

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Hepatitis C virus) | BDBM123407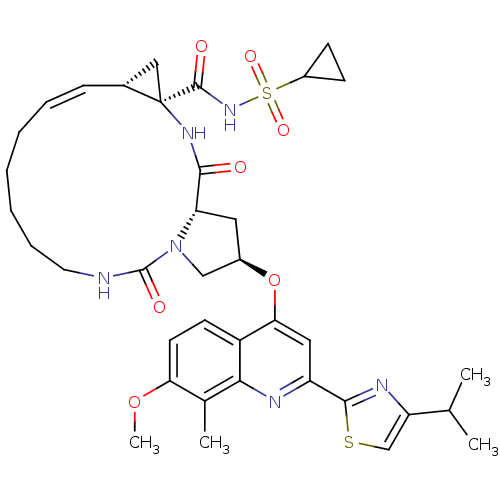 (US8741926, 91) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0500 | -59.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US8741926 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123410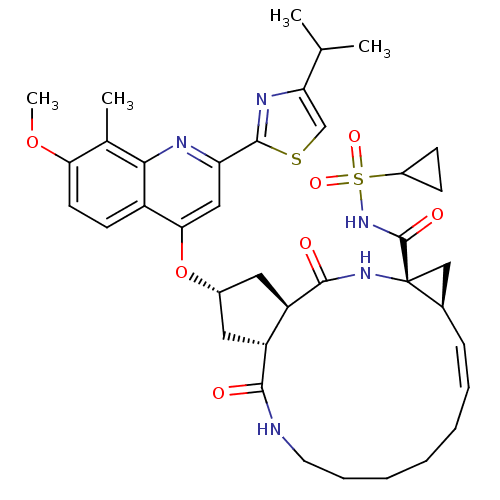 (US8741926, 82 | US8754106, 82 | US8754106, 91) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754106 (2014) BindingDB Entry DOI: 10.7270/Q2V40SWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123415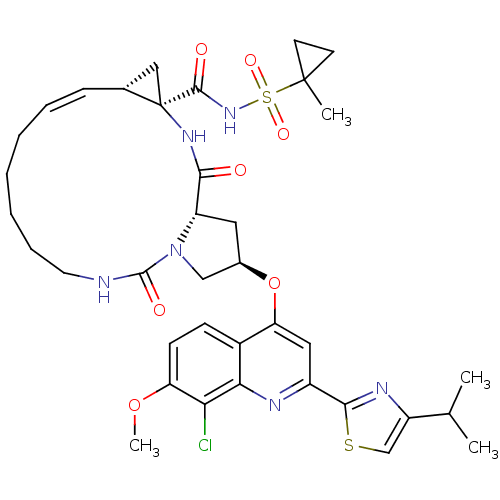 (US8741926, 95 | US8754106, 95) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | -58.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US8741926 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123413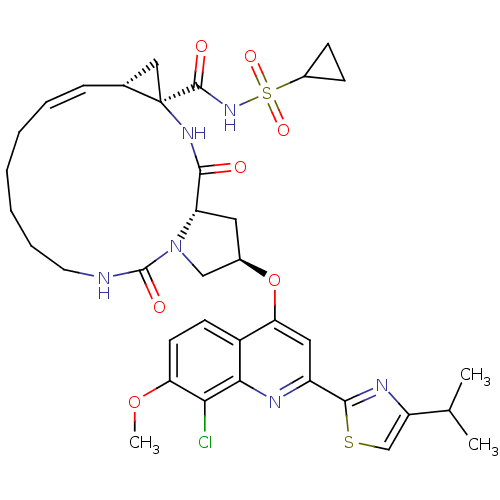 (US8741926, 94 | US8754106, 94) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | -58.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US8741926 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123411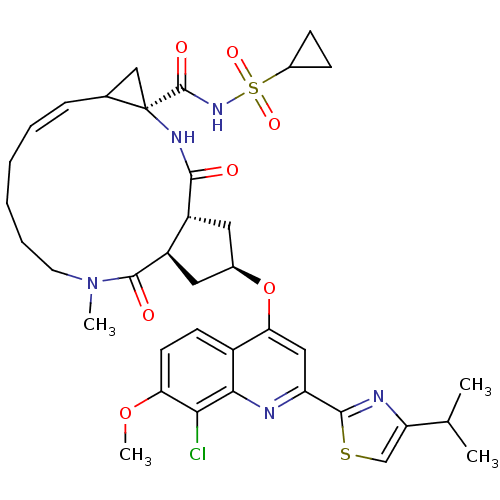 (US8741926, 56) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | -58.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US8741926 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123415 (US8741926, 95 | US8754106, 95) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754106 (2014) BindingDB Entry DOI: 10.7270/Q2V40SWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123413 (US8741926, 94 | US8754106, 94) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754106 (2014) BindingDB Entry DOI: 10.7270/Q2V40SWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM124106 (US8754106, 56) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754106 (2014) BindingDB Entry DOI: 10.7270/Q2V40SWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM124107 (US8754105, 23) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.200 | -56.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Medivir AB; Janssen R&D Ireland US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754105 (2014) BindingDB Entry DOI: 10.7270/Q2QC0250 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123414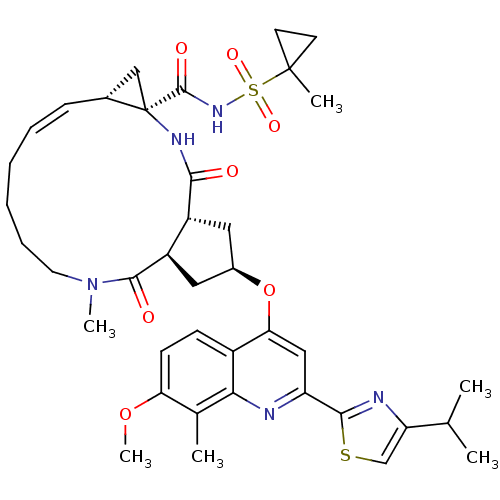 (US8741926, 48 | US8754106, 48) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.25 | -55.