

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50236961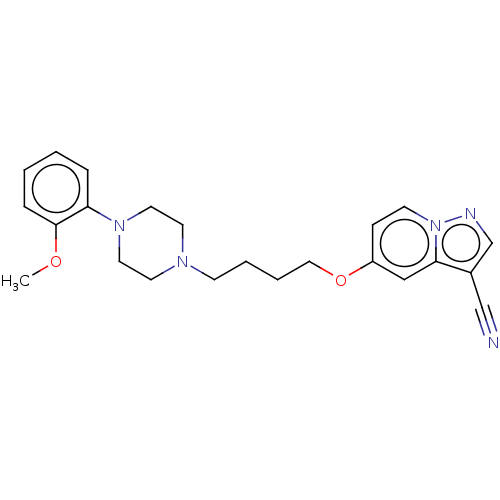 (CHEMBL4063235) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human Dopamine D2S receptor expressed in CHO cell membranes after 2 hrs by scintillation counting analysis | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50370684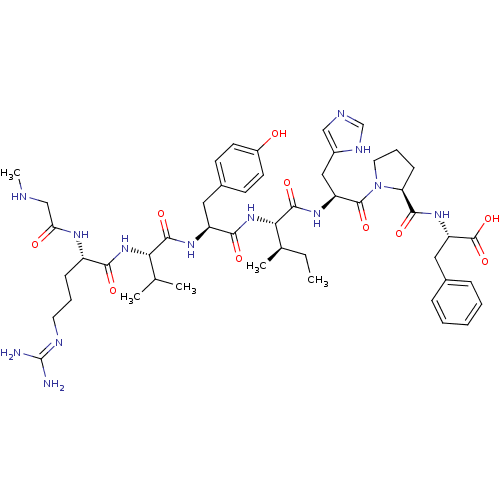 (CHEMBL1791349) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity for angiotensin II receptor, type 1 in rat liver membrane using [125I]-Ang II as radioligand, in pH 7.4 Tris-HCl buffer for 2 hr at ... | J Med Chem 48: 6620-31 (2005) Article DOI: 10.1021/jm050280z BindingDB Entry DOI: 10.7270/Q2HX1DG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50370684 (CHEMBL1791349) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity for angiotensin II receptor, type 2 in pig uterus myometrium using [125I]-Ang II as radioligand, in pH 7.4 Tris-HCl buffer for 1.5 h... | J Med Chem 48: 6620-31 (2005) Article DOI: 10.1021/jm050280z BindingDB Entry DOI: 10.7270/Q2HX1DG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50236961 (CHEMBL4063235) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human Dopamine D2L receptor expressed in CHO cell membranes after 2 hrs by scintillation counting analysis | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50370575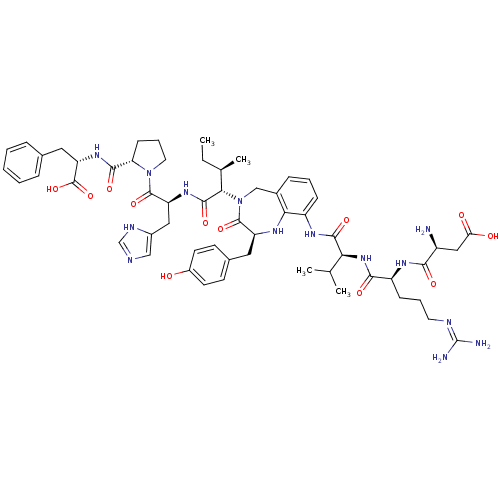 (CHEMBL1791308) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [125I]-Ang II from angiotensin II receptor type 2 in pig uterus myometrium | J Med Chem 48: 4009-24 (2005) Article DOI: 10.1021/jm0491492 BindingDB Entry DOI: 10.7270/Q2BR8SZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50370575 (CHEMBL1791308) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [125I]-Ang II from angiotensin II receptor type 2 in pig uterus myometrium | J Med Chem 48: 4009-24 (2005) Article DOI: 10.1021/jm0491492 BindingDB Entry DOI: 10.7270/Q2BR8SZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50236960 (CHEMBL4060403) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human Dopamine D2L receptor expressed in CHO cell membranes after 2 hrs by scintillation counting analysis | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50156173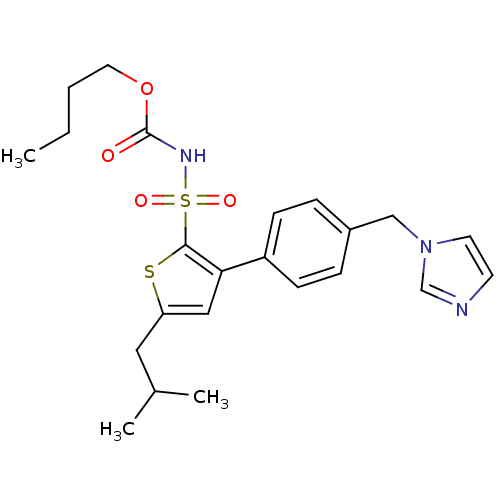 ((1-((2'-(1H-tetrazol-5-yl)biphenyl-4-yl)methyl)-2-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to AT2 receptor | Bioorg Med Chem 18: 4570-90 (2010) Article DOI: 10.1016/j.bmc.2010.03.064 BindingDB Entry DOI: 10.7270/Q2VD70F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50156173 ((1-((2'-(1H-tetrazol-5-yl)biphenyl-4-yl)methyl)-2-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [125I]-Ang II from pig uterus membrane angiotensin II type 2 (AT2) receptor | J Med Chem 47: 5995-6008 (2004) Article DOI: 10.1021/jm049715t BindingDB Entry DOI: 10.7270/Q29Z95PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Sus scrofa) | BDBM50156173 ((1-((2'-(1H-tetrazol-5-yl)biphenyl-4-yl)methyl)-2-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [125I]angiotensin 2 from AT2 receptor in pig uterus membrane after 1.5 hrs by gamma counting | Bioorg Med Chem 18: 4570-90 (2010) Article DOI: 10.1016/j.bmc.2010.03.064 BindingDB Entry DOI: 10.7270/Q2VD70F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50236960 (CHEMBL4060403) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human Dopamine D2S receptor expressed in CHO cell membranes after 2 hrs by scintillation counting analysis | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50237158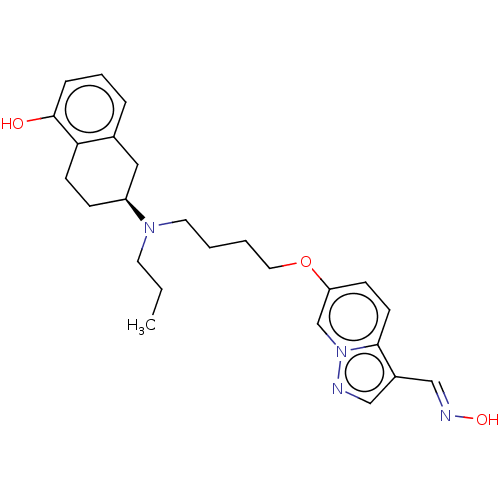 (CHEMBL4071868) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Agonist activity at human Dopamine D3 receptor expressed in HEK293T cell membranes coexpressing GalphaoA incubated for 30 mins measured after 75 mins... | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50130293 (7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Inhibition of FPP incorporation into biotinylated K-Ras-derived peptide by human farnesyl transferase with 5 nM ATP | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50130293 (7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Concentration required for the inhibitory activity against human Farnesyltransferase | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50236956 (CHEMBL4069091) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human Dopamine D2S receptor expressed in CHO cell membranes after 2 hrs by scintillation counting analysis | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50199311 (CHEMBL215160 | N-butyloxycarbonyl-2-(4-imidazol-1-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [125I]Ang2 from AT2 receptor in pig uterus membrane | J Med Chem 49: 7160-8 (2006) Article DOI: 10.1021/jm0606185 BindingDB Entry DOI: 10.7270/Q21C1WHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50156167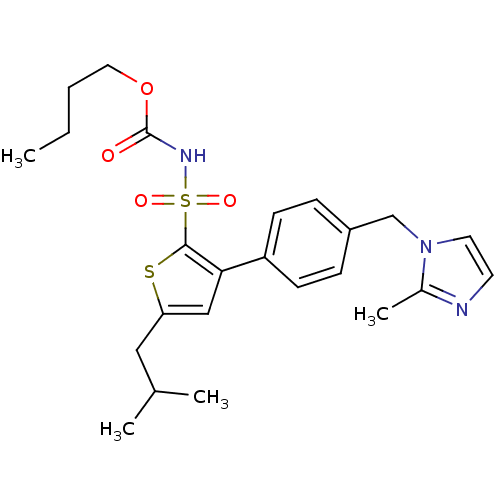 (CHEMBL361927 | butyl (5-isobutylthien-2-yl)sulfony...