 Found 524 hits with Last Name = 'olivier' and Initial = 'a'
Found 524 hits with Last Name = 'olivier' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gamma-aminobutyric acid receptor subunit alpha-5
(RAT) | BDBM26263
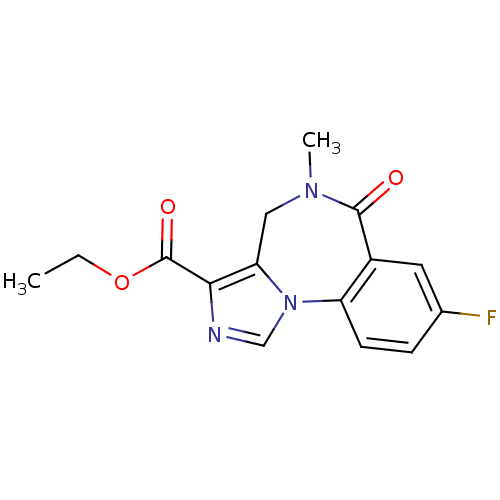
(Anexate | CHEMBL407 | FLUMAZENIL | Ro15-1788 | Rom...)Show InChI InChI=1S/C15H14FN3O3/c1-3-22-15(21)13-12-7-18(2)14(20)10-6-9(16)4-5-11(10)19(12)8-17-13/h4-6,8H,3,7H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synthélabo
Curated by PDSP Ki Database
| |
J Biol Chem 274: 13370-4 (1999)
Article DOI: 10.1074/jbc.274.19.13370
BindingDB Entry DOI: 10.7270/Q2765CWZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gamma-aminobutyric acid receptor subunit alpha-5
(RAT) | BDBM26263

(Anexate | CHEMBL407 | FLUMAZENIL | Ro15-1788 | Rom...)Show InChI InChI=1S/C15H14FN3O3/c1-3-22-15(21)13-12-7-18(2)14(20)10-6-9(16)4-5-11(10)19(12)8-17-13/h4-6,8H,3,7H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synthélabo
Curated by PDSP Ki Database
| |
J Biol Chem 274: 13370-4 (1999)
Article DOI: 10.1074/jbc.274.19.13370
BindingDB Entry DOI: 10.7270/Q2765CWZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gamma-aminobutyric acid receptor subunit alpha-1
(Rattus norvegicus (Rat)) | BDBM26263

(Anexate | CHEMBL407 | FLUMAZENIL | Ro15-1788 | Rom...)Show InChI InChI=1S/C15H14FN3O3/c1-3-22-15(21)13-12-7-18(2)14(20)10-6-9(16)4-5-11(10)19(12)8-17-13/h4-6,8H,3,7H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synthélabo
Curated by PDSP Ki Database
| |
J Biol Chem 274: 13370-4 (1999)
Article DOI: 10.1074/jbc.274.19.13370
BindingDB Entry DOI: 10.7270/Q2765CWZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gamma-aminobutyric acid receptor subunit alpha-5
(RAT) | BDBM26263

(Anexate | CHEMBL407 | FLUMAZENIL | Ro15-1788 | Rom...)Show InChI InChI=1S/C15H14FN3O3/c1-3-22-15(21)13-12-7-18(2)14(20)10-6-9(16)4-5-11(10)19(12)8-17-13/h4-6,8H,3,7H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synthélabo
Curated by PDSP Ki Database
| |
J Biol Chem 274: 13370-4 (1999)
Article DOI: 10.1074/jbc.274.19.13370
BindingDB Entry DOI: 10.7270/Q2765CWZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50236369
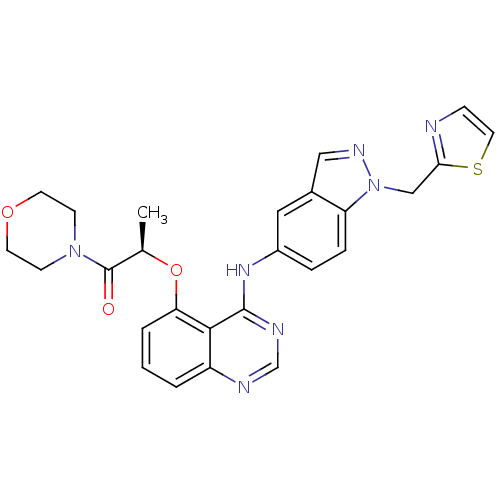
((R)-1-morpholino-2-(4-(1-(thiazol-2-ylmethyl)-1H-i...)Show SMILES C[C@@H](Oc1cccc2ncnc(Nc3ccc4n(Cc5nccs5)ncc4c3)c12)C(=O)N1CCOCC1 Show InChI InChI=1S/C26H25N7O3S/c1-17(26(34)32-8-10-35-11-9-32)36-22-4-2-3-20-24(22)25(29-16-28-20)31-19-5-6-21-18(13-19)14-30-33(21)15-23-27-7-12-37-23/h2-7,12-14,16-17H,8-11,15H2,1H3,(H,28,29,31)/t17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 18: 1799-803 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.035
BindingDB Entry DOI: 10.7270/Q23T9H0F |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5
(RAT) | BDBM26263

(Anexate | CHEMBL407 | FLUMAZENIL | Ro15-1788 | Rom...)Show InChI InChI=1S/C15H14FN3O3/c1-3-22-15(21)13-12-7-18(2)14(20)10-6-9(16)4-5-11(10)19(12)8-17-13/h4-6,8H,3,7H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synthélabo
Curated by PDSP Ki Database
| |
J Biol Chem 274: 13370-4 (1999)
Article DOI: 10.1074/jbc.274.19.13370
BindingDB Entry DOI: 10.7270/Q2765CWZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50236369

((R)-1-morpholino-2-(4-(1-(thiazol-2-ylmethyl)-1H-i...)Show SMILES C[C@@H](Oc1cccc2ncnc(Nc3ccc4n(Cc5nccs5)ncc4c3)c12)C(=O)N1CCOCC1 Show InChI InChI=1S/C26H25N7O3S/c1-17(26(34)32-8-10-35-11-9-32)36-22-4-2-3-20-24(22)25(29-16-28-20)31-19-5-6-21-18(13-19)14-30-33(21)15-23-27-7-12-37-23/h2-7,12-14,16-17H,8-11,15H2,1H3,(H,28,29,31)/t17-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of erbB2 |
Bioorg Med Chem Lett 18: 1799-803 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.035
BindingDB Entry DOI: 10.7270/Q23T9H0F |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1
(Rattus norvegicus (Rat)) | BDBM26266
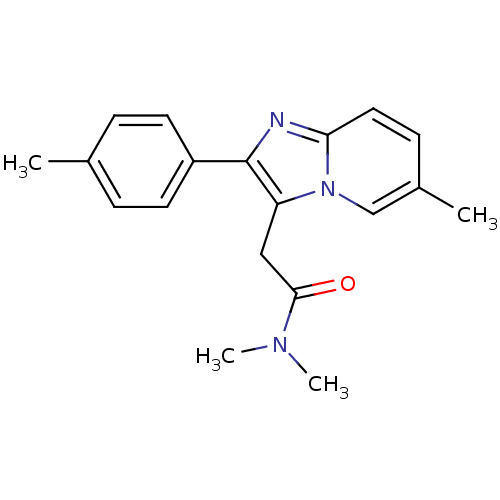
(Ambien | CHEMBL911 | Dalparan | N,N-dimethyl-2-[6-...)Show InChI InChI=1S/C19H21N3O/c1-13-5-8-15(9-6-13)19-16(11-18(23)21(3)4)22-12-14(2)7-10-17(22)20-19/h5-10,12H,11H2,1-4H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 29.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synthélabo
Curated by PDSP Ki Database
| |
J Biol Chem 274: 13370-4 (1999)
Article DOI: 10.1074/jbc.274.19.13370
BindingDB Entry DOI: 10.7270/Q2765CWZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gamma-aminobutyric acid receptor subunit alpha-5
(RAT) | BDBM26266