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US8741926 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123414 (US8741926, 48 | US8754106, 48) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754106 (2014) BindingDB Entry DOI: 10.7270/Q2V40SWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123412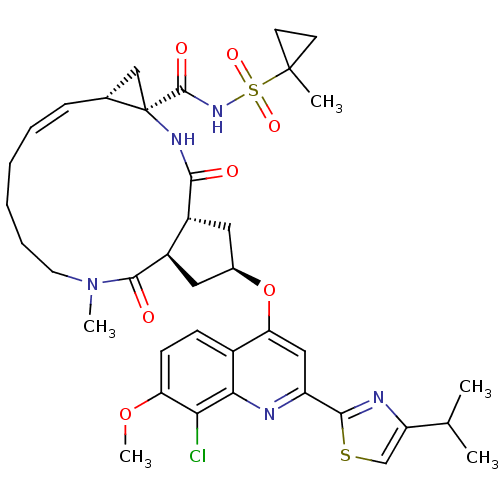 (US8741926, 57 | US8754106, 57) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.300 | -55.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US8741926 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123412 (US8741926, 57 | US8754106, 57) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754106 (2014) BindingDB Entry DOI: 10.7270/Q2V40SWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50336504 ((2R,3aR,10Z,11aS,12aR,14aR)-N-(cyclopropylsulfonyl...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754106 (2014) BindingDB Entry DOI: 10.7270/Q2V40SWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50336504 ((2R,3aR,10Z,11aS,12aR,14aR)-N-(cyclopropylsulfonyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB US Patent | 0.5 | -54.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US8741926 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XBC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM124112 (US8754105, 25) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.40 | -50.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Medivir AB; Janssen R&D Ireland US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754105 (2014) BindingDB Entry DOI: 10.7270/Q2QC0250 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940] (Rattus norvegicus (rat)) | BDBM24198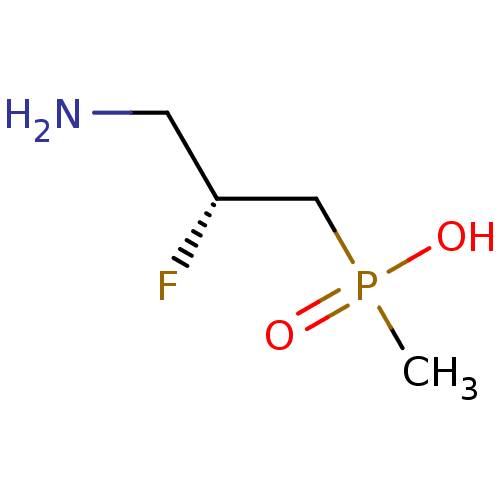 (3-aminopropylphosphinic derivative, (R)-8 | [(2R)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.30 | -47.3 | n/a | n/a | 14 | n/a | n/a | 7.4 | 22 |