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [125I]-Ang II from pig uterus membrane angiotensin II type 2 (AT2) receptor | J Med Chem 47: 5995-6008 (2004) Article DOI: 10.1021/jm049715t BindingDB Entry DOI: 10.7270/Q29Z95PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50237164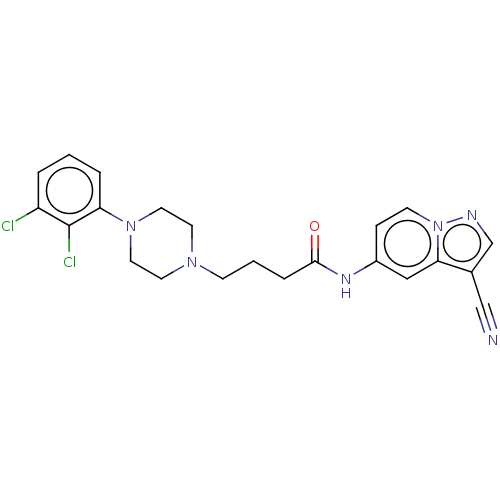 (CHEMBL4091064) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human Dopamine D2S receptor expressed in CHO cell membranes after 2 hrs by scintillation counting analysis | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50236962 (CHEMBL4096353) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human Dopamine D2S receptor expressed in CHO cell membranes after 2 hrs by scintillation counting analysis | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50236966 (CHEMBL4070091) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human Dopamine D2S receptor expressed in CHO cell membranes after 2 hrs by scintillation counting analysis | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50236966 (CHEMBL4070091) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human Dopamine D2S receptor expressed in CHO cell membranes after 2 hrs by scintillation counting analysis | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50236956 (CHEMBL4069091) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human Dopamine D2L receptor expressed in CHO cell membranes after 2 hrs by scintillation counting analysis | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50237165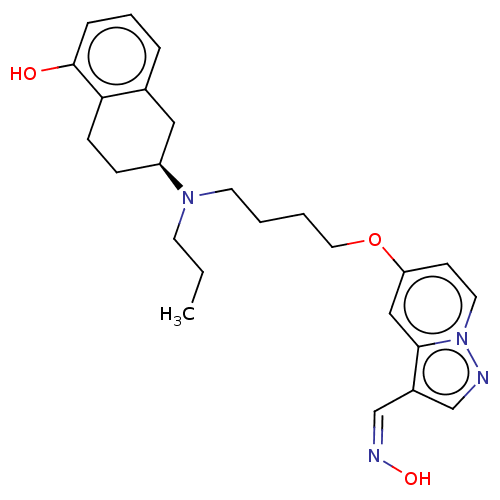 (CHEMBL4098859) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Concentration required for the inhibitory activity against human Farnesyltransferase | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50199326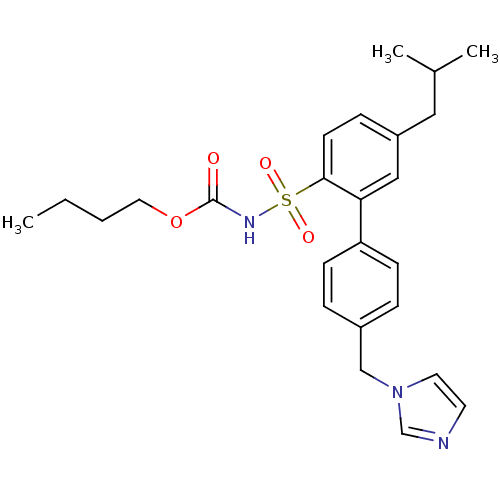 (CHEMBL217673 | N-butyloxycarbonyl-2-(4-imidazol-1-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [125I]Ang2 from AT2 receptor in pig uterus membrane | J Med Chem 49: 7160-8 (2006) Article DOI: 10.1021/jm0606185 