 Found 1420 hits with Last Name = 'parsons' and Initial = 'w'
Found 1420 hits with Last Name = 'parsons' and Initial = 'w' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50325767
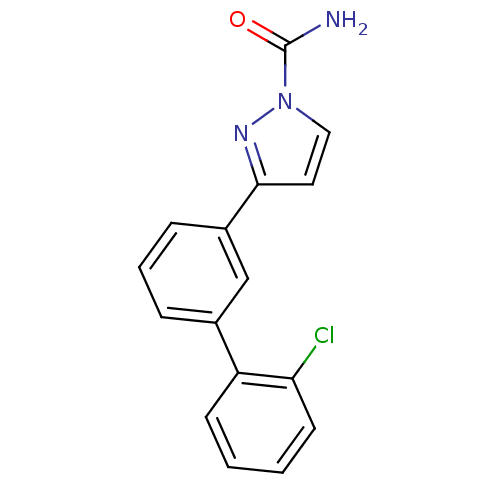
(3-(2'-chlorobiphenyl-3-yl)-1H-pyrazole-1-carboxami...)Show InChI InChI=1S/C16H12ClN3O/c17-14-7-2-1-6-13(14)11-4-3-5-12(10-11)15-8-9-20(19-15)16(18)21/h1-10H,(H2,18,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Nav 1.7 channel by electrophysiology |
Bioorg Med Chem Lett 20: 5480-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.080
BindingDB Entry DOI: 10.7270/Q2PV6KJX |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50325766
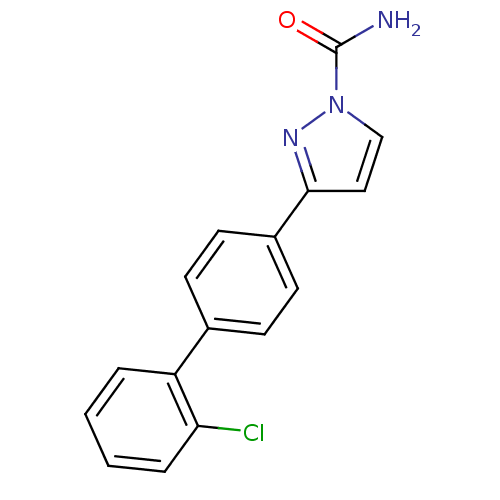
(3-(2'-chlorobiphenyl-4-yl)-1H-pyrazole-1-carboxami...)Show InChI InChI=1S/C16H12ClN3O/c17-14-4-2-1-3-13(14)11-5-7-12(8-6-11)15-9-10-20(19-15)16(18)21/h1-10H,(H2,18,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.111 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Nav 1.7 channel by electrophysiology |
Bioorg Med Chem Lett 20: 5480-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.080
BindingDB Entry DOI: 10.7270/Q2PV6KJX |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50280339

((S)-2-[4-(2-Benzenesulfonyl-ethoxy)-3-methoxy-5-(p...)Show SMILES CCCS(=O)(=O)c1cc(cc(OC)c1OCCS(=O)(=O)c1ccccc1)C1CC[C@H](O1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C31H38O10S2/c1-6-15-43(34,35)29-20-22(19-28(38-4)31(29)40-14-16-42(32,33)23-10-8-7-9-11-23)25-13-12-24(41-25)21-17-26(36-2)30(39-5)27(18-21)37-3/h7-11,17-20,24-25H,6,12-16H2,1-5H3/t24-,25?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of binding of [3H]-C18 PAF to human platelet membrane Platelet activating factor receptor |
Bioorg Med Chem Lett 2: 181-184 (1992)
Article DOI: 10.1016/S0960-894X(01)80446-7
BindingDB Entry DOI: 10.7270/Q24X588Z |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50002827

(1-{3-(3-Hydroxy-propoxy)-2-propoxy-5-[(2S,5S)-5-(3...)Show SMILES CCCOc1c(OCCCO)cc(cc1S(=O)(=O)CC(C)=O)[C@@H]1CC[C@H](O1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C28H38O10S/c1-6-11-37-28-25(36-12-7-10-29)15-20(16-26(28)39(31,32)17-18(2)30)22-9-8-21(38-22)19-13-23(33-3)27(35-5)24(14-19)34-4/h13-16,21-22,29H,6-12,17H2,1-5H3/t21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of the binding of [3H]C18-Platelet activating factor to human platelet membrane preparation |
J Med Chem 35: 3474-82 (1992)
BindingDB Entry DOI: 10.7270/Q2445N3B |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50002828
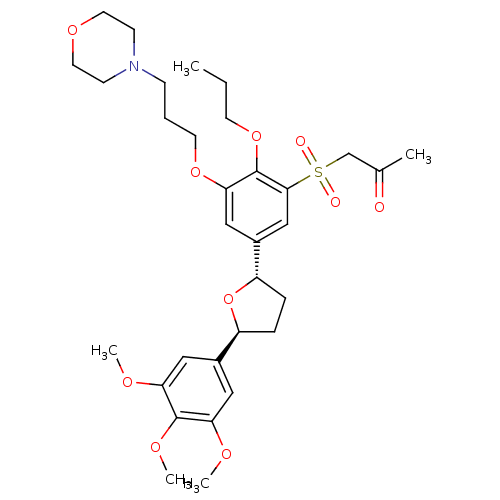
(1-{3-(3-Morpholin-4-yl-propoxy)-2-propoxy-5-[(2S,5...)Show SMILES CCCOc1c(OCCCN2CCOCC2)cc(cc1S(=O)(=O)CC(C)=O)[C@@H]1CC[C@H](O1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C32H45NO10S/c1-6-13-42-32-29(41-14-7-10-33-11-15-40-16-12-33)19-24(20-30(32)44(35,36)21-22(2)34)26-9-8-25(43-26)23-17-27(37-3)31(39-5)28(18-23)38-4/h17-20,25-26H,6-16,21H2,1-5H3/t25-,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of the binding of [3H]C18-Platelet activating factor to human platelet membrane preparation |
J Med Chem 35: 3474-82 (1992)
BindingDB Entry DOI: 10.7270/Q2445N3B |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50002824
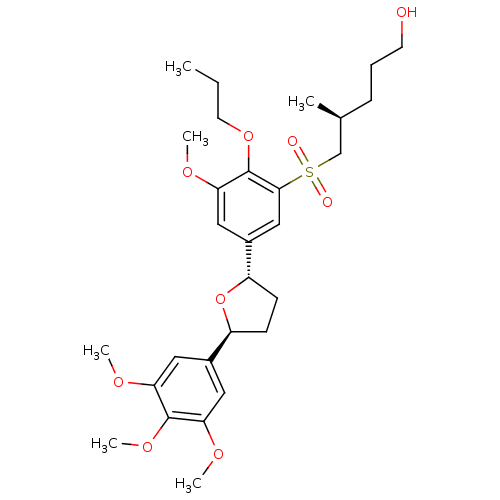
((S)-5-{3-Methoxy-2-propoxy-5-[(2S,5S)-5-(3,4,5-tri...)Show SMILES CCCOc1c(OC)cc(cc1S(=O)(=O)C[C@@H](C)CCCO)[C@@H]1CC[C@H](O1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C29H42O9S/c1-7-13-37-29-26(35-5)16-21(17-27(29)39(31,32)18-19(2)9-8-12-30)23-11-10-22(38-23)20-14-24(33-3)28(36-6)25(15-20)34-4/h14-17,19,22-23,30H,7-13,18H2,1-6H3/t19-,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of the binding of [3H]C18-Platelet activating factor to human platelet membrane preparation |
J Med Chem 35: 3474-82 (1992)
BindingDB Entry DOI: 10.7270/Q2445N3B |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50002827

