

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutamyl aminopeptidase (Homo sapiens (Human)) | BDBM50083386 (1-{[1-(2,3-Dicarboxy-pyrrolidine-1-carbonyl)-2-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.873 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Binding affinity against recombinant Aminopeptidase A | J Med Chem 42: 5197-211 (2000) BindingDB Entry DOI: 10.7270/Q2028S8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068901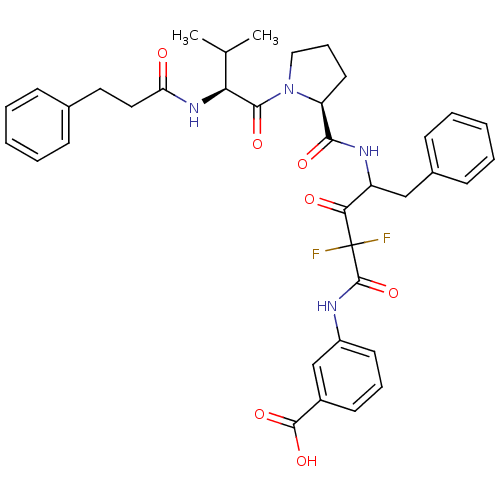 (3-[2,2-Difluoro-4-({(S)-1-[(S)-3-methyl-2-(3-pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068918 ((S)-4-((2S,3S)-2-Benzyloxycarbonylamino-3-methyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 919-24 (1999) BindingDB Entry DOI: 10.7270/Q2BZ656D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068899 (3-(4-{[(S)-1-((S)-2-Benzoylamino-3-methyl-butyryl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamyl aminopeptidase (Homo sapiens (Human)) | BDBM50083394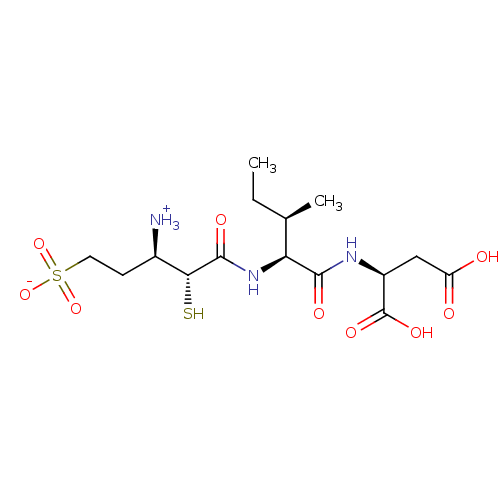 (1-{[1-(1,2-Dicarboxy-ethylcarbamoyl)-2-methyl-buty...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Binding affinity against recombinant Aminopeptidase A | J Med Chem 42: 5197-211 (2000) BindingDB Entry DOI: 10.7270/Q2028S8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamyl aminopeptidase (Homo sapiens (Human)) | BDBM50083376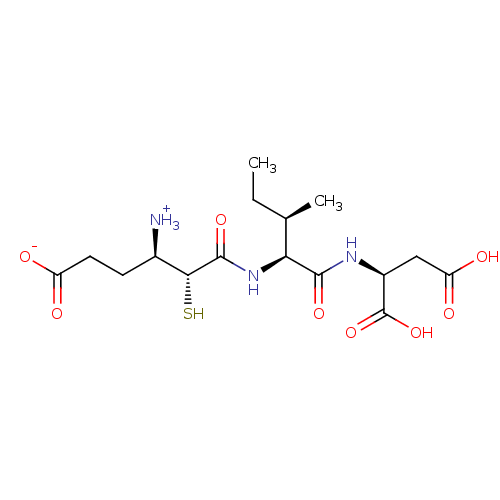 (3-Carboxy-1-{[1-(1,2-dicarboxy-ethylcarbamoyl)-2-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Binding affinity against recombinant Aminopeptidase A | J Med Chem 42: 5197-211 (2000) BindingDB