(Ambien | CHEMBL911 | Dalparan | N,N-dimethyl-2-[6-...)Show InChI InChI=1S/C19H21N3O/c1-13-5-8-15(9-6-13)19-16(11-18(23)21(3)4)22-12-14(2)7-10-17(22)20-19/h5-10,12H,11H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synthélabo
Curated by PDSP Ki Database
| |
J Biol Chem 274: 13370-4 (1999)
Article DOI: 10.1074/jbc.274.19.13370
BindingDB Entry DOI: 10.7270/Q2765CWZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5
(RAT) | BDBM50026756
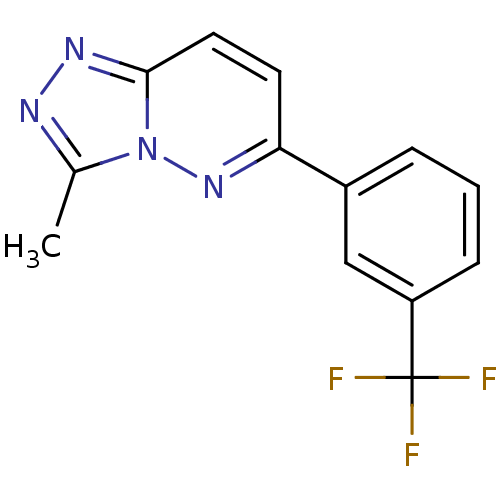
(3-Methyl-6-(3-trifluoromethyl-phenyl)-[1,2,4]triaz...)Show InChI InChI=1S/C13H9F3N4/c1-8-17-18-12-6-5-11(19-20(8)12)9-3-2-4-10(7-9)13(14,15)16/h2-7H,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 127 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synthélabo
Curated by PDSP Ki Database
| |
J Biol Chem 274: 13370-4 (1999)
Article DOI: 10.1074/jbc.274.19.13370
BindingDB Entry DOI: 10.7270/Q2765CWZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5
(RAT) | BDBM50026756

(3-Methyl-6-(3-trifluoromethyl-phenyl)-[1,2,4]triaz...)Show InChI InChI=1S/C13H9F3N4/c1-8-17-18-12-6-5-11(19-20(8)12)9-3-2-4-10(7-9)13(14,15)16/h2-7H,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 157 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synthélabo
Curated by PDSP Ki Database
| |
J Biol Chem 274: 13370-4 (1999)
Article DOI: 10.1074/jbc.274.19.13370
BindingDB Entry DOI: 10.7270/Q2765CWZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5
(RAT) | BDBM50026756

(3-Methyl-6-(3-trifluoromethyl-phenyl)-[1,2,4]triaz...)Show InChI InChI=1S/C13H9F3N4/c1-8-17-18-12-6-5-11(19-20(8)12)9-3-2-4-10(7-9)13(14,15)16/h2-7H,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 333 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synthélabo
Curated by PDSP Ki Database
| |
J Biol Chem 274: 13370-4 (1999)
Article DOI: 10.1074/jbc.274.19.13370
BindingDB Entry DOI: 10.7270/Q2765CWZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5
(RAT) | BDBM50026756

(3-Methyl-6-(3-trifluoromethyl-phenyl)-[1,2,4]triaz...)Show InChI InChI=1S/C13H9F3N4/c1-8-17-18-12-6-5-11(19-20(8)12)9-3-2-4-10(7-9)13(14,15)16/h2-7H,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 447 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synthélabo
Curated by PDSP Ki Database
| |
J Biol Chem 274: 13370-4 (1999)
Article DOI: 10.1074/jbc.274.19.13370
BindingDB Entry DOI: 10.7270/Q2765CWZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5
(RAT) | BDBM26266

(Ambien | CHEMBL911 | Dalparan | N,N-dimethyl-2-[6-...)Show InChI InChI=1S/C19H21N3O/c1-13-5-8-15(9-6-13)19-16(11-18(23)21(3)4)22-12-14(2)7-10-17(22)20-19/h5-10,12H,11H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 829 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synthélabo
Curated by PDSP Ki Database
| |
J Biol Chem 274: 13370-4 (1999)
Article DOI: 10.1074/jbc.274.19.13370
BindingDB Entry DOI: 10.7270/Q2765CWZ |
More data for this
Ligand-Target Pair | |
Membrane primary amine oxidase
(Homo sapiens (Human)) | BDBM50246766
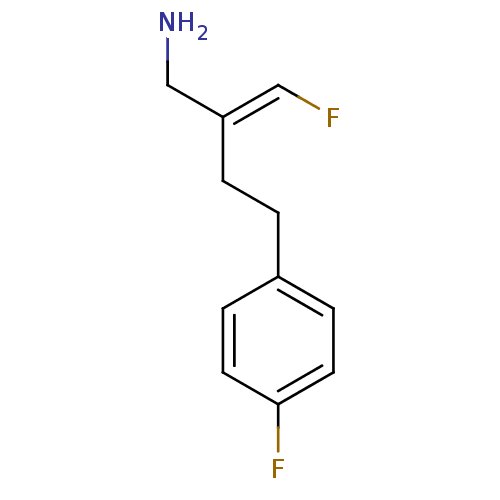
(CHEMBL489079 | Mofegiline | US9302986, Mofegiline)Show InChI InChI=1S/C11H13F2N/c12-7-10(8-14)2-1-9-3-5-11(13)6-4-9/h3-7H,1-2,8,14H2/b10-7+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaxis Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human SSAO/VAP1 measuring H2O2 production by Kitz and Wilson plot analysis |
Bioorg Med Chem Lett 22: 3935-40 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.111
BindingDB Entry DOI: 10.7270/Q2SQ91D6 |
More data for this
Ligand-Target Pair | |
Membrane primary amine oxidase
(Homo sapiens (Human)) | BDBM50384084
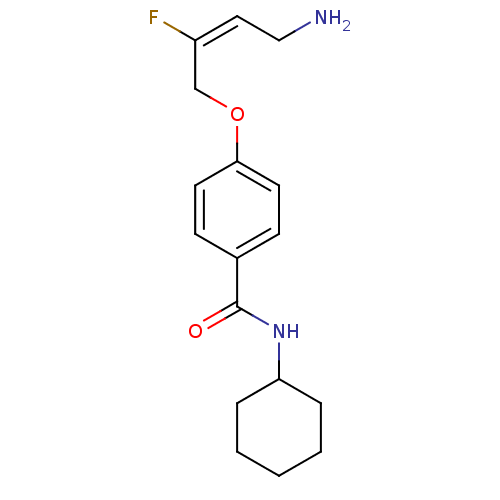
(CHEMBL2029546)Show InChI InChI=1S/C17H23FN2O2/c18-14(10-11-19)12-22-16-8-6-13(7-9-16)17(21)20-15-4-2-1-3-5-15/h6-10,15H,1-5,11-12,19H2,(H,20,21)/b14-10+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaxis Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human SSAO/VAP1 measuring H2O2 production by Kitz and Wilson plot analysis |
Bioorg Med Chem Lett 22: 3935-40 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.111
BindingDB Entry DOI: 10.7270/Q2SQ91D6 |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5
(RAT) | BDBM26266