(1-{3-(3-Hydroxy-propoxy)-2-propoxy-5-[(2S,5S)-5-(3...)Show SMILES CCCOc1c(OCCCO)cc(cc1S(=O)(=O)CC(C)=O)[C@@H]1CC[C@H](O1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C28H38O10S/c1-6-11-37-28-25(36-12-7-10-29)15-20(16-26(28)39(31,32)17-18(2)30)22-9-8-21(38-22)19-13-23(33-3)27(35-5)24(14-19)34-4/h13-16,21-22,29H,6-12,17H2,1-5H3/t21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro effect on inhibition of the binding of [3H]C18-Platelet activating factor to human PMN membranes preparation |
J Med Chem 35: 3474-82 (1992)
BindingDB Entry DOI: 10.7270/Q2445N3B |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50002826
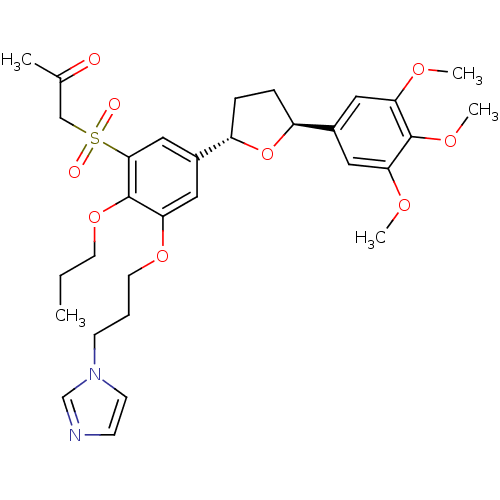
(1-{3-(3-Imidazol-1-yl-propoxy)-2-propoxy-5-[(2S,5S...)Show SMILES CCCOc1c(OCCCn2ccnc2)cc(cc1S(=O)(=O)CC(C)=O)[C@@H]1CC[C@H](O1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C31H40N2O9S/c1-6-13-41-31-28(40-14-7-11-33-12-10-32-20-33)17-23(18-29(31)43(35,36)19-21(2)34)25-9-8-24(42-25)22-15-26(37-3)30(39-5)27(16-22)38-4/h10,12,15-18,20,24-25H,6-9,11,13-14,19H2,1-5H3/t24-,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of the binding of [3H]C18-Platelet activating factor to human platelet membrane preparation |
J Med Chem 35: 3474-82 (1992)
BindingDB Entry DOI: 10.7270/Q2445N3B |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50002823
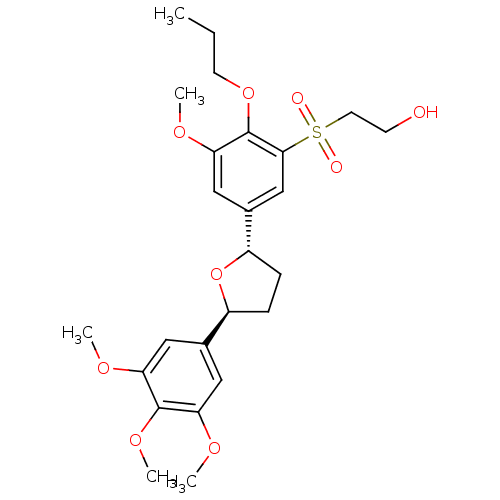
(2-{3-Methoxy-2-propoxy-5-[(2S,5S)-5-(3,4,5-trimeth...)Show SMILES CCCOc1c(OC)cc(cc1S(=O)(=O)CCO)[C@@H]1CC[C@H](O1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C25H34O9S/c1-6-10-33-25-22(31-4)14-17(15-23(25)35(27,28)11-9-26)19-8-7-18(34-19)16-12-20(29-2)24(32-5)21(13-16)30-3/h12-15,18-19,26H,6-11H2,1-5H3/t18-,19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro effect on inhibition of the binding of [3H]C18-Platelet activating factor to human PMN membranes preparation |
J Med Chem 35: 3474-82 (1992)
BindingDB Entry DOI: 10.7270/Q2445N3B |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50002824

((S)-5-{3-Methoxy-2-propoxy-5-[(2S,5S)-5-(3,4,5-tri...)Show SMILES CCCOc1c(OC)cc(cc1S(=O)(=O)C[C@@H](C)CCCO)[C@@H]1CC[C@H](O1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C29H42O9S/c1-7-13-37-29-26(35-5)16-21(17-27(29)39(31,32)18-19(2)9-8-12-30)23-11-10-22(38-23)20-14-24(33-3)28(36-6)25(15-20)34-4/h14-17,19,22-23,30H,7-13,18H2,1-6H3/t19-,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro effect on inhibition of the binding of [3H]C18-Platelet activating factor to human PMN membranes preparation |
J Med Chem 35: 3474-82 (1992)
BindingDB Entry DOI: 10.7270/Q2445N3B |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50002825

((R)-5-{3-Methoxy-2-propoxy-5-[(2S,5S)-5-(3,4,5-tri...)Show SMILES CCCOc1c(OC)cc(cc1S(=O)(=O)C[C@H](C)CCCO)[C@@H]1CC[C@H](O1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C29H42O9S/c1-7-13-37-29-26(35-5)16-21(17-27(29)39(31,32)18-19(2)9-8-12-30)23-11-10-22(38-23)20-14-24(33-3)28(36-6)25(15-20)34-4/h14-17,19,22-23,30H,7-13,18H2,1-6H3/t19-,22+,23+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of the binding of [3H]C18-Platelet activating factor to human platelet membrane preparation |
J Med Chem 35: 3474-82 (1992)
BindingDB Entry DOI: 10.7270/Q2445N3B |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50002825

((R)-5-{3-Methoxy-2-propoxy-5-[(2S,5S)-5-(3,4,5-tri...)Show SMILES CCCOc1c(OC)cc(cc1S(=O)(=O)C[C@H](C)CCCO)[C@@H]1CC[C@H](O1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C29H42O9S/c1-7-13-37-29-26(35-5)16-21(17-27(29)39(31,32)18-19(2)9-8-12-30)23-11-10-22(38-23)20-14-24(33-3)28(36-6)25(15-20)34-4/h14-17,19,22-23,30H,7-13,18H2,1-6H3/t19-,22+,23+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of the binding of [3H]C18-Platelet activating factor to human platelet membrane preparation |
J Med Chem 35: 3474-82 (1992)
BindingDB Entry DOI: 10.7270/Q2445N3B |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50002823

(2-{3-Methoxy-2-propoxy-5-[(2S,5S)-5-(3,4,5-trimeth...)Show SMILES CCCOc1c(OC)cc(cc1S(=O)(=O)CCO)[C@@H]1CC[C@H](O1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C25H34O9S/c1-6-10-33-25-22(31-4)14-17(15-23(25)35(27,28)11-9-26)19-8-7-18(34-19)16-12-20(29-2)24(32-5)21(13-16)30-3/h12-15,18-19,26H,6-11H2,1-5H3/t18-,19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of [3H]-C18 PAF binding to human platelet membrane Platelet activating factor receptor was determined |
Bioorg Med Chem Lett 2: 181-184 (1992)
Article DOI: 10.1016/S0960-894X(01)80446-7
BindingDB Entry DOI: 10.7270/Q24X588Z |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50002823

(2-{3-Methoxy-2-propoxy-5-[(2S,5S)-5-(3,4,5-trimeth...)Show SMILES CCCOc1c(OC)cc(cc1S(=O)(=O)CCO)[C@@H]1CC[C@H](O1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C25H34O9S/c1-6-10-33-25-22(31-4)14-17(15-23(25)35(27,28)11-9-26)19-8-7-18(34-19)16-12-20(29-2)24(32-5)21(13-16)30-3/h12-15,18-19,26H,6-11H2,1-5H3/t18-,19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of the binding of [3H]C18-Platelet activating factor to human platelet membrane preparation |
J Med Chem 35: 3474-82 (1992)
BindingDB Entry DOI: 10.7270/Q2445N3B |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50086717

