

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18207 ((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at glucocorticoid receptor in human foreskin fibroblasts assessed as inhibition of IL-1-induced IL-6 production by trans-repression ... | Bioorg Med Chem Lett 24: 1934-40 (2014) Article DOI: 10.1016/j.bmcl.2014.03.005 BindingDB Entry DOI: 10.7270/Q2251KPB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50330100 (CHEMBL1271550 | N-(4-(3-(pyridin-3-yl)-5-(trifluor...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-cDPPO from human Ephx2 by cell-based assay | Bioorg Med Chem Lett 20: 6379-83 (2010) Article DOI: 10.1016/j.bmcl.2010.09.095 BindingDB Entry DOI: 10.7270/Q2V69JT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM32416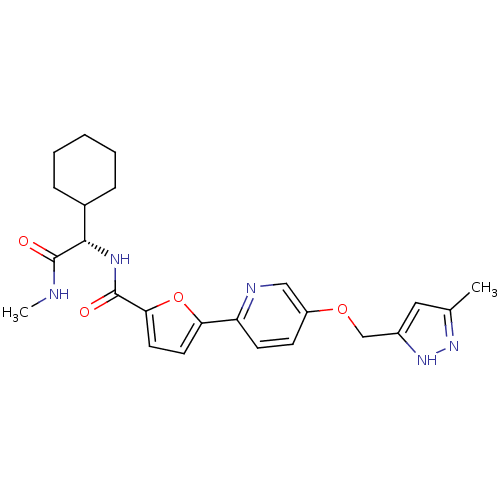 (furan-2-carboxamide deriv., 28) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Boehringer Ingelheim Pharmaceuticals | Assay Description MMP-13 was assessed by using the EnzoLyte 520 Generic MMP Assay Kit (AnaSpec Inc.). This kit uses a 5-FAM/QXL 520 fluorescence resonance 10 energy tr... | Bioorg Med Chem Lett 19: 5321-4 (2009) Article DOI: 10.1016/j.bmcl.2009.07.151 BindingDB Entry DOI: 10.7270/Q22B8WCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50001768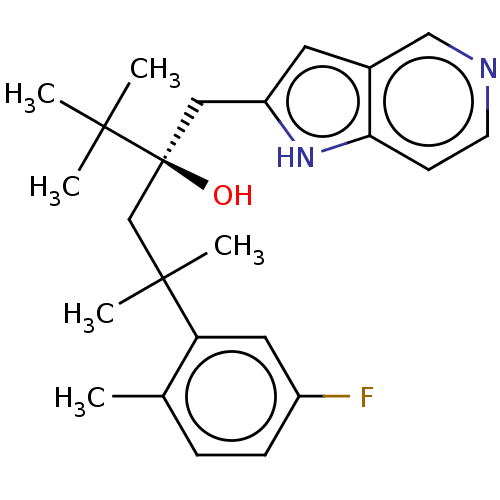 (CHEMBL3233281) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of TAMRA-labeled dexamethasone from glucocorticoid receptor (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 24: 1934-40 (2014) Article DOI: 10.1016/j.bmcl.2014.03.005 BindingDB Entry DOI: 10.7270/Q2251KPB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18207 ((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of tetramethylrhodamine labeled dexamethosone binding to GR by FP assay | J Med Chem 49: 7887-96 (2006) Article DOI: 10.1021/jm061273t BindingDB Entry DOI: 10.7270/Q2BG2PTB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18207 ((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of TAMRA-labeled dexamethasone from glucocorticoid receptor (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 24: 1934-40 (2014) Article DOI: 10.1016/j.bmcl.2014.03.005 BindingDB Entry DOI: 10.7270/Q2251KPB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50201093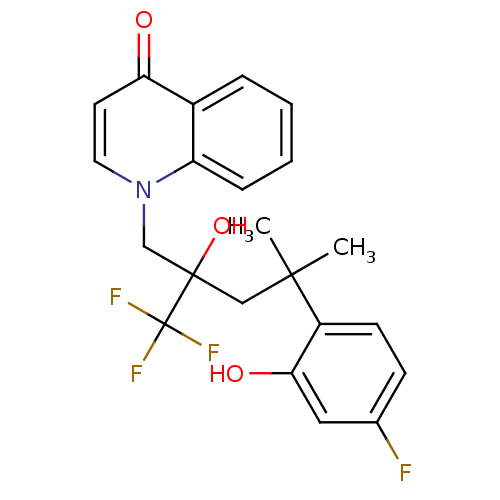 (1-[4-(4-fluoro-2-hydroxyphenyl)-2-hydroxy-4-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of