

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aurora kinase A (Mus musculus (mouse)) | BDBM14192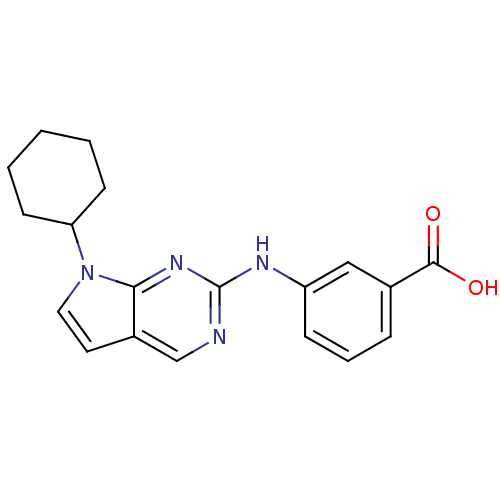 (3-({7-cyclohexyl-7H-pyrrolo[2,3-d]pyrimidin-2-yl}a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Johnson & Johnson Pharmaceutical | Assay Description The compounds were tested for their ability to inhibit the phosphorylation of serine 10 of histone-H3 by purified recombinant murine Aurora-A enzyme.... | Bioorg Med Chem Lett 16: 5778-83 (2006) Article DOI: 10.1016/j.bmcl.2006.08.080 BindingDB Entry DOI: 10.7270/Q2D21VVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Mus musculus (mouse)) | BDBM13534 (CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Johnson & Johnson Pharmaceutical | Assay Description The compounds were tested for their ability to inhibit the phosphorylation of serine 10 of histone-H3 by purified recombinant murine Aurora-A enzyme.... | Bioorg Med Chem Lett 16: 5778-83 (2006) Article DOI: 10.1016/j.bmcl.2006.08.080 BindingDB Entry DOI: 10.7270/Q2D21VVZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase A (Mus musculus (mouse)) | BDBM14208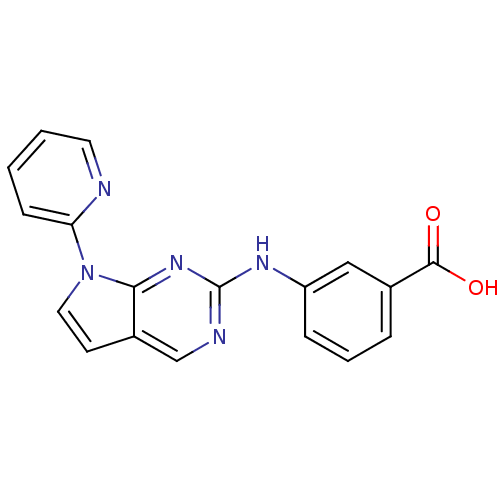 (3-{[7-(pyridin-2-yl)-7H-pyrrolo[2,3-d]pyrimidin-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Johnson & Johnson Pharmaceutical | Assay Description The compounds were tested for their ability to inhibit the phosphorylation of serine 10 of histone-H3 by purified recombinant murine Aurora-A enzyme.... | Bioorg Med Chem Lett 16: 5778-83 (2006) Article DOI: 10.1016/j.bmcl.2006.08.080 BindingDB Entry DOI: 10.7270/Q2D21VVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Mus musculus (mouse)) | BDBM14209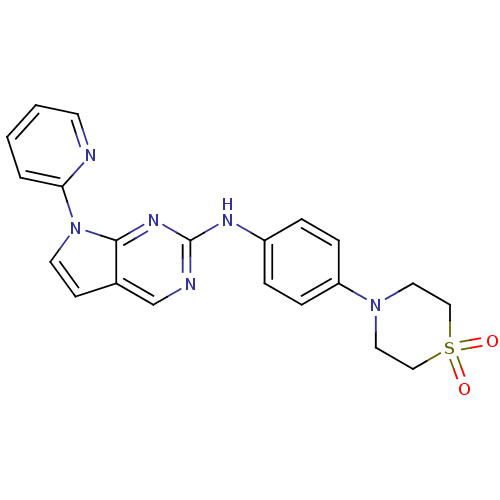 (4-(4-{[7-(pyridin-2-yl)-7H-pyrrolo[2,3-d]pyrimidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Johnson & Johnson Pharmaceutical | Assay Description The compounds were tested for their ability to inhibit the phosphorylation of serine 10 of histone-H3 by purified recombinant murine Aurora-A enzyme.... | Bioorg Med Chem Lett 16: 5778-83 (2006) Article DOI: 10.1016/j.bmcl.2006.08.080 BindingDB Entry DOI: 10.7270/Q2D21VVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM14209 (4-(4-{[7-(pyridin-2-yl)-7H-pyrrolo[2,3-d]pyrimidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description The compounds were tested for their ability to inhibit the phosphorylation of poly(Glu:Tyr) by purified recombinant human FLT3. The extent of phospho... | Bioorg Med Chem Lett 16: 5778-83 (2006) Article DOI: 10.1016/j.bmcl.2006.08.080 BindingDB Entry DOI: 