(JNJ-39439335 | Mavatrep)Show SMILES CC(C)(O)c1ccccc1-c1ccc2[nH]c(\C=C\c3ccc(cc3)C(F)(F)F)nc2c1 Show InChI InChI=1S/C25H21F3N2O/c1-24(2,31)20-6-4-3-5-19(20)17-10-13-21-22(15-17)30-23(29-21)14-9-16-7-11-18(12-8-16)25(26,27)28/h3-15,31H,1-2H3,(H,29,30)/b14-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-RTX from human TRPV1 transfected in HEK293 cells after 60 mins by scintillation spectroscopic analysis |
J Med Chem 58: 3859-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00132
BindingDB Entry DOI: 10.7270/Q2154JS3 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM81972
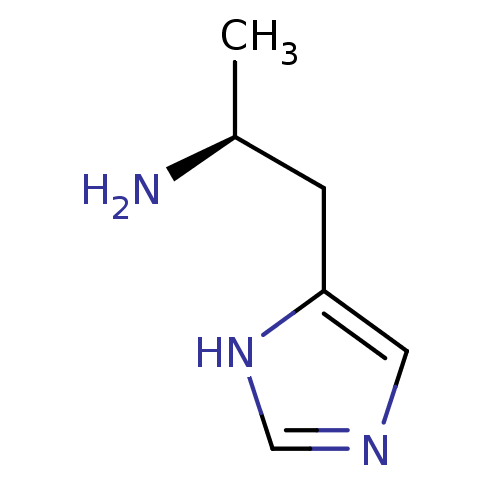
((S)-(+)-ALPHA-METHYL-1H-IMIDAZOLE-4-ETHANAMINE DIH...)Show InChI InChI=1S/C6H11N3/c1-5(7)2-6-3-8-4-9-6/h3-5H,2,7H2,1H3,(H,8,9)/t5-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of the binding of [3H]C18-Platelet activating factor to human platelet membrane preparation |
J Med Chem 35: 3474-82 (1992)
BindingDB Entry DOI: 10.7270/Q2445N3B |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50325765
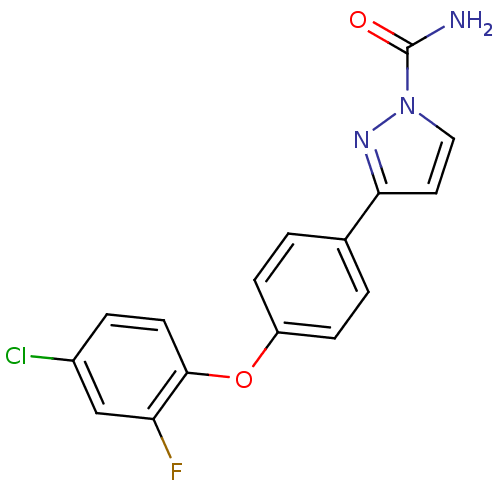
(3-(4-(4-chloro-2-fluorophenoxy)phenyl)-1H-pyrazole...)Show InChI InChI=1S/C16H11ClFN3O2/c17-11-3-6-15(13(18)9-11)23-12-4-1-10(2-5-12)14-7-8-21(20-14)16(19)22/h1-9H,(H2,19,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Nav 1.7 channel by electrophysiology |
Bioorg Med Chem Lett 20: 5480-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.080
BindingDB Entry DOI: 10.7270/Q2PV6KJX |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM81972

((S)-(+)-ALPHA-METHYL-1H-IMIDAZOLE-4-ETHANAMINE DIH...)Show InChI InChI=1S/C6H11N3/c1-5(7)2-6-3-8-4-9-6/h3-5H,2,7H2,1H3,(H,8,9)/t5-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
| 14.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of [3H]-C18 PAF binding to human platelet membrane Platelet activating factor receptor was determined |
Bioorg Med Chem Lett 2: 181-184 (1992)
Article DOI: 10.1016/S0960-894X(01)80446-7
BindingDB Entry DOI: 10.7270/Q24X588Z |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50325764
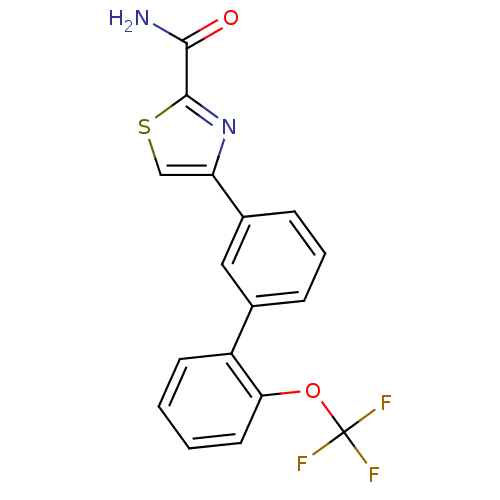
(4-(2'-(trifluoromethoxy)biphenyl-3-yl)thiazole-2-c...)Show SMILES NC(=O)c1nc(cs1)-c1cccc(c1)-c1ccccc1OC(F)(F)F Show InChI InChI=1S/C17H11F3N2O2S/c18-17(19,20)24-14-7-2-1-6-12(14)10-4-3-5-11(8-10)13-9-25-16(22-13)15(21)23/h1-9H,(H2,21,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Nav 1.7 channel by electrophysiology |
Bioorg Med Chem Lett 20: 5480-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.080
BindingDB Entry DOI: 10.7270/Q2PV6KJX |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50325819

((Z)-5-((2'-(trifluoromethoxy)biphenyl-3-yl)methyle...)Show SMILES OC1=NC(=O)C(S1)=Cc1cccc(c1)-c1ccccc1OC(F)(F)F |w:7.8,t:1| Show InChI InChI=1S/C17H10F3NO3S/c18-17(19,20)24-13-7-2-1-6-12(13)11-5-3-4-10(8-11)9-14-15(22)21-16(23)25-14/h1-9H,(H,21,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human Nav1.7 |
Bioorg Med Chem Lett 20: 5536-40 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.064
BindingDB Entry DOI: 10.7270/Q2FB535G |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50187039

(1-(3,4-dichlorobenzyl)-5-hydroxy-1H-indole-2-carbo...)Show InChI InChI=1S/C16H11Cl2NO3/c17-12-3-1-9(5-13(12)18)8-19-14-4-2-11(20)6-10(14)7-15(19)16(21)22/h1-7,20H,8H2,(H,21,22) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCP1 from human CCR2 expressed in CHO cells |
Bioorg Med Chem Lett 16: 3735-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.045
BindingDB Entry DOI: 10.7270/Q2N01645 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50164039

((1S,2S)-Cyclopentane-1,2-dicarboxylic acid 1-[(bip...)Show SMILES NS(=O)(=O)c1ccccc1-c1ccc(CNC(=O)[C@H]2CCC[C@@H]2C(=O)NCc2ccc(cc2)-c2ccccc2)cc1 Show InChI InChI=1S/C33H33N3O4S/c34-41(39,40)31-12-5-4-9-28(31)27-19-15-24(16-20-27)22-36-33(38)30-11-6-10-29(30)32(37)35-21-23-13-17-26(18-14-23)25-7-2-1-3-8-25/h1-5,7-9,12-20,29-30H,6,10-11,21-22H2,(H,35,37)(H,36,38)(H2,34,39,40)/t29-,30-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against voltage-gated sodium channel Nav1.7 in electrophysiology assays (EP) |
Bioorg Med Chem Lett 15: 1901-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.002
BindingDB Entry DOI: 10.7270/Q2HH6JMD |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50216671
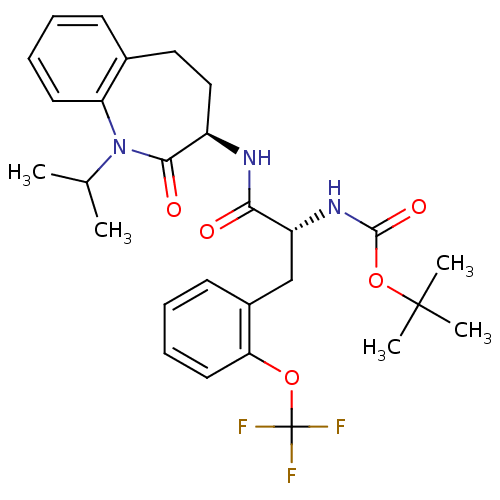
(CHEMBL247828 | tert-butyl (R)-1-((R)-1-isopropyl-2...)Show SMILES CC(C)N1c2ccccc2CC[C@@H](NC(=O)[C@@H](Cc2ccccc2OC(F)(F)F)NC(=O)OC(C)(C)C)C1=O Show InChI InChI=1S/C28H34F3N3O5/c1-17(2)34-22-12-8-6-10-18(22)14-15-20(25(34)36)32-24(35)21(33-26(37)39-27(3,4)5)16-19-11-7-9-13-23(19)38-28(29,30)31/h6-13,17,20-21H,14-16H2,1-5H3,(H,32,35)(H,33,37)/t20-,21-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity at human inactive state Nav1.7 by whole cell electrophysiology |
Bioorg Med Chem Lett 17: 4630-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.076
BindingDB Entry DOI: 10.7270/Q2HX1CCF |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50091438

