](CO)NC(=O)[C@@H](CS)NC(=O)[C@@H](CS)NC(=O)CN)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](CS)C(=O)N[C@@H](C)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CS)C(N)=O Show InChI InChI=1S/C65H99N19O22S4/c1-29(2)18-37(76-57(98)39(24-85)78-60(101)43(28-110)80-59(100)41(26-108)72-49(89)23-66)63(104)84-17-7-10-46(84)65(106)83-16-6-9-45(83)62(103)81-42(27-109)58(99)71-30(3)52(93)70-31(4)53(94)73-35(20-47(67)87)55(96)77-38(21-48(68)88)64(105)82-15-5-8-44(82)61(102)75-36(22-50(90)91)56(97)74-34(19-32-11-13-33(86)14-12-32)54(95)79-40(25-107)51(69)92/h11-14,29-31,34-46,85-86,107-110H,5-10,15-28,66H2,1-4H3,(H2,67,87)(H2,68,88)(H2,69,92)(H,70,93)(H,71,99)(H,72,89)(H,73,94)(H,74,97)(H,75,102)(H,76,98)(H,77,96)(H,78,101)(H,79,95)(H,80,100)(H,81,103)(H,90,91)/t30-,31-,34-,35-,36-,37+,38-,39+,40-,41+,42-,43+,44+,45-,46-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of rat Nicotinic acetylcholine receptor alpha7 (nAChR) in Xenopus Oocytes |
J Med Chem 47: 1234-41 (2004)
Article DOI: 10.1021/jm031010o
BindingDB Entry DOI: 10.7270/Q2RN378M |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3/beta-2
(Rattus norvegicus (Rat)) | BDBM50547503

(CHEMBL4753712)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H]1CSSC[C@@H]3NC(=O)[C@H](CO)NC(=O)[C@H](Cc4ccccc4)NC(=O)[C@H](Cc4c[nH]cn4)NC(=O)[C@H](CCCCN)NC(=O)CNC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)CNC(=O)[C@H](CC(N)=O)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)CNC3=O)C(=O)N1)NC(=O)[C@H](C)NC2=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rat alpha3beta2 expressed in Xenopus laevis oocytes assessed as inhibition of acetylcholine-induced channel current response at -70 mV ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00957
BindingDB Entry DOI: 10.7270/Q27S7SCT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50239250

(CHEMBL4086080)Show SMILES CNC(=O)c1c(oc2ccc(cc12)-c1cc(ccc1C)C(=O)NC1(CC1)c1ccncc1)-c1ccc(F)cc1 Show InChI InChI=1S/C32H26FN3O3/c1-19-3-4-22(30(37)36-32(13-14-32)23-11-15-35-16-12-23)18-25(19)21-7-10-27-26(17-21)28(31(38)34-2)29(39-27)20-5-8-24(33)9-6-20/h3-12,15-18H,13-14H2,1-2H3,(H,34,38)(H,36,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of microsomal CYP2C8 (unknown origin) |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
G2/mitotic-specific cyclin-B1
(Rattus norvegicus) | BDBM50140091