AstraZeneca | Assay Description Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... | J Med Chem 51: 4315-20 (2008) Article DOI: 10.1021/jm701425k BindingDB Entry DOI: 10.7270/Q2M32T2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM124105 (US8754106, 55) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754106 (2014) BindingDB Entry DOI: 10.7270/Q2V40SWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123408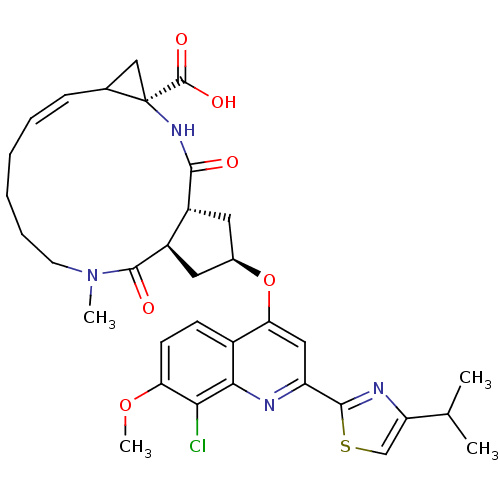 (US8741926, 55) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 5 | -48.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US8741926 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940] (Rattus norvegicus (rat)) | BDBM24195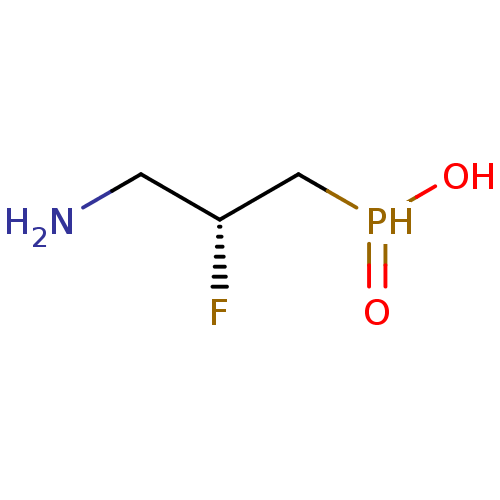 (3-aminopropylphosphinic derivative, (R)-7 | AZD335...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 5.10 | -46.9 | n/a | n/a | 8.64 | n/a | n/a | 7.4 | 22 |
AstraZeneca | Assay Description Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... | J Med Chem 51: 4315-20 (2008) Article DOI: 10.1021/jm701425k BindingDB Entry DOI: 10.7270/Q2M32T2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123410 (US8741926, 82 | US8754106, 82 | US8754106, 91) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754106 (2014) BindingDB Entry DOI: 10.7270/Q2V40SWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123410 (US8741926, 82 | US8754106, 82 | US8754106, 91) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 9.40 | -46.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US8741926 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940] (Rattus norvegicus (rat)) | BDBM24193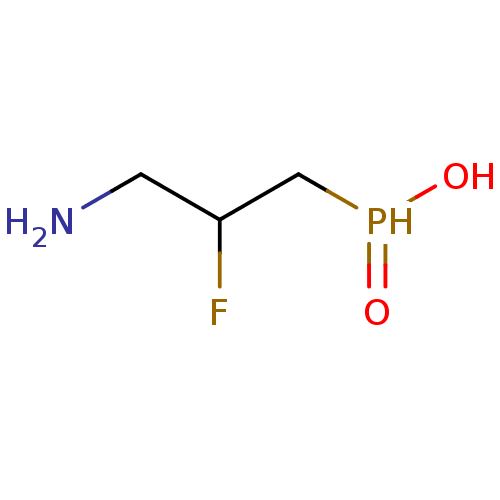 ((3-amino-2-fluoropropyl)phosphinic acid | 3-aminop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 10 | -45.2 | n/a | n/a | 15 | n/a | n/a | 7.4 | 22 |
AstraZeneca | Assay Description Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... | J Med Chem 51: 4315-20 (2008) Article DOI: 10.1021/jm701425k BindingDB Entry DOI: 10.7270/Q2M32T2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940] (Rattus norvegicus (rat)) | BDBM24196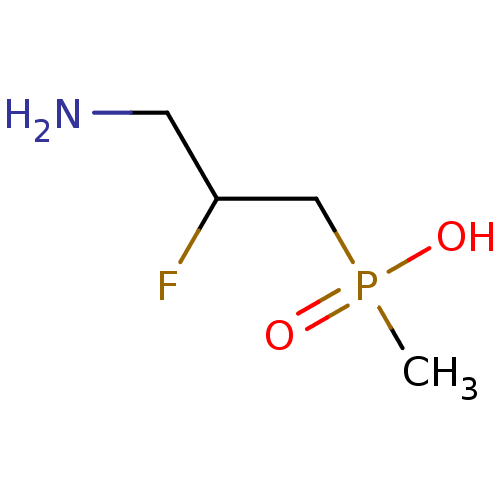 ((3-amino-2-fluoropropyl)(methyl)phosphinic acid | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 14 | -44.4 | n/a | n/a | 23 | n/a | n/a | 7.4 | 22 |
