

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10882 (6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding to human erythrocyte carbonic anhydrase was determined by fluorescence competition assay employing the fluorescent CA inhibitor dans... | J Med Chem 30: 591-7 (1987) BindingDB Entry DOI: 10.7270/Q2KW5GMM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50017729 (4-Ethylamino-7,7-dioxo-4,5,6,7-tetrahydro-7lambda*...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme-Chibret Curated by ChEMBL | Assay Description The equilibrium dissociation constant of the inhibitor-enzyme complex of human Carbonic anhydrase II | J Med Chem 32: 2510-3 (1989) BindingDB Entry DOI: 10.7270/Q29W0G22 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50041029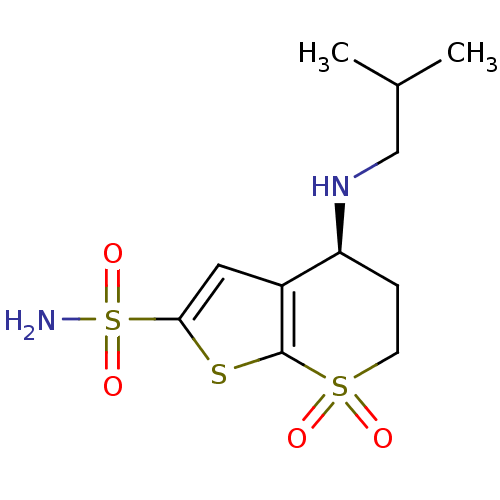 ((S)-4-Isobutylamino-7,7-dioxo-4,5,6,7-tetrahydro-7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme-Chibret Curated by ChEMBL | Assay Description The equilibrium dissociation constant of the inhibitor-enzyme complex of human Carbonic anhydrase II | J Med Chem 32: 2510-3 (1989) BindingDB Entry DOI: 10.7270/Q29W0G22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50041029 ((S)-4-Isobutylamino-7,7-dioxo-4,5,6,7-tetrahydro-7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme-Chibret Curated by ChEMBL | Assay Description 50% inhibitory concentration against human carbonic anhydrase II (HCA II) after pre-incubation for 4 min at 37 degree C | J Med Chem 32: 2510-3 (1989) BindingDB Entry DOI: 10.7270/Q29W0G22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50367851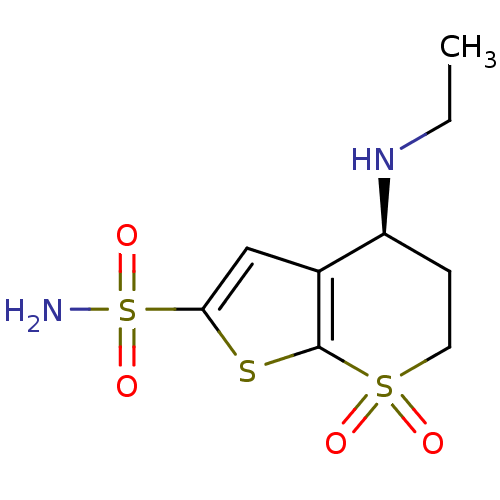 (CHEMBL1788291) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme-Chibret Curated by ChEMBL | Assay Description 50% inhibitory concentration against human carbonic anhydrase II (HCA II) after pre-incubation at 3 degree C | J Med Chem 32: 2510-3 (1989) BindingDB Entry DOI: 10.7270/Q29W0G22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50017728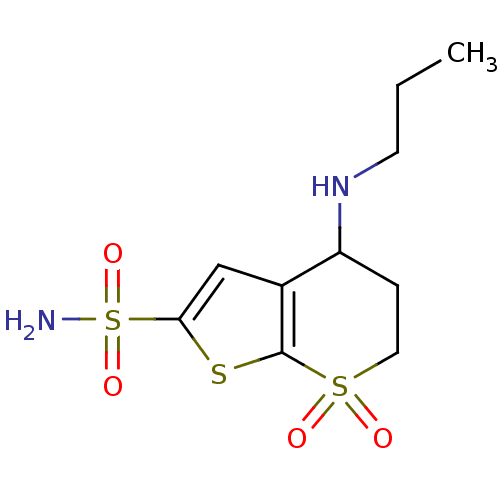 (7,7-Dioxo-4-propylamino-4,5,6,7-tetrahydro-7lambda...