(CHEMBL413505 | IRDgammaCCSNPACRVNNOHVC#)Show SMILES CC[C@H](C)[C@H](N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(O)=O)C(=O)NC(CC(C(O)=O)C(O)=O)C(=O)N[C@@H](CS)C(=O)N[C@@H](CS)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N1CC(O)CC1C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CS)C(N)=O Show InChI InChI=1S/C84H137N31O30S4/c1-8-35(6)59(88)76(137)100-40(12-9-15-95-83(90)91)64(125)102-45(24-58(121)122)68(129)101-43(20-39(81(142)143)82(144)145)66(127)110-52(31-149)73(134)111-51(30-148)72(133)107-48(27-116)70(131)106-46(22-56(86)119)79(140)114-17-11-14-53(114)74(135)98-36(7)63(124)109-50(29-147)71(132)99-41(13-10-16-96-84(92)93)65(126)112-60(33(2)3)77(138)104-44(21-55(85)118)67(128)105-47(23-57(87)120)80(141)115-26-38(117)19-54(115)75(136)103-42(18-37-25-94-32-97-37)69(130)113-61(34(4)5)78(139)108-49(28-146)62(89)123/h25,32-36,38-54,59-61,116-117,146-149H,8-24,26-31,88H2,1-7H3,(H2,85,118)(H2,86,119)(H2,87,120)(H2,89,123)(H,94,97)(H,98,135)(H,99,132)(H,100,137)(H,101,129)(H,102,125)(H,103,136)(H,104,138)(H,105,128)(H,106,131)(H,107,133)(H,108,139)(H,109,124)(H,110,127)(H,111,134)(H,112,126)(H,113,130)(H,121,122)(H,142,143)(H,144,145)(H4,90,91,95)(H4,92,93,96)/t35-,36-,38?,40-,41-,42-,43?,44-,45-,46-,47-,48-,49-,50-,51-,52-,53-,54?,59-,60-,61-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of rat Nicotinic acetylcholine receptor alpha7 (nAChR) in Xenopus Oocytes |
J Med Chem 47: 1234-41 (2004)
Article DOI: 10.1021/jm031010o
BindingDB Entry DOI: 10.7270/Q2RN378M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50239251

(CHEMBL4096241)Show SMILES CNC(=O)c1c(oc2ccc(cc12)-c1cc(ccc1C)C(=O)NC1(CC1)c1cccnc1)-c1ccc(F)cc1 Show InChI InChI=1S/C32H26FN3O3/c1-19-5-6-22(30(37)36-32(13-14-32)23-4-3-15-35-18-23)17-25(19)21-9-12-27-26(16-21)28(31(38)34-2)29(39-27)20-7-10-24(33)11-8-20/h3-12,15-18H,13-14H2,1-2H3,(H,34,38)(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of microsomal CYP3A4 (unknown origin) |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3/beta-2
(Rattus norvegicus (Rat)) | BDBM50547500

(CHEMBL4759352)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc3ccccc3)NC(=O)[C@H](Cc3c[nH]cn3)NC(=O)[C@H](CCCCN)NC(=O)CNC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)CNC(=O)[C@@H](N)CC(N)=O)C(=O)N1)NC(=O)[C@H](C)NC2=O)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rat alpha3beta2 expressed in Xenopus laevis oocytes assessed as inhibition of acetylcholine-induced channel current response at -70 mV ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00957
BindingDB Entry DOI: 10.7270/Q27S7SCT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50239250

(CHEMBL4086080)Show SMILES CNC(=O)c1c(oc2ccc(cc12)-c1cc(ccc1C)C(=O)NC1(CC1)c1ccncc1)-c1ccc(F)cc1 Show InChI InChI=1S/C32H26FN3O3/c1-19-3-4-22(30(37)36-32(13-14-32)23-11-15-35-16-12-23)18-25(19)21-7-10-27-26(17-21)28(31(38)34-2)29(39-27)20-5-8-24(33)9-6-20/h3-12,15-18H,13-14H2,1-2H3,(H,34,38)(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of microsomal CYP3A4 (unknown origin) |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50140092

(CHEMBL437662 | GGCCSHPACAANNQDYC*)Show SMILES C[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CS)NC(=O)[C@H](C)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CO)NC(=O)[C@H](CS)NC(=O)[C@H](CS)NC(=O)CNC(=O)CN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CS)C(N)=O Show InChI InChI=1S/C65H97N23O24S4/c1-27(75-61(108)42(24-115)86-54(101)29(3)76-64(111)44-5-4-12-88(44)65(112)38(14-31-19-71-26-73-31)83-60(107)39(21-89)84-63(110)43(25-116)87-62(109)41(23-114)77-49(95)20-72-48(94)18-66)52(99)74-28(2)53(100)79-35(15-46(68)92)58(105)81-36(16-47(69)93)57(104)78-33(10-11-45(67)91)55(102)82-37(17-50(96)97)59(106)80-34(13-30-6-8-32(90)9-7-30)56(103)85-40(22-113)51(70)98/h6-9,19,26-29,33-44,89-90,113-116H,4-5,10-18,20-25,66H2,1-3H3,(H2,67,91)(H2,68,92)(H2,69,93)(H2,70,98)(H,71,73)(H,72,94)(H,74,99)(H,75,108)(H,76,111)(H,77,95)(H,78,104)(H,79,100)(H,80,106)(H,81,105)(H,82,102)(H,83,107)(H,84,110)(H,85,103)(H,86,101)(H,87,109)(H,96,97)/t27-,28-,29-,33-,34-,35-,36-,37-,38-,39-,40-,41-,42-,43-,44+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of rat Nicotinic acetylcholine receptor alpha7 (nAChR) in Xenopus Oocytes |
J Med Chem 47: 1234-41 (2004)
Article DOI: 10.1021/jm031010o
BindingDB Entry DOI: 10.7270/Q2RN378M |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3/beta-2
(Rattus norvegicus (Rat)) | BDBM50140090