10.7270/Q2D21VVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50375155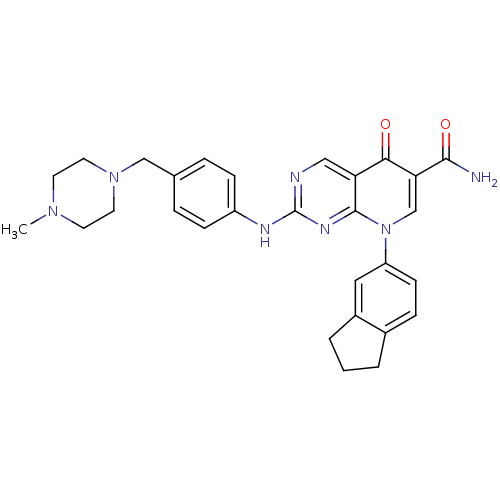 (CHEMBL256893) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of FLT3 | Bioorg Med Chem Lett 18: 2355-61 (2008) Article DOI: 10.1016/j.bmcl.2008.02.070 BindingDB Entry DOI: 10.7270/Q26W9BX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50371914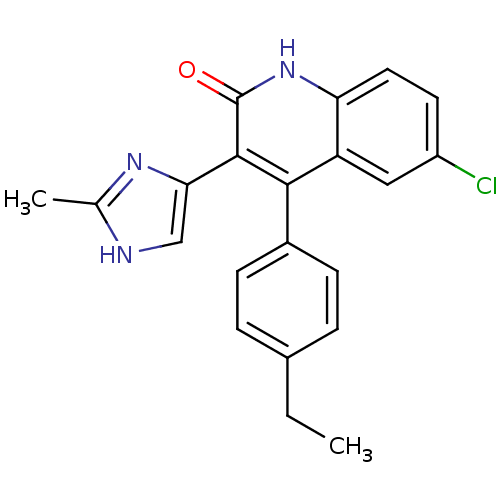 (CHEMBL403661) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human cytoplasmic macrophage colony-stimulating factor 1 receptor by fluorescence polarization | Bioorg Med Chem Lett 18: 2097-102 (2008) Article DOI: 10.1016/j.bmcl.2008.01.088 BindingDB Entry DOI: 10.7270/Q23779JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50371915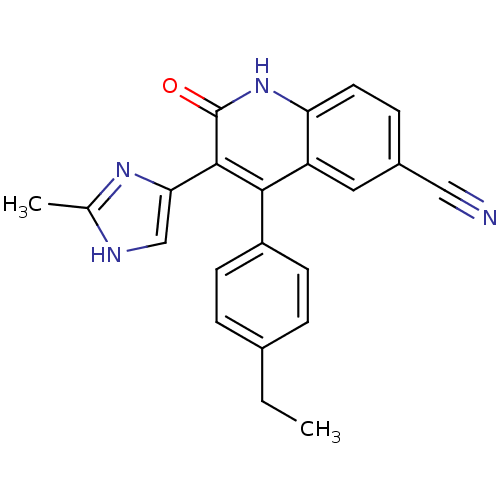 (CHEMBL273198) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human cytoplasmic macrophage colony-stimulating factor 1 receptor by fluorescence polarization | Bioorg Med Chem Lett 18: 2097-102 (2008) Article DOI: 10.1016/j.bmcl.2008.01.088 BindingDB Entry DOI: 10.7270/Q23779JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM27889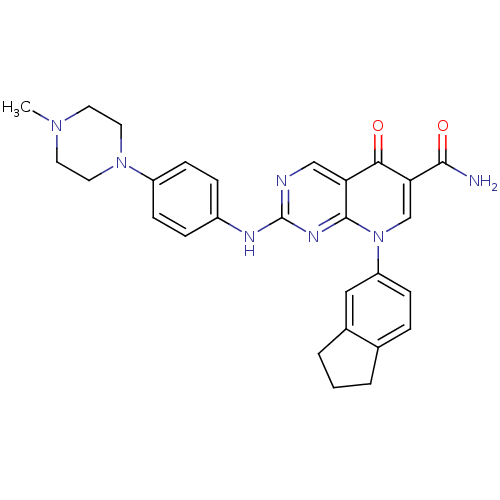 (8-(2,3-dihydro-1H-inden-5-yl)-2-{[4-(4-methylpiper...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of FLT3 | Bioorg Med Chem Lett 18: 2355-61 (2008) Article DOI: 10.1016/j.bmcl.2008.02.070 BindingDB Entry DOI: 10.7270/Q26W9BX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50375139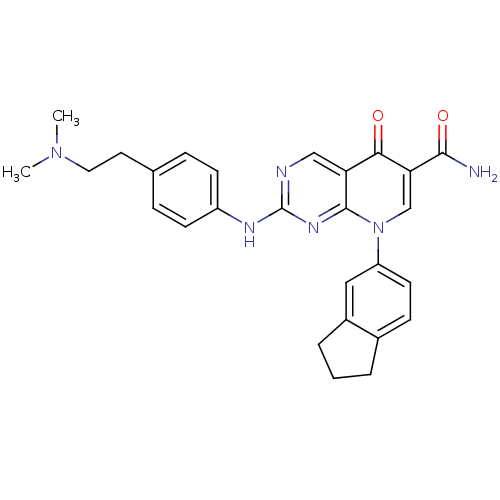 (CHEMBL256459) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of ATP-induced CSF1R autophosphorylation | Bioorg Med Chem Lett 18: 2355-61 (2008) Article DOI: 10.1016/j.bmcl.2008.02.070 BindingDB Entry DOI: 10.7270/Q26W9BX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Mus musculus (mouse)) | BDBM14201 (N-[3-(4-methylpiperazin-1-yl)phenyl]-7-phenyl-7H-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Johnson & Johnson Pharmaceutical | Assay Description The compounds were tested for their ability to inhibit the phosphorylation of serine 10 of histone-H3 by purified recombinant murine Aurora-A enzyme.... | Bioorg Med Chem Lett 16: 5778-83 (2006) Article DOI: 10.1016/j.bmcl.2006.08.080 BindingDB Entry DOI: 10.7270/Q2D21VVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50375138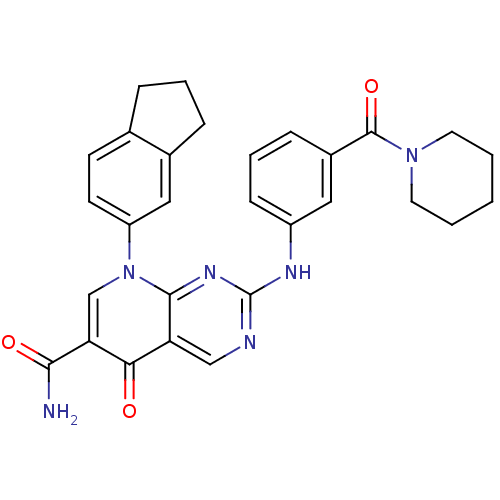 (CHEMBL402852) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of ATP-induced CSF1R autophosphorylation | Bioorg Med Chem Lett 18: 2355-61 (2008) Article DOI: 10.1016/j.bmcl.2008.02.070 BindingDB Entry DOI: 10.7270/Q26W9BX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50375145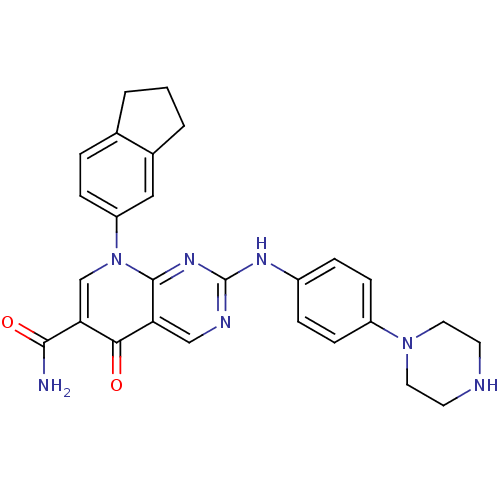 (CHEMBL271461) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of ATP-induced CSF1R autophosphorylation | Bioorg Med Chem Lett 18: 2355-61 (2008) Article DOI: 10.1016/j.bmcl.2008.02.070 BindingDB Entry DOI: 10.7270/Q26W9BX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Mus musculus (mouse)) | BDBM14207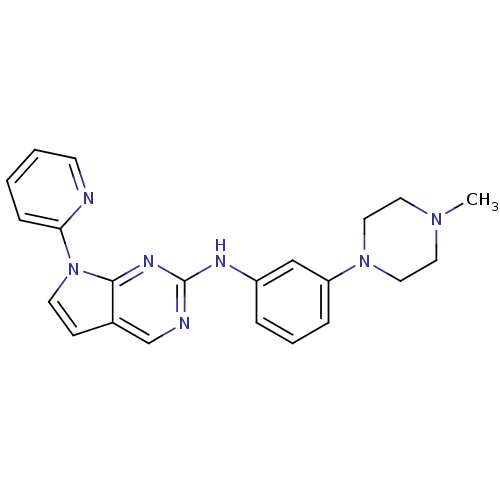 (N-[3-(4-methylpiperazin-1-yl)phenyl]-7-(pyridin-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Johnson & Johnson Pharmaceutical | Assay Description The compounds were tested for their ability to inhibit the phosphorylation of serine 10 of histone-H3 by purified recombinant murine Aurora-A enzyme.... | Bioorg Med Chem Lett 16: 5778-83 (2006) Article DOI: 10.1016/j.bmcl.2006.08.080 BindingDB Entry DOI: 10.7270/Q2D21VVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Mus musculus (mouse)) | BDBM14203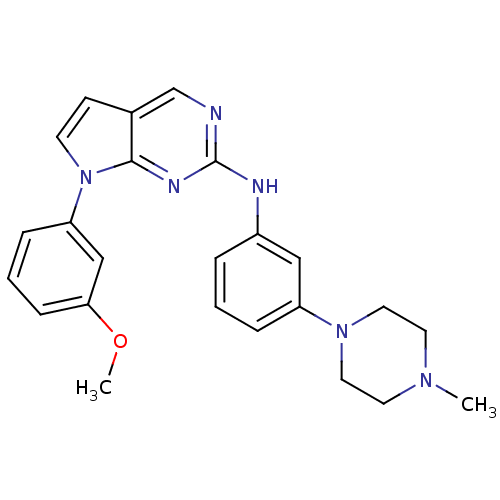 (7-(3-methoxyphenyl)-N-[3-(4-methylpiperazin-1-yl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Johnson & Johnson Pharmaceutical | Assay Description The compounds were tested for their ability to inhibit the phosphorylation of serine 10 of histone-H3 by purified recombinant murine Aurora-A enzyme.... | Bioorg Med Chem Lett 16: 5778-83 (2006) Article DOI: 10.1016/j.bmcl.2006.08.080 BindingDB Entry DOI: 10.7270/Q2D21VVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Mus musculus (mouse)) | BDBM14191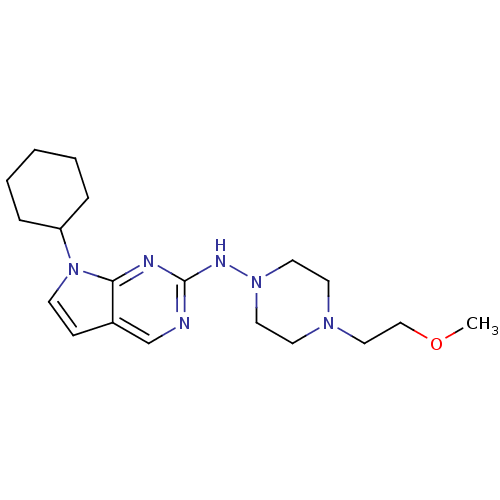 (N-{7-cyclohexyl-7H-pyrrolo[2,3-d]pyrimidin-2-yl}-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Johnson & Johnson Pharmaceutical | Assay Description The compounds were tested for their ability to inhibit the phosphorylation of serine 10 of histone-H3 by purified recombinant murine Aurora-A enzyme.... | Bioorg Med Chem Lett 16: 5778-83 (2006) Article DOI: 10.1016/j.bmcl.2006.08.080 BindingDB Entry DOI: 10.7270/Q2D21VVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50375159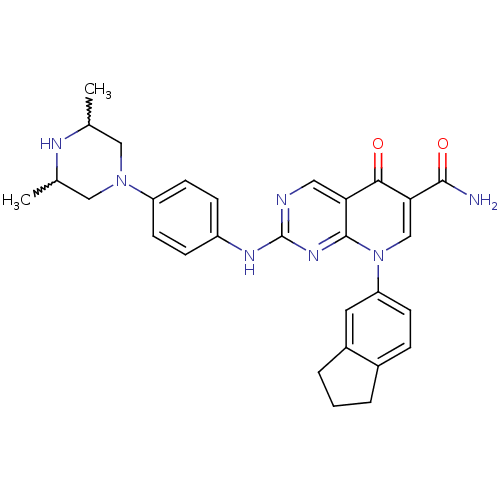 (CHEMBL255794) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of ATP-induced CSF1R autophosphorylation | Bioorg Med Chem Lett 18: 2355-61 (2008) Article DOI: 10.1016/j.bmcl.2008.02.070 BindingDB Entry DOI: 10.7270/Q26W9BX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50375158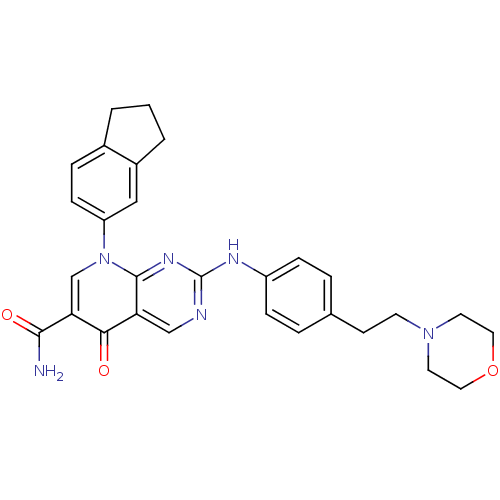 (CHEMBL404188) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of ATP-induced CSF1R autophosphorylation | Bioorg Med Chem Lett 18: 2355-61 (2008) Article DOI: 10.1016/j.bmcl.2008.02.070 BindingDB Entry DOI: 10.7270/Q26W9BX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50375144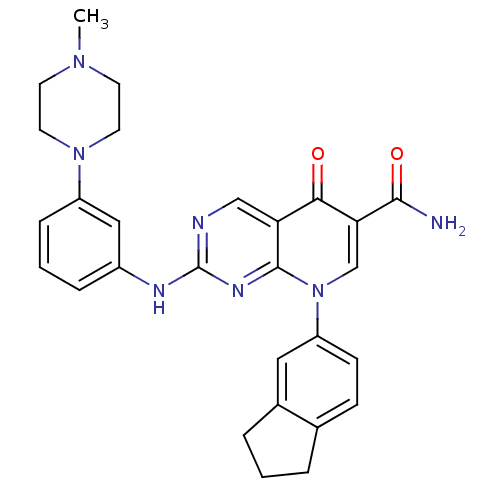 (CHEMBL255584) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of ATP-induced CSF1R autophosphorylation | Bioorg Med Chem Lett 18: 2355-61 (2008) Article DOI: 10.1016/j.bmcl.2008.02.070 BindingDB Entry DOI: 10.7270/Q26W9BX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50375142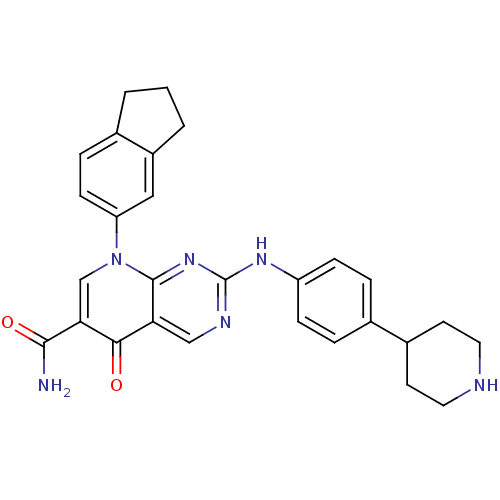 (CHEMBL257528) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of ATP-induced CSF1R autophosphorylation | Bioorg Med Chem Lett 18: 2355-61 (2008) Article DOI: 10.1016/j.bmcl.2008.02.070 BindingDB Entry DOI: 10.7270/Q26W9BX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM27890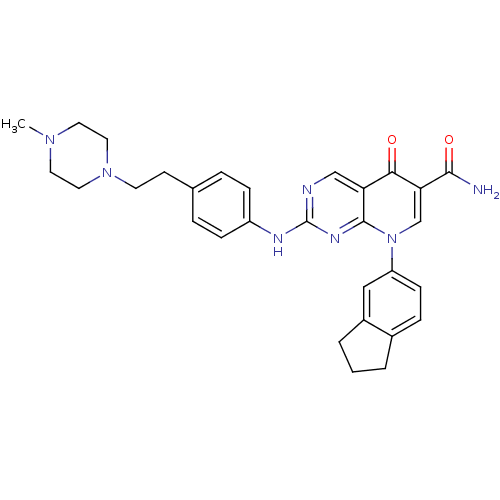 (8-(2,3-dihydro-1H-inden-5-yl)-2-({4-[2-(4-methylpi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of ATP-induced CSF1R autophosphorylation | Bioorg Med Chem Lett 18: 2355-61 (2008) Article DOI: 10.1016/j.bmcl.2008.02.070 BindingDB Entry DOI: 10.7270/Q26W9BX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Mus musculus (mouse)) | BDBM14197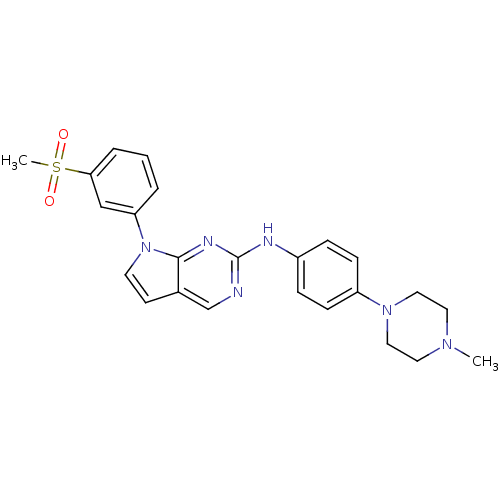 (7-(3-methanesulfonylphenyl)-N-[4-(4-methylpiperazi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Johnson & Johnson Pharmaceutical | Assay Description The compounds were tested for their ability to inhibit the phosphorylation of serine 10 of histone-H3 by purified recombinant murine Aurora-A enzyme.... | Bioorg Med Chem Lett 16: 5778-83 (2006) Article DOI: 10.1016/j.bmcl.2006.08.080 BindingDB Entry DOI: 10.7270/Q2D21VVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Mus musculus (mouse)) | BDBM14200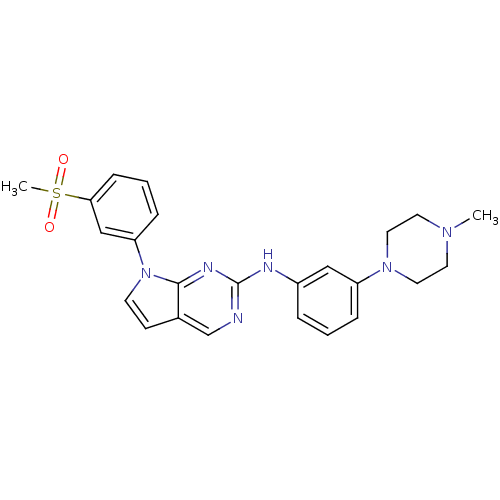 (7-(3-methanesulfonylphenyl)-N-[3-(4-methylpiperazi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Johnson & Johnson Pharmaceutical | Assay Description The compounds were tested for their ability to inhibit the phosphorylation of serine 10 of histone-H3 by purified recombinant murine Aurora-A enzyme.... | Bioorg Med Chem Lett 16: 5778-83 (2006) Article DOI: 10.1016/j.bmcl.2006.08.080 BindingDB Entry DOI: 10.7270/Q2D21VVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Mus musculus (mouse)) | BDBM14205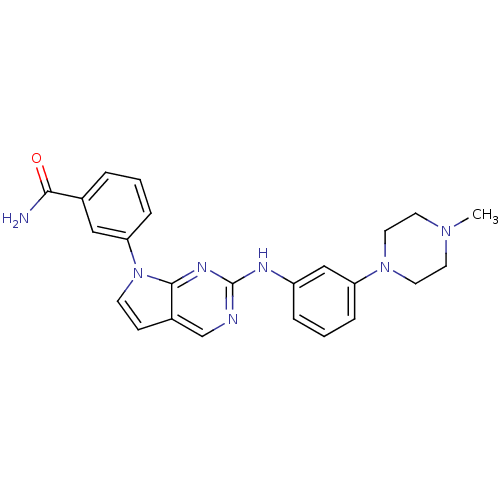 (3-(2-{[3-(4-methylpiperazin-1-yl)phenyl]amino}-7H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Johnson & Johnson Pharmaceutical | Assay Description The compounds were tested for their ability to inhibit the phosphorylation of serine 10 of histone-H3 by purified recombinant murine Aurora-A enzyme.... | Bioorg Med Chem Lett 16: 5778-83 (2006) Article DOI: 10.1016/j.bmcl.2006.08.080 BindingDB Entry DOI: 