Entry DOI: 10.7270/Q2028S8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamyl aminopeptidase (Homo sapiens (Human)) | BDBM50083378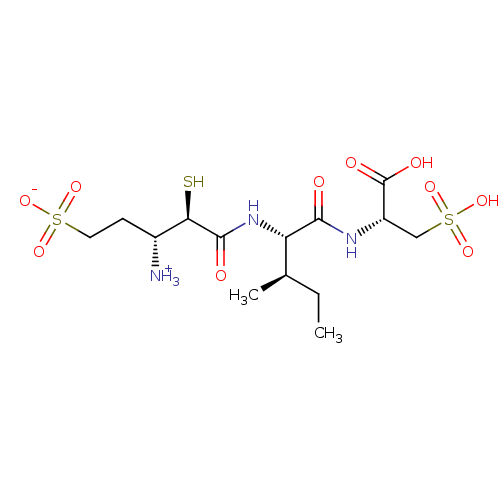 (1-{[1-(1-Carboxy-2-sulfo-ethylcarbamoyl)-2-methyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Binding affinity against recombinant Aminopeptidase A | J Med Chem 42: 5197-211 (2000) BindingDB Entry DOI: 10.7270/Q2028S8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamyl aminopeptidase (Homo sapiens (Human)) | BDBM50083385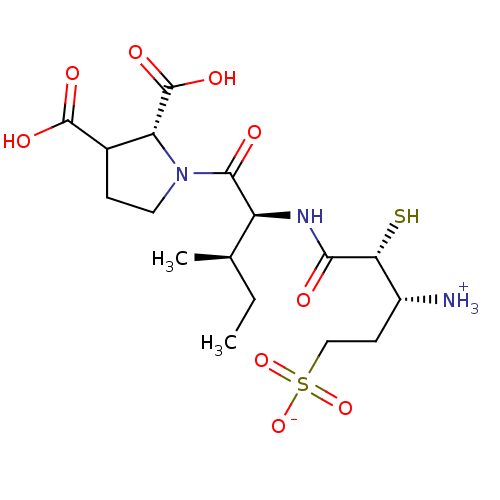 (1-{[1-(2,3-Dicarboxy-pyrrolidine-1-carbonyl)-2-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Binding affinity against recombinant Aminopeptidase A | J Med Chem 42: 5197-211 (2000) BindingDB Entry DOI: 10.7270/Q2028S8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068919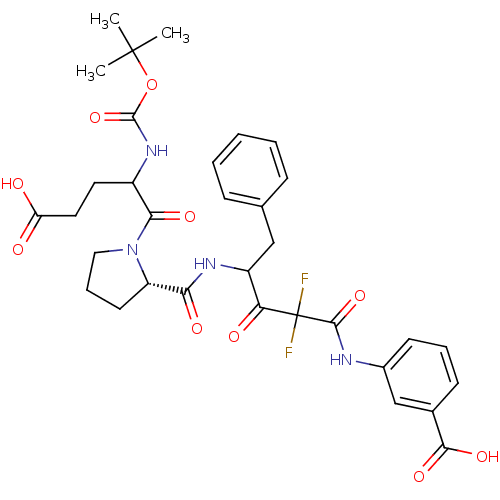 (3-(4-{[(S)-1-(2-tert-Butoxycarbonylamino-4-carboxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 919-24 (1999) BindingDB Entry DOI: 10.7270/Q2BZ656D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50083376 (3-Carboxy-1-{[1-(1,2-dicarboxy-ethylcarbamoyl)-2-m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Binding affinity against NEP from rabbit kidney(neutral endopeptidase) | J Med Chem 42: 5197-211 (2000) BindingDB Entry DOI: 10.7270/Q2028S8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamyl aminopeptidase (Homo sapiens (Human)) | BDBM50083393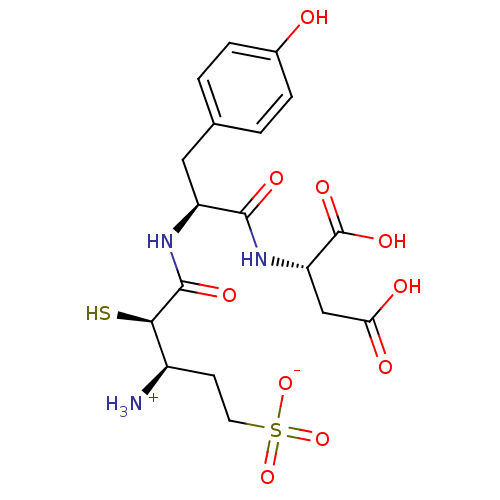 (1-{[1-(1,2-Dicarboxy-ethylcarbamoyl)-2-(4-hydroxy-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Binding