(CHEMBL217150 | RDPCCSNPVCTVHNPQIC*)Show SMILES CC(C)C[C@@H](NC(=O)[C@@H](CO)NC(=O)[C@@H](CS)NC(=O)[C@@H](CS)NC(=O)CN)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](CS)C(=O)N[C@@H](C)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CS)C(N)=O Show InChI InChI=1S/C65H99N19O22S4/c1-29(2)18-37(76-57(98)39(24-85)78-60(101)43(28-110)80-59(100)41(26-108)72-49(89)23-66)63(104)84-17-7-10-46(84)65(106)83-16-6-9-45(83)62(103)81-42(27-109)58(99)71-30(3)52(93)70-31(4)53(94)73-35(20-47(67)87)55(96)77-38(21-48(68)88)64(105)82-15-5-8-44(82)61(102)75-36(22-50(90)91)56(97)74-34(19-32-11-13-33(86)14-12-32)54(95)79-40(25-107)51(69)92/h11-14,29-31,34-46,85-86,107-110H,5-10,15-28,66H2,1-4H3,(H2,67,87)(H2,68,88)(H2,69,92)(H,70,93)(H,71,99)(H,72,89)(H,73,94)(H,74,97)(H,75,102)(H,76,98)(H,77,96)(H,78,101)(H,79,95)(H,80,100)(H,81,103)(H,90,91)/t30-,31-,34-,35-,36-,37+,38-,39+,40-,41+,42-,43+,44+,45-,46-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of rat Nicotinic acetylcholine receptor alpha3-beta2 expressed in Xenopus Oocytes |
J Med Chem 47: 1234-41 (2004)
Article DOI: 10.1021/jm031010o
BindingDB Entry DOI: 10.7270/Q2RN378M |
More data for this
Ligand-Target Pair | |
G2/mitotic-specific cyclin-B1
(Rattus norvegicus) | BDBM50140085

(CHEMBL266702 | GCCSLPPCAANNPDYC*)Show SMILES CC(C)C[C@@H](NC(=O)[C@@H](CO)NC(=O)[C@@H](CS)NC(=O)[C@@H](CS)NC(=O)CN)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](CS)C(=O)N[C@@H](C)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccc(S)cc1)C(=O)N[C@@H](CS)C(N)=O Show InChI InChI=1S/C65H99N19O21S5/c1-29(2)18-37(76-57(97)39(24-85)78-60(100)43(28-109)80-59(99)41(26-107)72-49(88)23-66)63(103)84-17-7-10-46(84)65(105)83-16-6-9-45(83)62(102)81-42(27-108)58(98)71-30(3)52(92)70-31(4)53(93)73-35(20-47(67)86)55(95)77-38(21-48(68)87)64(104)82-15-5-8-44(82)61(101)75-36(22-50(89)90)56(96)74-34(19-32-11-13-33(110)14-12-32)54(94)79-40(25-106)51(69)91/h11-14,29-31,34-46,85,106-110H,5-10,15-28,66H2,1-4H3,(H2,67,86)(H2,68,87)(H2,69,91)(H,70,92)(H,71,98)(H,72,88)(H,73,93)(H,74,96)(H,75,101)(H,76,97)(H,77,95)(H,78,100)(H,79,94)(H,80,99)(H,81,102)(H,89,90)/t30-,31-,34-,35-,36-,37+,38-,39+,40-,41+,42-,43+,44+,45-,46-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 252 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of rat Nicotinic acetylcholine receptor alpha7 (nAChR) in Xenopus Oocytes |
J Med Chem 47: 1234-41 (2004)
Article DOI: 10.1021/jm031010o
BindingDB Entry DOI: 10.7270/Q2RN378M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50239251