10.7270/Q2D21VVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50375157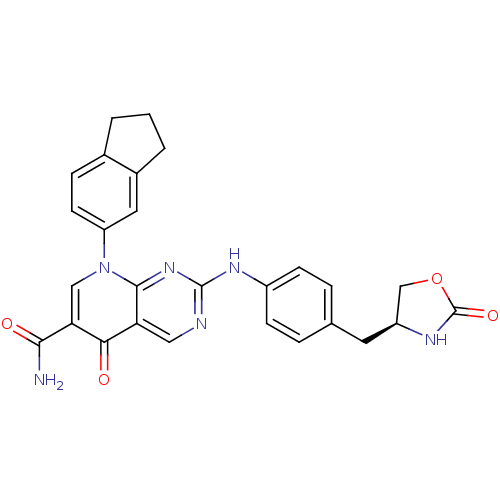 (CHEMBL273193) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of ATP-induced CSF1R autophosphorylation | Bioorg Med Chem Lett 18: 2355-61 (2008) Article DOI: 10.1016/j.bmcl.2008.02.070 BindingDB Entry DOI: 10.7270/Q26W9BX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Mus musculus (mouse)) | BDBM14199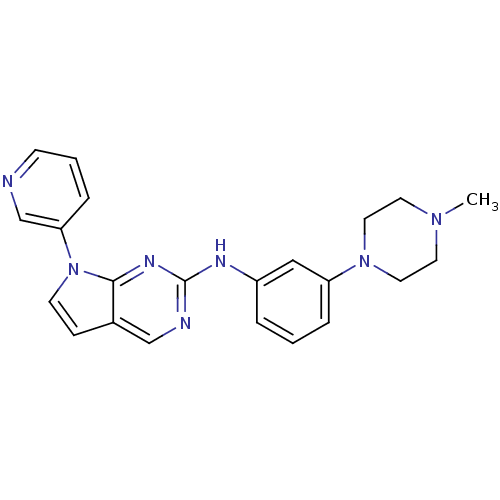 (N-[3-(4-methylpiperazin-1-yl)phenyl]-7-(pyridin-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Johnson & Johnson Pharmaceutical | Assay Description The compounds were tested for their ability to inhibit the phosphorylation of serine 10 of histone-H3 by purified recombinant murine Aurora-A enzyme.... | Bioorg Med Chem Lett 16: 5778-83 (2006) Article DOI: 10.1016/j.bmcl.2006.08.080 BindingDB Entry DOI: 10.7270/Q2D21VVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Mus musculus (mouse)) | BDBM14193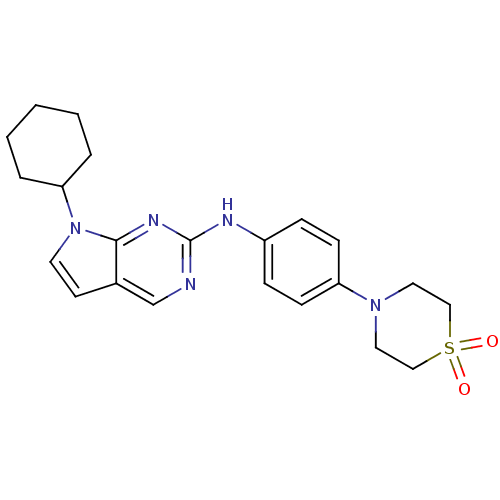 (4-[4-({7-cyclohexyl-7H-pyrrolo[2,3-d]pyrimidin-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Johnson & Johnson Pharmaceutical | Assay Description The compounds were tested for their ability to inhibit the phosphorylation of serine 10 of histone-H3 by purified recombinant murine Aurora-A enzyme.... | Bioorg Med Chem Lett 16: 5778-83 (2006) Article DOI: 10.1016/j.bmcl.2006.08.080 BindingDB Entry DOI: 10.7270/Q2D21VVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM27889 (8-(2,3-dihydro-1H-inden-5-yl)-2-{[4-(4-methylpiper...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of ATP-induced CSF1R autophosphorylation | Bioorg Med Chem Lett 18: 2355-61 (2008) Article DOI: 10.1016/j.bmcl.2008.02.070 BindingDB Entry DOI: 10.7270/Q26W9BX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Mus musculus (mouse)) | BDBM14202 (3-(2-{[3-(4-methylpiperazin-1-yl)phenyl]amino}-7H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Johnson & Johnson Pharmaceutical | Assay Description The compounds were tested for their ability to inhibit the phosphorylation of serine 10 of histone-H3 by purified recombinant murine Aurora-A enzyme.... | Bioorg Med Chem Lett 16: 5778-83 (2006) Article DOI: 10.1016/j.bmcl.2006.08.080 BindingDB Entry DOI: 10.7270/Q2D21VVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Mus musculus (mouse)) | BDBM14196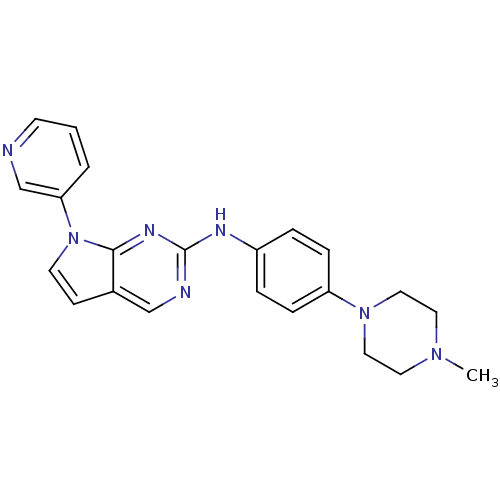 (N-[4-(4-methylpiperazin-1-yl)phenyl]-7-(pyridin-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Johnson & Johnson Pharmaceutical | Assay Description The compounds were tested for their ability to inhibit the phosphorylation of serine 10 of histone-H3 by purified recombinant murine Aurora-A enzyme.... | Bioorg Med Chem Lett 16: 5778-83 (2006) Article DOI: 10.1016/j.bmcl.2006.08.080 BindingDB Entry DOI: 10.7270/Q2D21VVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM27889 (8-(2,3-dihydro-1H-inden-5-yl)-2-{[4-(4-methylpiper...