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme-Chibret Curated by ChEMBL | Assay Description The equilibrium dissociation constant of the inhibitor-enzyme complex of human Carbonic anhydrase II | J Med Chem 32: 2510-3 (1989) BindingDB Entry DOI: 10.7270/Q29W0G22 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50024220 (7-Oxo-6,7-dihydro-5H-thieno[3,2-b]thiopyran-2-sulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition against purified human erythrocyte carbonic anhydrase II was determined (acetozolamide used as control.) | J Med Chem 30: 591-7 (1987) BindingDB Entry DOI: 10.7270/Q2KW5GMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50017726 (4-Butylamino-7,7-dioxo-4,5,6,7-tetrahydro-7lambda*...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme-Chibret Curated by ChEMBL | Assay Description The equilibrium dissociation constant of the inhibitor-enzyme complex of human Carbonic anhydrase II | J Med Chem 32: 2510-3 (1989) BindingDB Entry DOI: 10.7270/Q29W0G22 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50017732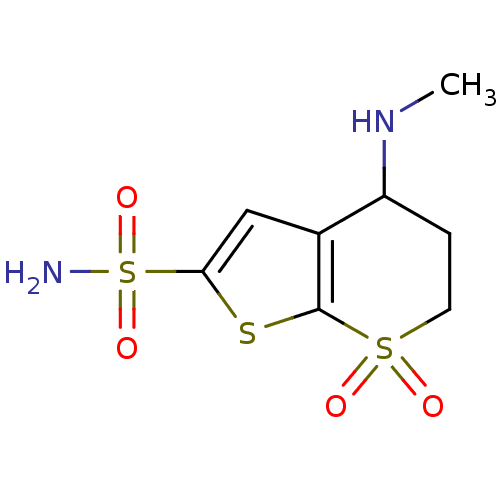 (4-Methylamino-7,7-dioxo-4,5,6,7-tetrahydro-7lambda...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme-Chibret Curated by ChEMBL | Assay Description The equilibrium dissociation constant of the inhibitor-enzyme complex of human Carbonic anhydrase II | J Med Chem 32: 2510-3 (1989) BindingDB Entry DOI: 10.7270/Q29W0G22 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50024224 (7,7-Dioxo-4,5,6,7-tetrahydro-7lambda*6*-thieno[2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding to human erythrocyte carbonic anhydrase was determined by fluorescence competition assay employing the fluorescent CA inhibitor dans... | J Med Chem 30: 591-7 (1987) BindingDB Entry DOI: 10.7270/Q2KW5GMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50024221 (4-Oxo-5,6-dihydro-4H-thieno[2,3-b]thiopyran-2-sulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition against purified human erythrocyte carbonic anhydrase II was determined (acetozolamide used as control.) | J Med Chem 30: 591-7 (1987) BindingDB Entry DOI: 10.7270/Q2KW5GMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50017731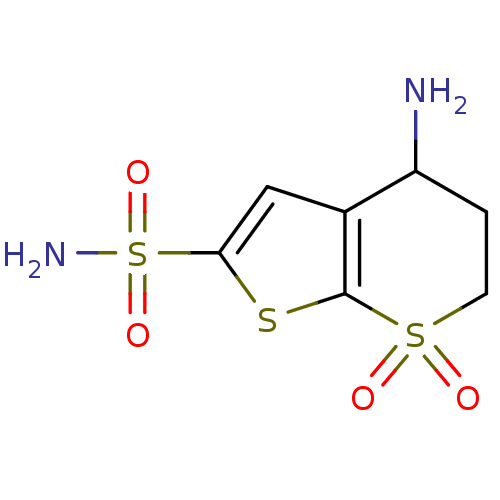 (4-Amino-7,7-dioxo-4,5,6,7-tetrahydro-7lambda*6*-th...