AstraZeneca | Assay Description Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... | J Med Chem 51: 4315-20 (2008) Article DOI: 10.1021/jm701425k BindingDB Entry DOI: 10.7270/Q2M32T2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940] (Rattus norvegicus (rat)) | BDBM24184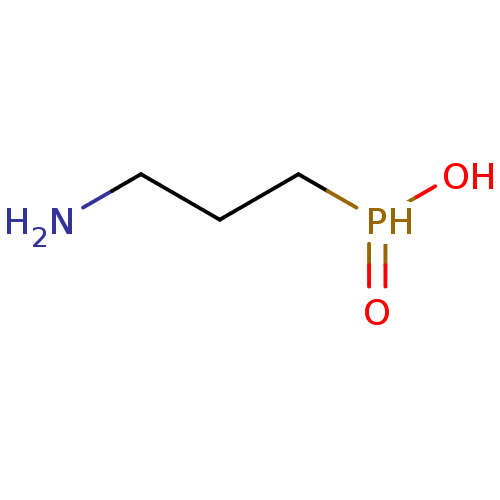 ((3-aminopropyl)phosphinic acid | 3-aminopropylphos...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 15 | -44.2 | n/a | n/a | 19 | n/a | n/a | 7.4 | 22 |
AstraZeneca | Assay Description Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... | J Med Chem 51: 4315-20 (2008) Article DOI: 10.1021/jm701425k BindingDB Entry DOI: 10.7270/Q2M32T2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940] (Rattus norvegicus (rat)) | BDBM24185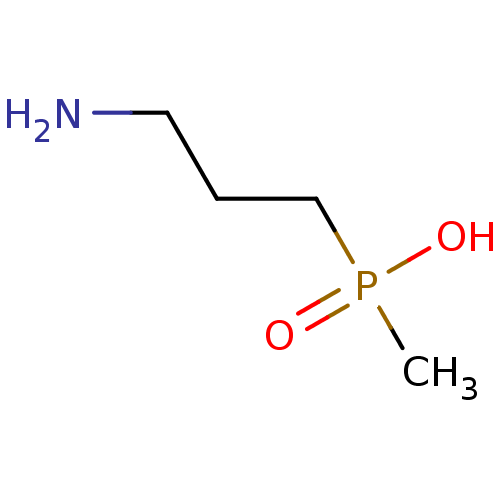 ((3-aminopropyl)(methyl)phosphinic acid | 3-Apmpa |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 33 | -42.3 | n/a | n/a | 41 | n/a | n/a | 7.4 | 22 |
AstraZeneca | Assay Description Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... | J Med Chem 51: 4315-20 (2008) Article DOI: 10.1021/jm701425k BindingDB Entry DOI: 10.7270/Q2M32T2T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM124111 (US8754105, 24) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 40 | -42.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Medivir AB; Janssen R&D Ireland US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754105 (2014) BindingDB Entry DOI: 10.7270/Q2QC0250 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM124110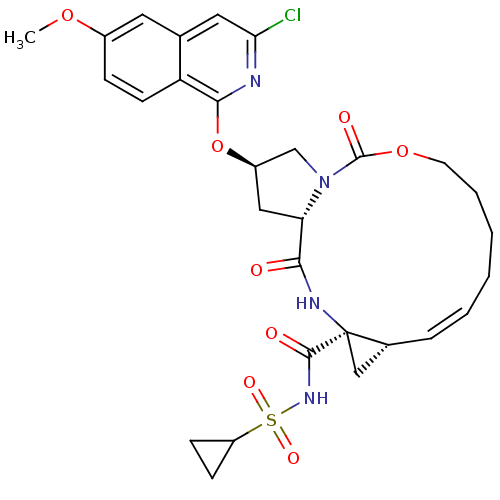 (US8754105, 17) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 43 | -42.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Medivir AB; Janssen R&D Ireland US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754105 (2014) BindingDB Entry DOI: 10.7270/Q2QC0250 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940] (Rattus norvegicus (rat)) | BDBM24191 ((3-amino-2-oxopropyl)phosphinic acid | (3-amino-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 48 | -41.4 | n/a | n/a | 81 | n/a | n/a | 7.4 | 22 |