tetramethylrhodamine labeled dexamethosone binding to GR by FP assay | J Med Chem 49: 7887-96 (2006) Article DOI: 10.1021/jm061273t BindingDB Entry DOI: 10.7270/Q2BG2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50201099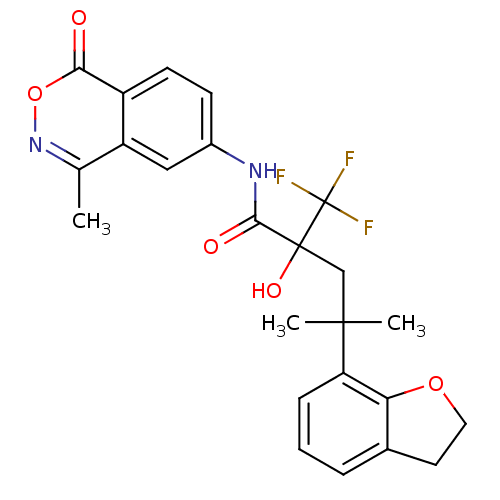 (4-(2,3-dihydrobenzofuran-7-yl)-2-hydroxy-4-methyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of tetramethylrhodamine labeled dexamethosone binding to GR by FP assay | J Med Chem 49: 7887-96 (2006) Article DOI: 10.1021/jm061273t BindingDB Entry DOI: 10.7270/Q2BG2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50001763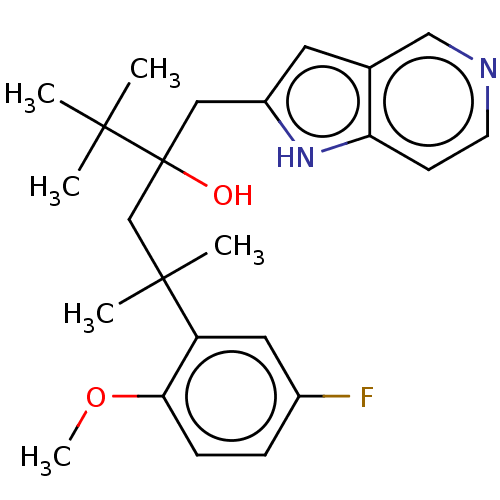 (CHEMBL3233277) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of TAMRA-labeled dexamethasone from glucocorticoid receptor (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 24: 1934-40 (2014) Article DOI: 10.1016/j.bmcl.2014.03.005 BindingDB Entry DOI: 10.7270/Q2251KPB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM32417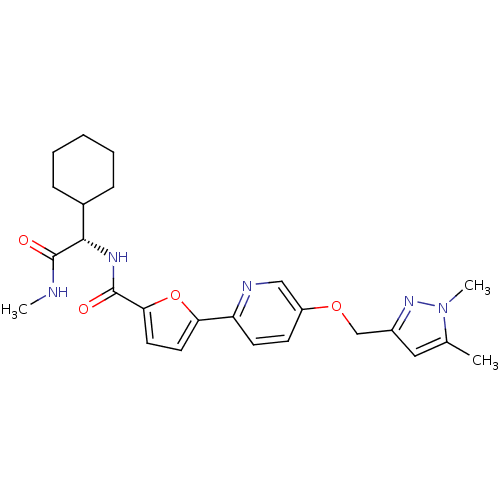 (furan-2-carboxamide deriv., 29) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Boehringer Ingelheim Pharmaceuticals | Assay Description MMP-13 was assessed by using the EnzoLyte 520 Generic MMP Assay Kit (AnaSpec Inc.). This kit uses a 5-FAM/QXL 520 fluorescence resonance 10 energy tr... | Bioorg Med Chem Lett 19: 5321-4 (2009) Article DOI: 10.1016/j.bmcl.2009.07.151 BindingDB Entry DOI: 10.7270/Q22B8WCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50201100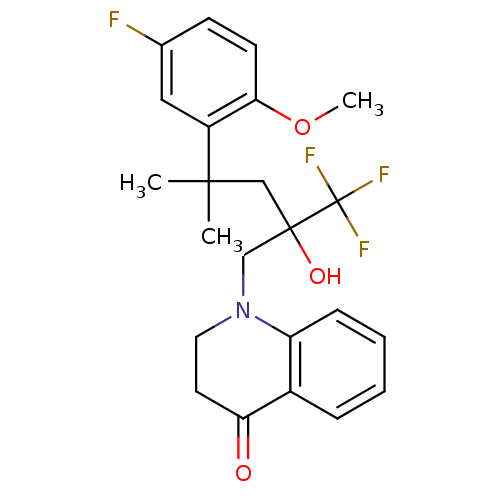 (1-[4-(5-fluoro-2-methoxyphenyl)-2-hydroxy-4-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of tetramethylrhodamine labeled dexamethosone binding to GR by FP assay | J Med Chem 49: 7887-96 (2006) Article DOI: 10.1021/jm061273t BindingDB Entry DOI: 10.7270/Q2BG2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50201081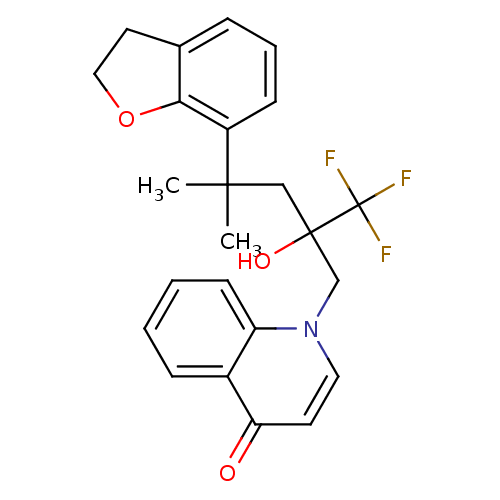 (1-[4-(2,3-dihydrobenzofuran-7-yl)-2-hydroxy-4-meth...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of tetramethylrhodamine labeled dexamethosone binding to GR by FP assay | J Med Chem 49: 7887-96 (2006) Article DOI: 10.1021/jm061273t BindingDB Entry DOI: 10.7270/Q2BG2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50326933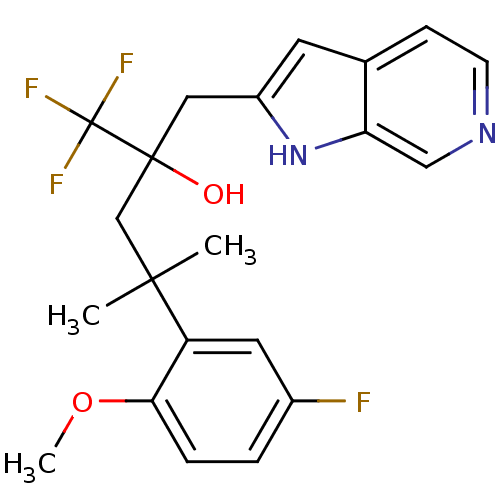 (1,1,1-Trifluoro-4-(5-fluoro-2-methoxyphenyl)-4-met...