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of CSF1R | Bioorg Med Chem Lett 18: 2355-61 (2008) Article DOI: 10.1016/j.bmcl.2008.02.070 BindingDB Entry DOI: 10.7270/Q26W9BX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50375155 (CHEMBL256893) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of ATP-induced CSF1R autophosphorylation | Bioorg Med Chem Lett 18: 2355-61 (2008) Article DOI: 10.1016/j.bmcl.2008.02.070 BindingDB Entry DOI: 10.7270/Q26W9BX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50375155 (CHEMBL256893) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of CSF1R | Bioorg Med Chem Lett 18: 2355-61 (2008) Article DOI: 10.1016/j.bmcl.2008.02.070 BindingDB Entry DOI: 10.7270/Q26W9BX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50223693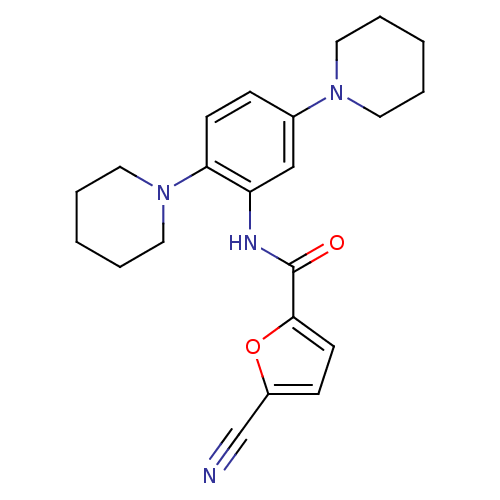 (5-cyano-N-(2,5-di(piperidin-1-yl)phenyl)furan-2-ca...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of ATP-induced autophosphorylation of cFMS tyrosine kinase | Bioorg Med Chem Lett 17: 6070-4 (2007) Article DOI: 10.1016/j.bmcl.2007.09.057 BindingDB Entry DOI: 10.7270/Q2028R9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50223698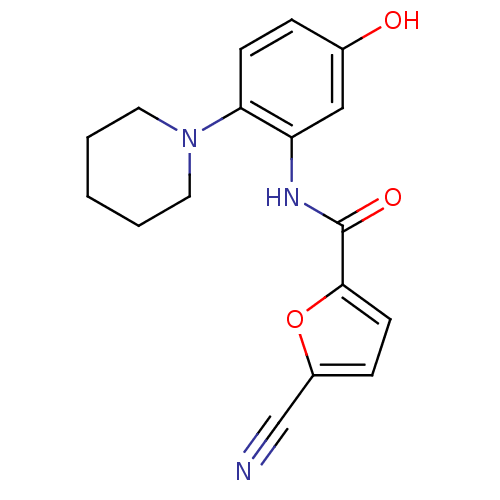 (5-cyano-N-(5-hydroxy-2-(piperidin-1-yl)phenyl)fura...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of ATP-induced autophosphorylation of cFMS tyrosine kinase | Bioorg Med Chem Lett 17: 6070-4 (2007) Article DOI: 10.1016/j.bmcl.2007.09.057 BindingDB Entry DOI: 10.7270/Q2028R9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Mus musculus (mouse)) | BDBM14195 (7-(3-methoxyphenyl)-N-[4-(4-methylpiperazin-1-yl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Johnson & Johnson Pharmaceutical | Assay Description The compounds were tested for their ability to inhibit the phosphorylation of serine 10 of histone-H3 by purified recombinant murine Aurora-A enzyme.... | Bioorg Med Chem Lett 16: 5778-83 (2006) Article DOI: 10.1016/j.bmcl.2006.08.080 BindingDB Entry DOI: 10.7270/Q2D21VVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50162972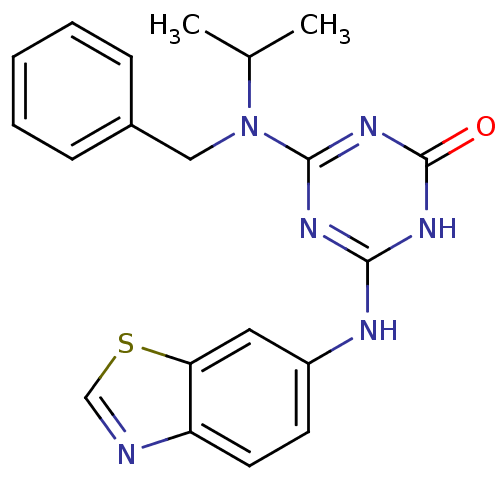 (4-(Benzothiazol-6-ylamino)-6-(benzyl-isopropyl-ami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of Vascular endothelial growth factor receptor 2 at 10 uM ATP | J Med Chem 48: 1717-20 (2005) Article DOI: 10.1021/jm049372z BindingDB Entry DOI: 10.7270/Q2GM86TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Mus musculus (mouse)) | BDBM14198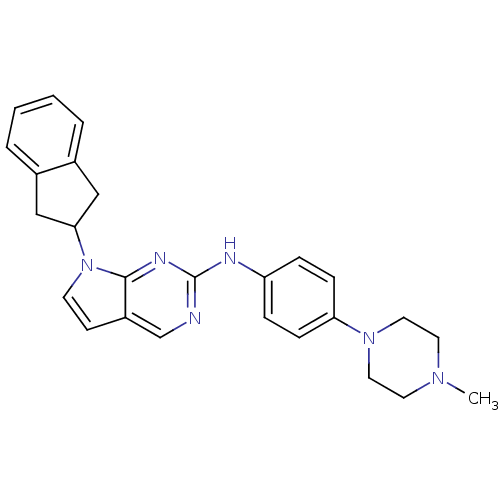 (7-(2,3-dihydro-1H-inden-2-yl)-N-[4-(4-methylpipera...