 Found 303 hits with Last Name = 'proulx' and Initial = 'c'
Found 303 hits with Last Name = 'proulx' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM31592
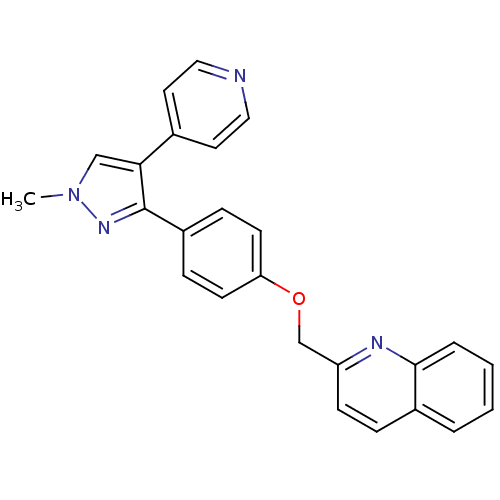
(PF-2545920 | US9138494, MP-10 | substituted pyraz...)Show SMILES Cn1cc(c(n1)-c1ccc(OCc2ccc3ccccc3n2)cc1)-c1ccncc1 Show InChI InChI=1S/C25H20N4O/c1-29-16-23(18-12-14-26-15-13-18)25(28-29)20-7-10-22(11-8-20)30-17-21-9-6-19-4-2-3-5-24(19)27-21/h2-16H,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Pfizer
| Assay Description
PDE activity was measured using a plate-based Scintillation Proximity Assay (SPA). For competitive enzyme inhibition assays, the substrate [3H]cAMP c... |
J Med Chem 52: 5188-96 (2009)
Article DOI: 10.1021/jm900521k
BindingDB Entry DOI: 10.7270/Q290223B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1C
(Homo sapiens (Human)) | BDBM50300099
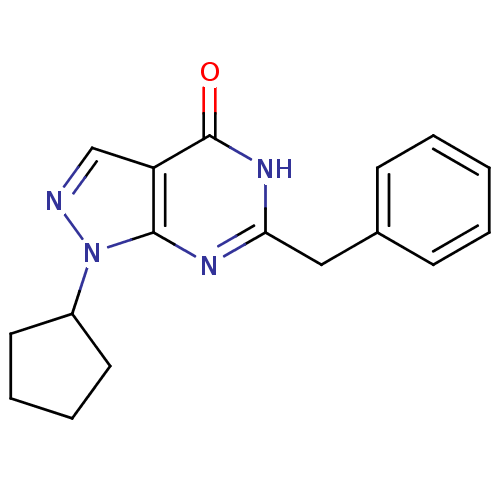
(6-benzyl-1-cyclopentyl-1,5-dihydro-4H-pyrazolo[3,4...)Show InChI InChI=1S/C17H18N4O/c22-17-14-11-18-21(13-8-4-5-9-13)16(14)19-15(20-17)10-12-6-2-1-3-7-12/h1-3,6-7,11,13H,4-5,8-10H2,(H,19,20,22) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE1C expressed in Sf9 cells using [3H]cAMP as substrate after 30 mins by scintillation proximity assay |
J Med Chem 55: 9045-54 (2012)
Article DOI: 10.1021/jm3007799
BindingDB Entry DOI: 10.7270/Q24J0G79 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM31591
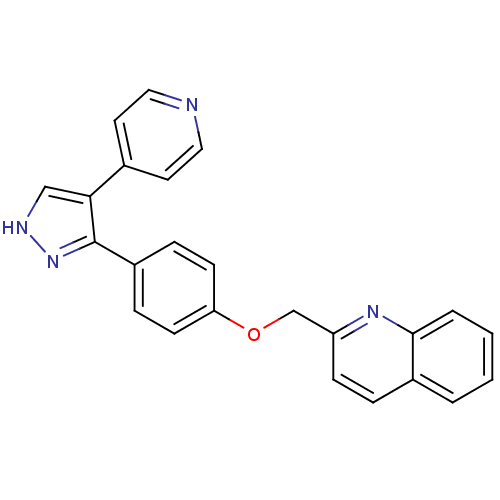
(pyrazole, 3)Show SMILES C(Oc1ccc(cc1)-c1n[nH]cc1-c1ccncc1)c1ccc2ccccc2n1 Show InChI InChI=1S/C24H18N4O/c1-2-4-23-18(3-1)5-8-20(27-23)16-29-21-9-6-19(7-10-21)24-22(15-26-28-24)17-11-13-25-14-12-17/h1-15H,16H2,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Pfizer
| Assay Description
PDE activity was measured using a plate-based Scintillation Proximity Assay (SPA). For competitive enzyme inhibition assays, the substrate [3H]cAMP c... |
J Med Chem 52: 5188-96 (2009)
Article DOI: 10.1021/jm900521k
BindingDB Entry DOI: 10.7270/Q290223B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM31606
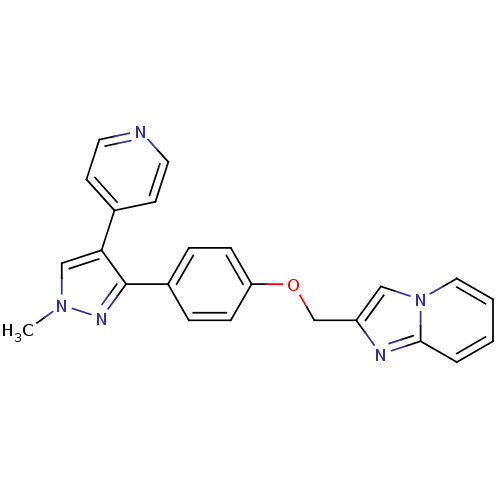
(methyl substituted pyrazole, 27)Show SMILES Cn1cc(c(n1)-c1ccc(OCc2cn3ccccc3n2)cc1)-c1ccncc1 Show InChI InChI=1S/C23H19N5O/c1-27-15-21(17-9-11-24-12-10-17)23(26-27)18-5-7-20(8-6-18)29-16-19-14-28-13-3-2-4-22(28)25-19/h2-15H,16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Pfizer
| Assay Description
PDE activity was measured using a plate-based Scintillation Proximity Assay (SPA). For competitive enzyme inhibition assays, the substrate [3H]cAMP c... |
J Med Chem 52: 5188-96 (2009)
Article DOI: 10.1021/jm900521k
BindingDB Entry DOI: 10.7270/Q290223B |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50300099

(6-benzyl-1-cyclopentyl-1,5-dihydro-4H-pyrazolo[3,4...)Show InChI InChI=1S/C17H18N4O/c22-17-14-11-18-21(13-8-4-5-9-13)16(14)19-15(20-17)10-12-6-2-1-3-7-12/h1-3,6-7,11,13H,4-5,8-10H2,(H,19,20,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE9A expressed in Sf9 cells using [3H]cGMP as substrate after 30 mins by scintillation proximity assay |
J Med Chem 55: 9045-54 (2012)
Article DOI: 10.1021/jm3007799
BindingDB Entry DOI: 10.7270/Q24J0G79 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50397844
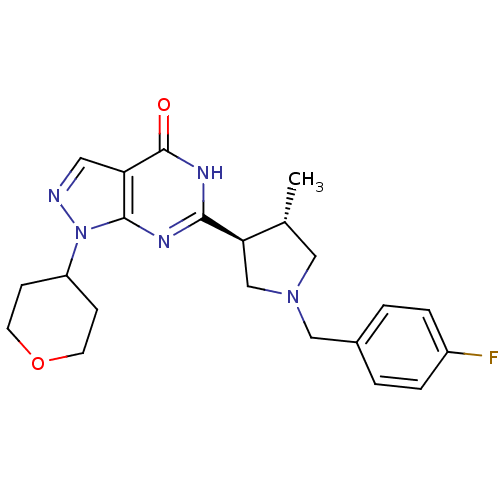
(CHEMBL2179099)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)C[C@H]1c1nc2n(ncc2c(=O)[nH]1)C1CCOCC1 |r| Show InChI InChI=1S/C22H26FN5O2/c1-14-11-27(12-15-2-4-16(23)5-3-15)13-19(14)20-25-21-18(22(29)26-20)10-24-28(21)17-6-8-30-9-7-17/h2-5,10,14,17,19H,6-9,11-13H2,1H3,(H,25,26,29)/t14-,19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE9A expressed in Sf9 cells using [3H]cGMP as substrate after 30 mins by scintillation proximity assay |
J Med Chem 55: 9045-54 (2012)
Article DOI: 10.1021/jm3007799