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of TAMRA-labeled dexamethasone from glucocorticoid receptor (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 24: 1934-40 (2014) Article DOI: 10.1016/j.bmcl.2014.03.005 BindingDB Entry DOI: 10.7270/Q2251KPB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50330103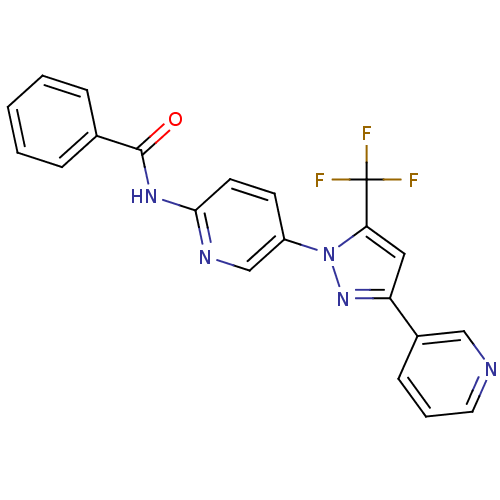 (CHEMBL1271608 | N-(5-(3-(pyridin-3-yl)-5-(trifluor...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of human Ephx2 after 30 mins by fluorescence polarization assay | Bioorg Med Chem Lett 20: 6379-83 (2010) Article DOI: 10.1016/j.bmcl.2010.09.095 BindingDB Entry DOI: 10.7270/Q2V69JT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50330103 (CHEMBL1271608 | N-(5-(3-(pyridin-3-yl)-5-(trifluor...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-cDPPO from human Ephx2 by cell-based assay | Bioorg Med Chem Lett 20: 6379-83 (2010) Article DOI: 10.1016/j.bmcl.2010.09.095 BindingDB Entry DOI: 10.7270/Q2V69JT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50201090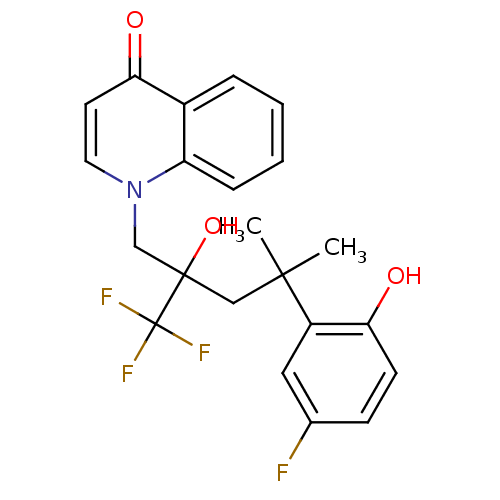 (1-[4-(5-fluoro-2-hydroxyphenyl)-2-hydroxy-4-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of tetramethylrhodamine labeled dexamethosone binding to GR by FP assay | J Med Chem 49: 7887-96 (2006) Article DOI: 10.1021/jm061273t BindingDB Entry DOI: 10.7270/Q2BG2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50001766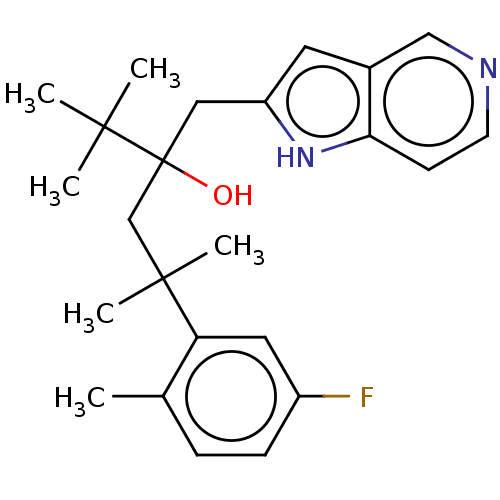 (CHEMBL3233279) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of TAMRA-labeled dexamethasone from glucocorticoid receptor (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 24: 1934-40 (2014) Article DOI: 10.1016/j.bmcl.2014.03.005 BindingDB Entry DOI: 10.7270/Q2251KPB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM32414 (furan-2-carboxamide deriv., 26) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Boehringer Ingelheim Pharmaceuticals | Assay Description MMP-13 was assessed by using the EnzoLyte 520 Generic MMP Assay Kit (AnaSpec Inc.). This kit uses a 5-FAM/QXL 520 fluorescence resonance 10 energy tr... | Bioorg Med Chem Lett 19: 5321-4 (2009) Article DOI: 10.1016/j.bmcl.2009.07.151 BindingDB Entry DOI: 10.7270/Q22B8WCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50001764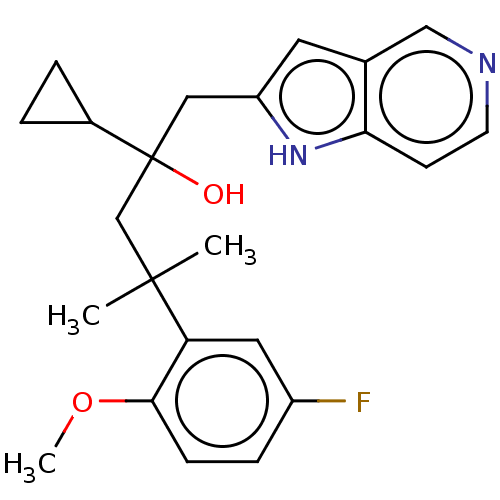 (CHEMBL3233278) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of TAMRA-labeled dexamethasone