(Ambien | CHEMBL911 | Dalparan | N,N-dimethyl-2-[6-...)Show InChI InChI=1S/C19H21N3O/c1-13-5-8-15(9-6-13)19-16(11-18(23)21(3)4)22-12-14(2)7-10-17(22)20-19/h5-10,12H,11H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synthélabo
Curated by PDSP Ki Database
| |
J Biol Chem 274: 13370-4 (1999)
Article DOI: 10.1074/jbc.274.19.13370
BindingDB Entry DOI: 10.7270/Q2765CWZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5
(RAT) | BDBM26266

(Ambien | CHEMBL911 | Dalparan | N,N-dimethyl-2-[6-...)Show InChI InChI=1S/C19H21N3O/c1-13-5-8-15(9-6-13)19-16(11-18(23)21(3)4)22-12-14(2)7-10-17(22)20-19/h5-10,12H,11H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synthélabo
Curated by PDSP Ki Database
| |
J Biol Chem 274: 13370-4 (1999)
Article DOI: 10.1074/jbc.274.19.13370
BindingDB Entry DOI: 10.7270/Q2765CWZ |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50329079
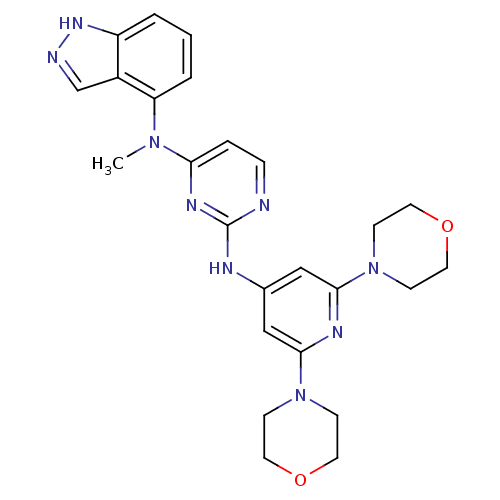
(CHEMBL1270280 | N2-(2,6-dimorpholinopyridin-4-yl)-...)Show SMILES CN(c1ccnc(Nc2cc(nc(c2)N2CCOCC2)N2CCOCC2)n1)c1cccc2[nH]ncc12 Show InChI InChI=1S/C25H29N9O2/c1-32(21-4-2-3-20-19(21)17-27-31-20)22-5-6-26-25(30-22)28-18-15-23(33-7-11-35-12-8-33)29-24(16-18)34-9-13-36-14-10-34/h2-6,15-17H,7-14H2,1H3,(H,27,31)(H,26,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 by acoustic dispensing assay |
Bioorg Med Chem Lett 20: 6242-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.100
BindingDB Entry DOI: 10.7270/Q2QR4XCB |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1/beta-2/gamma-2
(Homo sapiens (Human)) | BDBM50290077

(CHEMBL73416 | N-[2-(4-Chloro-phenyl)-imidazo[1,2-a...)Show InChI InChI=1S/C19H20ClN3O/c1-3-6-18(24)22(2)13-16-19(14-8-10-15(20)11-9-14)21-17-7-4-5-12-23(16)17/h4-5,7-12H,3,6,13H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro functional inhibitory potency prevention of ET-1 induced constriction of rat aortic rings (ETB receptors) |
Bioorg Med Chem Lett 7: 2277-2282 (1997)
Article DOI: 10.1016/S0960-894X(97)00404-6
BindingDB Entry DOI: 10.7270/Q2BK1CB0 |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1/beta-2/gamma-2
(Homo sapiens (Human)) | BDBM50290077

(CHEMBL73416 | N-[2-(4-Chloro-phenyl)-imidazo[1,2-a...)Show InChI InChI=1S/C19H20ClN3O/c1-3-6-18(24)22(2)13-16-19(14-8-10-15(20)11-9-14)21-17-7-4-5-12-23(16)17/h4-5,7-12H,3,6,13H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for in vitro functional inhibitory potency for prevention of ET-1 induced constriction of rat aortic rings (ETA receptors) |
Bioorg Med Chem Lett 7: 2277-2282 (1997)
Article DOI: 10.1016/S0960-894X(97)00404-6
BindingDB Entry DOI: 10.7270/Q2BK1CB0 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50329070
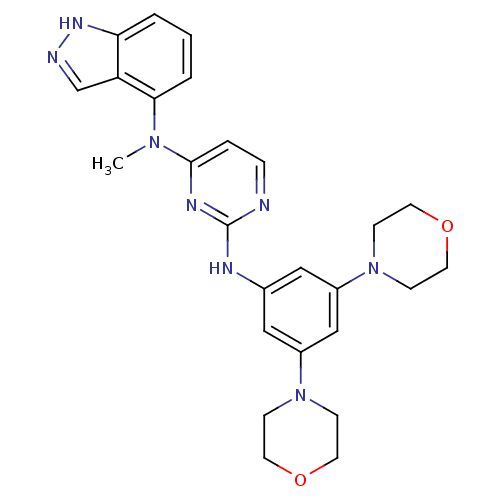
(CHEMBL1269858 | N2-(3,5-dimorpholinophenyl)-N4-(1H...)Show SMILES CN(c1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1)c1cccc2[nH]ncc12 Show InChI InChI=1S/C26H30N8O2/c1-32(24-4-2-3-23-22(24)18-28-31-23)25-5-6-27-26(30-25)29-19-15-20(33-7-11-35-12-8-33)17-21(16-19)34-9-13-36-14-10-34/h2-6,15-18H,7-14H2,1H3,(H,28,31)(H,27,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 by acoustic dispensing assay |
Bioorg Med Chem Lett 20: 6242-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.100
BindingDB Entry DOI: 10.7270/Q2QR4XCB |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50371358
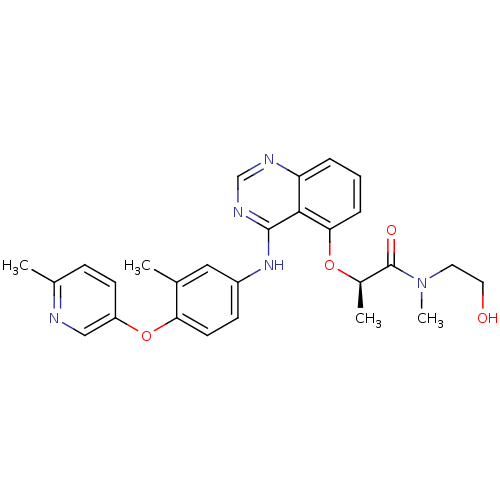
(CHEMBL257478)Show SMILES C[C@@H](Oc1cccc2ncnc(Nc3ccc(Oc4ccc(C)nc4)c(C)c3)c12)C(=O)N(C)CCO Show InChI InChI=1S/C27H29N5O4/c1-17-14-20(9-11-23(17)36-21-10-8-18(2)28-15-21)31-26-25-22(29-16-30-26)6-5-7-24(25)35-19(3)27(34)32(4)12-13-33/h5-11,14-16,19,33H,12-13H2,1-4H3,(H,29,30,31)/t19-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of ebrB2 |
Bioorg Med Chem Lett 18: 674-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.052
BindingDB Entry DOI: 10.7270/Q2736RSH |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50329080