BindingDB Entry DOI: 10.7270/Q24J0G79 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM31594
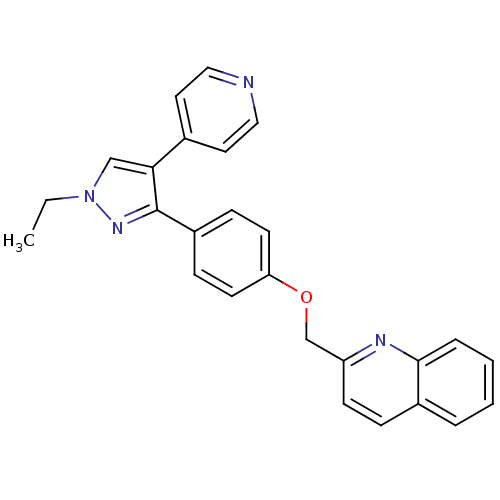
(substituted pyrazole, 11)Show SMILES CCn1cc(c(n1)-c1ccc(OCc2ccc3ccccc3n2)cc1)-c1ccncc1 Show InChI InChI=1S/C26H22N4O/c1-2-30-17-24(19-13-15-27-16-14-19)26(29-30)21-8-11-23(12-9-21)31-18-22-10-7-20-5-3-4-6-25(20)28-22/h3-17H,2,18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Pfizer
| Assay Description
PDE activity was measured using a plate-based Scintillation Proximity Assay (SPA). For competitive enzyme inhibition assays, the substrate [3H]cAMP c... |
J Med Chem 52: 5188-96 (2009)
Article DOI: 10.1021/jm900521k
BindingDB Entry DOI: 10.7270/Q290223B |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50397850
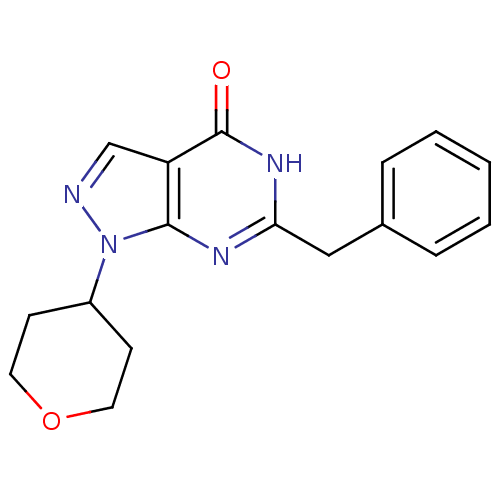
(CHEMBL2179093)Show InChI InChI=1S/C17H18N4O2/c22-17-14-11-18-21(13-6-8-23-9-7-13)16(14)19-15(20-17)10-12-4-2-1-3-5-12/h1-5,11,13H,6-10H2,(H,19,20,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE9A expressed in Sf9 cells using [3H]cGMP as substrate after 30 mins by scintillation proximity assay |
J Med Chem 55: 9045-54 (2012)
Article DOI: 10.1021/jm3007799
BindingDB Entry DOI: 10.7270/Q24J0G79 |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50397845
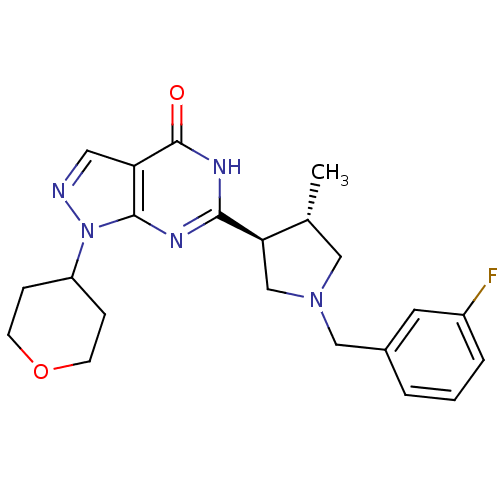
(CHEMBL2177125)Show SMILES C[C@@H]1CN(Cc2cccc(F)c2)C[C@H]1c1nc2n(ncc2c(=O)[nH]1)C1CCOCC1 |r| Show InChI InChI=1S/C22H26FN5O2/c1-14-11-27(12-15-3-2-4-16(23)9-15)13-19(14)20-25-21-18(22(29)26-20)10-24-28(21)17-5-7-30-8-6-17/h2-4,9-10,14,17,19H,5-8,11-13H2,1H3,(H,25,26,29)/t14-,19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE9A expressed in Sf9 cells using [3H]cGMP as substrate after 30 mins by scintillation proximity assay |
J Med Chem 55: 9045-54 (2012)
Article DOI: 10.1021/jm3007799
BindingDB Entry DOI: 10.7270/Q24J0G79 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM31605
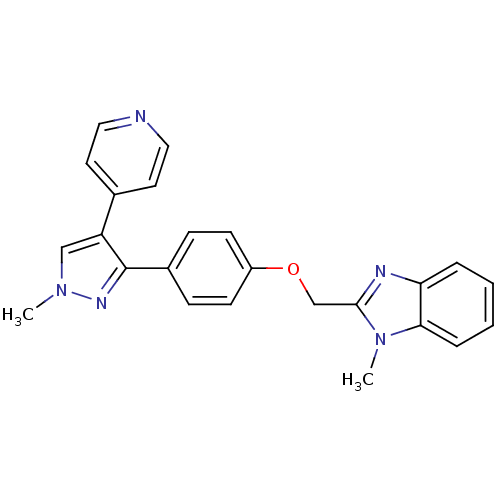
(methyl substituted pyrazole, 26)Show SMILES Cn1cc(c(n1)-c1ccc(OCc2nc3ccccc3n2C)cc1)-c1ccncc1 Show InChI InChI=1S/C24H21N5O/c1-28-15-20(17-11-13-25-14-12-17)24(27-28)18-7-9-19(10-8-18)30-16-23-26-21-5-3-4-6-22(21)29(23)2/h3-15H,16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.29 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Pfizer
| Assay Description
PDE activity was measured using a plate-based Scintillation Proximity Assay (SPA). For competitive enzyme inhibition assays, the substrate [3H]cAMP c... |
J Med Chem 52: 5188-96 (2009)
Article DOI: 10.1021/jm900521k
BindingDB Entry DOI: 10.7270/Q290223B |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM31596
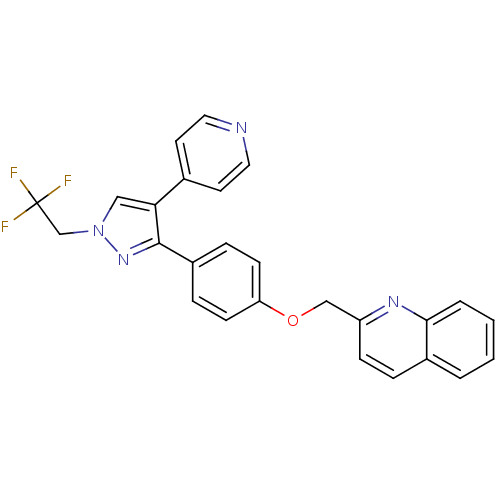
(substituted pyrazole, 13)Show SMILES FC(F)(F)Cn1cc(c(n1)-c1ccc(OCc2ccc3ccccc3n2)cc1)-c1ccncc1 Show InChI InChI=1S/C26H19F3N4O/c27-26(28,29)17-33-15-23(18-11-13-30-14-12-18)25(32-33)20-6-9-22(10-7-20)34-16-21-8-5-19-3-1-2-4-24(19)31-21/h1-15H,16-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.41 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Pfizer
| Assay Description
PDE activity was measured using a plate-based Scintillation Proximity Assay (SPA). For competitive enzyme inhibition assays, the substrate [3H]cAMP c... |
J Med Chem 52: 5188-96 (2009)
Article DOI: 10.1021/jm900521k
BindingDB Entry DOI: 10.7270/Q290223B |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM31593
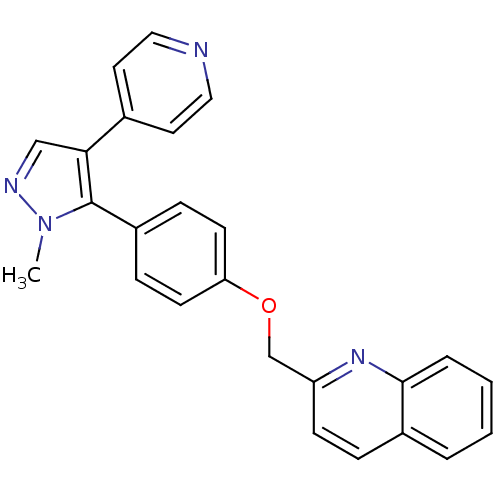
(substituted pyrazole, 10)Show SMILES Cn1ncc(c1-c1ccc(OCc2ccc3ccccc3n2)cc1)-c1ccncc1 Show InChI InChI=1S/C25H20N4O/c1-29-25(23(16-27-29)18-12-14-26-15-13-18)20-7-10-22(11-8-20)30-17-21-9-6-19-4-2-3-5-24(19)28-21/h2-16H,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.54 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Pfizer
| Assay Description