from glucocorticoid receptor (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 24: 1934-40 (2014) Article DOI: 10.1016/j.bmcl.2014.03.005 BindingDB Entry DOI: 10.7270/Q2251KPB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50201096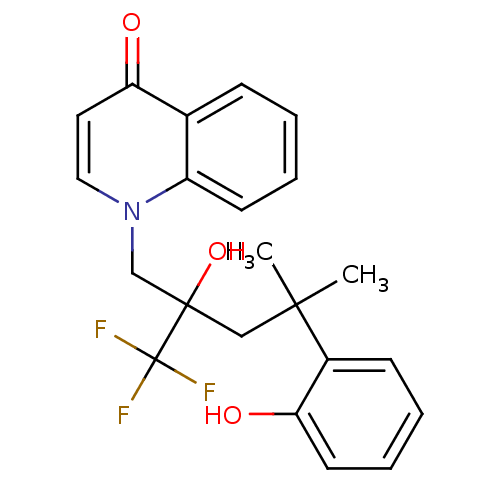 (1-[2-hydroxy-4-(2-hydroxyphenyl)-4-methyl-2-(trifl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of tetramethylrhodamine labeled dexamethosone binding to GR by FP assay | J Med Chem 49: 7887-96 (2006) Article DOI: 10.1021/jm061273t BindingDB Entry DOI: 10.7270/Q2BG2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM19190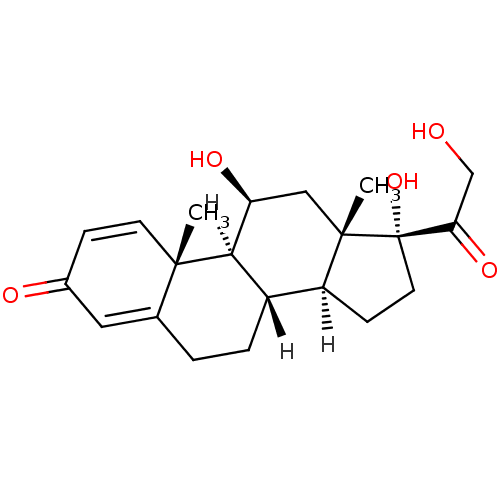 ((1S,2R,10S,11S,14R,15S,17S)-14,17-dihydroxy-14-(2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at glucocorticoid receptor in human foreskin fibroblasts assessed as inhibition of IL-1-induced IL-6 production by trans-repression ... | Bioorg Med Chem Lett 24: 1934-40 (2014) Article DOI: 10.1016/j.bmcl.2014.03.005 BindingDB Entry DOI: 10.7270/Q2251KPB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM32412 (furan-2-carboxamide deriv., 24) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Boehringer Ingelheim Pharmaceuticals | Assay Description MMP-13 was assessed by using the EnzoLyte 520 Generic MMP Assay Kit (AnaSpec Inc.). This kit uses a 5-FAM/QXL 520 fluorescence resonance 10 energy tr... | Bioorg Med Chem Lett 19: 5321-4 (2009) Article DOI: 10.1016/j.bmcl.2009.07.151 BindingDB Entry DOI: 10.7270/Q22B8WCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50330100 (CHEMBL1271550 | N-(4-(3-(pyridin-3-yl)-5-(trifluor...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of human Ephx2 after 30 mins by fluorescence polarization assay | Bioorg Med Chem Lett 20: 6379-83 (2010) Article DOI: 10.1016/j.bmcl.2010.09.095 BindingDB Entry DOI: 10.7270/Q2V69JT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50330099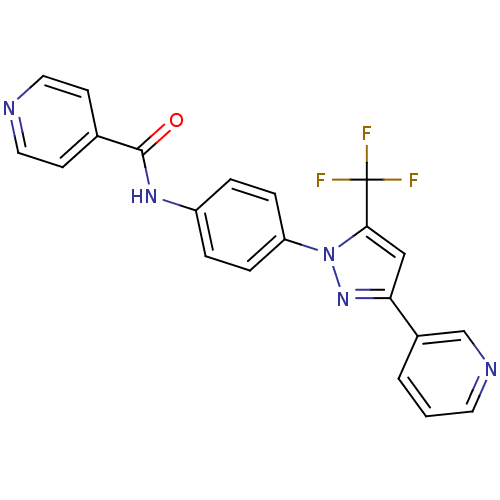 (CHEMBL1271498 | N-(4-(3-(pyridin-3-yl)-5-(trifluor...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of human Ephx2 after 30 mins by fluorescence polarization assay | Bioorg Med Chem Lett 20: 6379-83 (2010) Article DOI: 10.1016/j.bmcl.2010.09.095 BindingDB Entry DOI: 10.7270/Q2V69JT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50001764 (CHEMBL3233278) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at glucocorticoid receptor