(CHEMBL4096241)Show SMILES CNC(=O)c1c(oc2ccc(cc12)-c1cc(ccc1C)C(=O)NC1(CC1)c1cccnc1)-c1ccc(F)cc1 Show InChI InChI=1S/C32H26FN3O3/c1-19-5-6-22(30(37)36-32(13-14-32)23-4-3-15-35-18-23)17-25(19)21-9-12-27-26(16-21)28(31(38)34-2)29(39-27)20-7-10-24(33)11-8-20/h3-12,15-18H,13-14H2,1-2H3,(H,34,38)(H,36,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of microsomal CYP2C8 (unknown origin) |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
G2/mitotic-specific cyclin-B1
(Rattus norvegicus) | BDBM50140088

(CHEMBL451251 | GGCCSHPACAANNQDYC#)Show SMILES C[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CS)NC(=O)[C@H](C)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CO)NC(=O)[C@H](CS)NC(=O)[C@H](CS)NC(=O)CNC(=O)CN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CS)C(O)=O Show InChI InChI=1S/C65H96N22O25S4/c1-27(74-60(106)41(23-114)84-53(99)29(3)75-63(109)44-5-4-12-87(44)64(110)38(14-31-19-70-26-72-31)82-59(105)39(21-88)83-62(108)42(24-115)85-61(107)40(22-113)76-49(94)20-71-48(93)18-66)51(97)73-28(2)52(98)78-35(15-46(68)91)57(103)80-36(16-47(69)92)56(102)77-33(10-11-45(67)90)54(100)81-37(17-50(95)96)58(104)79-34(13-30-6-8-32(89)9-7-30)55(101)86-43(25-116)65(111)112/h6-9,19,26-29,33-44,88-89,113-116H,4-5,10-18,20-25,66H2,1-3H3,(H2,67,90)(H2,68,91)(H2,69,92)(H,70,72)(H,71,93)(H,73,97)(H,74,106)(H,75,109)(H,76,94)(H,77,102)(H,78,98)(H,79,104)(H,80,103)(H,81,100)(H,82,105)(H,83,108)(H,84,99)(H,85,107)(H,86,101)(H,95,96)(H,111,112)/t27-,28-,29-,33-,34-,35-,36-,37-,38-,39-,40-,41-,42-,43-,44+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 367 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of rat Nicotinic acetylcholine receptor alpha7 (nAChR) in Xenopus Oocytes |
J Med Chem 47: 1234-41 (2004)
Article DOI: 10.1021/jm031010o
BindingDB Entry DOI: 10.7270/Q2RN378M |
More data for this
Ligand-Target Pair | |
Casein kinase II subunit alpha
(Homo sapiens (Human)) | BDBM50252622