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme-Chibret Curated by ChEMBL | Assay Description The equilibrium dissociation constant of the inhibitor-enzyme complex of human Carbonic anhydrase II | J Med Chem 32: 2510-3 (1989) BindingDB Entry DOI: 10.7270/Q29W0G22 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50452418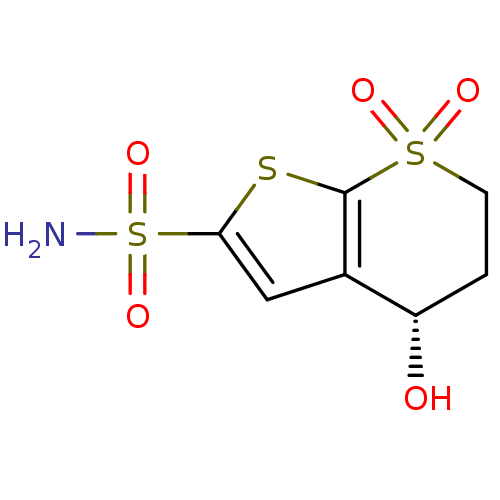 (CHEMBL2092886) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme-Chibret Curated by ChEMBL | Assay Description 50% inhibitory concentration against human carbonic anhydrase II (HCA II) after pre-incubation for 4 min at 37 degree C | J Med Chem 32: 2510-3 (1989) BindingDB Entry DOI: 10.7270/Q29W0G22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50452418 (CHEMBL2092886) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition against purified human erythrocyte carbonic anhydrase II was determined (acetozolamide used as control.) | J Med Chem 30: 591-7 (1987) BindingDB Entry DOI: 10.7270/Q2KW5GMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50017727 ((R) 4-Hydroxy-7,7-dioxo-4,5,6,7-tetrahydro-7lambda...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme-Chibret Curated by ChEMBL | Assay Description The equilibrium dissociation constant of the inhibitor-enzyme complex of human Carbonic anhydrase II | J Med Chem 32: 2510-3 (1989) BindingDB Entry DOI: 10.7270/Q29W0G22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50024222 (5-Hydroxy-7,7-dioxo-4,5,6,7-tetrahydro-7lambda*6*-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition against purified human erythrocyte carbonic anhydrase II was determined (acetozolamide used as control.) | J Med Chem 30: 591-7 (1987) BindingDB Entry DOI: 10.7270/Q2KW5GMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50017727 ((R) 4-Hydroxy-7,7-dioxo-4,5,6,7-tetrahydro-7lambda...