in human foreskin fibroblasts assessed as inhibition of IL-1-induced IL-6 production by trans-repression ... | Bioorg Med Chem Lett 24: 1934-40 (2014) Article DOI: 10.1016/j.bmcl.2014.03.005 BindingDB Entry DOI: 10.7270/Q2251KPB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50201082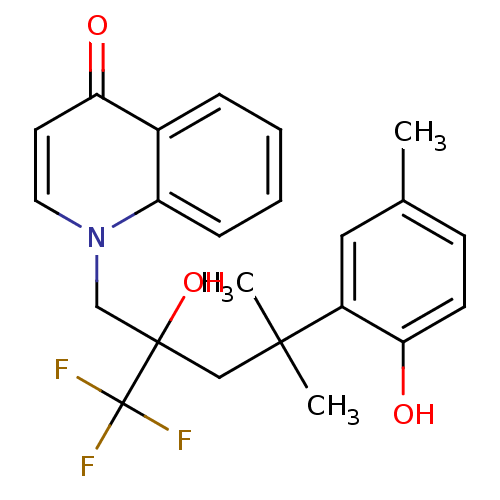 (1-[2-hydroxy-4-(2-hydroxy-5-methylphenyl)-4-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of tetramethylrhodamine labeled dexamethosone binding to GR by FP assay | J Med Chem 49: 7887-96 (2006) Article DOI: 10.1021/jm061273t BindingDB Entry DOI: 10.7270/Q2BG2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50001819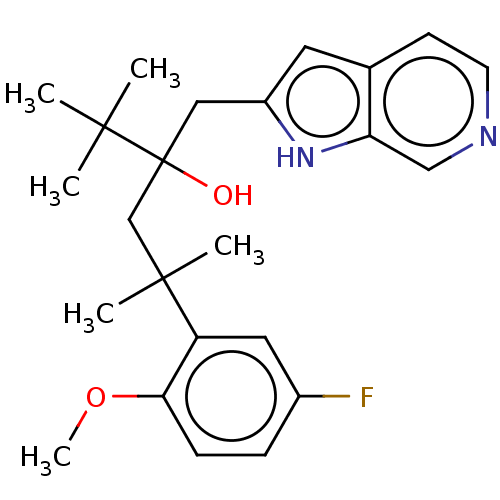 (CHEMBL3233272) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of TAMRA-labeled dexamethasone from glucocorticoid receptor (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 24: 1934-40 (2014) Article DOI: 10.1016/j.bmcl.2014.03.005 BindingDB Entry DOI: 10.7270/Q2251KPB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50201104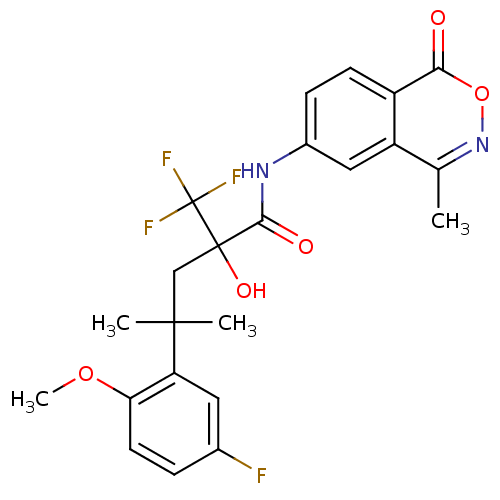 (4-(5-fluoro-2-methoxyphenyl)-2-hydroxy-4-methyl-N-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of tetramethylrhodamine labeled dexamethosone binding to GR by FP assay | J Med Chem 49: 7887-96 (2006) Article DOI: 10.1021/jm061273t BindingDB Entry DOI: 10.7270/Q2BG2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50330105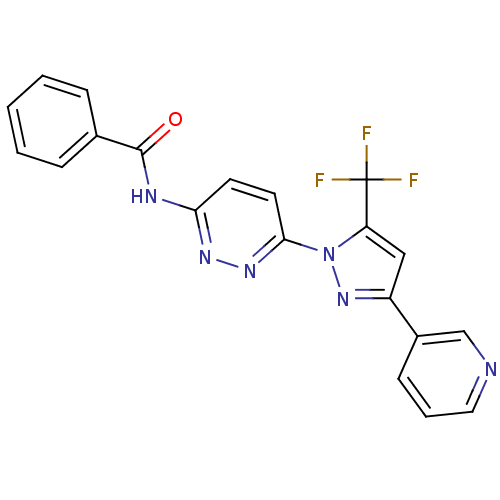 (CHEMBL1271659 | N-(6-(3-(pyridin-3-yl)-5-(trifluor...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of human Ephx2 after 30 mins by fluorescence polarization assay | Bioorg Med Chem Lett 20: 6379-83 (2010) Article DOI: 10.1016/j.bmcl.2010.09.095 BindingDB Entry DOI: 10.7270/Q2V69JT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50001767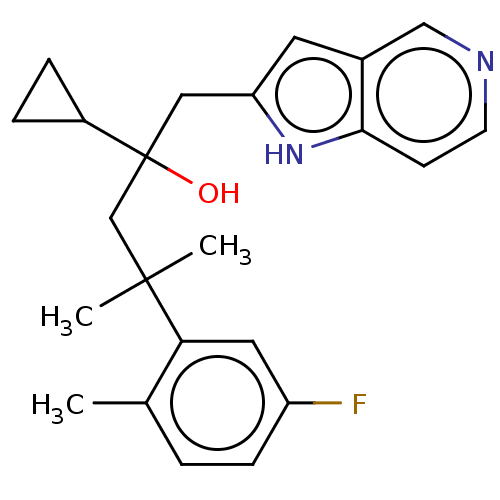 (CHEMBL3233280) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of TAMRA-labeled dexamethasone from glucocorticoid receptor (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 24: 1934-40 (2014) Article DOI: 10.1016/j.bmcl.2014.03.005 BindingDB Entry DOI: 10.7270/Q2251KPB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50001818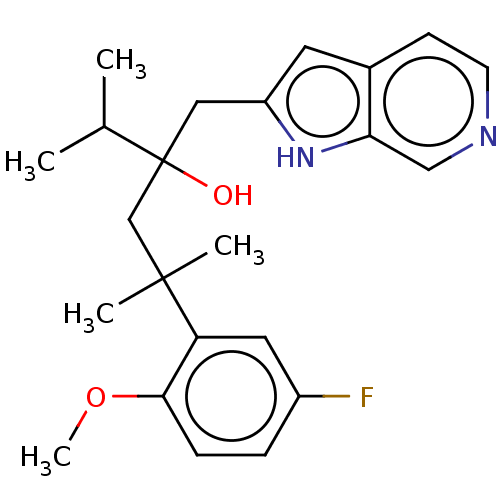 (CHEMBL3233271) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of TAMRA-labeled dexamethasone from glucocorticoid receptor (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 