((E)-3-(3,4-Dichloro-phenyl)-N-{5-[4-(5-hydroxy-1H-...)Show SMILES Oc1ccc2[nH]cc(C3CCN(CCCCCNC(=O)\C=C\c4ccc(Cl)c(Cl)c4)CC3)c2c1 Show InChI InChI=1S/C27H31Cl2N3O2/c28-24-7-4-19(16-25(24)29)5-9-27(34)30-12-2-1-3-13-32-14-10-20(11-15-32)23-18-31-26-8-6-21(33)17-22(23)26/h4-9,16-18,20,31,33H,1-3,10-15H2,(H,30,34)/b9-5+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCP1 from human CCR2 expressed in CHO cells |
Bioorg Med Chem Lett 16: 3735-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.045
BindingDB Entry DOI: 10.7270/Q2N01645 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50164048
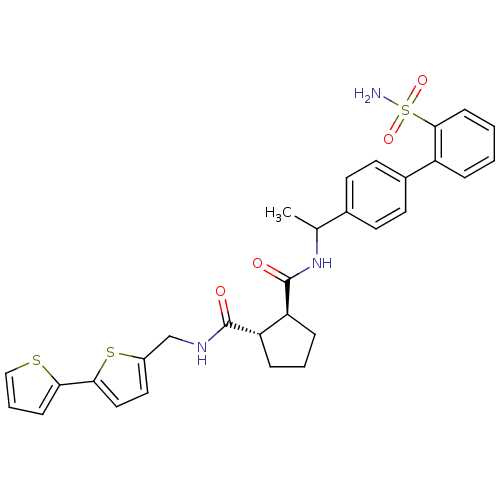
((1S,2S)-Cyclopentane-1,2-dicarboxylic acid 1-[([2,...)Show SMILES CC(NC(=O)[C@H]1CCC[C@@H]1C(=O)NCc1ccc(s1)-c1cccs1)c1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C30H31N3O4S3/c1-19(20-11-13-21(14-12-20)23-6-2-3-10-28(23)40(31,36)37)33-30(35)25-8-4-7-24(25)29(34)32-18-22-15-16-27(39-22)26-9-5-17-38-26/h2-3,5-6,9-17,19,24-25H,4,7-8,18H2,1H3,(H,32,34)(H,33,35)(H2,31,36,37)/t19?,24-,25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against voltage-gated sodium channel Nav1.7 in electrophysiology assays (EP) |
Bioorg Med Chem Lett 15: 1901-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.002
BindingDB Entry DOI: 10.7270/Q2HH6JMD |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50164043
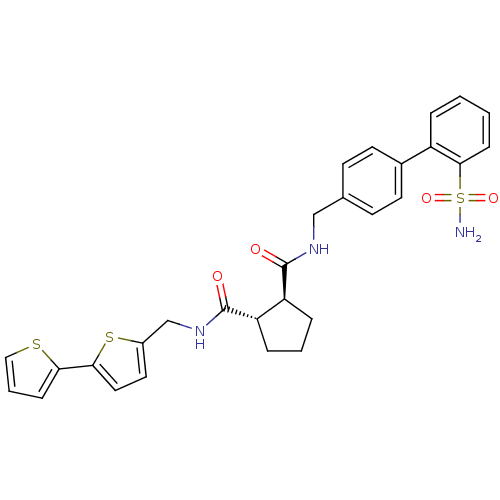
((1S,2S)-Cyclopentane-1,2-dicarboxylic acid 1-[([2,...)Show SMILES NS(=O)(=O)c1ccccc1-c1ccc(CNC(=O)[C@H]2CCC[C@@H]2C(=O)NCc2ccc(s2)-c2cccs2)cc1 Show InChI InChI=1S/C29H29N3O4S3/c30-39(35,36)27-9-2-1-5-22(27)20-12-10-19(11-13-20)17-31-28(33)23-6-3-7-24(23)29(34)32-18-21-14-15-26(38-21)25-8-4-16-37-25/h1-2,4-5,8-16,23-24H,3,6-7,17-18H2,(H,31,33)(H,32,34)(H2,30,35,36)/t23-,24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against voltage-gated sodium channel Nav1.7 in electrophysiology assays (EP) |
Bioorg Med Chem Lett 15: 1901-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.002
BindingDB Entry DOI: 10.7270/Q2HH6JMD |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50366241
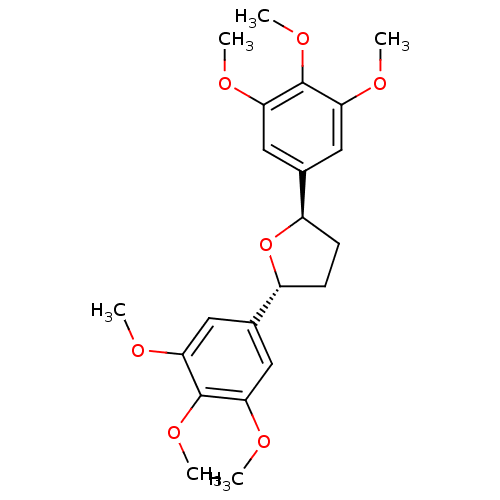
(CHEMBL297624 | L-652731)Show SMILES COc1cc(cc(OC)c1OC)[C@H]1CC[C@@H](O1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C22H28O7/c1-23-17-9-13(10-18(24-2)21(17)27-5)15-7-8-16(29-15)14-11-19(25-3)22(28-6)20(12-14)26-4/h9-12,15-16H,7-8H2,1-6H3/t15-,16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of the binding of [3H]C18-Platelet activating factor to human platelet membrane preparation |
J Med Chem 35: 3474-82 (1992)
BindingDB Entry DOI: 10.7270/Q2445N3B |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50164070
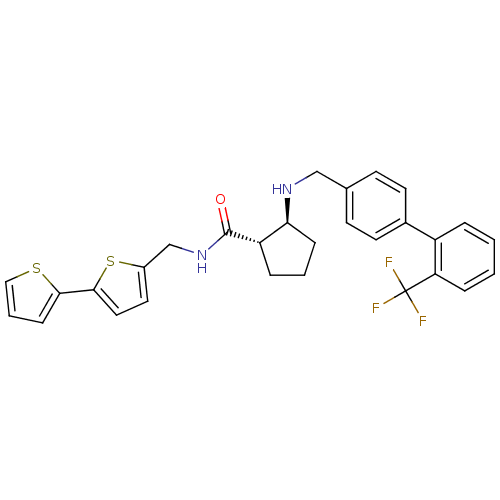
((1S,2S)-2-[(2''-Trifluoromethyl-biphenyl-4-ylmethy...)Show SMILES FC(F)(F)c1ccccc1-c1ccc(CN[C@H]2CCC[C@@H]2C(=O)NCc2ccc(s2)-c2cccs2)cc1 Show InChI InChI=1S/C29H27F3N2OS2/c30-29(31,32)24-7-2-1-5-22(24)20-12-10-19(11-13-20)17-33-25-8-3-6-23(25)28(35)34-18-21-14-15-27(37-21)26-9-4-16-36-26/h1-2,4-5,7,9-16,23,25,33H,3,6,8,17-18H2,(H,34,35)/t23-,25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against voltage-gated sodium channel Nav1.7 in electrophysiology assays (EP) |
Bioorg Med Chem Lett 15: 1901-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.002
BindingDB Entry DOI: 10.7270/Q2HH6JMD |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50002822