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding to human erythrocyte Carbonic anhydrase II was determined by fluorescence competition assay employing the fluorescent CA inhibitor d... | J Med Chem 30: 591-7 (1987) BindingDB Entry DOI: 10.7270/Q2KW5GMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50017730 (4-Diethylamino-7,7-dioxo-4,5,6,7-tetrahydro-7lambd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme-Chibret Curated by ChEMBL | Assay Description The equilibrium dissociation constant of the inhibitor-enzyme complex of human Carbonic anhydrase II | J Med Chem 32: 2510-3 (1989) BindingDB Entry DOI: 10.7270/Q29W0G22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50024227 (7-Hydroxy-6,7-dihydro-5H-thieno[3,2-b]thiopyran-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition against purified human erythrocyte carbonic anhydrase II was determined (acetozolamide used as control.) | J Med Chem 30: 591-7 (1987) BindingDB Entry DOI: 10.7270/Q2KW5GMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50024219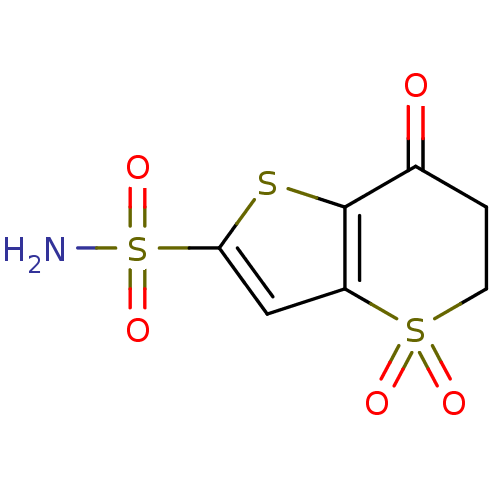 (4,4,7-Trioxo-4,5,6,7-tetrahydro-4lambda*6*-thieno[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition against purified human erythrocyte carbonic anhydrase II was determined (acetozolamide used as control.) | J Med Chem 30: 591-7 (1987) BindingDB Entry DOI: 10.7270/Q2KW5GMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50367483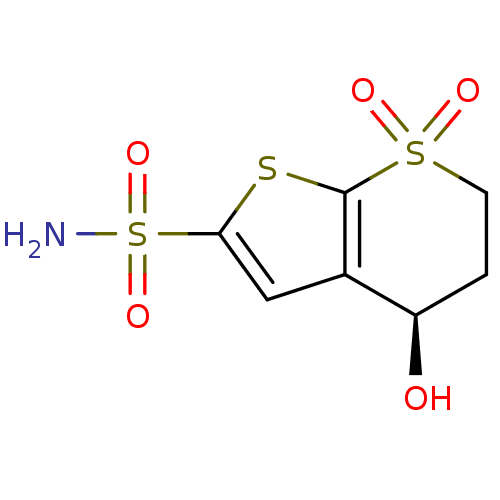 (CHEMBL1788205) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding to human erythrocyte carbonic anhydrase was determined by fluorescence competition assay employing the fluorescent CA inhibitor dans... | J Med Chem 30: 591-7 (1987) BindingDB Entry DOI: 10.7270/Q2KW5GMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50367483 (CHEMBL1788205) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme-Chibret Curated by ChEMBL | Assay Description The equilibrium dissociation constant of the inhibitor-enzyme complex of human carbonic anhydrase | J Med Chem 32: 2510-3 (1989) BindingDB Entry DOI: 10.7270/Q29W0G22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50452588 (CHEMBL2092885) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme-Chibret Curated by ChEMBL | Assay Description 50% inhibitory concentration against human carbonic anhydrase II (HCA II) after pre-incubation at 3 degree C | J Med Chem 32: 2510-3 (1989) BindingDB Entry DOI: 