24: 1934-40 (2014) Article DOI: 10.1016/j.bmcl.2014.03.005 BindingDB Entry DOI: 10.7270/Q2251KPB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50001763 (CHEMBL3233277) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at glucocorticoid receptor in human foreskin fibroblasts assessed as inhibition of IL-1-induced IL-6 production by trans-repression ... | Bioorg Med Chem Lett 24: 1934-40 (2014) Article DOI: 10.1016/j.bmcl.2014.03.005 BindingDB Entry DOI: 10.7270/Q2251KPB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50330104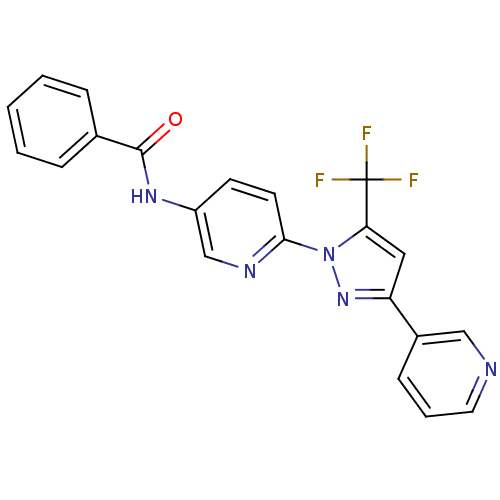 (CHEMBL1271658 | N-(6-(3-(pyridin-3-yl)-5-(trifluor...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of human Ephx2 after 30 mins by fluorescence polarization assay | Bioorg Med Chem Lett 20: 6379-83 (2010) Article DOI: 10.1016/j.bmcl.2010.09.095 BindingDB Entry DOI: 10.7270/Q2V69JT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50326933 (1,1,1-Trifluoro-4-(5-fluoro-2-methoxyphenyl)-4-met...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at glucocorticoid receptor in human foreskin fibroblasts assessed as inhibition of IL-1-induced IL-6 production by trans-repression ... | Bioorg Med Chem Lett 24: 1934-40 (2014) Article DOI: 10.1016/j.bmcl.2014.03.005 BindingDB Entry DOI: 10.7270/Q2251KPB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071365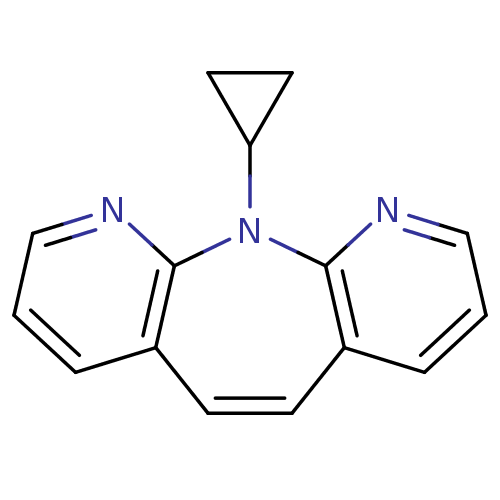 (5-Cyclopropyl-5H-4,5,6-triaza-dibenzo[a,d]cyclohep...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against HIV-1 mutant type reverse transcriptase (Y181C) | Bioorg Med Chem Lett 8: 2169-72 (1999) BindingDB Entry DOI: 10.7270/Q2028QP0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50201086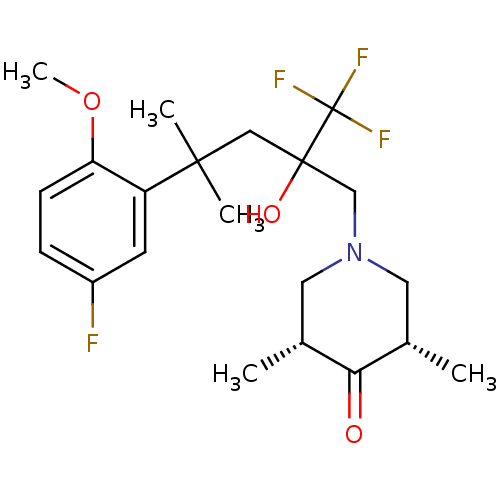 (CHEMBL216273 | cis-1-[4-(5-fluoro-2-methoxyphenyl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of tetramethylrhodamine labeled dexamethosone binding to GR by FP assay | J Med Chem 49: 7887-96 (2006) Article DOI: 10.1021/jm061273t BindingDB Entry DOI: 10.7270/Q2BG2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071364 (5-Ethyl-5H-4,5,6-triaza-dibenzo[a,d]cycloheptene |...