PDE activity was measured using a plate-based Scintillation Proximity Assay (SPA). For competitive enzyme inhibition assays, the substrate [3H]cAMP c... |
J Med Chem 52: 5188-96 (2009)
Article DOI: 10.1021/jm900521k
BindingDB Entry DOI: 10.7270/Q290223B |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50397848
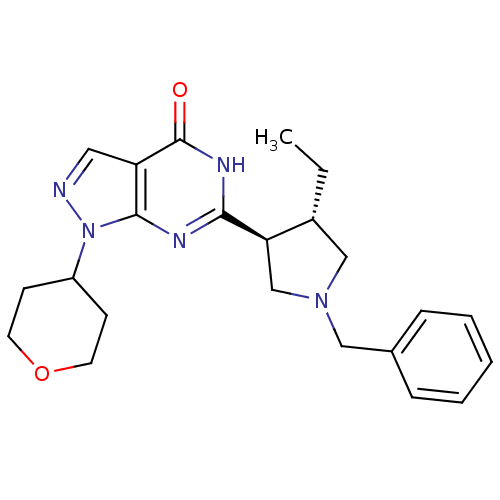
(CHEMBL2179096)Show SMILES CC[C@@H]1CN(Cc2ccccc2)C[C@H]1c1nc2n(ncc2c(=O)[nH]1)C1CCOCC1 |r| Show InChI InChI=1S/C23H29N5O2/c1-2-17-14-27(13-16-6-4-3-5-7-16)15-20(17)21-25-22-19(23(29)26-21)12-24-28(22)18-8-10-30-11-9-18/h3-7,12,17-18,20H,2,8-11,13-15H2,1H3,(H,25,26,29)/t17-,20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE9A expressed in Sf9 cells using [3H]cGMP as substrate after 30 mins by scintillation proximity assay |
J Med Chem 55: 9045-54 (2012)
Article DOI: 10.1021/jm3007799
BindingDB Entry DOI: 10.7270/Q24J0G79 |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50300113
(1-cyclopentyl-6-[(3S,4S)-4-methyl-1-(quinoxalin-6-...)Show SMILES C[C@@H]1CN(Cc2ccc3nccnc3c2)C[C@H]1c1nc2n(ncc2c(=O)[nH]1)C1CCCC1 |r| Show InChI InChI=1S/C24H27N7O/c1-15-12-30(13-16-6-7-20-21(10-16)26-9-8-25-20)14-19(15)22-28-23-18(24(32)29-22)11-27-31(23)17-4-2-3-5-17/h6-11,15,17,19H,2-5,12-14H2,1H3,(H,28,29,32)/t15-,19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE9A expressed in Sf9 cells by SPA |
J Med Chem 52: 7946-9 (2009)
Article DOI: 10.1021/jm9015334
BindingDB Entry DOI: 10.7270/Q2RV0NRS |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50300113

(1-cyclopentyl-6-[(3S,4S)-4-methyl-1-(quinoxalin-6-...)Show SMILES C[C@@H]1CN(Cc2ccc3nccnc3c2)C[C@H]1c1nc2n(ncc2c(=O)[nH]1)C1CCCC1 |r| Show InChI InChI=1S/C24H27N7O/c1-15-12-30(13-16-6-7-20-21(10-16)26-9-8-25-20)14-19(15)22-28-23-18(24(32)29-22)11-27-31(23)17-4-2-3-5-17/h6-11,15,17,19H,2-5,12-14H2,1H3,(H,28,29,32)/t15-,19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE9A expressed in Sf9 cells by SPA |
J Med Chem 52: 7946-9 (2009)
Article DOI: 10.1021/jm9015334
BindingDB Entry DOI: 10.7270/Q2RV0NRS |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50300112
(CHEMBL565667 | trans-1-cyclopentyl-6-(4-methyl-1-(...)Show SMILES C[C@H]1CN(Cc2ccc3nccnc3c2)C[C@@H]1c1nc2n(ncc2c(=O)[nH]1)C1CCCC1 |r| Show InChI InChI=1S/C24H27N7O/c1-15-12-30(13-16-6-7-20-21(10-16)26-9-8-25-20)14-19(15)22-28-23-18(24(32)29-22)11-27-31(23)17-4-2-3-5-17/h6-11,15,17,19H,2-5,12-14H2,1H3,(H,28,29,32)/t15-,19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE9A expressed in Sf9 cells by SPA |
J Med Chem 52: 7946-9 (2009)
Article DOI: 10.1021/jm9015334
BindingDB Entry DOI: 10.7270/Q2RV0NRS |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50300112

(CHEMBL565667 | trans-1-cyclopentyl-6-(4-methyl-1-(...)Show SMILES C[C@H]1CN(Cc2ccc3nccnc3c2)C[C@@H]1c1nc2n(ncc2c(=O)[nH]1)C1CCCC1 |r| Show InChI InChI=1S/C24H27N7O/c1-15-12-30(13-16-6-7-20-21(10-16)26-9-8-25-20)14-19(15)22-28-23-18(24(32)29-22)11-27-31(23)17-4-2-3-5-17/h6-11,15,17,19H,2-5,12-14H2,1H3,(H,28,29,32)/t15-,19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE9A expressed in Sf9 cells by SPA |
J Med Chem 52: 7946-9 (2009)
Article DOI: 10.1021/jm9015334
BindingDB Entry DOI: 10.7270/Q2RV0NRS |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1C
(Homo sapiens (Human)) | BDBM50300099

(6-benzyl-1-cyclopentyl-1,5-dihydro-4H-pyrazolo[3,4...)Show InChI InChI=1S/C17H18N4O/c22-17-14-11-18-21(13-8-4-5-9-13)16(14)19-15(20-17)10-12-6-2-1-3-7-12/h1-3,6-7,11,13H,4-5,8-10H2,(H,19,20,22) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE1C expressed in Sf9 cells by SPA |
J Med Chem 52: 7946-9 (2009)
Article DOI: 10.1021/jm9015334
BindingDB Entry DOI: 10.7270/Q2RV0NRS |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50300099