affinity against recombinant Aminopeptidase A | J Med Chem 42: 5197-211 (2000) BindingDB Entry DOI: 10.7270/Q2028S8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamyl aminopeptidase (Homo sapiens (Human)) | BDBM50083390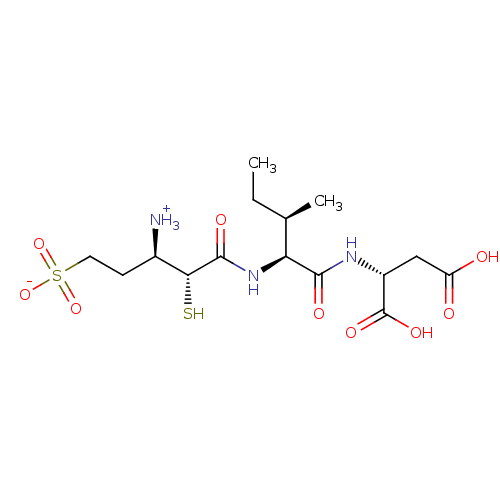 (1-{[1-(1,2-Dicarboxy-ethylcarbamoyl)-2-methyl-buty...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Binding affinity against recombinant Aminopeptidase A | J Med Chem 42: 5197-211 (2000) BindingDB Entry DOI: 10.7270/Q2028S8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50083382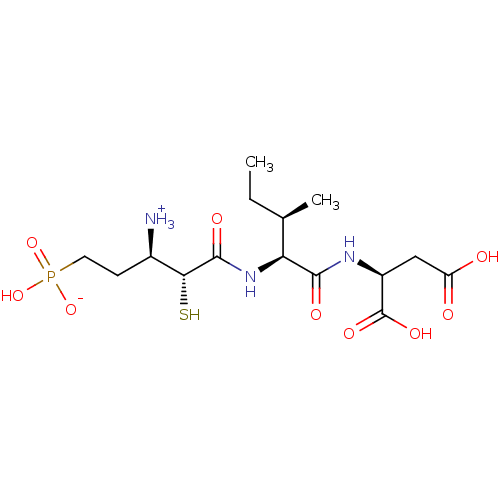 (1-{[1-(1,2-Dicarboxy-ethylcarbamoyl)-2-methyl-buty...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Binding affinity against NEP from rabbit kidney(neutral endopeptidase) | J Med Chem 42: 5197-211 (2000) BindingDB Entry DOI: 10.7270/Q2028S8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068911 (3-(4-{[(S)-1-(2-tert-Butoxycarbonylamino-3-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 919-24 (1999) BindingDB Entry DOI: 10.7270/Q2BZ656D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068894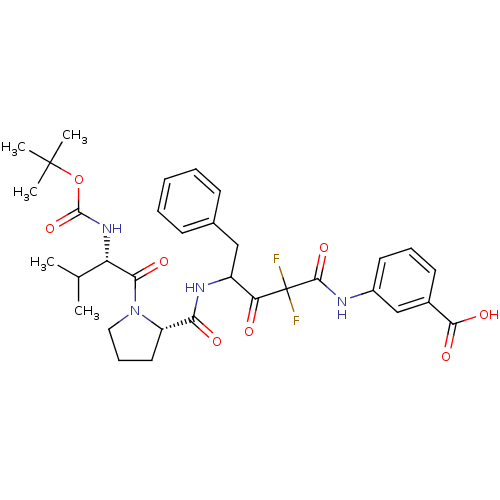 (3-(4-{[(S)-1-((S)-2-tert-Butoxycarbonylamino-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068917 (5-(4-{[(S)-1-(2-tert-Butoxycarbonylamino-4-carboxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 919-24 (1999) BindingDB Entry DOI: 10.7270/Q2BZ656D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068889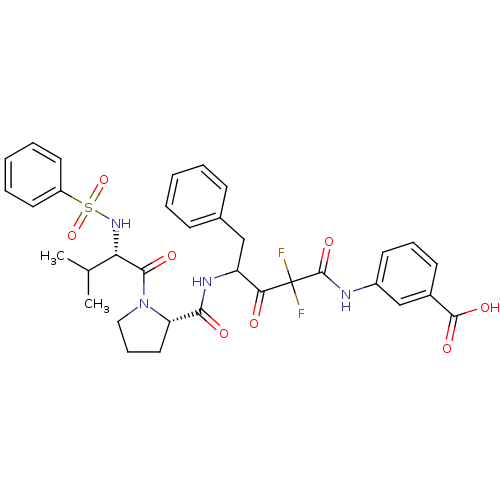 (3-(4-{[(S)-1-((S)-2-Benzenesulfonylamino-3-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068896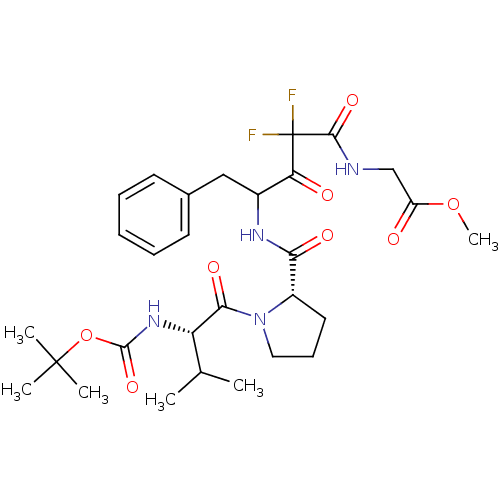 ((4-{[(S)-1-((S)-2-tert-Butoxycarbonylamino-3-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068915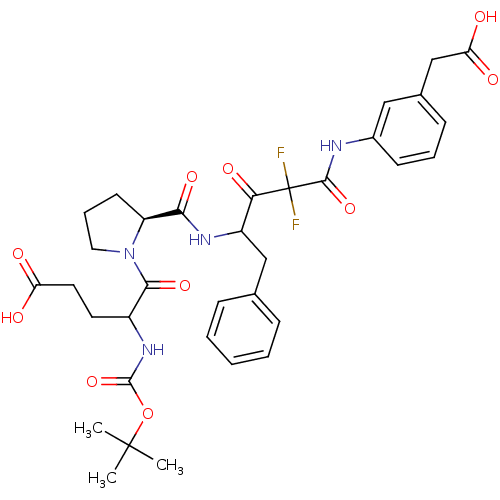 (5-{(S)-2-[1-Benzyl-3-(3-carboxymethyl-phenylcarbam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 919-24 (1999) BindingDB Entry DOI: 10.7270/Q2BZ656D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068887 (4-(4-{[(S)-1-((S)-2-tert-Butoxycarbonylamino-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068892 (3-(4-{[(S)-1-((S)-2-Acetylamino-3-methyl-butyryl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamyl aminopeptidase (Homo sapiens (Human)) | BDBM50083374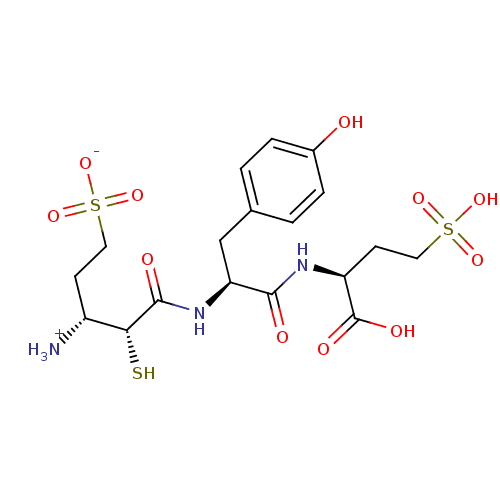 (1-{[1-(1-Carboxy-3-sulfo-propylcarbamoyl)-2-(4-hyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Binding affinity against recombinant Aminopeptidase A | J Med Chem 42: 5197-211 (2000) BindingDB Entry DOI: 10.7270/Q2028S8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50068892 (3-(4-{[(S)-1-((S)-2-Acetylamino-3-methyl-butyryl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against Chymotrypsinogen | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamyl aminopeptidase (Homo sapiens (Human)) | BDBM50083382 (1-{[1-(1,2-Dicarboxy-ethylcarbamoyl)-2-methyl-buty...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Binding affinity against recombinant Aminopeptidase A | J Med Chem 42: 5197-211 (2000) BindingDB Entry DOI: 10.7270/Q2028S8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamyl aminopeptidase (Homo