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against HIV-1 mutant type reverse transcriptase (P236L) | Bioorg Med Chem Lett 8: 2169-72 (1999) BindingDB Entry DOI: 10.7270/Q2028QP0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50201109 (1-[4-(5-fluoro-2-methoxyphenyl)-2-hydroxy-4-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of tetramethylrhodamine labeled dexamethosone binding to GR by FP assay | J Med Chem 49: 7887-96 (2006) Article DOI: 10.1021/jm061273t BindingDB Entry DOI: 10.7270/Q2BG2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1528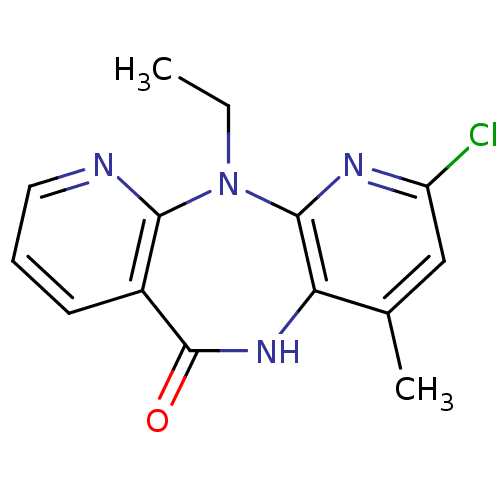 (5-chloro-2-ethyl-7-methyl-2,4,9,15-tetraazatricycl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.8 | 25 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 38: 4830-8 (1995) Article DOI: 10.1021/jm00024a010 BindingDB Entry DOI: 10.7270/Q23B5XBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1539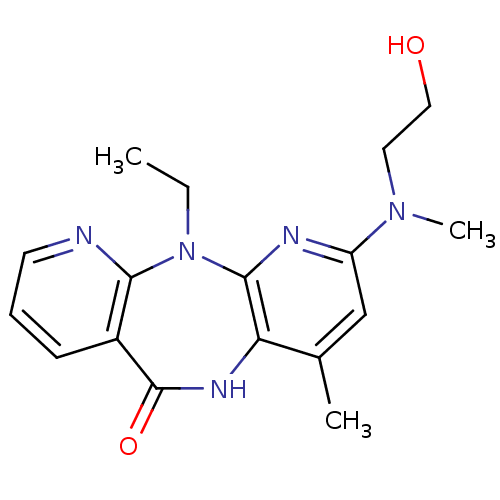 (2-ethyl-5-[(2-hydroxyethyl)(methyl)amino]-7-methyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 38: 4830-8 (1995) Article DOI: 10.1021/jm00024a010 BindingDB Entry DOI: 10.7270/Q23B5XBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50201094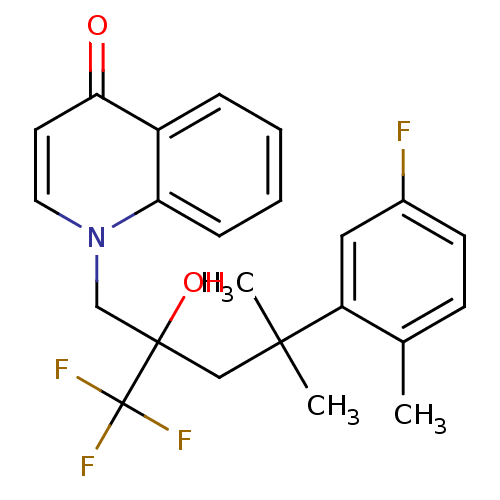 (1-[4-(5-fluoro-2-methylphenyl)-2-hydroxy-4-methyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of tetramethylrhodamine labeled dexamethosone binding to GR by FP assay | J Med Chem 49: 7887-96 (2006) Article DOI: 10.1021/jm061273t BindingDB Entry DOI: 10.7270/Q2BG2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50201083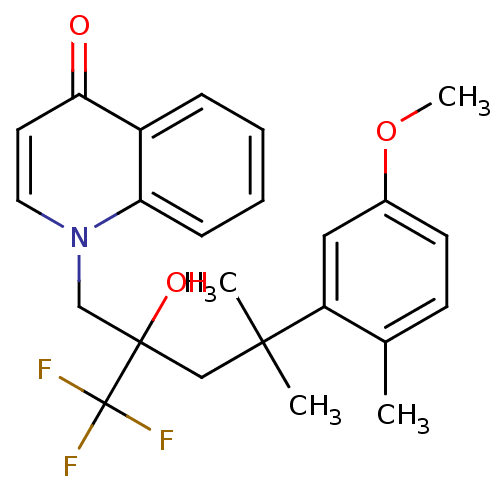 (1-[2-hydroxy-4-(5-methoxy-2-methylphenyl)-4-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of tetramethylrhodamine labeled dexamethosone binding to GR by FP assay | J Med Chem 49: 7887-96 (2006) Article DOI: 10.1021/jm061273t BindingDB Entry DOI: 10.7270/Q2BG2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50001820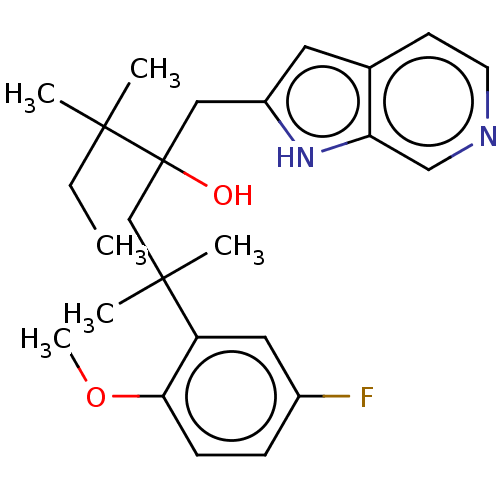 (CHEMBL3233273) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of TAMRA-labeled dexamethasone from glucocorticoid receptor (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 24: 1934-40 (2014) Article DOI: 10.1016/j.bmcl.2014.03.005 BindingDB Entry DOI: 10.7270/Q2251KPB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50201111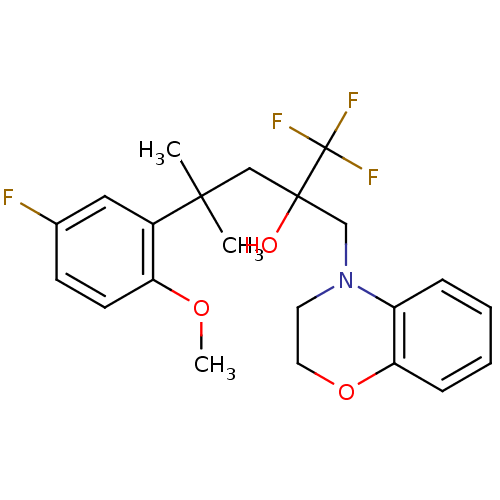 (2-[(2,3-dihydrobenzo[1,4]oxazin-4-yl)methyl]-1,1,1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of tetramethylrhodamine labeled dexamethosone binding to GR by FP assay | J Med Chem 49: 7887-96 (2006) Article DOI: 10.1021/jm061273t