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Johnson & Johnson Pharmaceutical | Assay Description The compounds were tested for their ability to inhibit the phosphorylation of serine 10 of histone-H3 by purified recombinant murine Aurora-A enzyme.... | Bioorg Med Chem Lett 16: 5778-83 (2006) Article DOI: 10.1016/j.bmcl.2006.08.080 BindingDB Entry DOI: 10.7270/Q2D21VVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50223670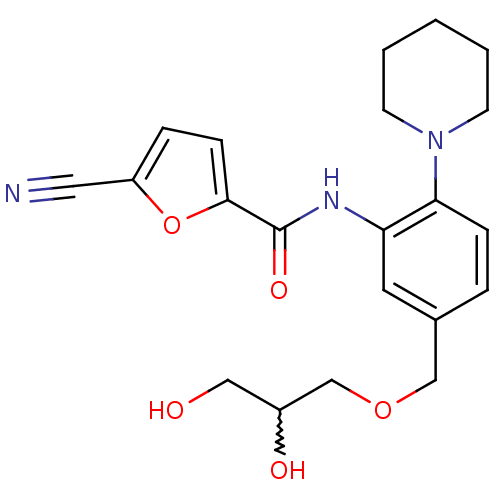 (5-cyano-N-(5-((2,3-dihydroxypropoxy)methyl)-2-(pip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of ATP-induced autophosphorylation of cFMS tyrosine kinase | Bioorg Med Chem Lett 17: 6070-4 (2007) Article DOI: 10.1016/j.bmcl.2007.09.057 BindingDB Entry DOI: 10.7270/Q2028R9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50375140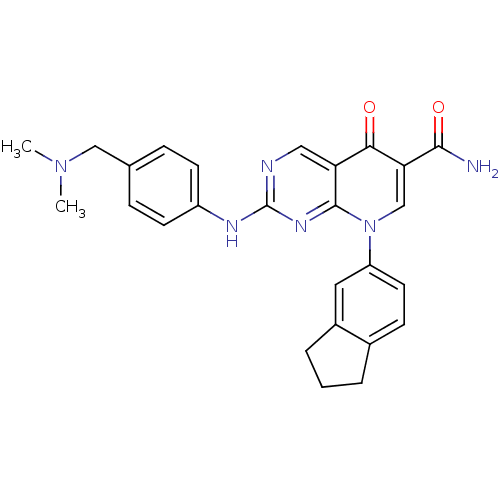 (CHEMBL256405) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of ATP-induced CSF1R autophosphorylation | Bioorg Med Chem Lett 18: 2355-61 (2008) Article DOI: 10.1016/j.bmcl.2008.02.070 BindingDB Entry DOI: 10.7270/Q26W9BX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50375141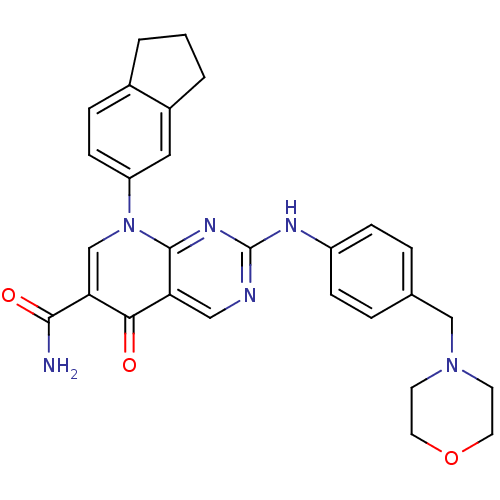 (CHEMBL256406) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of ATP-induced CSF1R autophosphorylation | Bioorg Med Chem Lett 18: 2355-61 (2008) Article DOI: 10.1016/j.bmcl.2008.02.070 BindingDB Entry DOI: 10.7270/Q26W9BX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM17750 (5-cyano-N-[5-(hydroxymethyl)-2-(4-methylpiperidin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of ATP-induced autophosphorylation of cFMS tyrosine kinase | Bioorg Med Chem Lett 17: 6070-4 (2007) Article DOI: 10.1016/j.bmcl.2007.09.057 BindingDB Entry DOI: 10.7270/Q2028R9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM17750 (5-cyano-N-[5-(hydroxymethyl)-2-(4-methylpiperidin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Johnson & Johnson Pharmaceutical | Assay Description The full-length cFMS cytoplasmic domain (FMS.538-972.6His) was incubated with compound in reaction buffer. Control reactions were run in each plate. ... | J Biol Chem 282: 4094-101 (2007) Article DOI: 10.1074/jbc.M608183200 BindingDB Entry DOI: 10.7270/Q2HT2MKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50223700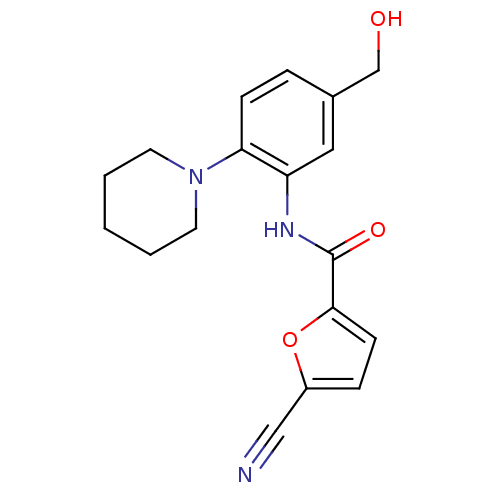 (5-cyano-N-(5-(hydroxymethyl)-2-(piperidin-1-yl)phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of