(CHEMBL1270378 | N2-(2,6-dimorpholinopyrimidin-4-yl...)Show SMILES CN(c1ccnc(Nc2cc(nc(n2)N2CCOCC2)N2CCOCC2)n1)c1cccc2[nH]ncc12 Show InChI InChI=1S/C24H28N10O2/c1-32(19-4-2-3-18-17(19)16-26-31-18)21-5-6-25-23(29-21)27-20-15-22(33-7-11-35-12-8-33)30-24(28-20)34-9-13-36-14-10-34/h2-6,15-16H,7-14H2,1H3,(H,26,31)(H,25,27,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 by acoustic dispensing assay |
Bioorg Med Chem Lett 20: 6242-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.100
BindingDB Entry DOI: 10.7270/Q2QR4XCB |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM12255

(AZD0530 | CHEMBL217092 | Compound 33 | N-(5-chloro...)Show SMILES CN1CCN(CCOc2cc(OC3CCOCC3)c3c(Nc4c5OCOc5ccc4Cl)ncnc3c2)CC1 Show InChI InChI=1S/C27H32ClN5O5/c1-32-6-8-33(9-7-32)10-13-35-19-14-21-24(23(15-19)38-18-4-11-34-12-5-18)27(30-16-29-21)31-25-20(28)2-3-22-26(25)37-17-36-22/h2-3,14-16,18H,4-13,17H2,1H3,(H,29,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
| Assay Description
This assay determines the ability of test compounds to inhibit Src kinase activity that catalyzes the transfer of the terminal phosphate to the immob... |
J Med Chem 49: 6465-88 (2006)
Article DOI: 10.1021/jm060434q
BindingDB Entry DOI: 10.7270/Q2J67F5X |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5
(Homo sapiens (Human)) | BDBM50290077

(CHEMBL73416 | N-[2-(4-Chloro-phenyl)-imidazo[1,2-a...)Show InChI InChI=1S/C19H20ClN3O/c1-3-6-18(24)22(2)13-16-19(14-8-10-15(20)11-9-14)21-17-7-4-5-12-23(16)17/h4-5,7-12H,3,6,13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Receptor binding affinity was determined in a radioligand binding assay against [125I]ET1 with recombinant human ETA receptor, expressed in baculovir... |
Bioorg Med Chem Lett 7: 2277-2282 (1997)
Article DOI: 10.1016/S0960-894X(97)00404-6
BindingDB Entry DOI: 10.7270/Q2BK1CB0 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM12241