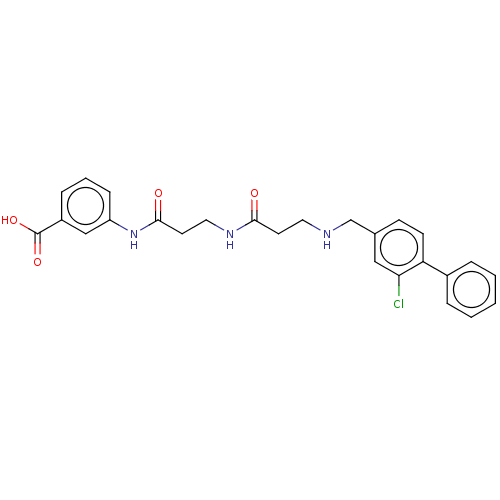
(CHEMBL4070389)Show SMILES OC(=O)c1cccc(NC(=O)CCNC(=O)CCNCc2ccc(c(Cl)c2)-c2ccccc2)c1 Show InChI InChI=1S/C26H26ClN3O4/c27-23-15-18(9-10-22(23)19-5-2-1-3-6-19)17-28-13-11-24(31)29-14-12-25(32)30-21-8-4-7-20(16-21)26(33)34/h1-10,15-16,28H,11-14,17H2,(H,29,31)(H,30,32)(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00063
BindingDB Entry DOI: 10.7270/Q2ZP4B53 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
G2/mitotic-specific cyclin-B1
(Rattus norvegicus) | BDBM50409922
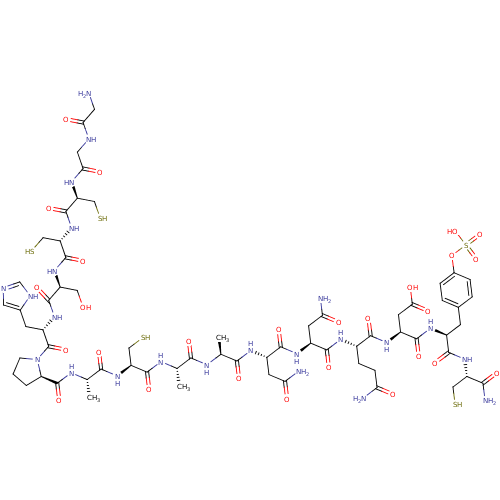
(CHEMBL2092998)Show SMILES C[C@H](NC(=O)[C@H](CS)NC(=O)[C@H](C)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CO)NC(=O)[C@H](CS)NC(=O)[C@H](CS)NC(=O)CNC(=O)CN)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccc(OS(O)(=O)=O)cc1)C(=O)N[C@@H](CS)C(N)=O Show InChI InChI=1S/C65H97N23O27S5/c1-27(75-61(107)42(24-118)86-54(100)29(3)76-64(110)44-5-4-12-88(44)65(111)38(14-31-19-71-26-73-31)83-60(106)39(21-89)84-63(109)43(25-119)87-62(108)41(23-117)77-49(94)20-72-48(93)18-66)52(98)74-28(2)53(99)79-35(15-46(68)91)58(104)81-36(16-47(69)92)57(103)78-33(10-11-45(67)90)55(101)82-37(17-50(95)96)59(105)80-34(56(102)85-40(22-116)51(70)97)13-30-6-8-32(9-7-30)115-120(112,113)114/h6-9,19,26-29,33-44,89,116-119H,4-5,10-18,20-25,66H2,1-3H3,(H2,67,90)(H2,68,91)(H2,69,92)(H2,70,97)(H,71,73)(H,72,93)(H,74,98)(H,75,107)(H,76,110)(H,77,94)(H,78,103)(H,79,99)(H,80,105)(H,81,104)(H,82,101)(H,83,106)(H,84,109)(H,85,102)(H,86,100)(H,87,108)(H,95,96)(H,112,113,114)/t27-,28-,29-,33-,34-,35-,36-,37-,38-,39-,40-,41-,42-,43-,44+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 836 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of rat Nicotinic acetylcholine receptor alpha7 (nAChR) in Xenopus Oocytes |
J Med Chem 47: 1234-41 (2004)
Article DOI: 10.1021/jm031010o
BindingDB Entry DOI: 10.7270/Q2RN378M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50239252