ATP-induced autophosphorylation of cFMS tyrosine kinase | Bioorg Med Chem Lett 17: 6070-4 (2007) Article DOI: 10.1016/j.bmcl.2007.09.057 BindingDB Entry DOI: 10.7270/Q2028R9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50375155 (CHEMBL256893) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of KIT | Bioorg Med Chem Lett 18: 2355-61 (2008) Article DOI: 10.1016/j.bmcl.2008.02.070 BindingDB Entry DOI: 10.7270/Q26W9BX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM14194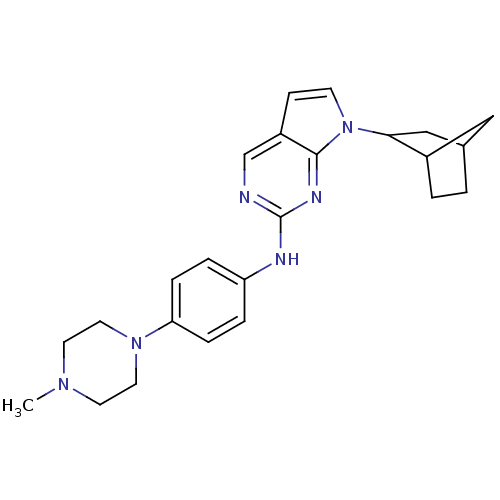 (7-{bicyclo[2.2.1]heptan-2-yl}-N-[4-(4-methylpipera...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description The compounds were tested for their ability to inhibit the phosphorylation of serine 795 of Rb by purified recombinant CDK4. The phosphorylation of s... | Bioorg Med Chem Lett 16: 5778-83 (2006) Article DOI: 10.1016/j.bmcl.2006.08.080 BindingDB Entry DOI: 10.7270/Q2D21VVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Mus musculus (mouse)) | BDBM14204 (N-[3-(4-methylpiperazin-1-yl)phenyl]-7-[3-(propan-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Johnson & Johnson Pharmaceutical | Assay Description The compounds were tested for their ability to inhibit the phosphorylation of serine 10 of histone-H3 by purified recombinant murine Aurora-A enzyme.... | Bioorg Med Chem Lett 16: 5778-83 (2006) Article DOI: 10.1016/j.bmcl.2006.08.080 BindingDB Entry DOI: 10.7270/Q2D21VVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM14189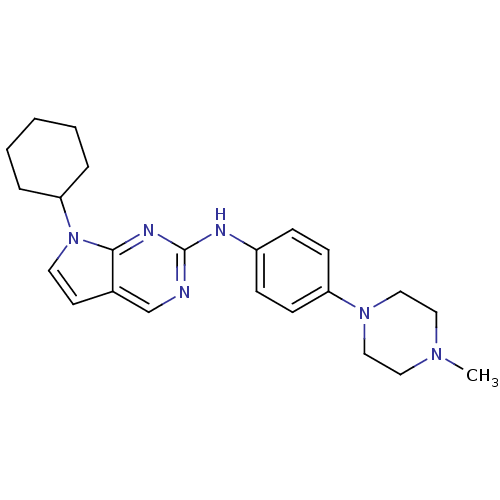 (7-cyclohexyl-N-[4-(4-methylpiperazin-1-yl)phenyl]-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description The compounds were tested for their ability to inhibit the phosphorylation of serine 795 of Rb by purified recombinant CDK4. The phosphorylation of s... | Bioorg Med Chem Lett 16: 5778-83 (2006) Article DOI: 10.1016/j.bmcl.2006.08.080 BindingDB Entry DOI: 10.7270/Q2D21VVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50375152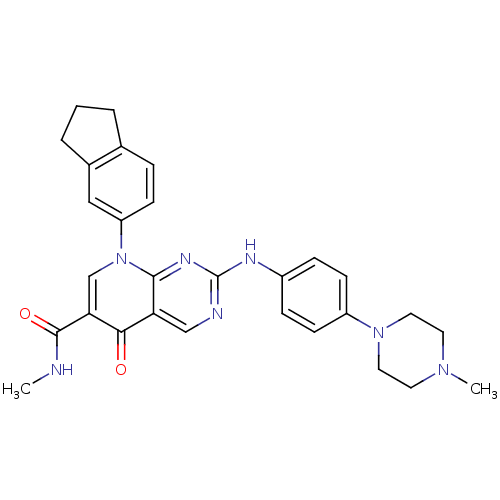 (CHEMBL255182) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of ATP-induced CSF1R autophosphorylation | Bioorg Med Chem Lett 18: 2355-61 (2008) Article DOI: 10.1016/j.bmcl.2008.02.070 BindingDB Entry DOI: 10.7270/Q26W9BX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50371914 (CHEMBL403661) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of FLT3 | Bioorg Med Chem Lett 18: 2097-102 (2008) Article DOI: 10.1016/j.bmcl.2008.01.088 BindingDB Entry DOI: 10.7270/Q23779JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 253 total ) | Next | Last >> |