(6-benzyl-1-cyclopentyl-1,5-dihydro-4H-pyrazolo[3,4...)Show InChI InChI=1S/C17H18N4O/c22-17-14-11-18-21(13-8-4-5-9-13)16(14)19-15(20-17)10-12-6-2-1-3-7-12/h1-3,6-7,11,13H,4-5,8-10H2,(H,19,20,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE9A expressed in Sf9 cells by SPA |
J Med Chem 52: 7946-9 (2009)
Article DOI: 10.1021/jm9015334
BindingDB Entry DOI: 10.7270/Q2RV0NRS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1C
(Homo sapiens (Human)) | BDBM50300099

(6-benzyl-1-cyclopentyl-1,5-dihydro-4H-pyrazolo[3,4...)Show InChI InChI=1S/C17H18N4O/c22-17-14-11-18-21(13-8-4-5-9-13)16(14)19-15(20-17)10-12-6-2-1-3-7-12/h1-3,6-7,11,13H,4-5,8-10H2,(H,19,20,22) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE1C expressed in Sf9 cells by SPA |
J Med Chem 52: 7946-9 (2009)
Article DOI: 10.1021/jm9015334
BindingDB Entry DOI: 10.7270/Q2RV0NRS |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50300099

(6-benzyl-1-cyclopentyl-1,5-dihydro-4H-pyrazolo[3,4...)Show InChI InChI=1S/C17H18N4O/c22-17-14-11-18-21(13-8-4-5-9-13)16(14)19-15(20-17)10-12-6-2-1-3-7-12/h1-3,6-7,11,13H,4-5,8-10H2,(H,19,20,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE9A expressed in Sf9 cells by SPA |
J Med Chem 52: 7946-9 (2009)
Article DOI: 10.1021/jm9015334
BindingDB Entry DOI: 10.7270/Q2RV0NRS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50397843
(CHEMBL2179100)Show SMILES COc1ccc(CN2C[C@@H](C)[C@@H](C2)c2nc3n(ncc3c(=O)[nH]2)C2CCOCC2)cc1 |r| Show InChI InChI=1S/C23H29N5O3/c1-15-12-27(13-16-3-5-18(30-2)6-4-16)14-20(15)21-25-22-19(23(29)26-21)11-24-28(22)17-7-9-31-10-8-17/h3-6,11,15,17,20H,7-10,12-14H2,1-2H3,(H,25,26,29)/t15-,20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE9A expressed in Sf9 cells using [3H]cGMP as substrate after 30 mins by scintillation proximity assay |
J Med Chem 55: 9045-54 (2012)
Article DOI: 10.1021/jm3007799
BindingDB Entry DOI: 10.7270/Q24J0G79 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM31607

(methyl substituted pyrazole, 28)Show SMILES Cn1cc(c(n1)-c1ccc(OCc2nc3ccccn3n2)cc1)-c1ccncc1 Show InChI InChI=1S/C22H18N6O/c1-27-14-19(16-9-11-23-12-10-16)22(26-27)17-5-7-18(8-6-17)29-15-20-24-21-4-2-3-13-28(21)25-20/h2-14H,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Pfizer
| Assay Description
PDE activity was measured using a plate-based Scintillation Proximity Assay (SPA). For competitive enzyme inhibition assays, the substrate [3H]cAMP c... |
J Med Chem 52: 5188-96 (2009)
Article DOI: 10.1021/jm900521k
BindingDB Entry DOI: 10.7270/Q290223B |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50397841
(CHEMBL2179102)Show SMILES C[C@@H]1CN(Cc2ccc(C)nc2)C[C@H]1c1nc2n(ncc2c(=O)[nH]1)C1CCOCC1 |r| Show InChI InChI=1S/C22H28N6O2/c1-14-11-27(12-16-4-3-15(2)23-9-16)13-19(14)20-25-21-18(22(29)26-20)10-24-28(21)17-5-7-30-8-6-17/h3-4,9-10,14,17,19H,5-8,11-13H2,1-2H3,(H,25,26,29)/t14-,19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE9A expressed in Sf9 cells using [3H]cGMP as substrate after 30 mins by scintillation proximity assay |
J Med Chem 55: 9045-54 (2012)
Article DOI: 10.1021/jm3007799
BindingDB Entry DOI: 10.7270/Q24J0G79 |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50397846
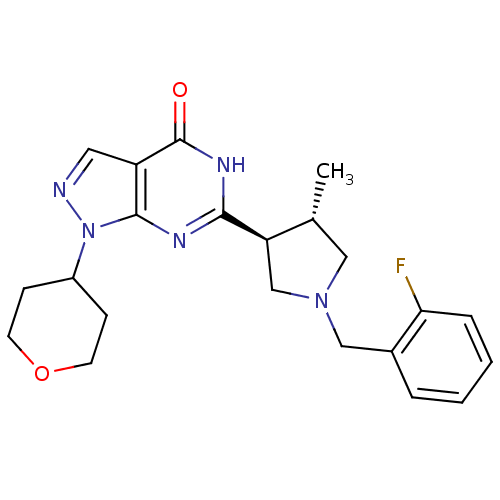
(CHEMBL2179098)Show SMILES C[C@@H]1CN(Cc2ccccc2F)C[C@H]1c1nc2n(ncc2c(=O)[nH]1)C1CCOCC1 |r| Show InChI InChI=1S/C22H26FN5O2/c1-14-11-27(12-15-4-2-3-5-19(15)23)13-18(14)20-25-21-17(22(29)26-20)10-24-28(21)16-6-8-30-9-7-16/h2-5,10,14,16,18H,6-9,11-13H2,1H3,(H,25,26,29)/t14-,18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE9A expressed in Sf9 cells using [3H]cGMP as substrate after 30 mins by scintillation proximity assay |
J Med Chem 55: 9045-54 (2012)
Article DOI: 10.1021/jm3007799
BindingDB Entry DOI: 10.7270/Q24J0G79 |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50397839
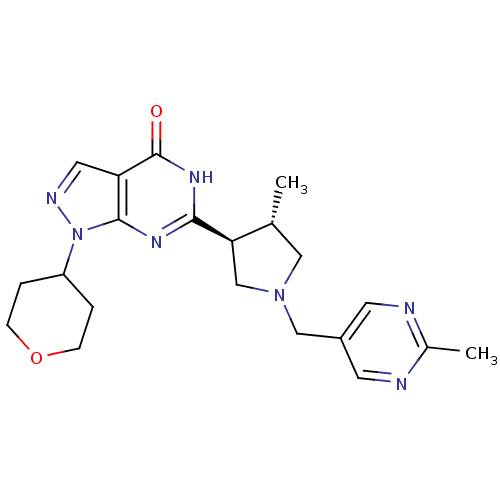