BindingDB Entry DOI: 10.7270/Q2BG2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM19190 ((1S,2R,10S,11S,14R,15S,17S)-14,17-dihydroxy-14-(2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of TAMRA-labeled dexamethasone from glucocorticoid receptor (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 24: 1934-40 (2014) Article DOI: 10.1016/j.bmcl.2014.03.005 BindingDB Entry DOI: 10.7270/Q2251KPB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM19190 ((1S,2R,10S,11S,14R,15S,17S)-14,17-dihydroxy-14-(2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of tetramethylrhodamine labeled dexamethosone binding to GR by FP assay | J Med Chem 49: 7887-96 (2006) Article DOI: 10.1021/jm061273t BindingDB Entry DOI: 10.7270/Q2BG2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50001844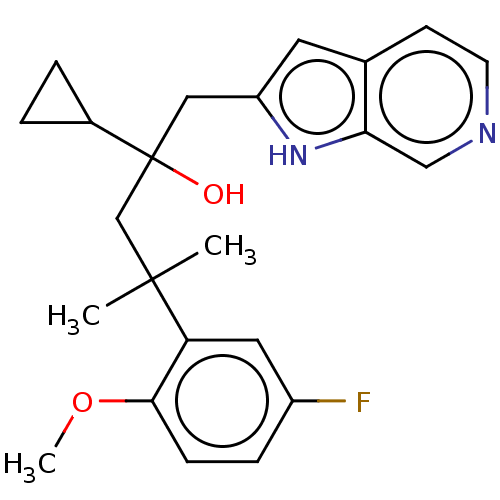 (CHEMBL3233274) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of TAMRA-labeled dexamethasone from glucocorticoid receptor (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 24: 1934-40 (2014) Article DOI: 10.1016/j.bmcl.2014.03.005 BindingDB Entry DOI: 10.7270/Q2251KPB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM32402 (furan-2-carboxamide deriv., 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Boehringer Ingelheim Pharmaceuticals | Assay Description MMP-13 was assessed by using the EnzoLyte 520 Generic MMP Assay Kit (AnaSpec Inc.). This kit uses a 5-FAM/QXL 520 fluorescence resonance 10 energy tr... | Bioorg Med Chem Lett 19: 5321-4 (2009) Article DOI: 10.1016/j.bmcl.2009.07.151 BindingDB Entry DOI: 10.7270/Q22B8WCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50001865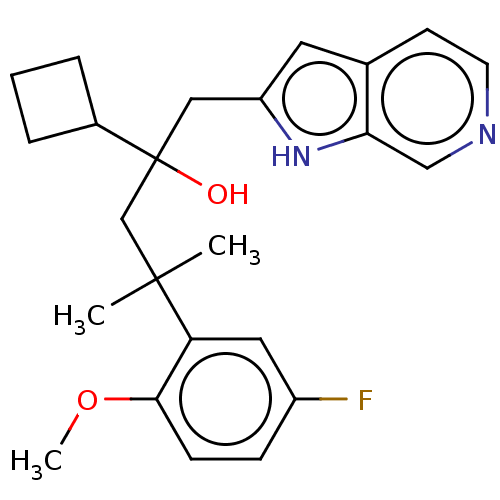 (CHEMBL3233275) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of TAMRA-labeled dexamethasone from glucocorticoid receptor (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 24: 1934-40 (2014) Article DOI: 10.1016/j.bmcl.2014.03.005 BindingDB Entry DOI: 10.7270/Q2251KPB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50201099 (4-(2,3-dihydrobenzofuran-7-yl)-2-hydroxy-4-methyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of tetramethylrhodamine labeled RU486 binding to PR by FP assay | J Med Chem 49: 7887-96 (2006) Article DOI: 10.1021/jm061273t BindingDB Entry DOI: 10.7270/Q2BG2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 478 total ) | Next | Last >> |