BindingDB Entry DOI: 10.7270/Q21C1WHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50237166 (CHEMBL4097330) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description In vitro binding affinity against Tachykinin receptor 1 in guinea pig ileum using organ bath assay | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50236737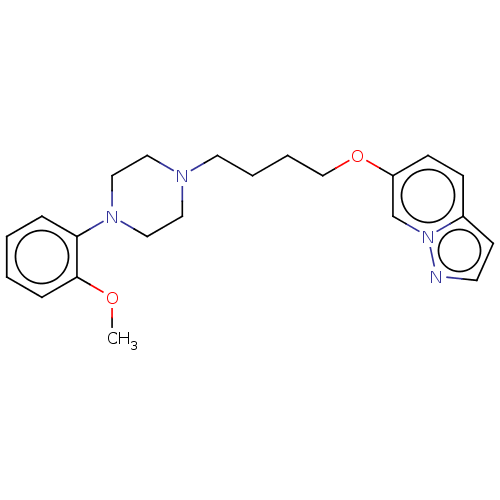 (CHEMBL4098803) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human Dopamine D4 receptor expressed in CHO cell membranes after 2 hrs by scintillation counting analysis | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50370686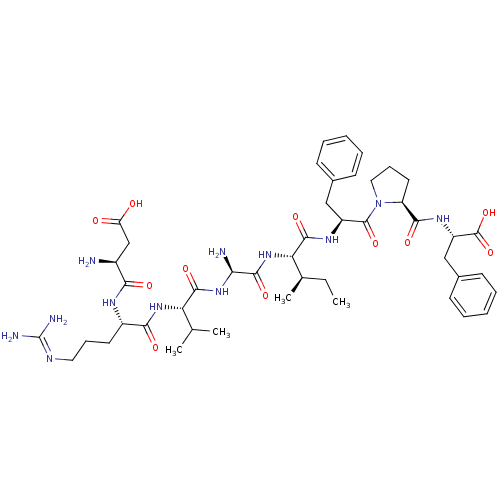 (CHEMBL1791350) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity for angiotensin II receptor, type 2 in pig uterus myometrium using [125I]-Ang II as radioligand, in pH 7.4 Tris-HCl buffer for 1.5 h... | J Med Chem 48: 6620-31 (2005) Article DOI: 10.1021/jm050280z BindingDB Entry DOI: 10.7270/Q2HX1DG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50236740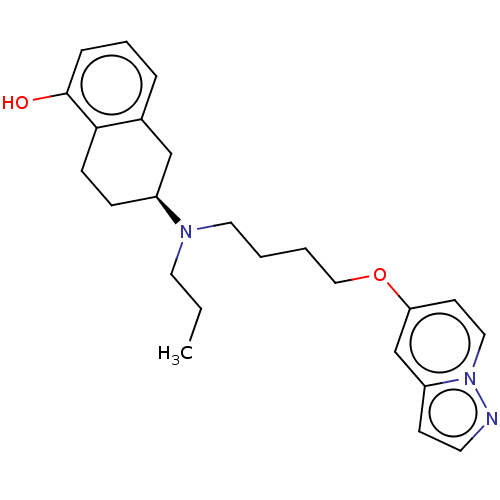 (CHEMBL4078348) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human Dopamine D2S receptor expressed in CHO cell membranes after 2 hrs by scintillation counting analysis | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50237166 (CHEMBL4097330) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Agonist activity at human Dopamine D3 receptor expressed in HEK293T cell membranes coexpressing GalphaoA incubated for 30 mins measured after 75 mins... | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50236959 (CHEMBL4060461) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human Dopamine D2S receptor expressed in CHO cell membranes after 2 hrs by scintillation counting analysis | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50236959 (CHEMBL4060461) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human Dopamine D2S receptor expressed in CHO cell membranes after 2 hrs by scintillation counting analysis | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50236737 (CHEMBL4098803) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human Dopamine D2S receptor expressed in CHO cell membranes after 2 hrs by scintillation counting analysis | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50237166 (CHEMBL4097330) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human Dopamine D2S receptor expressed in CHO cell membranes after 2 hrs by scintillation counting analysis | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236740 (CHEMBL4078348) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Agonist activity at human Dopamine D3 receptor expressed in HEK293T cell membranes coexpressing GalphaoA incubated for 30 mins measured after 75 mins... | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50168428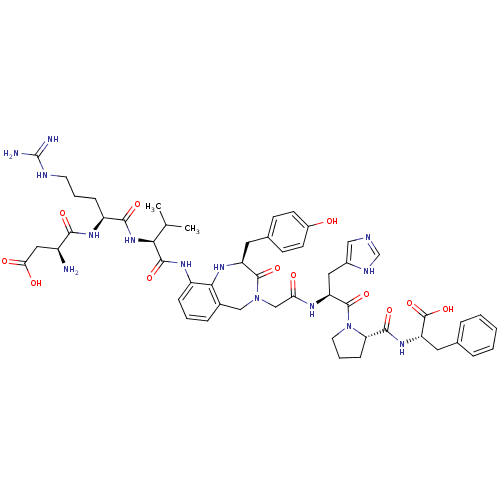 ((3S)-3-amino-3-{[(1S)-1-{[(1S)-1-{[(2S)-4-({[(2S)-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [125I]-Ang II from angiotensin II receptor type 2 in pig uterus myometrium | J Med Chem 48: 4009-24 (2005) Article DOI: 10.1021/jm0491492 BindingDB Entry DOI: 10.7270/Q2BR8SZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50237158 (CHEMBL4071868) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human Dopamine D2S receptor expressed in CHO cell membranes after 2 hrs by scintillation counting analysis | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50237165 (CHEMBL4098859) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human Dopamine D2S receptor expressed in CHO cell membranes after 2 hrs by scintillation counting analysis | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50370375 (CHEMBL268815) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [125I]-Ang II from angiotensin II receptor type 2 in pig uterus myometrium | J Med Chem 48: 4009-24 (2005) Article DOI: 10.1021/jm0491492 BindingDB Entry DOI: 10.7270/Q2BR8SZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50237162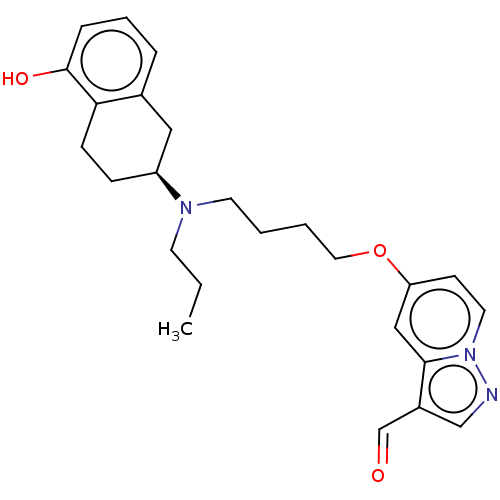 (CHEMBL4090208) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human Dopamine D2S receptor expressed in CHO cell membranes after 2 hrs by scintillation counting analysis | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50237164 (CHEMBL4091064) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human Dopamine D2L receptor expressed in CHO cell membranes after 2 hrs by scintillation counting analysis | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50237169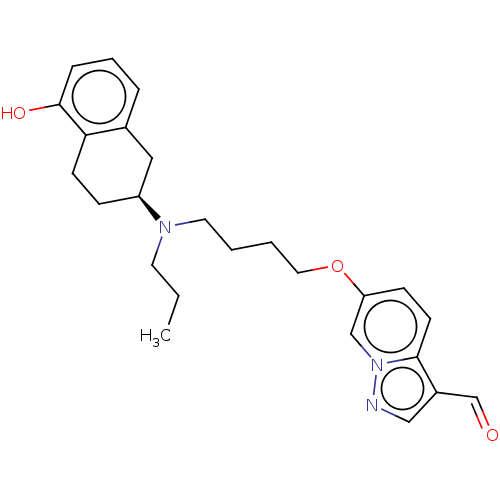 (CHEMBL4071185) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Inhibitory constant on human somatostatin receptor 5 | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50236962 (CHEMBL4096353) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human Dopamine D2L receptor expressed in CHO cell membranes after 2 hrs by scintillation counting analysis | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50199331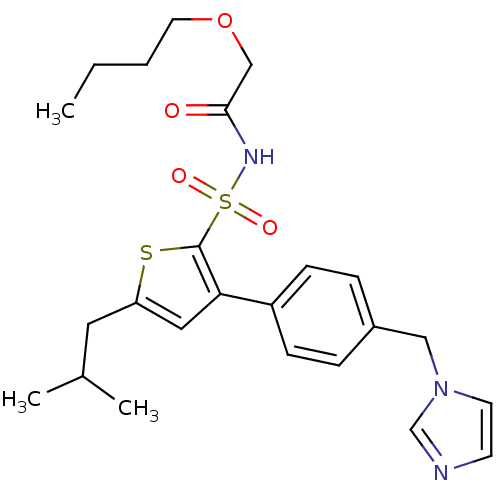 (CHEMBL216250 | N-(1-butyloxy-methylcarbonyl)-3-(4-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [125I]Ang2 from AT2 receptor in pig uterus membrane | J Med Chem 49: 7160-8 (2006) Article DOI: 10.1021/jm0606185 BindingDB Entry DOI: 10.7270/Q21C1WHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50237162 (CHEMBL4090208) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Inhibition of FPP incorporation into biotinylated K-Ras-derived peptide by human farnesyl transferase 5 nM ATP | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50236740 (CHEMBL4078348) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human Dopamine D4 receptor expressed in CHO cell membranes after 2 hrs by scintillation counting analysis | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50236959 (CHEMBL4060461) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human Dopamine D2L receptor expressed in CHO cell membranes after 2 hrs by scintillation counting analysis | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50236959 (CHEMBL4060461) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human Dopamine D2L receptor expressed in CHO cell membranes after 2 hrs by scintillation counting analysis | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50236961 (CHEMBL4063235) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human Dopamine D4 receptor expressed in CHO cell membranes after 2 hrs by scintillation counting analysis | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50237159 (CHEMBL4088639) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Ability to displace [3H]spiperone radioligand from cloned human Dopamine receptor D2 in CHO cells | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50237159 (CHEMBL4088639) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human Dopamine D2S receptor expressed in CHO cell membranes after 2 hrs by scintillation counting analysis | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 529 total ) | Next | Last >> |