(AZD0530 analogue 19 | N-(5-chloro-2H-1,3-benzodiox...)Show SMILES Clc1ccc2OCOc2c1Nc1ncnc2cc(OCCOc3ccncc3)cc(OC3CCOCC3)c12 Show InChI InChI=1S/C27H25ClN4O6/c28-20-1-2-22-26(37-16-36-22)25(20)32-27-24-21(30-15-31-27)13-19(14-23(24)38-18-5-9-33-10-6-18)35-12-11-34-17-3-7-29-8-4-17/h1-4,7-8,13-15,18H,5-6,9-12,16H2,(H,30,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
| Assay Description
This assay determines the ability of test compounds to inhibit Src kinase activity that catalyzes the transfer of the terminal phosphate to the immob... |
J Med Chem 49: 6465-88 (2006)
Article DOI: 10.1021/jm060434q
BindingDB Entry DOI: 10.7270/Q2J67F5X |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50179758
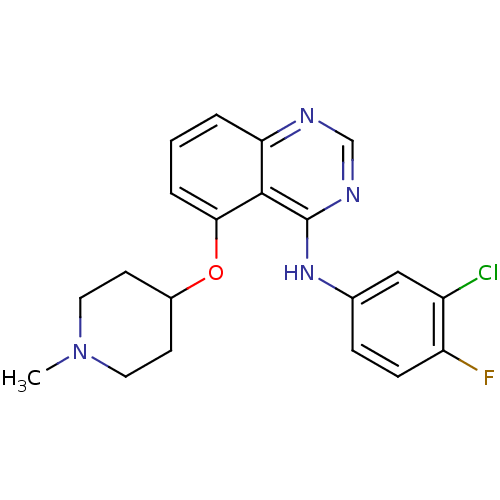
(CHEMBL203725 | N-(3-chloro-4-fluorophenyl)-5-(1-me...)Show SMILES CN1CCC(CC1)Oc1cccc2ncnc(Nc3ccc(F)c(Cl)c3)c12 Show InChI InChI=1S/C20H20ClFN4O/c1-26-9-7-14(8-10-26)27-18-4-2-3-17-19(18)20(24-12-23-17)25-13-5-6-16(22)15(21)11-13/h2-6,11-12,14H,7-10H2,1H3,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibitory activity against EGFR |
Bioorg Med Chem Lett 16: 1633-7 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.028
BindingDB Entry DOI: 10.7270/Q2P84BGH |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM12231
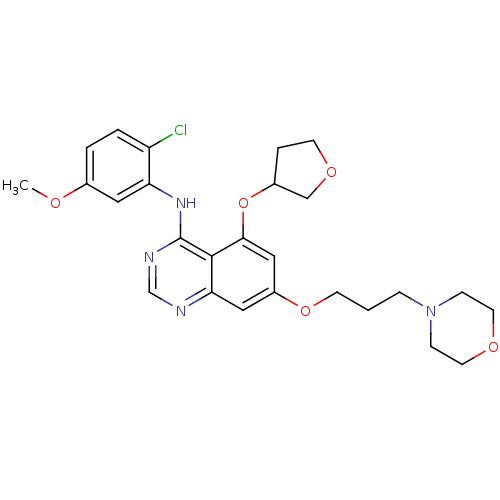
(AZD0530 analogue 9 | N-(2-chloro-5-methoxyphenyl)-...)Show SMILES COc1ccc(Cl)c(Nc2ncnc3cc(OCCCN4CCOCC4)cc(OC4CCOC4)c23)c1 Show InChI InChI=1S/C26H31ClN4O5/c1-32-18-3-4-21(27)22(13-18)30-26-25-23(28-17-29-26)14-20(15-24(25)36-19-5-10-34-16-19)35-9-2-6-31-7-11-33-12-8-31/h3-4,13-15,17,19H,2,5-12,16H2,1H3,(H,28,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
This assay determines the ability of test compounds to inhibit Src kinase activity that catalyzes the transfer of the terminal phosphate to the immob... |
J Med Chem 49: 6465-88 (2006)
Article DOI: 10.1021/jm060434q
BindingDB Entry DOI: 10.7270/Q2J67F5X |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM12237
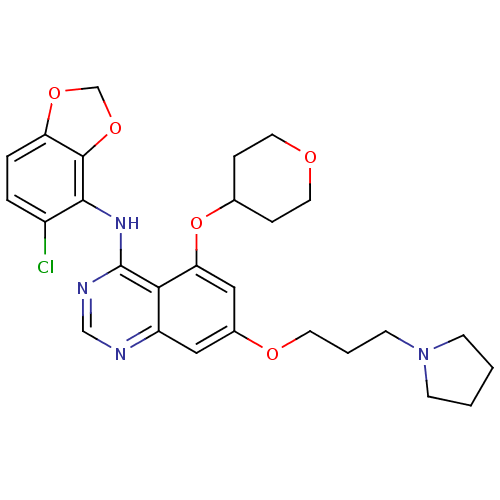
(AZD0530 analogue 15 | N-(5-chloro-2H-1,3-benzodiox...)Show SMILES Clc1ccc2OCOc2c1Nc1ncnc2cc(OCCCN3CCCC3)cc(OC3CCOCC3)c12 Show InChI InChI=1S/C27H31ClN4O5/c28-20-4-5-22-26(36-17-35-22)25(20)31-27-24-21(29-16-30-27)14-19(34-11-3-10-32-8-1-2-9-32)15-23(24)37-18-6-12-33-13-7-18/h4-5,14-16,18H,1-3,6-13,17H2,(H,29,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
| Assay Description
This assay determines the ability of test compounds to inhibit Src kinase activity that catalyzes the transfer of the terminal phosphate to the immob... |
J Med Chem 49: 6465-88 (2006)
Article DOI: 10.1021/jm060434q
BindingDB Entry DOI: 10.7270/Q2J67F5X |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM12243
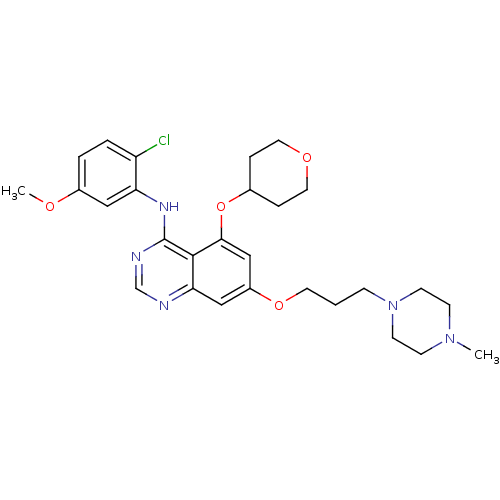
(AZD0530 analogue 21 | N-(2-chloro-5-methoxyphenyl)...)Show SMILES COc1ccc(Cl)c(Nc2ncnc3cc(OCCCN4CCN(C)CC4)cc(OC4CCOCC4)c23)c1 Show InChI InChI=1S/C28H36ClN5O4/c1-33-9-11-34(12-10-33)8-3-13-37-22-17-25-27(26(18-22)38-20-6-14-36-15-7-20)28(31-19-30-25)32-24-16-21(35-2)4-5-23(24)29/h4-5,16-20H,3,6-15H2,1-2H3,(H,30,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
| Assay Description
This assay determines the ability of test compounds to inhibit Src kinase activity that catalyzes the transfer of the terminal phosphate to the immob... |
J Med Chem 49: 6465-88 (2006)
Article DOI: 10.1021/jm060434q
BindingDB Entry DOI: 10.7270/Q2J67F5X |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50293247
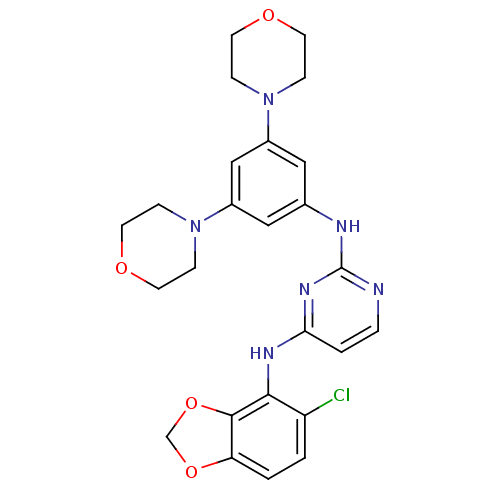
(CHEMBL468970 | N4-(5-chlorobenzo[d][1,3]dioxol-4-y...)Show SMILES Clc1ccc2OCOc2c1Nc1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1 Show InChI InChI=1S/C25H27ClN6O4/c26-20-1-2-21-24(36-16-35-21)23(20)29-22-3-4-27-25(30-22)28-17-13-18(31-5-9-33-10-6-31)15-19(14-17)32-7-11-34-12-8-32/h1-4,13-15H,5-12,16H2,(H2,27,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 by acoustic dispensing assay |
Bioorg Med Chem Lett 20: 6242-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.100
BindingDB Entry DOI: 10.7270/Q2QR4XCB |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM12250