(CHEMBL4078188)Show SMILES CNC(=O)c1c(oc2ccc(cc12)-c1cc(ccc1C)C(=O)NC1(CC1)c1ccccn1)-c1ccc(F)cc1 Show InChI InChI=1S/C32H26FN3O3/c1-19-6-7-22(30(37)36-32(14-15-32)27-5-3-4-16-35-27)18-24(19)21-10-13-26-25(17-21)28(31(38)34-2)29(39-26)20-8-11-23(33)12-9-20/h3-13,16-18H,14-15H2,1-2H3,(H,34,38)(H,36,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of microsomal CYP2C8 (unknown origin) |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50239253
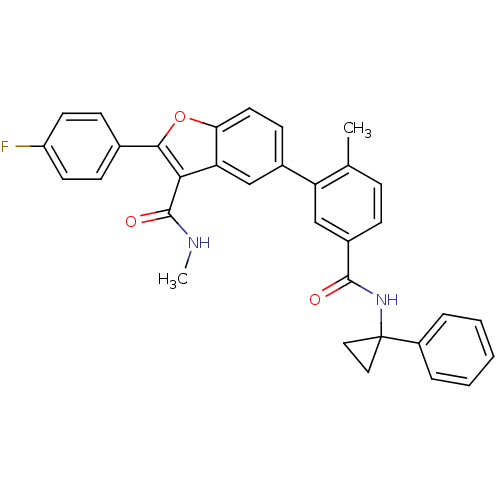
(CHEMBL4088517)Show SMILES CNC(=O)c1c(oc2ccc(cc12)-c1cc(ccc1C)C(=O)NC1(CC1)c1ccccc1)-c1ccc(F)cc1 Show InChI InChI=1S/C33H27FN2O3/c1-20-8-9-23(31(37)36-33(16-17-33)24-6-4-3-5-7-24)19-26(20)22-12-15-28-27(18-22)29(32(38)35-2)30(39-28)21-10-13-25(34)14-11-21/h3-15,18-19H,16-17H2,1-2H3,(H,35,38)(H,36,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of microsomal CYP2C8 (unknown origin) |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50239253

(CHEMBL4088517)Show SMILES CNC(=O)c1c(oc2ccc(cc12)-c1cc(ccc1C)C(=O)NC1(CC1)c1ccccc1)-c1ccc(F)cc1 Show InChI InChI=1S/C33H27FN2O3/c1-20-8-9-23(31(37)36-33(16-17-33)24-6-4-3-5-7-24)19-26(20)22-12-15-28-27(18-22)29(32(38)35-2)30(39-28)21-10-13-25(34)14-11-21/h3-15,18-19H,16-17H2,1-2H3,(H,35,38)(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of microsomal CYP3A4 (unknown origin) |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50239249

(CHEMBL4104000)Show SMILES CNC(=O)c1c(oc2ccc(cc12)-c1cc(ccc1C)C(=O)NC1(CC1)c1ncccn1)-c1ccc(F)cc1 Show InChI InChI=1S/C31H25FN4O3/c1-18-4-5-21(28(37)36-31(12-13-31)30-34-14-3-15-35-30)17-23(18)20-8-11-25-24(16-20)26(29(38)33-2)27(39-25)19-6-9-22(32)10-7-19/h3-11,14-17H,12-13H2,1-2H3,(H,33,38)(H,36,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of microsomal CYP2C8 (unknown origin) |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50239252

(CHEMBL4078188)Show SMILES CNC(=O)c1c(oc2ccc(cc12)-c1cc(ccc1C)C(=O)NC1(CC1)c1ccccn1)-c1ccc(F)cc1 Show InChI InChI=1S/C32H26FN3O3/c1-19-6-7-22(30(37)36-32(14-15-32)27-5-3-4-16-35-27)18-24(19)21-10-13-26-25(17-21)28(31(38)34-2)29(39-26)20-8-11-23(33)12-9-20/h3-13,16-18H,14-15H2,1-2H3,(H,34,38)(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of microsomal CYP3A4 (unknown origin) |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50239249

(CHEMBL4104000)Show SMILES CNC(=O)c1c(oc2ccc(cc12)-c1cc(ccc1C)C(=O)NC1(CC1)c1ncccn1)-c1ccc(F)cc1 Show InChI InChI=1S/C31H25FN4O3/c1-18-4-5-21(28(37)36-31(12-13-31)30-34-14-3-15-35-30)17-23(18)20-8-11-25-24(16-20)26(29(38)33-2)27(39-25)19-6-9-22(32)10-7-19/h3-11,14-17H,12-13H2,1-2H3,(H,33,38)(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by thallium flux assay |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50239252