(CHEMBL2179104)Show SMILES C[C@@H]1CN(Cc2cnc(C)nc2)C[C@H]1c1nc2n(ncc2c(=O)[nH]1)C1CCOCC1 |r| Show InChI InChI=1S/C21H27N7O2/c1-13-10-27(11-15-7-22-14(2)23-8-15)12-18(13)19-25-20-17(21(29)26-19)9-24-28(20)16-3-5-30-6-4-16/h7-9,13,16,18H,3-6,10-12H2,1-2H3,(H,25,26,29)/t13-,18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE9A expressed in Sf9 cells using [3H]cGMP as substrate after 30 mins by scintillation proximity assay |
J Med Chem 55: 9045-54 (2012)
Article DOI: 10.1021/jm3007799
BindingDB Entry DOI: 10.7270/Q24J0G79 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM31595

(substituted pyrazole, 12)Show SMILES CCn1ncc(c1-c1ccc(OCc2ccc3ccccc3n2)cc1)-c1ccncc1 Show InChI InChI=1S/C26H22N4O/c1-2-30-26(24(17-28-30)19-13-15-27-16-14-19)21-8-11-23(12-9-21)31-18-22-10-7-20-5-3-4-6-25(20)29-22/h3-17H,2,18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.76 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Pfizer
| Assay Description
PDE activity was measured using a plate-based Scintillation Proximity Assay (SPA). For competitive enzyme inhibition assays, the substrate [3H]cAMP c... |
J Med Chem 52: 5188-96 (2009)
Article DOI: 10.1021/jm900521k
BindingDB Entry DOI: 10.7270/Q290223B |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50397851
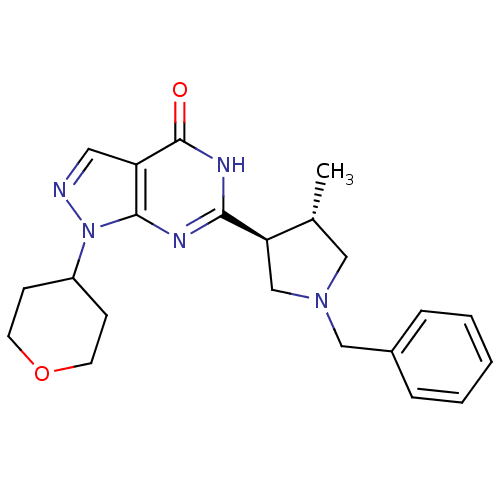
(CHEMBL2179094)Show SMILES C[C@@H]1CN(Cc2ccccc2)C[C@H]1c1nc2n(ncc2c(=O)[nH]1)C1CCOCC1 |r| Show InChI InChI=1S/C22H27N5O2/c1-15-12-26(13-16-5-3-2-4-6-16)14-19(15)20-24-21-18(22(28)25-20)11-23-27(21)17-7-9-29-10-8-17/h2-6,11,15,17,19H,7-10,12-14H2,1H3,(H,24,25,28)/t15-,19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE9A expressed in Sf9 cells using [3H]cGMP as substrate after 30 mins by scintillation proximity assay |
J Med Chem 55: 9045-54 (2012)
Article DOI: 10.1021/jm3007799
BindingDB Entry DOI: 10.7270/Q24J0G79 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM31604
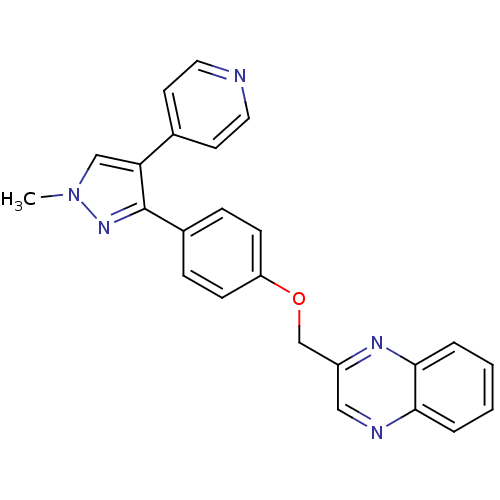
(methyl substituted pyrazole, 25)Show SMILES Cn1cc(c(n1)-c1ccc(OCc2cnc3ccccc3n2)cc1)-c1ccncc1 Show InChI InChI=1S/C24H19N5O/c1-29-15-21(17-10-12-25-13-11-17)24(28-29)18-6-8-20(9-7-18)30-16-19-14-26-22-4-2-3-5-23(22)27-19/h2-15H,16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.09 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Pfizer
| Assay Description
PDE activity was measured using a plate-based Scintillation Proximity Assay (SPA). For competitive enzyme inhibition assays, the substrate [3H]cAMP c... |
J Med Chem 52: 5188-96 (2009)
Article DOI: 10.1021/jm900521k
BindingDB Entry DOI: 10.7270/Q290223B |
More data for this
Ligand-Target Pair | |
Estrogen receptor [D538G]
(Homo sapiens (Human)) | BDBM50370294
(Examorelin | HEXARELIN | US9708370, Hexareline)Show SMILES C[C@H](NC(=O)[C@@H](Cc1c(C)[nH]c2ccccc12)NC(=O)[C@@H](N)Cc1cnc[nH]1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCCN)C(N)=O Show InChI InChI=1S/C47H58N12O6/c1-27-34(33-15-7-9-17-37(33)54-27)23-41(58-44(62)35(49)22-31-25-51-26-53-31)45(63)55-28(2)43(61)57-40(21-30-24-52-36-16-8-6-14-32(30)36)47(65)59-39(20-29-12-4-3-5-13-29)46(64)56-38(42(50)60)18-10-11-19-48/h3-9,12-17,24-26,28,35,38-41,52,54H,10-11,18-23,48-49H2,1-2H3,(H2,50,60)(H,51,53)(H,55,63)(H,56,64)(H,57,61)(H,58,62)(H,59,65)/t28-,35-,38-,39+,40-,41+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6.08 | n/a | n/a | n/a | n/a | n/a | n/a |
VALORISATION-RECHERCHE, LIMITED PARTNERSHIP; VALORISATION HSJ, LIMITED PARTNERSHIP
US Patent
| Assay Description
GHS-R1a radioreceptor assay using LLCPK-1 membranes overexpressing GSH-R1a and radioiodinated (1-28) rat ghrelin as tracer. |
US Patent US9708370 (2017)
BindingDB Entry DOI: 10.7270/Q2XP76ZK |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50049479