AstraZeneca | Assay Description Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... | J Med Chem 51: 4315-20 (2008) Article DOI: 10.1021/jm701425k BindingDB Entry DOI: 10.7270/Q2M32T2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940] (Rattus norvegicus (rat)) | BDBM24186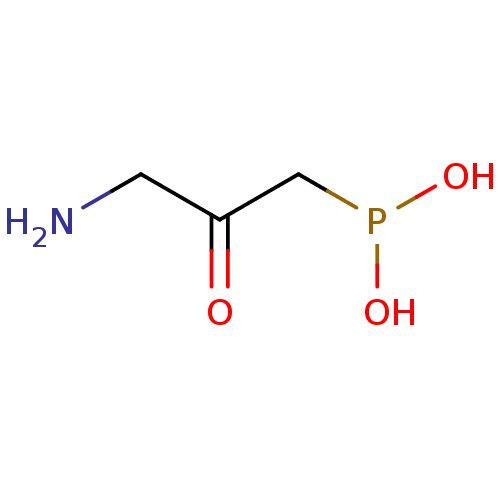 ((3-amino-2-hydroxypropyl)phosphinic acid | 3-amino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 50 | -41.3 | n/a | n/a | 130 | n/a | n/a | 7.4 | 22 |
AstraZeneca | Assay Description Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... | J Med Chem 51: 4315-20 (2008) Article DOI: 10.1021/jm701425k BindingDB Entry DOI: 10.7270/Q2M32T2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940] (Rattus norvegicus (rat)) | BDBM24194 (3-aminopropylphosphinic derivative, (S)-7 | [(2S)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 70 | -40.4 | n/a | n/a | 250 | n/a | n/a | 7.4 | 22 |
AstraZeneca | Assay Description Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... | J Med Chem 51: 4315-20 (2008) Article DOI: 10.1021/jm701425k BindingDB Entry DOI: 10.7270/Q2M32T2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940] (Rattus norvegicus (rat)) | BDBM24186 ((3-amino-2-hydroxypropyl)phosphinic acid | 3-amino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 94 | -39.7 | n/a | n/a | 220 | n/a | n/a | 7.4 | 22 |
AstraZeneca | Assay Description Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... | J Med Chem 51: 4315-20 (2008) Article DOI: 10.1021/jm701425k BindingDB Entry DOI: 10.7270/Q2M32T2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940] (Rattus norvegicus (rat)) | BDBM24189 (3-aminopropylphosphinic derivative, (R)-4 | 3-amin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 180 | -38.1 | n/a | n/a | 150 | n/a | n/a | 7.4 | 22 |
AstraZeneca | Assay Description Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... | J Med Chem 51: 4315-20 (2008) Article DOI: 10.1021/jm701425k BindingDB Entry DOI: 10.7270/Q2M32T2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940] (Rattus norvegicus (rat)) | BDBM24192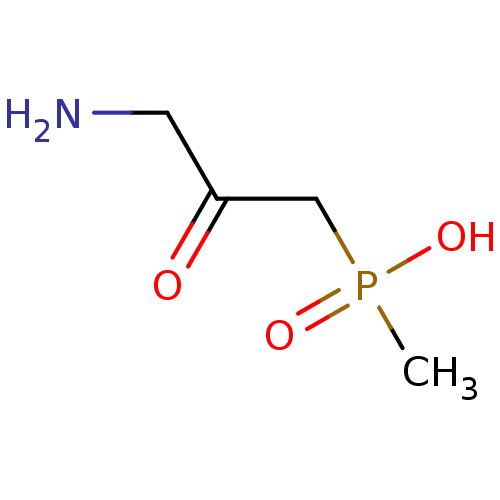 ((3-amino-2-oxopropyl)(methyl)phosphinic acid | (3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 200 | -37.9 | n/a | n/a | 270 | n/a | n/a | 7.4 | 22 |