10.7270/Q29W0G22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50024223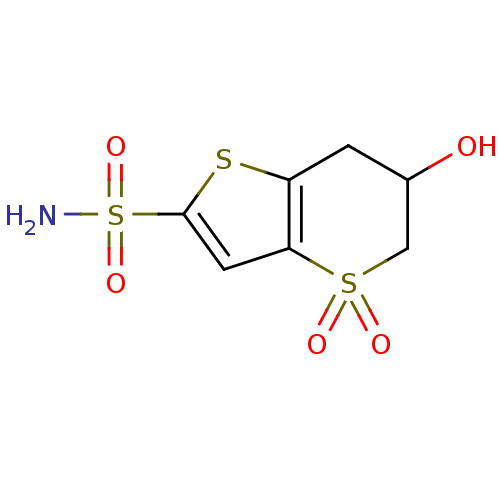 (6-Hydroxy-4,4-dioxo-4,5,6,7-tetrahydro-4lambda*6*-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding to human erythrocyte carbonic anhydrase was determined by fluorescence competition assay employing the fluorescent CA inhibitor dans... | J Med Chem 30: 591-7 (1987) BindingDB Entry DOI: 10.7270/Q2KW5GMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme-Chibret Curated by ChEMBL | Assay Description The equilibrium dissociation constant of the inhibitor-enzyme complex of human carbonic anhydrase | J Med Chem 32: 2510-3 (1989) BindingDB Entry DOI: 10.7270/Q29W0G22 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50024225 (4-Hydroxy-5,6-dihydro-4H-thieno[2,3-b]thiopyran-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding to human erythrocyte Carbonic anhydrase II was determined by fluorescence competition assay employing the fluorescent CA inhibitor d... | J Med Chem 30: 591-7 (1987) BindingDB Entry DOI: 10.7270/Q2KW5GMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50024218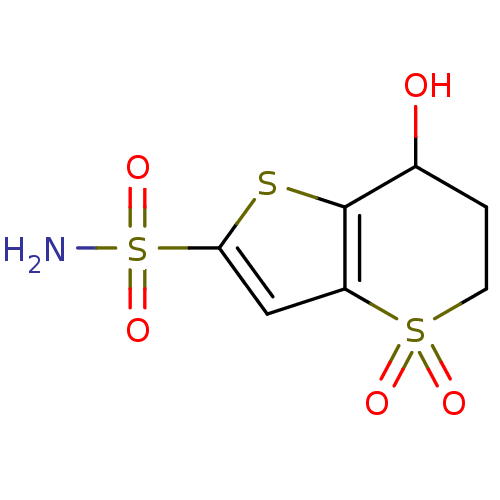 (7-Hydroxy-4,4-dioxo-4,5,6,7-tetrahydro-4lambda*6*-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition against purified human erythrocyte carbonic anhydrase II was determined (acetozolamide used as control.) | J Med Chem 30: 591-7 (1987) BindingDB Entry DOI: 10.7270/Q2KW5GMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070791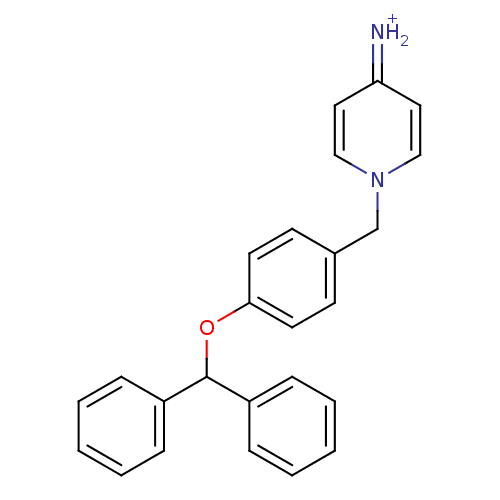 (4-Amino-1-(4-benzhydryloxy-benzyl)-pyridinium | CH...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human thrombin | Bioorg Med Chem Lett 8: 1697-702 (1999) BindingDB Entry DOI: 10.7270/Q26T0KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50041028 ((R)-4-Isobutylamino-7,7-dioxo-4,5,6,7-tetrahydro-7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme-Chibret Curated by ChEMBL | Assay Description 50% inhibitory concentration against human carbonic anhydrase II (HCA II) after pre-incubation for 4 min at 37 degree C | J Med Chem 32: 2510-3 (1989) BindingDB Entry DOI: 10.7270/Q29W0G22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50024226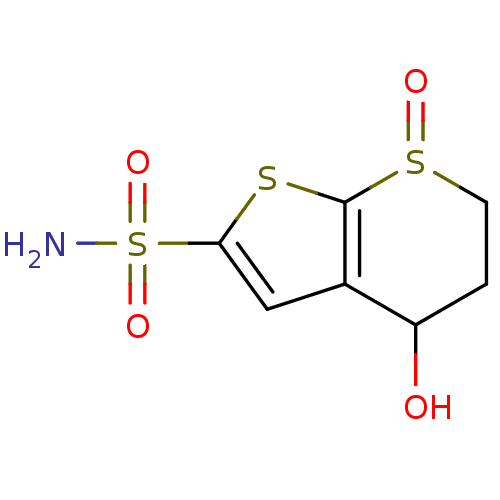 (4-Hydroxy-7-oxo-4,5,6,7-tetrahydro-7lambda*4*-thie...