(AZD0530 analogue 28 | N-(5-Chloro-1,3-benzodioxol-...)Show SMILES CN1CCC(CC1)Oc1cc(OCCF)cc2ncnc(Nc3c4OCOc4ccc3Cl)c12 Show InChI InChI=1S/C23H24ClFN4O4/c1-29-7-4-14(5-8-29)33-19-11-15(30-9-6-25)10-17-20(19)23(27-12-26-17)28-21-16(24)2-3-18-22(21)32-13-31-18/h2-3,10-12,14H,4-9,13H2,1H3,(H,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
| Assay Description
This assay determines the ability of test compounds to inhibit Src kinase activity that catalyzes the transfer of the terminal phosphate to the immob... |
J Med Chem 49: 6465-88 (2006)
Article DOI: 10.1021/jm060434q
BindingDB Entry DOI: 10.7270/Q2J67F5X |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM12252
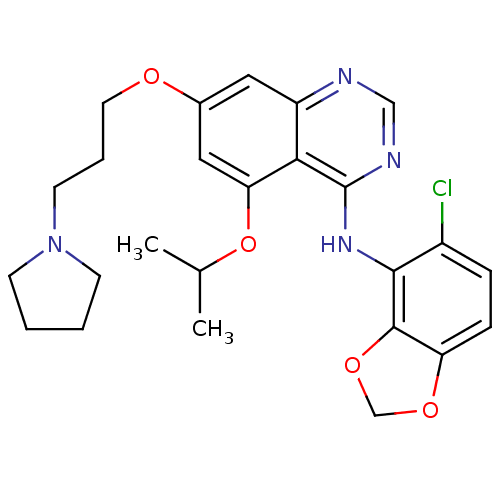
(AZD0530 analogue 30 | N-(5-Chloro-1,3-benzodioxol-...)Show SMILES CC(C)Oc1cc(OCCCN2CCCC2)cc2ncnc(Nc3c4OCOc4ccc3Cl)c12 Show InChI InChI=1S/C25H29ClN4O4/c1-16(2)34-21-13-17(31-11-5-10-30-8-3-4-9-30)12-19-22(21)25(28-14-27-19)29-23-18(26)6-7-20-24(23)33-15-32-20/h6-7,12-14,16H,3-5,8-11,15H2,1-2H3,(H,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
| Assay Description
This assay determines the ability of test compounds to inhibit Src kinase activity that catalyzes the transfer of the terminal phosphate to the immob... |
J Med Chem 49: 6465-88 (2006)
Article DOI: 10.1021/jm060434q
BindingDB Entry DOI: 10.7270/Q2J67F5X |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM12247
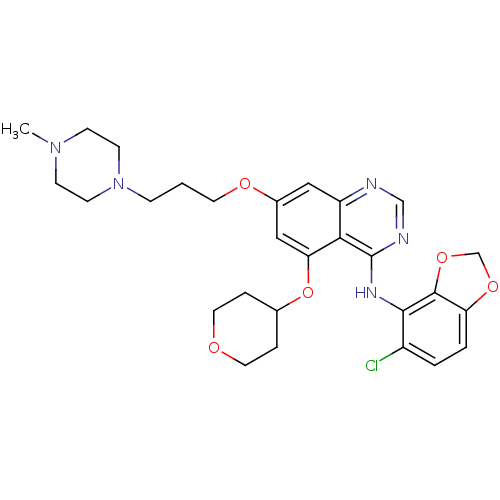
(AZD0530 analogue 25 | N-(5-chloro-2H-1,3-benzodiox...)Show SMILES CN1CCN(CCCOc2cc(OC3CCOCC3)c3c(Nc4c5OCOc5ccc4Cl)ncnc3c2)CC1 Show InChI InChI=1S/C28H34ClN5O5/c1-33-8-10-34(11-9-33)7-2-12-36-20-15-22-25(24(16-20)39-19-5-13-35-14-6-19)28(31-17-30-22)32-26-21(29)3-4-23-27(26)38-18-37-23/h3-4,15-17,19H,2,5-14,18H2,1H3,(H,30,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
| Assay Description
This assay determines the ability of test compounds to inhibit Src kinase activity that catalyzes the transfer of the terminal phosphate to the immob... |
J Med Chem 49: 6465-88 (2006)
Article DOI: 10.1021/jm060434q
BindingDB Entry DOI: 10.7270/Q2J67F5X |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5
(Homo sapiens (Human)) | BDBM50290077

(CHEMBL73416 | N-[2-(4-Chloro-phenyl)-imidazo[1,2-a...)Show InChI InChI=1S/C19H20ClN3O/c1-3-6-18(24)22(2)13-16-19(14-8-10-15(20)11-9-14)21-17-7-4-5-12-23(16)17/h4-5,7-12H,3,6,13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-Flumazenil binding to recombinant GABA-A receptor alpha-5 subunit in spinal cord |
Bioorg Med Chem Lett 7: 2277-2282 (1997)
Article DOI: 10.1016/S0960-894X(97)00404-6
BindingDB Entry DOI: 10.7270/Q2BK1CB0 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM12253

(AZD0530 analogue 31 | N-(5-chloro-2H-1,3-benzodiox...)Show SMILES CC(C)Oc1cc(OCCN2CCOCC2)cc2ncnc(Nc3c4OCOc4ccc3Cl)c12 Show InChI InChI=1S/C24H27ClN4O5/c1-15(2)34-20-12-16(31-10-7-29-5-8-30-9-6-29)11-18-21(20)24(27-13-26-18)28-22-17(25)3-4-19-23(22)33-14-32-19/h3-4,11-13,15H,5-10,14H2,1-2H3,(H,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
| Assay Description
This assay determines the ability of test compounds to inhibit Src kinase activity that catalyzes the transfer of the terminal phosphate to the immob... |
J Med Chem 49: 6465-88 (2006)
Article DOI: 10.1021/jm060434q
BindingDB Entry DOI: 10.7270/Q2J67F5X |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM6218
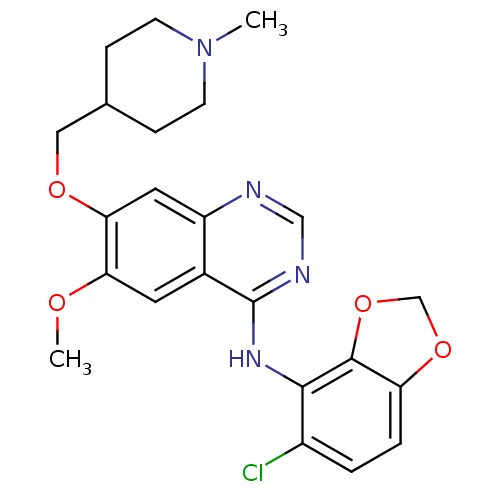
(AZD0530 analogue 34 | Chlorobenzodioxole deriv. 27...)Show SMILES COc1cc2c(Nc3c4OCOc4ccc3Cl)ncnc2cc1OCC1CCN(C)CC1 Show InChI InChI=1S/C23H25ClN4O4/c1-28-7-5-14(6-8-28)11-30-20-10-17-15(9-19(20)29-2)23(26-12-25-17)27-21-16(24)3-4-18-22(21)32-13-31-18/h3-4,9-10,12,14H,5-8,11,13H2,1-2H3,(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
| Assay Description
This assay determines the ability of test compounds to inhibit Src kinase activity that catalyzes the transfer of the terminal phosphate to the immob... |
J Med Chem 49: 6465-88 (2006)
Article DOI: 10.1021/jm060434q
BindingDB Entry DOI: 10.7270/Q2J67F5X |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM12259
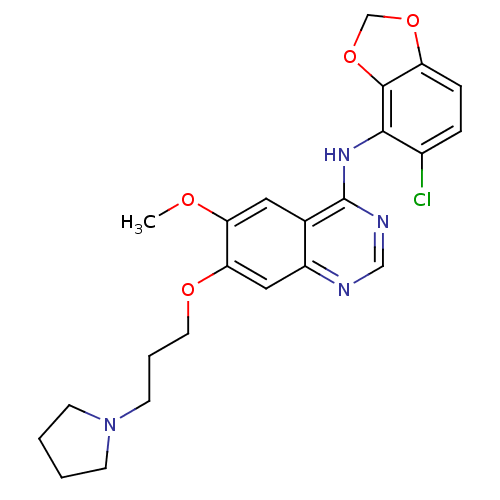
(AZD0530 analogue 37 | N-(5-chloro-2H-1,3-benzodiox...)Show SMILES COc1cc2c(Nc3c4OCOc4ccc3Cl)ncnc2cc1OCCCN1CCCC1 Show InChI InChI=1S/C23H25ClN4O4/c1-29-19-11-15-17(12-20(19)30-10-4-9-28-7-2-3-8-28)25-13-26-23(15)27-21-16(24)5-6-18-22(21)32-14-31-18/h5-6,11-13H,2-4,7-10,14H2,1H3,(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
| Assay Description
This assay determines the ability of test compounds to inhibit Src kinase activity that catalyzes the transfer of the terminal phosphate to the immob... |
J Med Chem 49: 6465-88 (2006)
Article DOI: 10.1021/jm060434q
BindingDB Entry DOI: 10.7270/Q2J67F5X |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50329079