((S)-6-Amino-2-{(R)-2-[(S)-2-{(S)-2-[(R)-2-[(S)-2-a...)Show SMILES C[C@H](NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](N)Cc1cnc[nH]1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCCN)C(N)=O Show InChI InChI=1S/C46H56N12O6/c1-27(54-44(62)39(20-29-23-51-35-15-7-5-13-32(29)35)57-43(61)34(48)22-31-25-50-26-53-31)42(60)56-40(21-30-24-52-36-16-8-6-14-33(30)36)46(64)58-38(19-28-11-3-2-4-12-28)45(63)55-37(41(49)59)17-9-10-18-47/h2-8,11-16,23-27,34,37-40,51-52H,9-10,17-22,47-48H2,1H3,(H2,49,59)(H,50,53)(H,54,62)(H,55,63)(H,56,60)(H,57,61)(H,58,64)/t27-,34-,37-,38+,39+,40-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.08 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Displacement of [125I]ghrelin expressed in in LLC-PK1 cells incubated for 1 hr by gamma counting method |
J Med Chem 55: 6502-11 (2012)
Article DOI: 10.1021/jm300557t
BindingDB Entry DOI: 10.7270/Q22J6D00 |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50397842
(CHEMBL2179101)Show SMILES C[C@@H]1CN(Cc2ccccn2)C[C@H]1c1nc2n(ncc2c(=O)[nH]1)C1CCOCC1 |r| Show InChI InChI=1S/C21H26N6O2/c1-14-11-26(12-15-4-2-3-7-22-15)13-18(14)19-24-20-17(21(28)25-19)10-23-27(20)16-5-8-29-9-6-16/h2-4,7,10,14,16,18H,5-6,8-9,11-13H2,1H3,(H,24,25,28)/t14-,18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE9A expressed in Sf9 cells using [3H]cGMP as substrate after 30 mins by scintillation proximity assay |
J Med Chem 55: 9045-54 (2012)
Article DOI: 10.1021/jm3007799
BindingDB Entry DOI: 10.7270/Q24J0G79 |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50397838
(CHEMBL2179105 | PF-04447943 | US10513524, Referenc...)Show SMILES C[C@@H]1CN(Cc2ncccn2)C[C@H]1c1nc2n(ncc2c(=O)[nH]1)C1CCOCC1 |r| Show InChI InChI=1S/C20H25N7O2/c1-13-10-26(12-17-21-5-2-6-22-17)11-16(13)18-24-19-15(20(28)25-18)9-23-27(19)14-3-7-29-8-4-14/h2,5-6,9,13-14,16H,3-4,7-8,10-12H2,1H3,(H,24,25,28)/t13-,16-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE9A expressed in Sf9 cells using [3H]cGMP as substrate after 30 mins by scintillation proximity assay |
J Med Chem 55: 9045-54 (2012)
Article DOI: 10.1021/jm3007799
BindingDB Entry DOI: 10.7270/Q24J0G79 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50300109
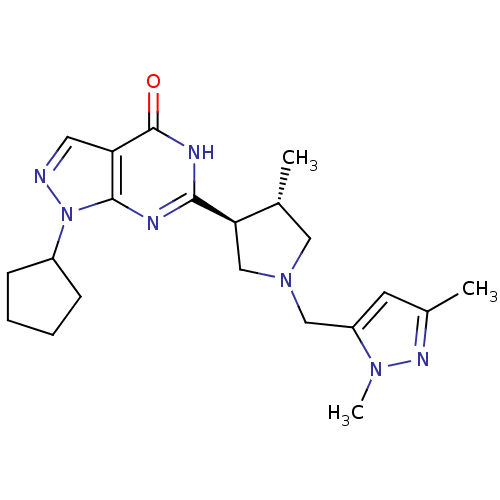
(CHEMBL575791 | trans-1-cyclopentyl-6-(1-((1,3-dime...)Show SMILES C[C@@H]1CN(Cc2cc(C)nn2C)C[C@H]1c1nc2n(ncc2c(=O)[nH]1)C1CCCC1 |r| Show InChI InChI=1S/C21H29N7O/c1-13-10-27(11-16-8-14(2)25-26(16)3)12-18(13)19-23-20-17(21(29)24-19)9-22-28(20)15-6-4-5-7-15/h8-9,13,15,18H,4-7,10-12H2,1-3H3,(H,23,24,29)/t13-,18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE9A expressed in Sf9 cells by SPA |
J Med Chem 52: 7946-9 (2009)
Article DOI: 10.1021/jm9015334
BindingDB Entry DOI: 10.7270/Q2RV0NRS |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50300110
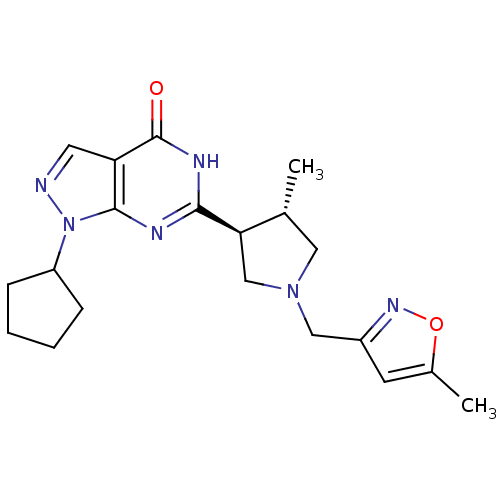
(CHEMBL583064 | trans-1-cyclopentyl-6-(4-methyl-1-(...)Show SMILES C[C@@H]1CN(Cc2cc(C)on2)C[C@H]1c1nc2n(ncc2c(=O)[nH]1)C1CCCC1 |r| Show InChI InChI=1S/C20H26N6O2/c1-12-9-25(10-14-7-13(2)28-24-14)11-17(12)18-22-19-16(20(27)23-18)8-21-26(19)15-5-3-4-6-15/h7-8,12,15,17H,3-6,9-11H2,1-2H3,(H,22,23,27)/t12-,17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE9A expressed in Sf9 cells by SPA |
J Med Chem 52: 7946-9 (2009)
Article DOI: 10.1021/jm9015334
BindingDB Entry DOI: 10.7270/Q2RV0NRS |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50300107

(CHEMBL578033 | trans-1-cyclopentyl-6-(4-methyl-1-(...)Show SMILES C[C@@H]1CN(Cc2cnc(C)nc2)C[C@H]1c1nc2n(ncc2c(=O)[nH]1)C1CCCC1 |r| Show InChI InChI=1S/C21H27N7O/c1-13-10-27(11-15-7-22-14(2)23-8-15)12-18(13)19-25-20-17(21(29)26-19)9-24-28(20)16-5-3-4-6-16/h7-9,13,16,18H,3-6,10-12H2,1-2H3,(H,25,26,29)/t13-,18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE9A expressed in Sf9 cells by SPA |
J Med Chem 52: 7946-9 (2009)
Article DOI: 10.1021/jm9015334
BindingDB Entry DOI: 10.7270/Q2RV0NRS |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM31590