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 104 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition against purified human erythrocyte carbonic anhydrase II was determined (acetozolamide used as control.) | J Med Chem 30: 591-7 (1987) BindingDB Entry DOI: 10.7270/Q2KW5GMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070787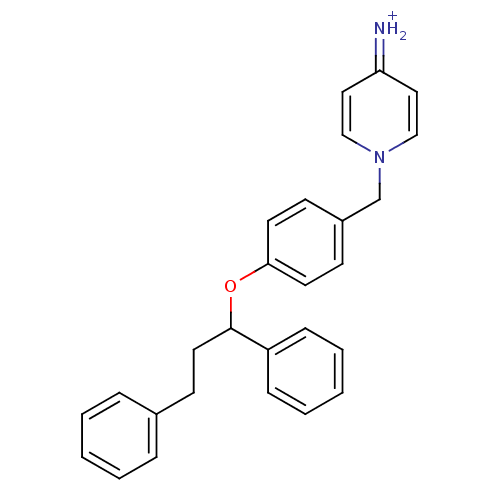 (4-Amino-1-[4-(1,3-diphenyl-propoxy)-benzyl]-pyridi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human thrombin | Bioorg Med Chem Lett 8: 1697-702 (1999) BindingDB Entry DOI: 10.7270/Q26T0KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50024228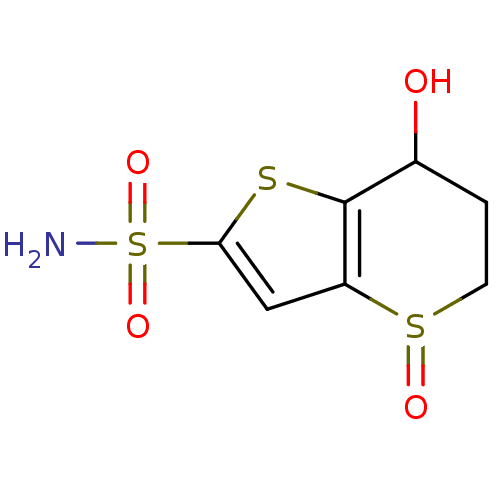 (7-Hydroxy-4-oxo-4,5,6,7-tetrahydro-4lambda*4*-thie...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 309 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition against purified human erythrocyte carbonic anhydrase II was determined (acetozolamide used as control.) | J Med Chem 30: 591-7 (1987) BindingDB Entry DOI: 10.7270/Q2KW5GMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070786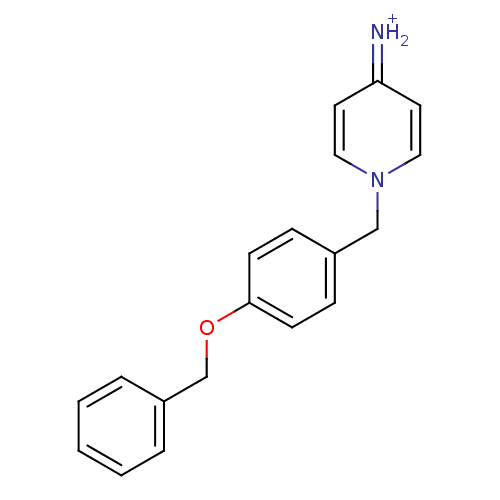 (4-Amino-1-(4-benzyloxy-benzyl)-pyridinium | CHEMBL...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human thrombin | Bioorg Med Chem Lett 8: 1697-702 (1999) BindingDB Entry DOI: 10.7270/Q26T0KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070793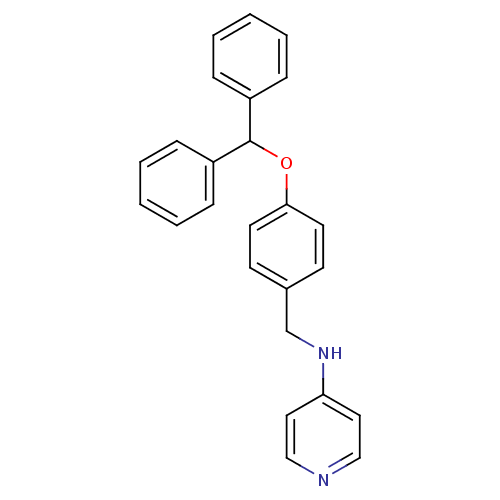 ((4-Benzhydryloxy-benzyl)-pyridin-4-yl-amine | CHEM...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human thrombin | Bioorg Med Chem Lett 8: 1697-702 (1999) BindingDB Entry DOI: 10.7270/Q26T0KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070790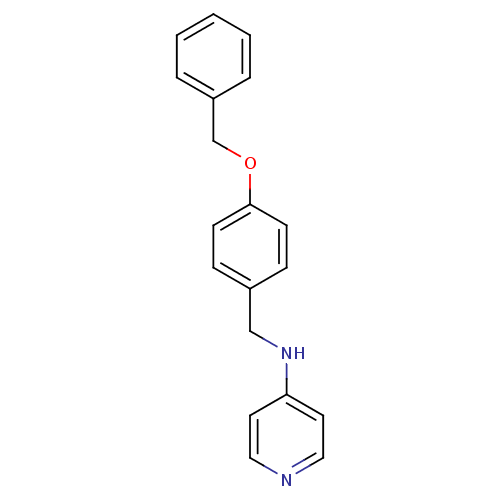 ((4-Benzyloxy-benzyl)-pyridin-4-yl-amine | CHEMBL47...