((-)-trans 42s,5S)-2-[3-[(2-Oxopropyl)sulfony1]-4-n...)Show SMILES CCCOc1c(OCCCOP(O)([O-])=O)cc(cc1S(=O)(=O)CC(C)=O)[C@@H]1CC[C@H](O1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C28H39O13PS/c1-6-10-39-28-25(38-11-7-12-40-42(30,31)32)15-20(16-26(28)43(33,34)17-18(2)29)22-9-8-21(41-22)19-13-23(35-3)27(37-5)24(14-19)36-4/h13-16,21-22H,6-12,17H2,1-5H3,(H2,30,31,32)/p-1/t21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of the binding of [3H]C18-Platelet activating factor to human platelet membrane preparation |
J Med Chem 35: 3474-82 (1992)
BindingDB Entry DOI: 10.7270/Q2445N3B |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50164042
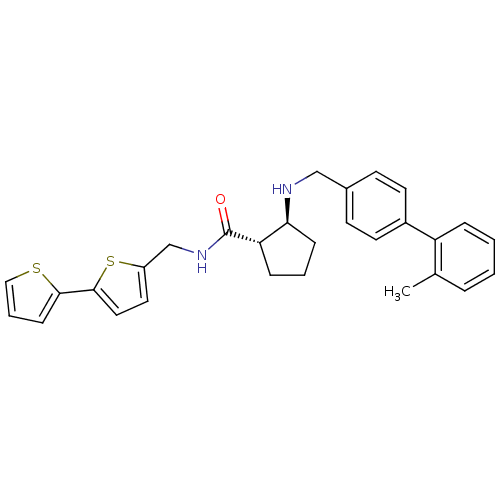
((1S,2S)-2-[(2''-Methyl-biphenyl-4-ylmethyl)-amino]...)Show SMILES Cc1ccccc1-c1ccc(CN[C@H]2CCC[C@@H]2C(=O)NCc2ccc(s2)-c2cccs2)cc1 Show InChI InChI=1S/C29H30N2OS2/c1-20-6-2-3-7-24(20)22-13-11-21(12-14-22)18-30-26-9-4-8-25(26)29(32)31-19-23-15-16-28(34-23)27-10-5-17-33-27/h2-3,5-7,10-17,25-26,30H,4,8-9,18-19H2,1H3,(H,31,32)/t25-,26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against voltage-gated sodium channel Nav1.7 in electrophysiology assays (EP) |
Bioorg Med Chem Lett 15: 1901-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.002
BindingDB Entry DOI: 10.7270/Q2HH6JMD |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50164067
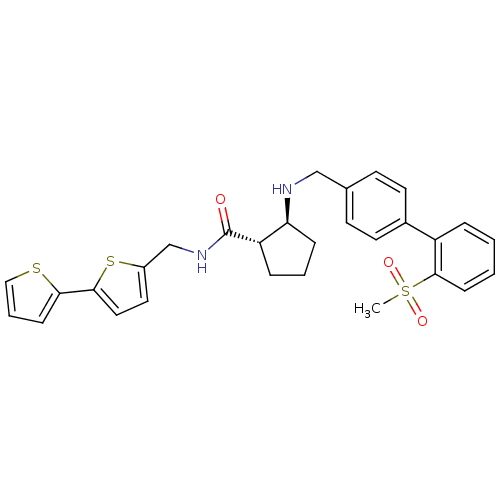
((1S,2S)-2-[(2''-Methanesulfonyl-biphenyl-4-ylmethy...)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(CN[C@H]2CCC[C@@H]2C(=O)NCc2ccc(s2)-c2cccs2)cc1 Show InChI InChI=1S/C29H30N2O3S3/c1-37(33,34)28-10-3-2-6-23(28)21-13-11-20(12-14-21)18-30-25-8-4-7-24(25)29(32)31-19-22-15-16-27(36-22)26-9-5-17-35-26/h2-3,5-6,9-17,24-25,30H,4,7-8,18-19H2,1H3,(H,31,32)/t24-,25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against voltage-gated sodium channel Nav1.7 in electrophysiology assays (EP) |
Bioorg Med Chem Lett 15: 1901-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.002
BindingDB Entry DOI: 10.7270/Q2HH6JMD |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50164058

(CHEMBL360307 | N-[2,2'']Bithiophenyl-5-ylmethyl-N'...)Show SMILES NS(=O)(=O)c1ccccc1-c1ccc(CNC(=O)c2ccccc2C(=O)NCc2ccc(s2)-c2cccs2)cc1 Show InChI InChI=1S/C30H25N3O4S3/c31-40(36,37)28-10-4-3-6-23(28)21-13-11-20(12-14-21)18-32-29(34)24-7-1-2-8-25(24)30(35)33-19-22-15-16-27(39-22)26-9-5-17-38-26/h1-17H,18-19H2,(H,32,34)(H,33,35)(H2,31,36,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against voltage-gated sodium channel Nav1.7 in electrophysiology assays (EP) |
Bioorg Med Chem Lett 15: 1901-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.002
BindingDB Entry DOI: 10.7270/Q2HH6JMD |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50166790
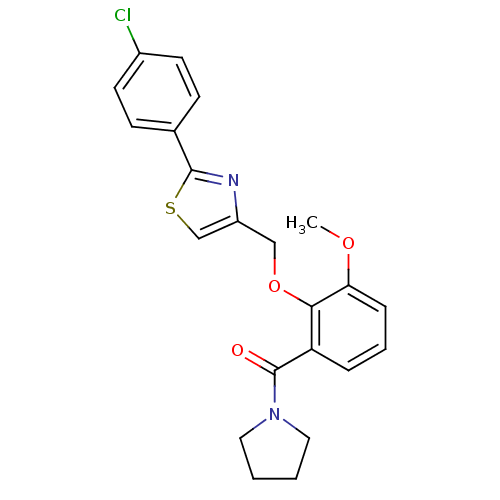
(CHEMBL364912 | {2-[2-(4-Chloro-phenyl)-thiazol-4-y...)Show SMILES COc1cccc(C(=O)N2CCCC2)c1OCc1csc(n1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C22H21ClN2O3S/c1-27-19-6-4-5-18(22(26)25-11-2-3-12-25)20(19)28-13-17-14-29-21(24-17)15-7-9-16(23)10-8-15/h4-10,14H,2-3,11-13H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against Na v1.7 channel electrophysiology in inactivated state expressed in HEK293 cells using Voltage/Ion Probe Re... |
Bioorg Med Chem Lett 15: 2943-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.093
BindingDB Entry DOI: 10.7270/Q2X929TB |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50164047
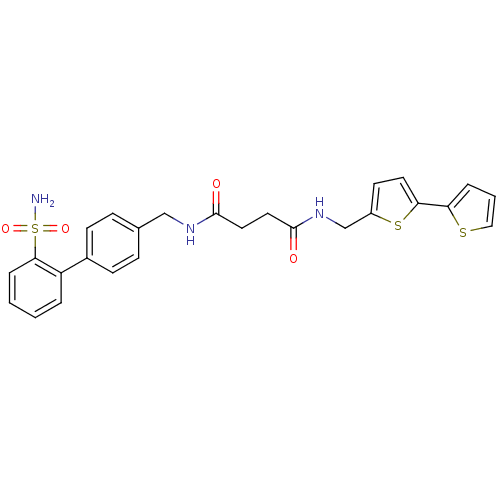
(CHEMBL361097 | N-[2,2'']Bithiophenyl-5-ylmethyl-N'...)Show SMILES NS(=O)(=O)c1ccccc1-c1ccc(CNC(=O)CCC(=O)NCc2ccc(s2)-c2cccs2)cc1 Show InChI InChI=1S/C26H25N3O4S3/c27-36(32,33)24-6-2-1-4-21(24)19-9-7-18(8-10-19)16-28-25(30)13-14-26(31)29-17-20-11-12-23(35-20)22-5-3-15-34-22/h1-12,15H,13-14,16-17H2,(H,28,30)(H,29,31)(H2,27,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of peripheral nerve sodium channel NaV1.7 |
Bioorg Med Chem Lett 16: 1358-61 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.051
BindingDB Entry DOI: 10.7270/Q2FX791S |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50164047