(pyrazole, 2)Show SMILES C(Oc1ccc(cc1)-c1n[nH]cc1Cc1ccncc1)c1ccc2ccccc2n1 Show InChI InChI=1S/C25H20N4O/c1-2-4-24-19(3-1)5-8-22(28-24)17-30-23-9-6-20(7-10-23)25-21(16-27-29-25)15-18-11-13-26-14-12-18/h1-14,16H,15,17H2,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 11.5 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Pfizer
| Assay Description
PDE activity was measured using a plate-based Scintillation Proximity Assay (SPA). For competitive enzyme inhibition assays, the substrate [3H]cAMP c... |
J Med Chem 52: 5188-96 (2009)
Article DOI: 10.1021/jm900521k
BindingDB Entry DOI: 10.7270/Q290223B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM31597
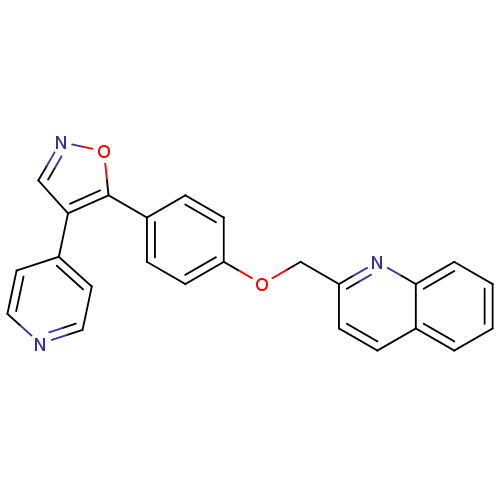
(isoxazole, 14)Show SMILES C(Oc1ccc(cc1)-c1oncc1-c1ccncc1)c1ccc2ccccc2n1 Show InChI InChI=1S/C24H17N3O2/c1-2-4-23-18(3-1)5-8-20(27-23)16-28-21-9-6-19(7-10-21)24-22(15-26-29-24)17-11-13-25-14-12-17/h1-15H,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11.7 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Pfizer
| Assay Description
PDE activity was measured using a plate-based Scintillation Proximity Assay (SPA). For competitive enzyme inhibition assays, the substrate [3H]cAMP c... |
J Med Chem 52: 5188-96 (2009)
Article DOI: 10.1021/jm900521k
BindingDB Entry DOI: 10.7270/Q290223B |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM31600
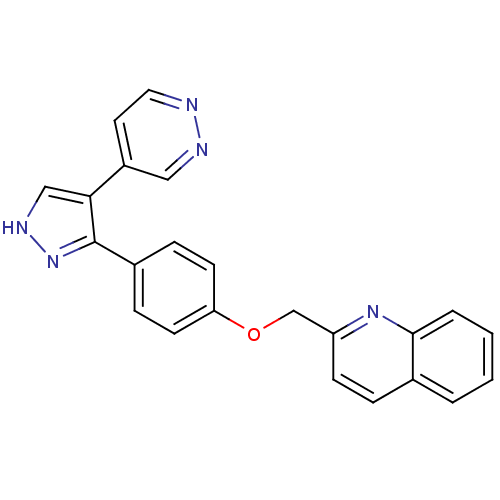
(pyrazole, 17)Show SMILES C(Oc1ccc(cc1)-c1n[nH]cc1-c1ccnnc1)c1ccc2ccccc2n1 Show InChI InChI=1S/C23H17N5O/c1-2-4-22-16(3-1)5-8-19(27-22)15-29-20-9-6-17(7-10-20)23-21(14-26-28-23)18-11-12-24-25-13-18/h1-14H,15H2,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11.9 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Pfizer
| Assay Description
PDE activity was measured using a plate-based Scintillation Proximity Assay (SPA). For competitive enzyme inhibition assays, the substrate [3H]cAMP c... |
J Med Chem 52: 5188-96 (2009)
Article DOI: 10.1021/jm900521k
BindingDB Entry DOI: 10.7270/Q290223B |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50397840
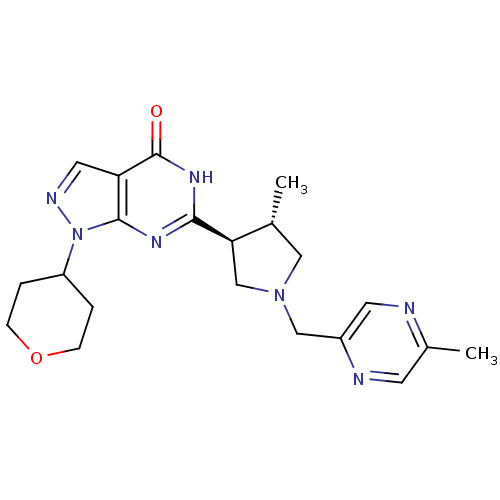
(CHEMBL2179103)Show SMILES C[C@@H]1CN(Cc2cnc(C)cn2)C[C@H]1c1nc2n(ncc2c(=O)[nH]1)C1CCOCC1 |r| Show InChI InChI=1S/C21H27N7O2/c1-13-10-27(11-15-8-22-14(2)7-23-15)12-18(13)19-25-20-17(21(29)26-19)9-24-28(20)16-3-5-30-6-4-16/h7-9,13,16,18H,3-6,10-12H2,1-2H3,(H,25,26,29)/t13-,18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE9A expressed in Sf9 cells using [3H]cGMP as substrate after 30 mins by scintillation proximity assay |
J Med Chem 55: 9045-54 (2012)
Article DOI: 10.1021/jm3007799
BindingDB Entry DOI: 10.7270/Q24J0G79 |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50300111
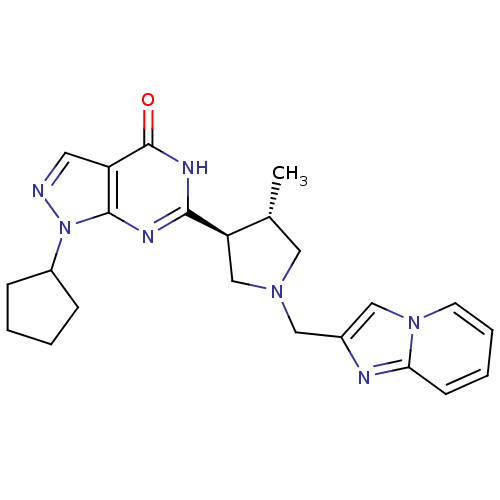
(CHEMBL566300 | trans-1-cyclopentyl-6-(1-(imidazo[1...)Show SMILES C[C@@H]1CN(Cc2cn3ccccc3n2)C[C@H]1c1nc2n(ncc2c(=O)[nH]1)C1CCCC1 |r| Show InChI InChI=1S/C23H27N7O/c1-15-11-28(12-16-13-29-9-5-4-8-20(29)25-16)14-19(15)21-26-22-18(23(31)27-21)10-24-30(22)17-6-2-3-7-17/h4-5,8-10,13,15,17,19H,2-3,6-7,11-12,14H2,1H3,(H,26,27,31)/t15-,19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE9A expressed in Sf9 cells by SPA |
J Med Chem 52: 7946-9 (2009)
Article DOI: 10.1021/jm9015334
BindingDB Entry DOI: 10.7270/Q2RV0NRS |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50300111

(CHEMBL566300 | trans-1-cyclopentyl-6-(1-(imidazo[1...)Show SMILES C[C@@H]1CN(Cc2cn3ccccc3n2)C[C@H]1c1nc2n(ncc2c(=O)[nH]1)C1CCCC1 |r| Show InChI InChI=1S/C23H27N7O/c1-15-11-28(12-16-13-29-9-5-4-8-20(29)25-16)14-19(15)21-26-22-18(23(31)27-21)10-24-30(22)17-6-2-3-7-17/h4-5,8-10,13,15,17,19H,2-3,6-7,11-12,14H2,1H3,(H,26,27,31)/t15-,19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE9A expressed in Sf9 cells by SPA |