(CHEMBL4078188)Show SMILES CNC(=O)c1c(oc2ccc(cc12)-c1cc(ccc1C)C(=O)NC1(CC1)c1ccccn1)-c1ccc(F)cc1 Show InChI InChI=1S/C32H26FN3O3/c1-19-6-7-22(30(37)36-32(14-15-32)27-5-3-4-16-35-27)18-24(19)21-10-13-26-25(17-21)28(31(38)34-2)29(39-26)20-8-11-23(33)12-9-20/h3-13,16-18H,14-15H2,1-2H3,(H,34,38)(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by thallium flux assay |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50557382
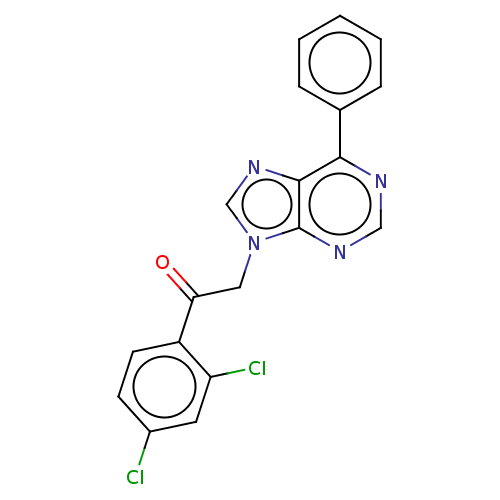
(CHEMBL4780724)Show SMILES Clc1ccc(C(=O)Cn2cnc3c(ncnc23)-c2ccccc2)c(Cl)c1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human P2X7 receptor expressed in HEK293 cells assessed as reduction in benzoyl-ATP-induced YO-PRO-1 dye uptake pre-incubated f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02145
BindingDB Entry DOI: 10.7270/Q2Z323BT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50239248
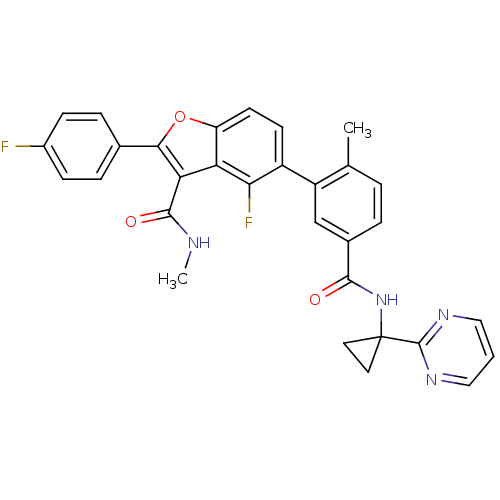
(CHEMBL4093031)Show SMILES CNC(=O)c1c(oc2ccc(c(F)c12)-c1cc(ccc1C)C(=O)NC1(CC1)c1ncccn1)-c1ccc(F)cc1 Show InChI InChI=1S/C31H24F2N4O3/c1-17-4-5-19(28(38)37-31(12-13-31)30-35-14-3-15-36-30)16-22(17)21-10-11-23-24(26(21)33)25(29(39)34-2)27(40-23)18-6-8-20(32)9-7-18/h3-11,14-16H,12-13H2,1-2H3,(H,34,39)(H,37,38) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of microsomal CYP2C8 (unknown origin) |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50557380