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human thrombin | Bioorg Med Chem Lett 8: 1697-702 (1999) BindingDB Entry DOI: 10.7270/Q26T0KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070788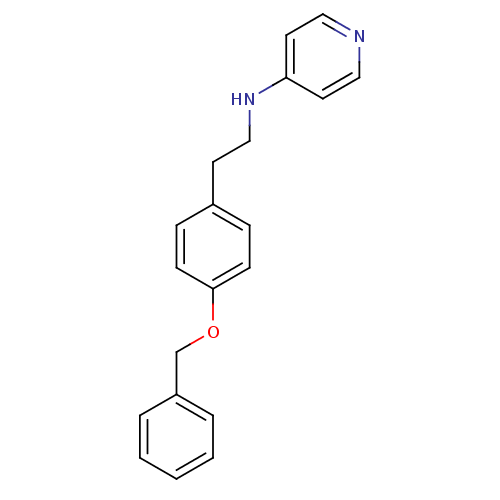 (CHEMBL48029 | N-(4-(benzyloxy)phenethyl)pyridin-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human thrombin | Bioorg Med Chem Lett 8: 1697-702 (1999) BindingDB Entry DOI: 10.7270/Q26T0KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50070792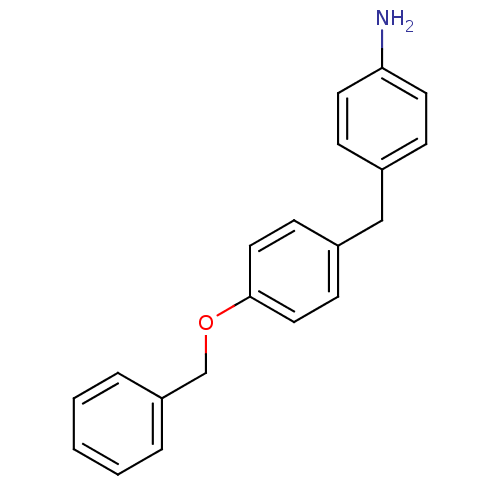 (4-(4-Benzyloxy-benzyl)-phenylamine | CHEMBL45648) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against trypsin | Bioorg Med Chem Lett 8: 1697-702 (1999) BindingDB Entry DOI: 10.7270/Q26T0KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070792 (4-(4-Benzyloxy-benzyl)-phenylamine | CHEMBL45648) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human thrombin | Bioorg Med Chem Lett 8: 1697-702 (1999) BindingDB Entry DOI: 10.7270/Q26T0KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50070787 (4-Amino-1-[4-(1,3-diphenyl-propoxy)-benzyl]-pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against trypsin | Bioorg Med Chem Lett 8: 1697-702 (1999) BindingDB Entry DOI: 10.7270/Q26T0KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50070786 (4-Amino-1-(4-benzyloxy-benzyl)-pyridinium | CHEMBL...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against trypsin | Bioorg Med Chem Lett 8: 1697-702 (1999) BindingDB Entry DOI: 10.7270/Q26T0KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50070793 ((4-Benzhydryloxy-benzyl)-pyridin-4-yl-amine | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against trypsin | Bioorg Med Chem Lett 8: 1697-702 (1999) BindingDB Entry DOI: 10.7270/Q26T0KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50070790 ((4-Benzyloxy-benzyl)-pyridin-4-yl-amine | CHEMBL47...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against trypsin | Bioorg Med Chem Lett 8: 1697-702 (1999) BindingDB Entry DOI: 10.7270/Q26T0KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070789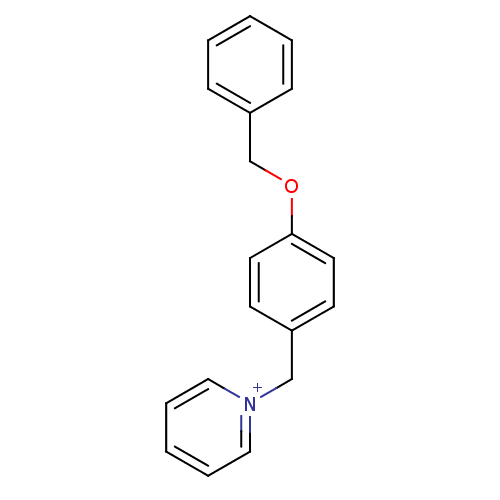 (1-(4-Benzyloxy-benzyl)-pyridinium | CHEMBL296280) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human thrombin | Bioorg Med Chem Lett 8: 1697-702 (1999) BindingDB Entry DOI: 10.7270/Q26T0KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50070791 (4-Amino-1-(4-benzhydryloxy-benzyl)-pyridinium | CH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.17E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against trypsin | Bioorg Med Chem Lett 8: 1697-702 (1999) BindingDB Entry DOI: 10.7270/Q26T0KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50070788 (CHEMBL48029 | N-(4-(benzyloxy)phenethyl)pyridin-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 5.68E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against trypsin | Bioorg Med Chem Lett 8: 1697-702 (1999) BindingDB Entry DOI: 10.7270/Q26T0KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070794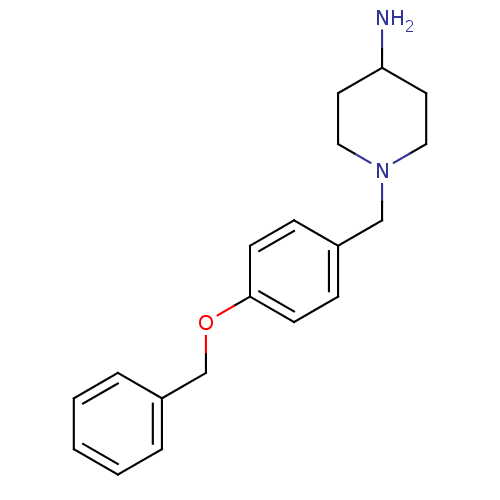 (1-(4-Benzyloxy-benzyl)-piperidin-4-ylamine | CHEMB...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.81E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human thrombin | Bioorg Med Chem Lett 8: 1697-702 (1999) BindingDB Entry DOI: 10.7270/Q26T0KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 11/12/13/14 (Homo sapiens (Human)) | BDBM15240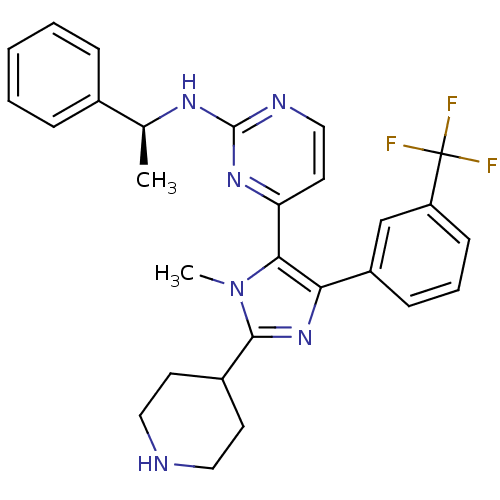 (4-[1-methyl-2-(piperidin-4-yl)-4-[3-(trifluorometh...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Mitogen-activated protein kinase p38 | Bioorg Med Chem Lett 12: 689-92 (2002) BindingDB Entry DOI: 10.7270/Q2930TQS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 11/12/13/14 (Homo sapiens (Human)) | BDBM50219316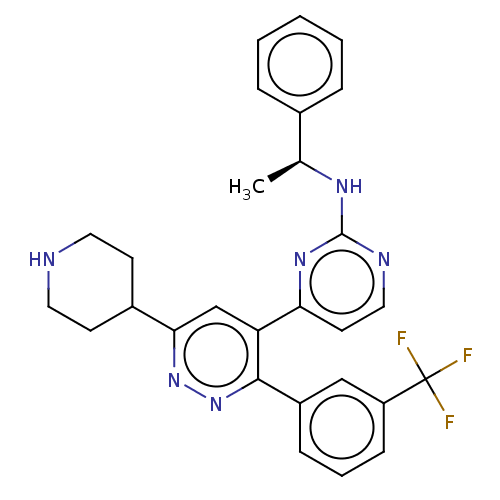 (CHEMBL545353) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Mitogen-activated protein kinase p38 | Bioorg Med Chem Lett 12: 689-92 (2002) BindingDB Entry DOI: 10.7270/Q2930TQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 11/12/13/14 (Homo sapiens (Human)) | BDBM50219323 (CHEMBL161276) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Mitogen-activated protein kinase p38 | Bioorg Med Chem Lett 12: 689-92 (2002) BindingDB Entry DOI: 10.7270/Q2930TQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 11/12/13/14 (Homo sapiens (Human)) | BDBM50219315 (CHEMBL161477) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Mitogen-activated protein kinase p38 | Bioorg Med Chem Lett 12: 689-92 (2002) BindingDB Entry DOI: 10.7270/Q2930TQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 114 total ) | Next | Last >> |