AstraZeneca | Assay Description Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... | J Med Chem 51: 4315-20 (2008) Article DOI: 10.1021/jm701425k BindingDB Entry DOI: 10.7270/Q2M32T2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940] (Rattus norvegicus (rat)) | BDBM24182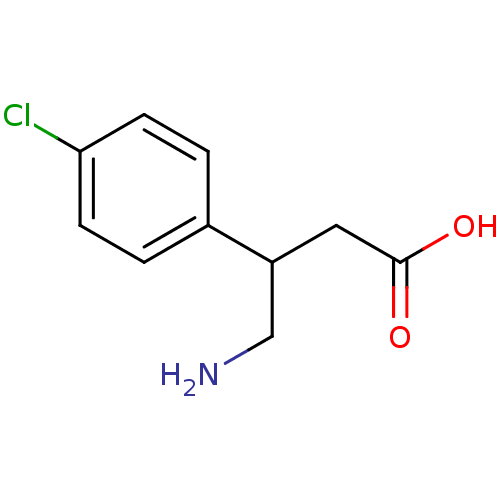 (4-amino-3-(4-chlorophenyl)butanoic acid | Baclofen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 220 | -37.6 | n/a | n/a | 750 | n/a | n/a | 7.4 | 22 |
AstraZeneca | Assay Description Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... | J Med Chem 51: 4315-20 (2008) Article DOI: 10.1021/jm701425k BindingDB Entry DOI: 10.7270/Q2M32T2T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940] (Rattus norvegicus (rat)) | BDBM24197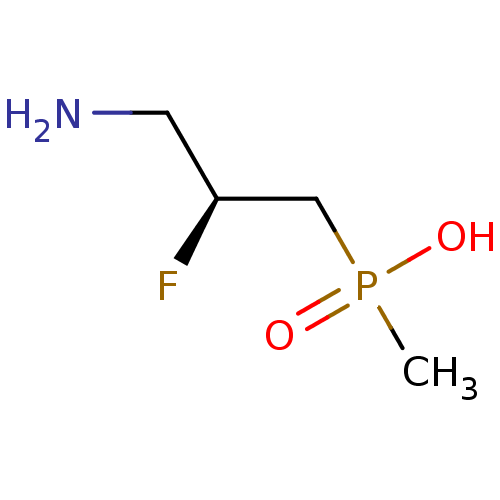 (3-aminopropylphosphinic derivative, (S)-8 | [(2S)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 480 | -35.7 | n/a | n/a | 1.70E+3 | n/a | n/a | 7.4 | 22 |
AstraZeneca | Assay Description Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... | J Med Chem 51: 4315-20 (2008) Article DOI: 10.1021/jm701425k BindingDB Entry DOI: 10.7270/Q2M32T2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940] (Rattus norvegicus (rat)) | BDBM24189 (3-aminopropylphosphinic derivative, (R)-4 | 3-amin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 840 | -34.3 | n/a | n/a | 600 | n/a | n/a | 7.4 | 22 |
AstraZeneca | Assay Description Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... | J Med Chem 51: 4315-20 (2008) Article DOI: 10.1021/jm701425k BindingDB Entry DOI: 10.7270/Q2M32T2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123409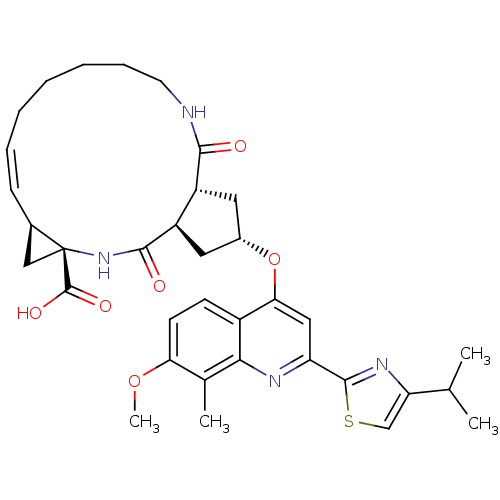 (US8741926, 81) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.00E+3 | -34.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US8741926 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM124109 (US8754105, 16) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.00E+3 | -34.