(CHEMBL4751055) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human P2X7 receptor expressed in HEK293 cells assessed as reduction in benzoyl-ATP-induced YO-PRO-1 dye uptake pre-incubated f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02145
BindingDB Entry DOI: 10.7270/Q2Z323BT |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50557385
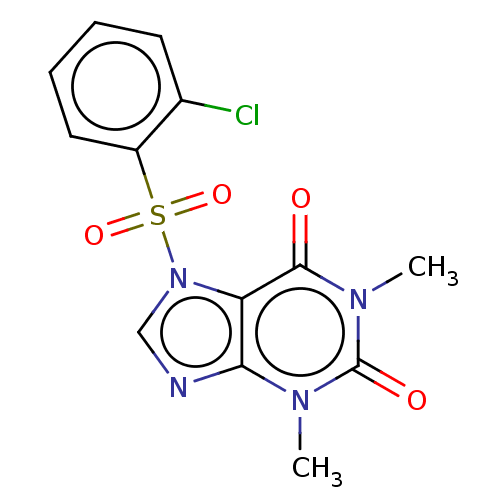
(CHEMBL4780725)Show SMILES Cn1c2ncn(c2c(=O)n(C)c1=O)S(=O)(=O)c1ccccc1Cl | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human P2X7 receptor expressed in HEK293 cells assessed as reduction in benzoyl-ATP-induced YO-PRO-1 dye uptake pre-incubated f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02145
BindingDB Entry DOI: 10.7270/Q2Z323BT |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50557381
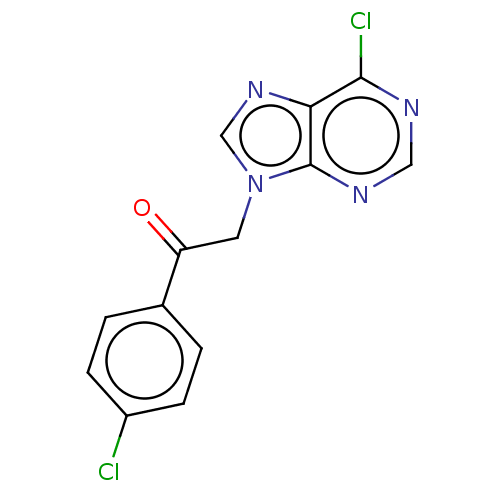
(CHEMBL4757539) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human P2X7 receptor expressed in HEK293 cells assessed as reduction in benzoyl-ATP-induced YO-PRO-1 dye uptake pre-incubated f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02145
BindingDB Entry DOI: 10.7270/Q2Z323BT |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50557384
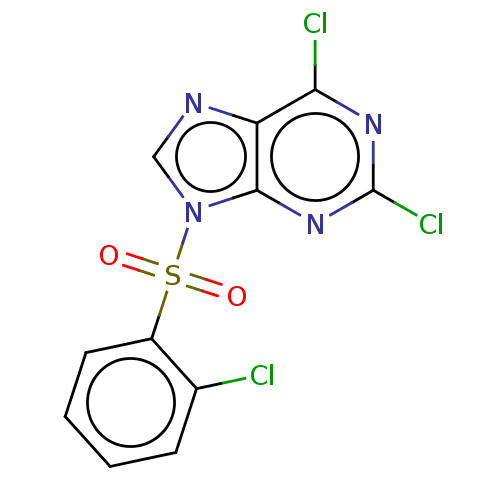
(CHEMBL4743642) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human P2X7 receptor expressed in HEK293 cells assessed as reduction in benzoyl-ATP-induced YO-PRO-1 dye uptake pre-incubated f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02145
BindingDB Entry DOI: 10.7270/Q2Z323BT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50239249

(CHEMBL4104000)Show SMILES CNC(=O)c1c(oc2ccc(cc12)-c1cc(ccc1C)C(=O)NC1(CC1)c1ncccn1)-c1ccc(F)cc1 Show InChI InChI=1S/C31H25FN4O3/c1-18-4-5-21(28(37)36-31(12-13-31)30-34-14-3-15-35-30)17-23(18)20-8-11-25-24(16-20)26(29(38)33-2)27(39-25)19-6-9-22(32)10-7-19/h3-11,14-17H,12-13H2,1-2H3,(H,33,38)(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of microsomal CYP3A4 (unknown origin) |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50557383
(CHEMBL4753833)Show SMILES Clc1ccc(C(=O)Cn2cnc3c(Cl)nc(Cl)nc23)c(Cl)c1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human P2X7 receptor expressed in HEK293 cells assessed as reduction in benzoyl-ATP-induced YO-PRO-1 dye uptake pre-incubated f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02145
BindingDB Entry DOI: 10.7270/Q2Z323BT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data