(CHEMBL1270280 | N2-(2,6-dimorpholinopyridin-4-yl)-...)Show SMILES CN(c1ccnc(Nc2cc(nc(c2)N2CCOCC2)N2CCOCC2)n1)c1cccc2[nH]ncc12 Show InChI InChI=1S/C25H29N9O2/c1-32(21-4-2-3-20-19(21)17-27-31-20)22-5-6-26-25(30-22)28-18-15-23(33-7-11-35-12-8-33)29-24(16-18)34-9-13-36-14-10-34/h2-6,15-17H,7-14H2,1H3,(H,27,31)(H,26,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 autophosphorylation expressed in CHOK1 cells |
Bioorg Med Chem Lett 20: 6242-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.100
BindingDB Entry DOI: 10.7270/Q2QR4XCB |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50171250
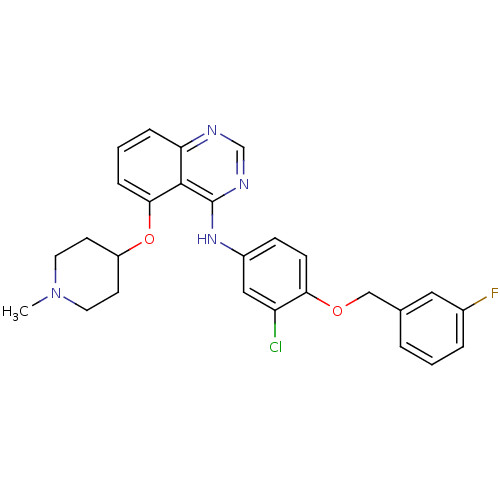
(CHEMBL197640 | [3-Chloro-4-(3-fluoro-benzyloxy)-ph...)Show SMILES CN1CCC(CC1)Oc1cccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c12 Show InChI InChI=1S/C27H26ClFN4O2/c1-33-12-10-21(11-13-33)35-25-7-3-6-23-26(25)27(31-17-30-23)32-20-8-9-24(22(28)15-20)34-16-18-4-2-5-19(29)14-18/h2-9,14-15,17,21H,10-13,16H2,1H3,(H,30,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibitory concentration against EGFR kinase phosphorylation using synthetic peptide as a substrate |
Bioorg Med Chem Lett 15: 4226-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.068
BindingDB Entry DOI: 10.7270/Q2TQ612W |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50174932

(CHEMBL197287 | N-(2-chloro-5-methoxypyridin-3-yl)-...)Show SMILES COc1cnc(Cl)c(Nc2ncnc3cc(OCCCN4CCOCC4)cc(OC(C)C)c23)c1 Show InChI InChI=1S/C24H30ClN5O4/c1-16(2)34-21-13-17(33-8-4-5-30-6-9-32-10-7-30)11-19-22(21)24(28-15-27-19)29-20-12-18(31-3)14-26-23(20)25/h11-16H,4-10H2,1-3H3,(H,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibitory activity against c-Src kinase |
Bioorg Med Chem Lett 15: 5446-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.106
BindingDB Entry DOI: 10.7270/Q2765DWC |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50171256

(CHEMBL444619 | [3-Chloro-4-(pyridin-2-ylmethoxy)-p...)Show SMILES COc1cc(ON2CCC(C)CC2)c2c(Nc3ccc(OCc4ccccn4)c(Cl)c3)ncnc2c1 Show InChI InChI=1S/C27H28ClN5O3/c1-18-8-11-33(12-9-18)36-25-15-21(34-2)14-23-26(25)27(31-17-30-23)32-19-6-7-24(22(28)13-19)35-16-20-5-3-4-10-29-20/h3-7,10,13-15,17-18H,8-9,11-12,16H2,1-2H3,(H,30,31,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibitory concentration against erbB2 kinase phosphorylation using synthetic peptide as a substrate |
Bioorg Med Chem Lett 15: 4226-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.068
BindingDB Entry DOI: 10.7270/Q2TQ612W |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50171256