(CHEMBL361097 | N-[2,2'']Bithiophenyl-5-ylmethyl-N'...)Show SMILES NS(=O)(=O)c1ccccc1-c1ccc(CNC(=O)CCC(=O)NCc2ccc(s2)-c2cccs2)cc1 Show InChI InChI=1S/C26H25N3O4S3/c27-36(32,33)24-6-2-1-4-21(24)19-9-7-18(8-10-19)16-28-25(30)13-14-26(31)29-17-20-11-12-23(35-20)22-5-3-15-34-22/h1-12,15H,13-14,16-17H2,(H,28,30)(H,29,31)(H2,27,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against voltage-gated sodium channel Nav1.7 in electrophysiology assays (EP) |
Bioorg Med Chem Lett 15: 1901-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.002
BindingDB Entry DOI: 10.7270/Q2HH6JMD |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50164051
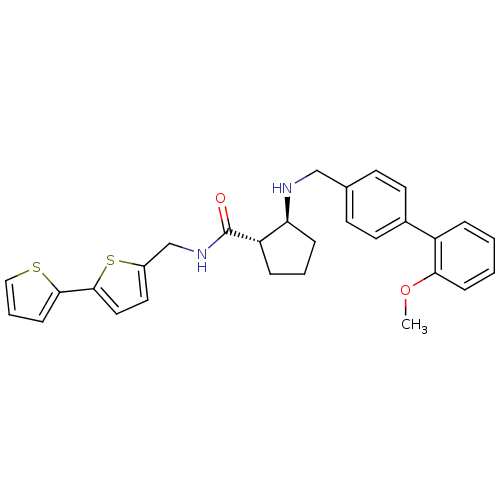
((1S,2S)-2-[(2''-Methoxy-biphenyl-4-ylmethyl)-amino...)Show SMILES COc1ccccc1-c1ccc(CN[C@H]2CCC[C@@H]2C(=O)NCc2ccc(s2)-c2cccs2)cc1 Show InChI InChI=1S/C29H30N2O2S2/c1-33-26-9-3-2-6-23(26)21-13-11-20(12-14-21)18-30-25-8-4-7-24(25)29(32)31-19-22-15-16-28(35-22)27-10-5-17-34-27/h2-3,5-6,9-17,24-25,30H,4,7-8,18-19H2,1H3,(H,31,32)/t24-,25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against voltage-gated sodium channel Nav1.7 in electrophysiology assays (EP) |
Bioorg Med Chem Lett 15: 1901-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.002
BindingDB Entry DOI: 10.7270/Q2HH6JMD |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50166787
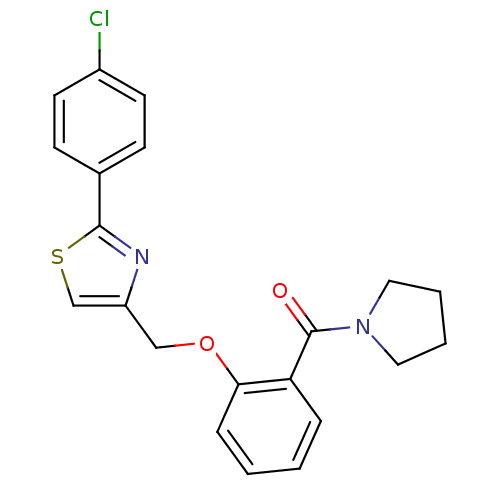
(CHEMBL195235 | {2-[2-(4-Chloro-phenyl)-thiazol-4-y...)Show SMILES Clc1ccc(cc1)-c1nc(COc2ccccc2C(=O)N2CCCC2)cs1 Show InChI InChI=1S/C21H19ClN2O2S/c22-16-9-7-15(8-10-16)20-23-17(14-27-20)13-26-19-6-2-1-5-18(19)21(25)24-11-3-4-12-24/h1-2,5-10,14H,3-4,11-13H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against Na v1.7 channel electrophysiology in inactivated state expressed in HEK293 cells using Voltage/Ion Probe Re... |
Bioorg Med Chem Lett 15: 2943-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.093
BindingDB Entry DOI: 10.7270/Q2X929TB |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50002822

((-)-trans 42s,5S)-2-[3-[(2-Oxopropyl)sulfony1]-4-n...)Show SMILES CCCOc1c(OCCCOP(O)([O-])=O)cc(cc1S(=O)(=O)CC(C)=O)[C@@H]1CC[C@H](O1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C28H39O13PS/c1-6-10-39-28-25(38-11-7-12-40-42(30,31)32)15-20(16-26(28)43(33,34)17-18(2)29)22-9-8-21(41-22)19-13-23(35-3)27(37-5)24(14-19)36-4/h13-16,21-22H,6-12,17H2,1-5H3,(H2,30,31,32)/p-1/t21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 174 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro effect on inhibition of the binding of [3H]C18-Platelet activating factor to human PMN membranes preparation |
J Med Chem 35: 3474-82 (1992)
BindingDB Entry DOI: 10.7270/Q2445N3B |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50164040
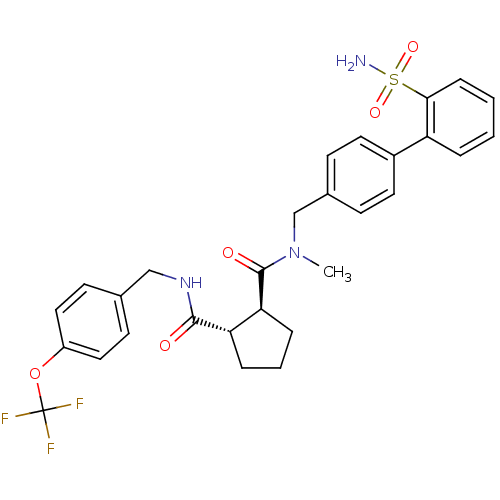
((1S,2S)-Cyclopentane-1,2-dicarboxylic acid 1-[meth...)Show SMILES CN(Cc1ccc(cc1)-c1ccccc1S(N)(=O)=O)C(=O)[C@H]1CCC[C@@H]1C(=O)NCc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C29H30F3N3O5S/c1-35(18-20-9-13-21(14-10-20)23-5-2-3-8-26(23)41(33,38)39)28(37)25-7-4-6-24(25)27(36)34-17-19-11-15-22(16-12-19)40-29(30,31)32/h2-3,5,8-16,24-25H,4,6-7,17-18H2,1H3,(H,34,36)(H2,33,38,39)/t24-,25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against voltage-gated sodium channel Nav1.7 in electrophysiology assays (EP) |
Bioorg Med Chem Lett 15: 1901-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.002
BindingDB Entry DOI: 10.7270/Q2HH6JMD |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50325764