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Medivir AB; Janssen R&D Ireland US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754105 (2014) BindingDB Entry DOI: 10.7270/Q2QC0250 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM124108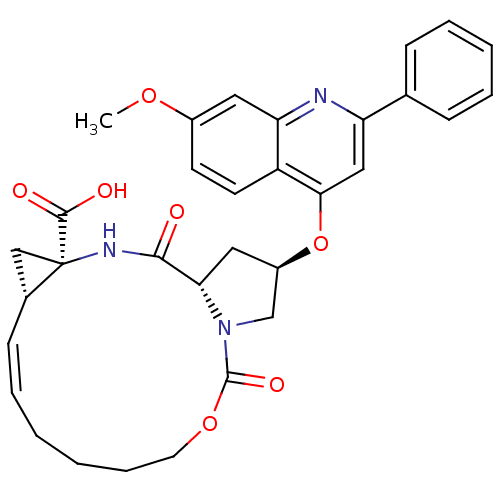 (US8754105, 22) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.07E+3 | -34.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Medivir AB; Janssen R&D Ireland US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754105 (2014) BindingDB Entry DOI: 10.7270/Q2QC0250 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940] (Rattus norvegicus (rat)) | BDBM24186 ((3-amino-2-hydroxypropyl)phosphinic acid | 3-amino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10E+3 | -32.1 | n/a | n/a | 1.10E+3 | n/a | n/a | 7.4 | 22 |
AstraZeneca | Assay Description Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... | J Med Chem 51: 4315-20 (2008) Article DOI: 10.1021/jm701425k BindingDB Entry DOI: 10.7270/Q2M32T2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50424479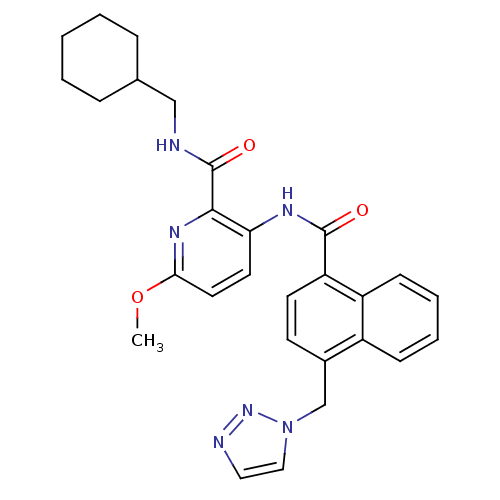 (CHEMBL2316376) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293S cell membranes by scintillation counting analysis | J Med Chem 56: 220-40 (2013) Article DOI: 10.1021/jm301511h BindingDB Entry DOI: 10.7270/Q2057H7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50424491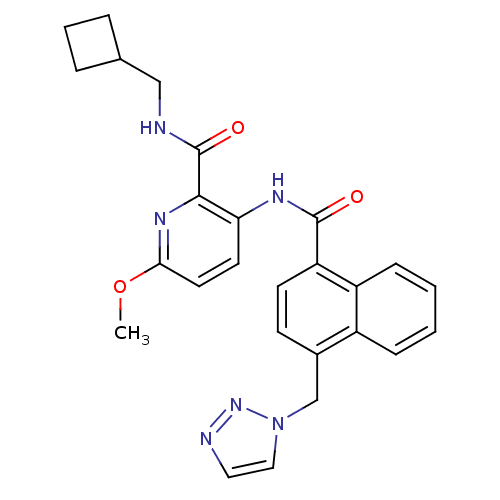 (CHEMBL2316377) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293S cell membranes by scintillation counting analysis | J Med Chem 56: 220-40 (2013) Article DOI: 10.1021/jm301511h BindingDB Entry DOI: 10.7270/Q2057H7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase (Homo sapiens (Human)) | BDBM50433276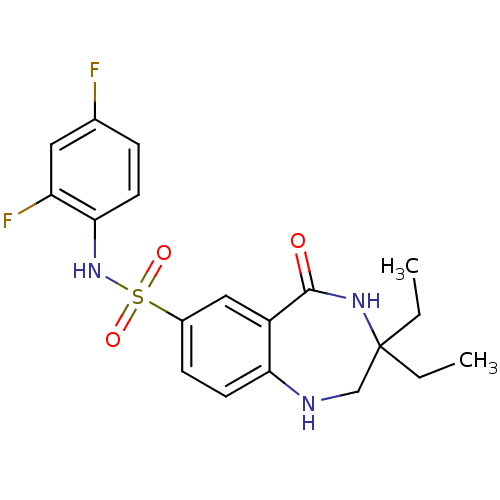 (CHEMBL2376316) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant MGAT2 expressed in insect cell membrane using 2-oleoyl-sn-glycerol and oleoyl coenzyme A as substrate after 40 mins b... | Bioorg Med Chem Lett 23: 2721-6 (2013) Article DOI: 10.1016/j.bmcl.2013.02.084 BindingDB Entry DOI: 10.7270/Q2GB25DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50424478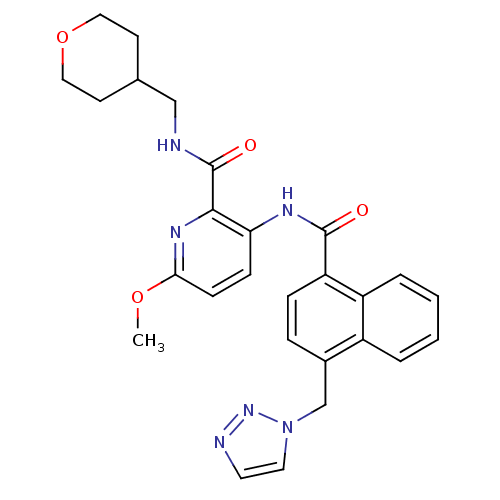 (CHEMBL2316378) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293S cell membranes by scintillation counting analysis | J Med Chem 56: 220-40 (2013) Article DOI: 10.1021/jm301511h BindingDB Entry DOI: 10.7270/Q2057H7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 [866-1154] (Homo sapiens (Human)) | BDBM264225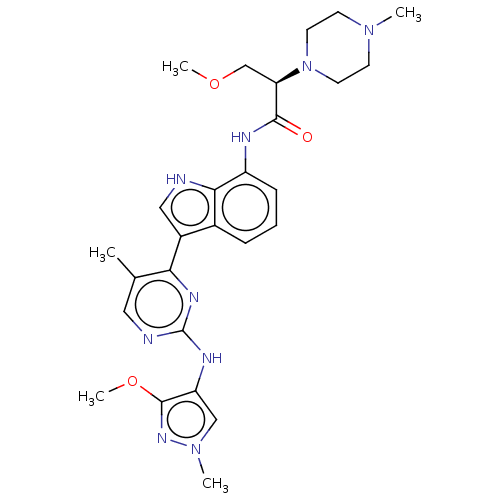 ((2R)-3-methoxy-N- (3-{2-[(3-methoxy- 1-methyl-1H-p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dizal (Jiangsu) Pharmaceutical Co., Ltd. US Patent | Assay Description Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, PV4774, Carlsbad, Calif.), JAK2 (amino acid... | US Patent US10654835 (2020) BindingDB Entry DOI: 10.7270/Q29C71F3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 [866-1154] (Homo sapiens (Human)) | BDBM489170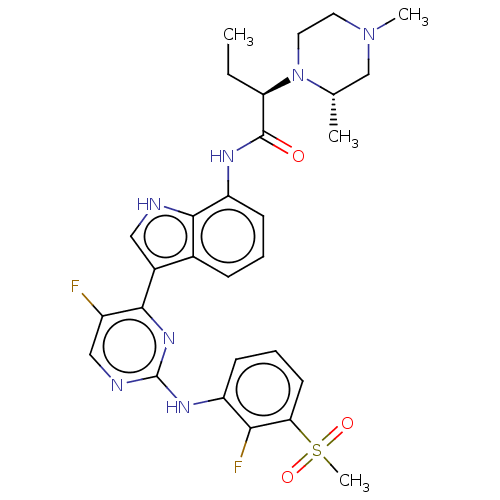 ((R)-2-((R)-2,4- dimethylpiperazin-1- yl)-N-(3-(5-f...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, #PV4774, Carlsbad, Calif.), JAK2 (amino aci... | US Patent US10961228 (2021) BindingDB Entry DOI: 10.7270/Q20G3P8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 [866-1154] (Homo sapiens (Human)) | BDBM489168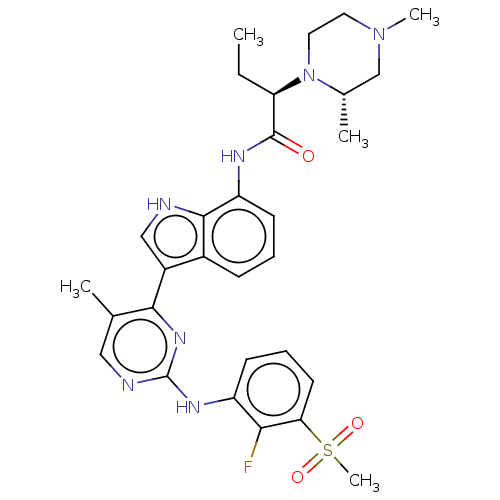 ((R)-2-((R)-2,4- dimethylpiperazin-1- yl)-N-(3-(2-(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, #PV4774, Carlsbad, Calif.), JAK2 (amino aci... | US Patent US10961228 (2021) BindingDB Entry DOI: 10.7270/Q20G3P8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 [866-1154] (Homo sapiens (Human)) | BDBM489165 ((R)-2-((R)-2,4- dimethylpiperazin-1- yl)-N-(3-(2-(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, #PV4774, Carlsbad, Calif.), JAK2 (amino aci... | US Patent US10961228 (2021) BindingDB Entry DOI: 10.7270/Q20G3P8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 [866-1154] (Homo sapiens (Human)) | BDBM489073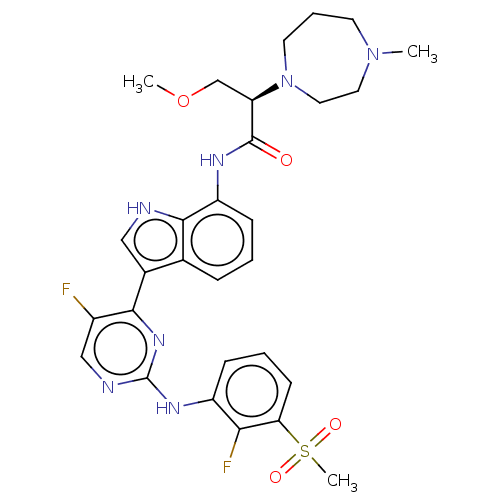 ((R)-N-(3-(5-fluoro-2- ((2-fluoro-3- (methylsulfony...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, #PV4774, Carlsbad, Calif.), JAK2 (amino aci... | US Patent US10961228 (2021) BindingDB Entry DOI: 10.7270/Q20G3P8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1497 total ) | Next | Last >> |