(CHEMBL444619 | [3-Chloro-4-(pyridin-2-ylmethoxy)-p...)Show SMILES COc1cc(ON2CCC(C)CC2)c2c(Nc3ccc(OCc4ccccn4)c(Cl)c3)ncnc2c1 Show InChI InChI=1S/C27H28ClN5O3/c1-18-8-11-33(12-9-18)36-25-15-21(34-2)14-23-26(25)27(31-17-30-23)32-19-6-7-24(22(28)13-19)35-16-20-5-3-4-10-29-20/h3-7,10,13-15,17-18H,8-9,11-12,16H2,1-2H3,(H,30,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibitory concentration against EGFR kinase phosphorylation using synthetic peptide as a substrate |
Bioorg Med Chem Lett 15: 4226-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.068
BindingDB Entry DOI: 10.7270/Q2TQ612W |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50174939
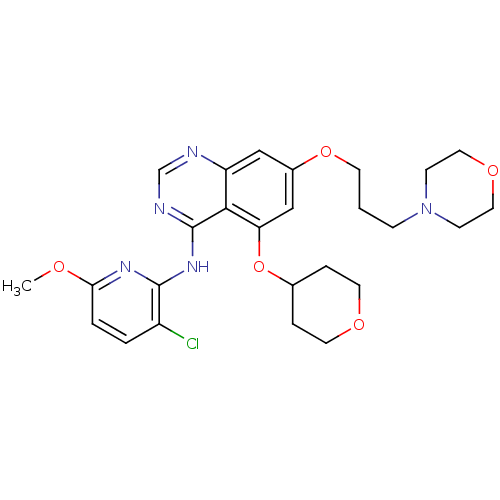
(CHEMBL424664 | N-(3-chloro-6-methoxypyridin-2-yl)-...)Show SMILES COc1ccc(Cl)c(Nc2ncnc3cc(OCCCN4CCOCC4)cc(OC4CCOCC4)c23)n1 Show InChI InChI=1S/C26H32ClN5O5/c1-33-23-4-3-20(27)25(30-23)31-26-24-21(28-17-29-26)15-19(16-22(24)37-18-5-11-34-12-6-18)36-10-2-7-32-8-13-35-14-9-32/h3-4,15-18H,2,5-14H2,1H3,(H,28,29,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibitory activity against c-Src kinase |
Bioorg Med Chem Lett 15: 5446-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.106
BindingDB Entry DOI: 10.7270/Q2765DWC |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM12238
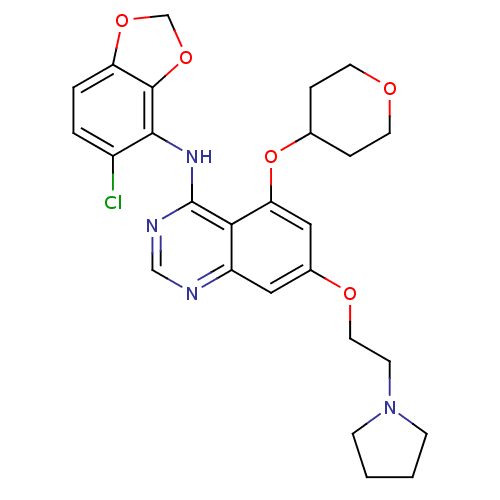
(AZD0530 analogue 16 | N-(5-chloro-2H-1,3-benzodiox...)Show SMILES Clc1ccc2OCOc2c1Nc1ncnc2cc(OCCN3CCCC3)cc(OC3CCOCC3)c12 Show InChI InChI=1S/C26H29ClN4O5/c27-19-3-4-21-25(35-16-34-21)24(19)30-26-23-20(28-15-29-26)13-18(33-12-9-31-7-1-2-8-31)14-22(23)36-17-5-10-32-11-6-17/h3-4,13-15,17H,1-2,5-12,16H2,(H,28,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
| Assay Description
This assay determines the ability of test compounds to inhibit Src kinase activity that catalyzes the transfer of the terminal phosphate to the immob... |
J Med Chem 49: 6465-88 (2006)
Article DOI: 10.1021/jm060434q
BindingDB Entry DOI: 10.7270/Q2J67F5X |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50174934
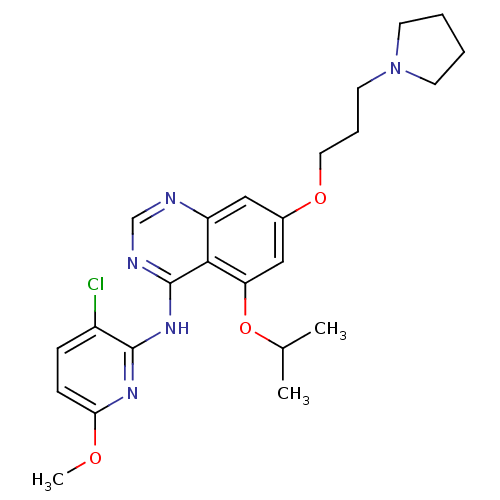
(CHEMBL424839 | N-(3-chloro-6-methoxypyridin-2-yl)-...)Show SMILES COc1ccc(Cl)c(Nc2ncnc3cc(OCCCN4CCCC4)cc(OC(C)C)c23)n1 Show InChI InChI=1S/C24H30ClN5O3/c1-16(2)33-20-14-17(32-12-6-11-30-9-4-5-10-30)13-19-22(20)24(27-15-26-19)29-23-18(25)7-8-21(28-23)31-3/h7-8,13-16H,4-6,9-12H2,1-3H3,(H,26,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibitory activity against c-Src kinase |
Bioorg Med Chem Lett 15: 5446-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.106
BindingDB Entry DOI: 10.7270/Q2765DWC |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50236375

((S)-2-(4-(1-(3-fluorobenzyl)-1H-indazol-5-ylamino)...)Show SMILES C[C@H](Oc1cccc2ncnc(Nc3ccc4n(Cc5cccc(F)c5)ncc4c3)c12)C(=O)N(C)C Show InChI InChI=1S/C27H25FN6O2/c1-17(27(35)33(2)3)36-24-9-5-8-22-25(24)26(30-16-29-22)32-21-10-11-23-19(13-21)14-31-34(23)15-18-6-4-7-20(28)12-18/h4-14,16-17H,15H2,1-3H3,(H,29,30,32)/t17-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of human erbB2 autophosphorylation in MCF7 cells |
Bioorg Med Chem Lett 18: 1799-803 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.035
BindingDB Entry DOI: 10.7270/Q23T9H0F |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM12240
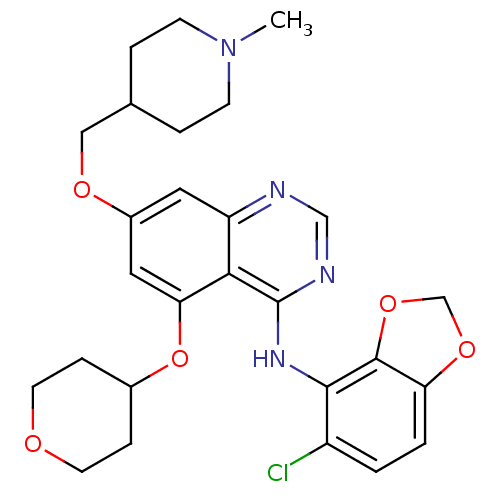
(AZD0530 analogue 18 | N-(5-Chloro-1,3-benzodioxol-...)Show SMILES CN1CCC(COc2cc(OC3CCOCC3)c3c(Nc4c5OCOc5ccc4Cl)ncnc3c2)CC1 Show InChI InChI=1S/C27H31ClN4O5/c1-32-8-4-17(5-9-32)14-34-19-12-21-24(23(13-19)37-18-6-10-33-11-7-18)27(30-15-29-21)31-25-20(28)2-3-22-26(25)36-16-35-22/h2-3,12-13,15,17-18H,4-11,14,16H2,1H3,(H,29,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
| Assay Description
This assay determines the ability of test compounds to inhibit Src kinase activity that catalyzes the transfer of the terminal phosphate to the immob... |
J Med Chem 49: 6465-88 (2006)
Article DOI: 10.1021/jm060434q
BindingDB Entry DOI: 10.7270/Q2J67F5X |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM12251
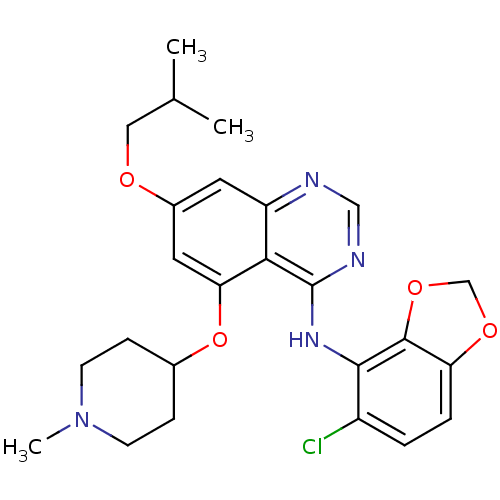
(AZD0530 analogue 29 | N-(5-chloro-2H-1,3-benzodiox...)Show SMILES CC(C)COc1cc(OC2CCN(C)CC2)c2c(Nc3c4OCOc4ccc3Cl)ncnc2c1 Show InChI InChI=1S/C25H29ClN4O4/c1-15(2)12-31-17-10-19-22(21(11-17)34-16-6-8-30(3)9-7-16)25(28-13-27-19)29-23-18(26)4-5-20-24(23)33-14-32-20/h4-5,10-11,13,15-16H,6-9,12,14H2,1-3H3,(H,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
| Assay Description
This assay determines the ability of test compounds to inhibit Src kinase activity that catalyzes the transfer of the terminal phosphate to the immob... |
J Med Chem 49: 6465-88 (2006)
Article DOI: 10.1021/jm060434q
BindingDB Entry DOI: 10.7270/Q2J67F5X |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data