sapiens (Human)) | BDBM50083403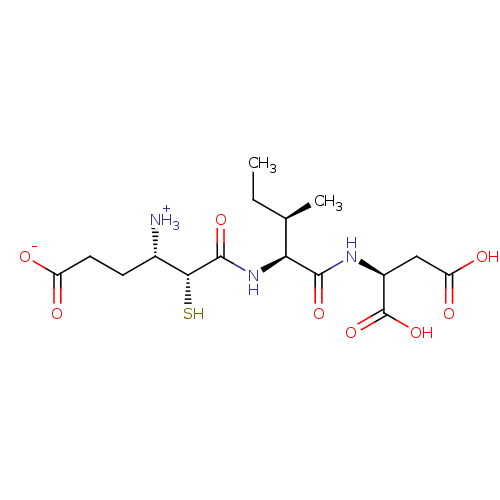 (3-Carboxy-1-{[1-(1,2-dicarboxy-ethylcarbamoyl)-2-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Binding affinity against recombinant Aminopeptidase A | J Med Chem 42: 5197-211 (2000) BindingDB Entry DOI: 10.7270/Q2028S8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068905 ((4-{[(S)-1-((S)-2-tert-Butoxycarbonylamino-3-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068903 ((4-{[(S)-1-((S)-2-tert-Butoxycarbonylamino-3-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068912 (3-(4-{[(S)-1-(2-tert-Butoxycarbonylamino-4-carboxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 919-24 (1999) BindingDB Entry DOI: 10.7270/Q2BZ656D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamyl aminopeptidase (Homo sapiens (Human)) | BDBM50083387 (1-{[1-(1-Carbamoyl-2-carboxy-ethylcarbamoyl)-2-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Binding affinity against recombinant Aminopeptidase A | J Med Chem 42: 5197-211 (2000) BindingDB Entry DOI: 10.7270/Q2028S8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamyl aminopeptidase (Homo sapiens (Human)) | BDBM50083392 (1-{[1-(1,2-Dicarboxy-ethylcarbamoyl)-2-methyl-buty...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Binding affinity against recombinant Aminopeptidase A | J Med Chem 42: 5197-211 (2000) BindingDB Entry DOI: 10.7270/Q2028S8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068908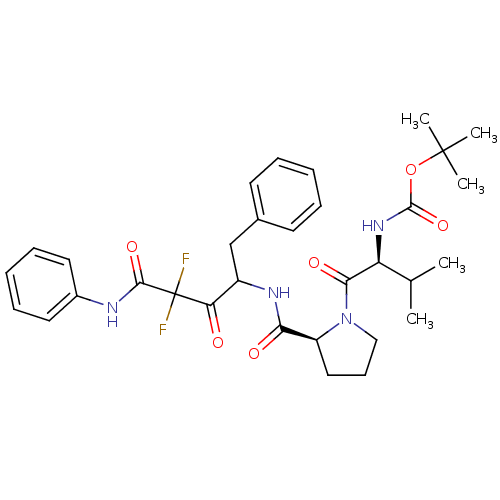 (CHEMBL352917 | {(S)-1-[(S)-2-(1-Benzyl-3,3-difluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamyl aminopeptidase (Homo sapiens (Human)) | BDBM50083400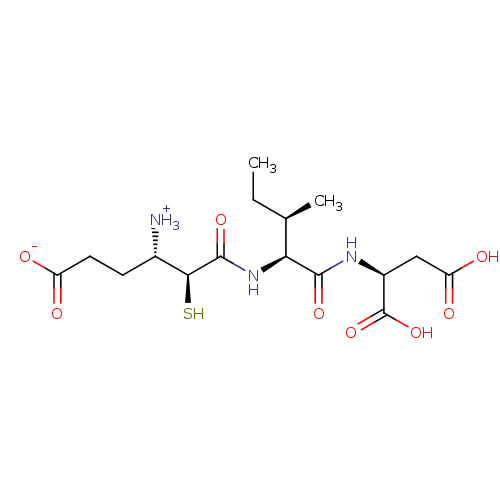 (3-Carboxy-1-{[1-(1,2-dicarboxy-ethylcarbamoyl)-2-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Binding affinity against recombinant Aminopeptidase A | J Med Chem 42: 5197-211 (2000) BindingDB Entry DOI: 10.7270/Q2028S8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068909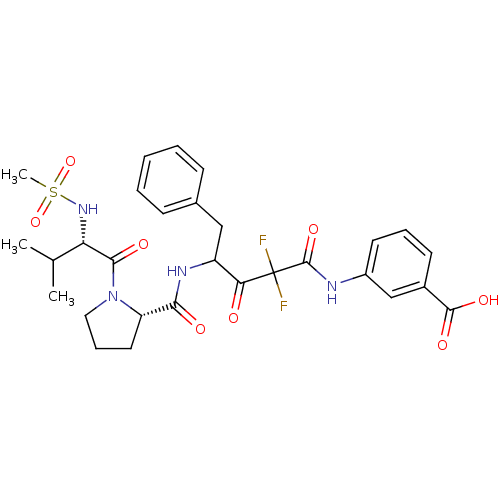 (3-(2,2-Difluoro-4-{[(S)-1-((S)-2-methanesulfonylam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50083378 (1-{[1-(1-Carboxy-2-sulfo-ethylcarbamoyl)-2-methyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Binding affinity against NEP from rabbit kidney(neutral endopeptidase) | J Med Chem 42: 5197-211 (2000) BindingDB Entry DOI: 10.7270/Q2028S8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068897 (CHEMBL171119 | {(S)-1-[(S)-2-(1-Benzyl-3,3-difluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50068901 (3-[2,2-Difluoro-4-({(S)-1-[(S)-3-methyl-2-(3-pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against Chymotrypsinogen | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamyl aminopeptidase (Homo sapiens (Human)) | BDBM50083384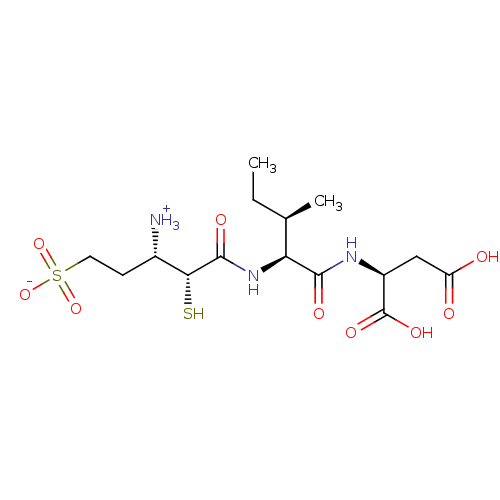 (1-{[1-(1,2-Dicarboxy-ethylcarbamoyl)-2-methyl-buty...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Binding affinity against recombinant Aminopeptidase A | J Med Chem 42: 5197-211 (2000) BindingDB Entry DOI: 10.7270/Q2028S8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068913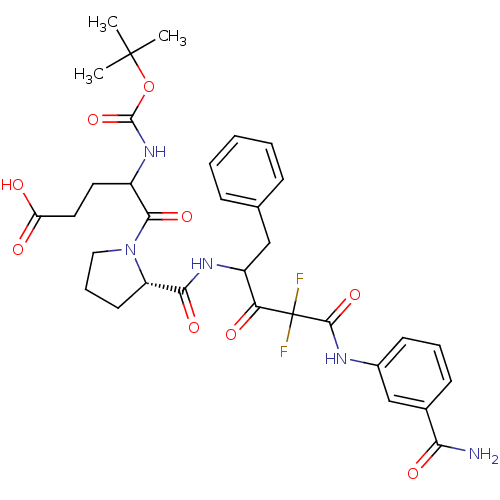 (5-{(S)-2-[1-Benzyl-3-(3-carbamoyl-phenylcarbamoyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 919-24 (1999) BindingDB Entry DOI: 10.7270/Q2BZ656D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50083394 (1-{[1-(1,2-Dicarboxy-ethylcarbamoyl)-2-methyl-buty...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Binding affinity against NEP from rabbit kidney(neutral endopeptidase) | J Med Chem 42: 5197-211 (2000) BindingDB Entry DOI: 10.7270/Q2028S8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068914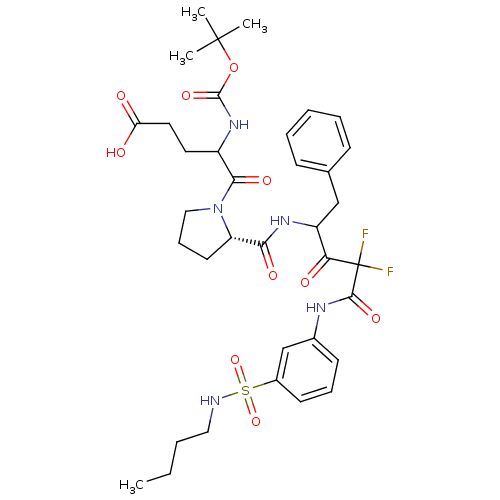 (5-{(S)-2-[1-Benzyl-3-(3-butylsulfamoyl-phenylcarba...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 919-24 (1999) BindingDB Entry DOI: 10.7270/Q2BZ656D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068885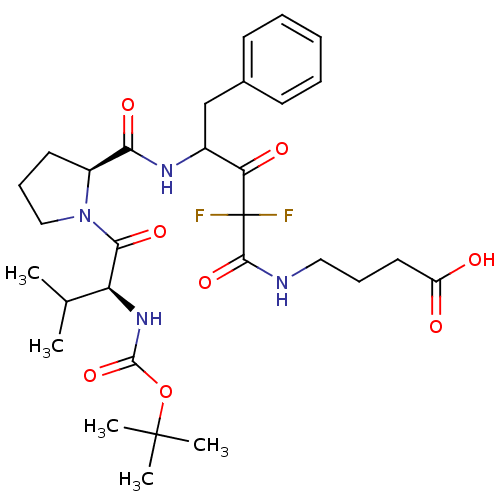 (4-(4-{[(S)-1-((S)-2-tert-Butoxycarbonylamino-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamyl aminopeptidase (Homo sapiens (Human)) | BDBM50083395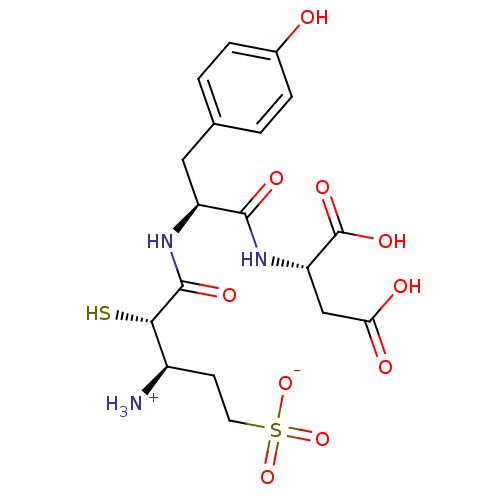 (1-{[1-(1,2-Dicarboxy-ethylcarbamoyl)-2-(4-hydroxy-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Binding affinity against recombinant Aminopeptidase A | J Med Chem 42: 5197-211 (2000) BindingDB Entry DOI: 10.7270/Q2028S8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068920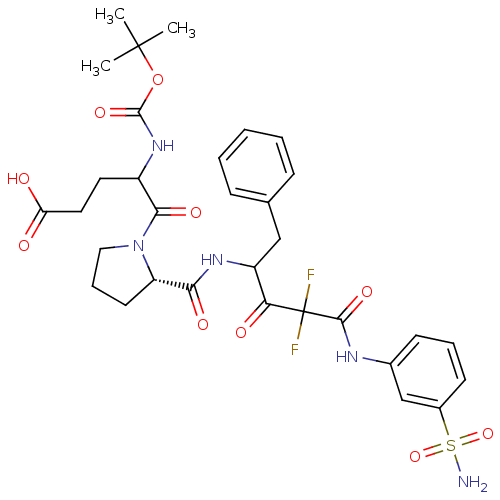 (5-{(S)-2-[1-Benzyl-3,3-difluoro-2-oxo-3-(3-sulfamo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 919-24 (1999) BindingDB Entry DOI: 10.7270/Q2BZ656D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068891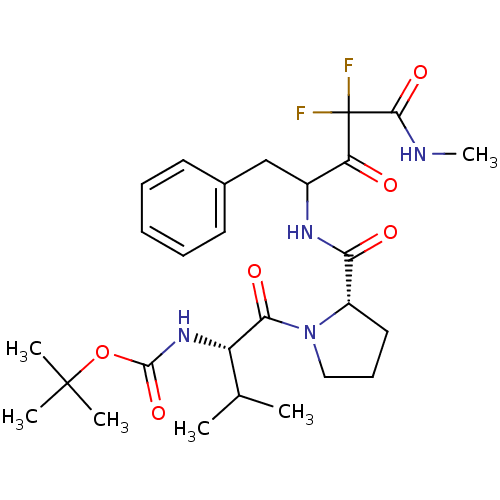 (CHEMBL170683 | {(S)-1-[(S)-2-(1-Benzyl-3,3-difluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068890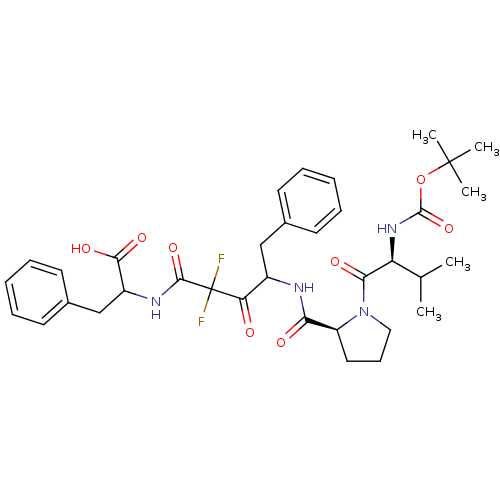 (2-(4-{[(S)-1-((S)-2-tert-Butoxycarbonylamino-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50068918 ((S)-4-((2S,3S)-2-Benzyloxycarbonylamino-3-methyl-p...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against Bovine Chymotrypsinogen | Bioorg Med Chem Lett 8: 919-24 (1999) BindingDB Entry DOI: 10.7270/Q2BZ656D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50083393 (1-{[1-(1,2-Dicarboxy-ethylcarbamoyl)-2-(4-hydroxy-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Binding affinity against NEP from rabbit kidney(neutral endopeptidase) | J Med Chem 42: 5197-211 (2000) BindingDB Entry DOI: 10.7270/Q2028S8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068904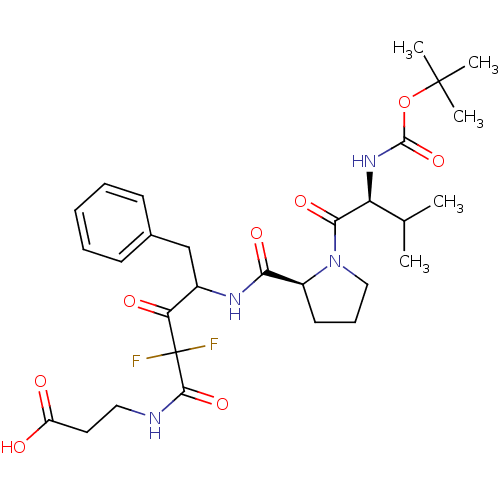 (3-(4-{[(S)-1-((S)-2-tert-Butoxycarbonylamino-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068916 (3-(4-{[(S)-1-(2-tert-Butoxycarbonylamino-3-carboxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 919-24 (1999) BindingDB Entry DOI: 10.7270/Q2BZ656D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamyl aminopeptidase (Homo sapiens (Human)) | BDBM50083398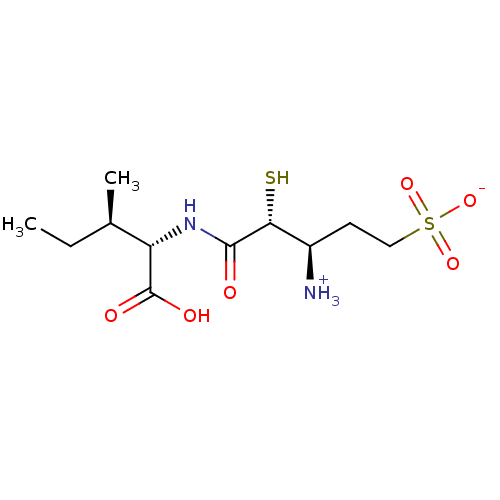 ((3R,4R)-3-ammonio-5-{[(1S)-1-carboxy-2-methylbutyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Binding affinity against recombinant Aminopeptidase A | J Med Chem 42: 5197-211 (2000) BindingDB Entry DOI: 10.7270/Q2028S8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 464 total ) | Next | Last >> |