(4-(2'-(trifluoromethoxy)biphenyl-3-yl)thiazole-2-c...)Show SMILES NC(=O)c1nc(cs1)-c1cccc(c1)-c1ccccc1OC(F)(F)F Show InChI InChI=1S/C17H11F3N2O2S/c18-17(19,20)24-14-7-2-1-6-12(14)10-4-3-5-11(8-10)13-9-25-16(22-13)15(21)23/h1-9H,(H2,21,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 by VIPR assay |
Bioorg Med Chem Lett 20: 7479-82 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.017
BindingDB Entry DOI: 10.7270/Q2MW2HDH |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50178865
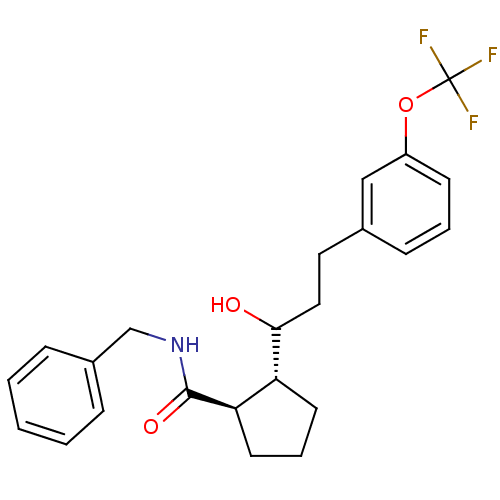
(CHEMBL203440 | trans-N-benzyl-2-(1-hydroxy-3-(3-(t...)Show SMILES OC(CCc1cccc(OC(F)(F)F)c1)[C@@H]1CCC[C@H]1C(=O)NCc1ccccc1 Show InChI InChI=1S/C23H26F3NO3/c24-23(25,26)30-18-9-4-8-16(14-18)12-13-21(28)19-10-5-11-20(19)22(29)27-15-17-6-2-1-3-7-17/h1-4,6-9,14,19-21,28H,5,10-13,15H2,(H,27,29)/t19-,20-,21?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of peripheral nerve sodium channel NaV1.7 |
Bioorg Med Chem Lett 16: 1358-61 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.051
BindingDB Entry DOI: 10.7270/Q2FX791S |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50164062
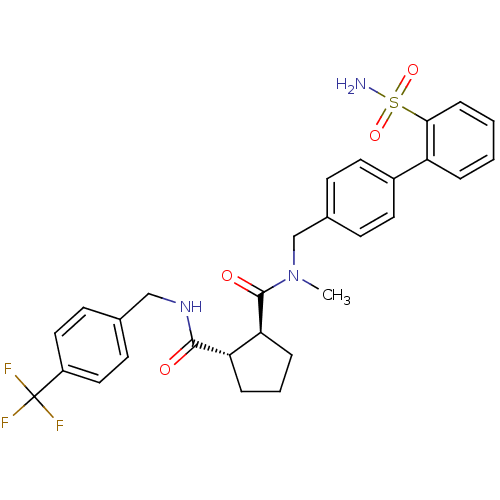
((1S,2S)-Cyclopentane-1,2-dicarboxylic acid 1-[meth...)Show SMILES CN(Cc1ccc(cc1)-c1ccccc1S(N)(=O)=O)C(=O)[C@H]1CCC[C@@H]1C(=O)NCc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C29H30F3N3O4S/c1-35(18-20-9-13-21(14-10-20)23-5-2-3-8-26(23)40(33,38)39)28(37)25-7-4-6-24(25)27(36)34-17-19-11-15-22(16-12-19)29(30,31)32/h2-3,5,8-16,24-25H,4,6-7,17-18H2,1H3,(H,34,36)(H2,33,38,39)/t24-,25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against voltage-gated sodium channel Nav1.7 in electrophysiology assays (EP) |
Bioorg Med Chem Lett 15: 1901-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.002
BindingDB Entry DOI: 10.7270/Q2HH6JMD |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50178861
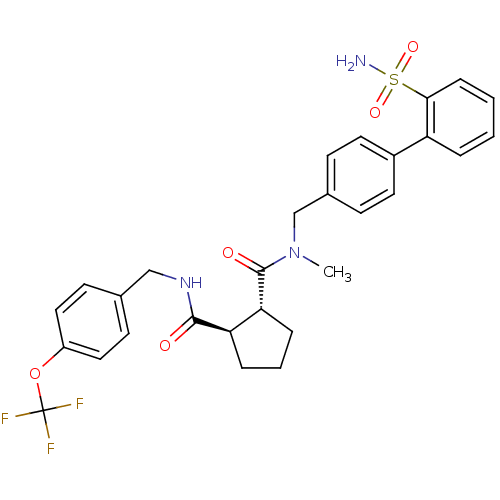
((1R,2R)-N1-methyl-N1-((2'-sulfamoylbiphenyl-4-yl)m...)Show SMILES CN(Cc1ccc(cc1)-c1ccccc1S(N)(=O)=O)C(=O)[C@@H]1CCC[C@H]1C(=O)NCc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C29H30F3N3O5S/c1-35(18-20-9-13-21(14-10-20)23-5-2-3-8-26(23)41(33,38)39)28(37)25-7-4-6-24(25)27(36)34-17-19-11-15-22(16-12-19)40-29(30,31)32/h2-3,5,8-16,24-25H,4,6-7,17-18H2,1H3,(H,34,36)(H2,33,38,39)/t24-,25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of peripheral nerve sodium channel NaV1.7 |
Bioorg Med Chem Lett 16: 1358-61 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.051
BindingDB Entry DOI: 10.7270/Q2FX791S |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50178859
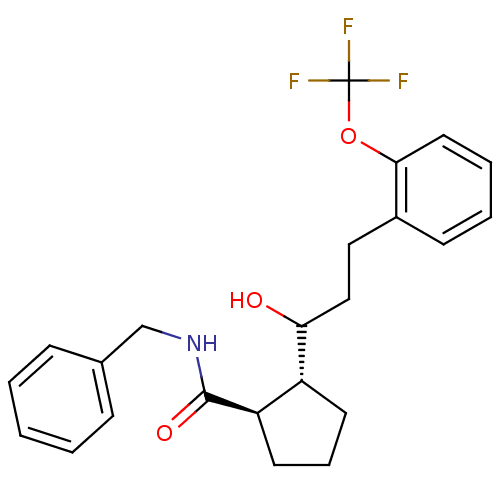
(CHEMBL205258 | trans-N-benzyl-2-(1-hydroxy-3-(2-(t...)Show SMILES OC(CCc1ccccc1OC(F)(F)F)[C@@H]1CCC[C@H]1C(=O)NCc1ccccc1 Show InChI InChI=1S/C23H26F3NO3/c24-23(25,26)30-21-12-5-4-9-17(21)13-14-20(28)18-10-6-11-19(18)22(29)27-15-16-7-2-1-3-8-16/h1-5,7-9,12,18-20,28H,6,10-11,13-15H2,(H,27,29)/t18-,19-,20?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of peripheral nerve sodium channel NaV1.7 |
Bioorg Med Chem Lett 16: 1358-61 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.051
BindingDB Entry DOI: 10.7270/Q2FX791S |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50164057
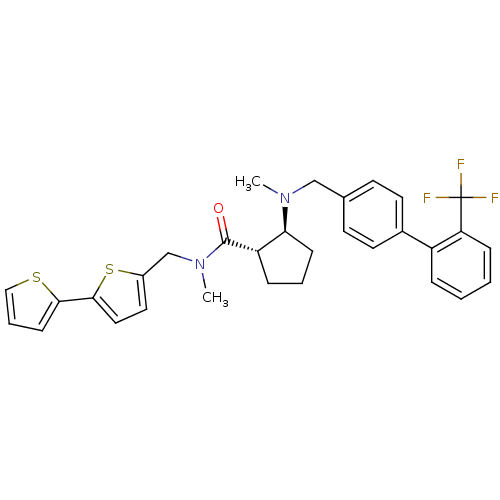
((1S,2S)-2-[Methyl-(2''-trifluoromethyl-biphenyl-4-...)Show SMILES CN(Cc1ccc(cc1)-c1ccccc1C(F)(F)F)[C@H]1CCC[C@@H]1C(=O)N(C)Cc1ccc(s1)-c1cccs1 Show InChI InChI=1S/C31H31F3N2OS2/c1-35(19-21-12-14-22(15-13-21)24-7-3-4-9-26(24)31(32,33)34)27-10-5-8-25(27)30(37)36(2)20-23-16-17-29(39-23)28-11-6-18-38-28/h3-4,6-7,9,11-18,25,27H,5,8,10,19-20H2,1-2H3/t25-,27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against voltage-gated sodium channel Nav1.7 in electrophysiology assays (EP) |
Bioorg Med Chem Lett 15: 1901-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.002
BindingDB Entry DOI: 10.7270/Q2HH6JMD |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50178867
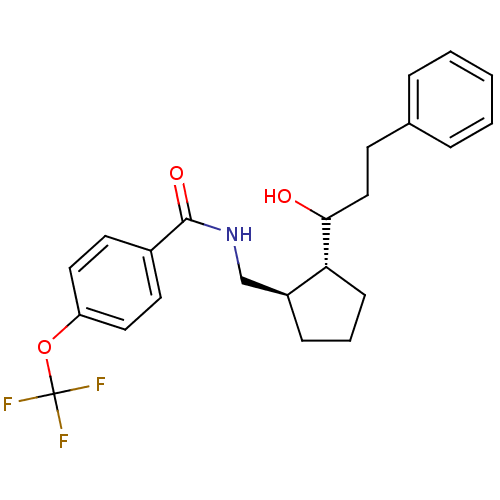
(CHEMBL206431 | N-((trans-2-(1-hydroxy-3-phenylprop...)Show SMILES OC(CCc1ccccc1)[C@@H]1CCC[C@H]1CNC(=O)c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C23H26F3NO3/c24-23(25,26)30-19-12-10-17(11-13-19)22(29)27-15-18-7-4-8-20(18)21(28)14-9-16-5-2-1-3-6-16/h1-3,5-6,10-13,18,20-21,28H,4,7-9,14-15H2,(H,27,29)/t18-,20+,21?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of peripheral nerve sodium channel NaV1.7 |
Bioorg Med Chem Lett 16: 1358-61 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.051
BindingDB Entry DOI: 10.7270/Q2FX791S |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50325762
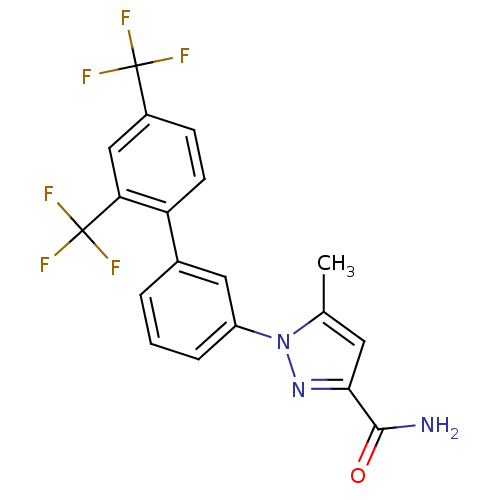
(1-(2',4'-bis(trifluoromethyl)biphenyl-3-yl)-5-meth...)Show SMILES Cc1cc(nn1-c1cccc(c1)-c1ccc(cc1C(F)(F)F)C(F)(F)F)C(N)=O Show InChI InChI=1S/C19H13F6N3O/c1-10-7-16(17(26)29)27-28(10)13-4-2-3-11(8-13)14-6-5-12(18(20,21)22)9-15(14)19(23,24)25/h2-9H,1H3,(H2,26,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 by VIPR assay |
Bioorg Med Chem Lett 20: 7479-82 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.017
BindingDB Entry DOI: 10.7270/Q2MW2HDH |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50141073
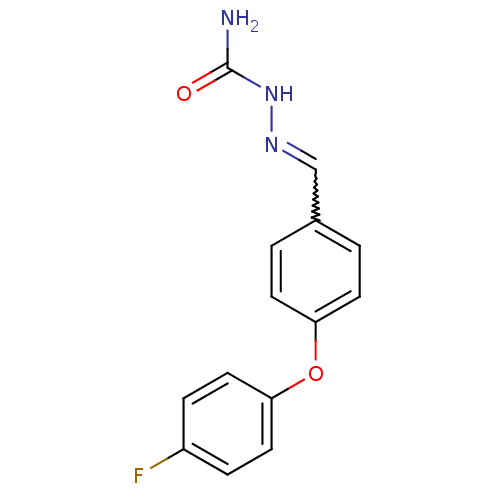
((E)-2-(4-(4-fluorophenoxy)benzylidene)hydrazinecar...)Show InChI InChI=1S/C14H12FN3O2/c15-11-3-7-13(8-4-11)20-12-5-1-10(2-6-12)9-17-18-14(16)19/h1-9H,(H3,16,18,19) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human Nav1.7 |
Bioorg Med Chem Lett 20: 5536-40 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.064
BindingDB Entry DOI: 10.7270/Q2FB535G |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50178863
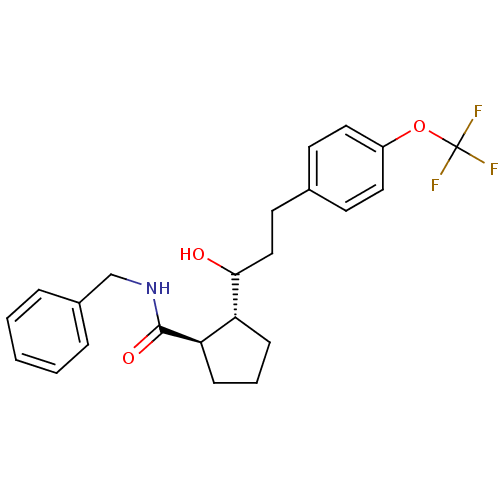
(CHEMBL203185 | trans-N-benzyl-2-((S)-1-hydroxy-3-(...)Show SMILES OC(CCc1ccc(OC(F)(F)F)cc1)[C@@H]1CCC[C@H]1C(=O)NCc1ccccc1 Show InChI InChI=1S/C23H26F3NO3/c24-23(25,26)30-18-12-9-16(10-13-18)11-14-21(28)19-7-4-8-20(19)22(29)27-15-17-5-2-1-3-6-17/h1-3,5-6,9-10,12-13,19-21,28H,4,7-8,11,14-15H2,(H,27,29)/t19-,20-,21?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of peripheral nerve sodium channel NaV1.7 |
Bioorg Med Chem Lett 16: 1358-61 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.051
BindingDB Entry DOI: 10.7270/Q2FX791S |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50164068
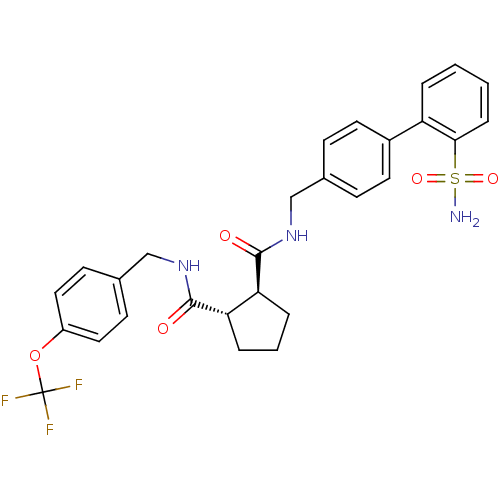
((1S,2S)-Cyclopentane-1,2-dicarboxylic acid 1-[(2''...)Show SMILES NS(=O)(=O)c1ccccc1-c1ccc(CNC(=O)[C@H]2CCC[C@@H]2C(=O)NCc2ccc(OC(F)(F)F)cc2)cc1 Show InChI InChI=1S/C28H28F3N3O5S/c29-28(30,31)39-21-14-10-19(11-15-21)17-34-27(36)24-6-3-5-23(24)26(35)33-16-18-8-12-20(13-9-18)22-4-1-2-7-25(22)40(32,37)38/h1-2,4,7-15,23-24H,3,5-6,16-17H2,(H,33,35)(H,34,36)(H2,32,37,38)/t23-,24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against voltage-gated sodium channel Nav1.7 in electrophysiology assays (EP) |
Bioorg Med Chem Lett 15: 1901-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.002
BindingDB Entry DOI: 10.7270/Q2HH6JMD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data