J Med Chem 52: 7946-9 (2009)
Article DOI: 10.1021/jm9015334
BindingDB Entry DOI: 10.7270/Q2RV0NRS |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1C
(Homo sapiens (Human)) | BDBM50397850

(CHEMBL2179093)Show InChI InChI=1S/C17H18N4O2/c22-17-14-11-18-21(13-6-8-23-9-7-13)16(14)19-15(20-17)10-12-4-2-1-3-5-12/h1-5,11,13H,6-10H2,(H,19,20,22) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE1C expressed in Sf9 cells using [3H]cAMP as substrate after 30 mins by scintillation proximity assay |
J Med Chem 55: 9045-54 (2012)
Article DOI: 10.1021/jm3007799
BindingDB Entry DOI: 10.7270/Q24J0G79 |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50300108
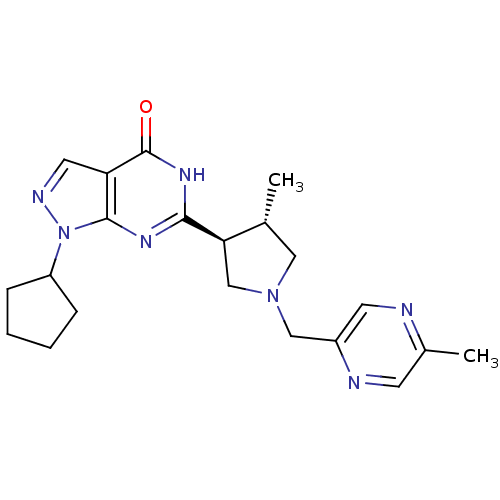
(CHEMBL575790 | trans-1-cyclopentyl-6-(4-methyl-1-(...)Show SMILES C[C@@H]1CN(Cc2cnc(C)cn2)C[C@H]1c1nc2n(ncc2c(=O)[nH]1)C1CCCC1 |r| Show InChI InChI=1S/C21H27N7O/c1-13-10-27(11-15-8-22-14(2)7-23-15)12-18(13)19-25-20-17(21(29)26-19)9-24-28(20)16-5-3-4-6-16/h7-9,13,16,18H,3-6,10-12H2,1-2H3,(H,25,26,29)/t13-,18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE9A expressed in Sf9 cells by SPA |
J Med Chem 52: 7946-9 (2009)
Article DOI: 10.1021/jm9015334
BindingDB Entry DOI: 10.7270/Q2RV0NRS |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50370294

(Examorelin | HEXARELIN | US9708370, Hexareline)Show SMILES C[C@H](NC(=O)[C@@H](Cc1c(C)[nH]c2ccccc12)NC(=O)[C@@H](N)Cc1cnc[nH]1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCCN)C(N)=O Show InChI InChI=1S/C47H58N12O6/c1-27-34(33-15-7-9-17-37(33)54-27)23-41(58-44(62)35(49)22-31-25-51-26-53-31)45(63)55-28(2)43(61)57-40(21-30-24-52-36-16-8-6-14-32(30)36)47(65)59-39(20-29-12-4-3-5-13-29)46(64)56-38(42(50)60)18-10-11-19-48/h3-9,12-17,24-26,28,35,38-41,52,54H,10-11,18-23,48-49H2,1-2H3,(H2,50,60)(H,51,53)(H,55,63)(H,56,64)(H,57,61)(H,58,62)(H,59,65)/t28-,35-,38-,39+,40-,41+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Displacement of [125I]ghrelin expressed in in LLC-PK1 cells incubated for 1 hr by gamma counting method |
J Med Chem 55: 6502-11 (2012)
Article DOI: 10.1021/jm300557t
BindingDB Entry DOI: 10.7270/Q22J6D00 |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50300106
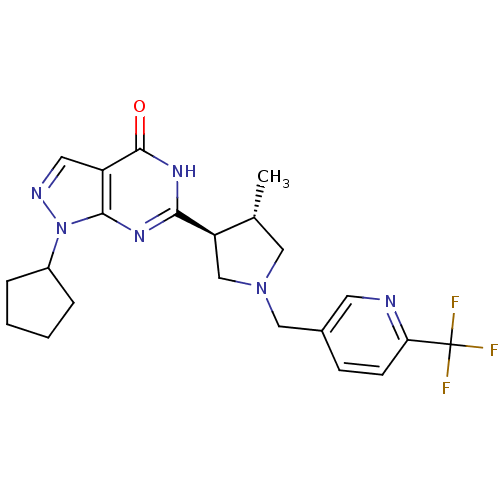
(CHEMBL574449 | trans-1-cyclopentyl-6-(4-methyl-1-(...)Show SMILES C[C@@H]1CN(Cc2ccc(nc2)C(F)(F)F)C[C@H]1c1nc2n(ncc2c(=O)[nH]1)C1CCCC1 |r| Show InChI InChI=1S/C22H25F3N6O/c1-13-10-30(11-14-6-7-18(26-8-14)22(23,24)25)12-17(13)19-28-20-16(21(32)29-19)9-27-31(20)15-4-2-3-5-15/h6-9,13,15,17H,2-5,10-12H2,1H3,(H,28,29,32)/t13-,17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE9A expressed in Sf9 cells by SPA |
J Med Chem 52: 7946-9 (2009)
Article DOI: 10.1021/jm9015334
BindingDB Entry DOI: 10.7270/Q2RV0NRS |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM31599
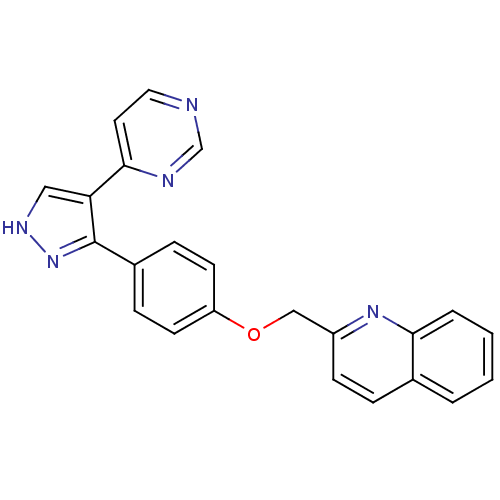
(pyrazole, 16)Show SMILES C(Oc1ccc(cc1)-c1n[nH]cc1-c1ccncn1)c1ccc2ccccc2n1 Show InChI InChI=1S/C23H17N5O/c1-2-4-21-16(3-1)5-8-18(27-21)14-29-19-9-6-17(7-10-19)23-20(13-26-28-23)22-11-12-24-15-25-22/h1-13,15H,14H2,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27.2 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Pfizer
| Assay Description
PDE activity was measured using a plate-based Scintillation Proximity Assay (SPA). For competitive enzyme inhibition assays, the substrate [3H]cAMP c... |
J Med Chem 52: 5188-96 (2009)
Article DOI: 10.1021/jm900521k
BindingDB Entry DOI: 10.7270/Q290223B |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50300105
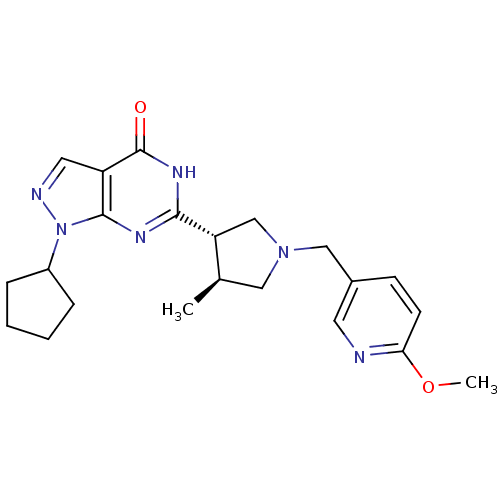
(CHEMBL575775 | trans-1-cyclopentyl-6-(1-((6-methox...)Show SMILES COc1ccc(CN2C[C@@H](C)[C@@H](C2)c2nc3n(ncc3c(=O)[nH]2)C2CCCC2)cn1 |r| Show InChI InChI=1S/C22H28N6O2/c1-14-11-27(12-15-7-8-19(30-2)23-9-15)13-18(14)20-25-21-17(22(29)26-20)10-24-28(21)16-5-3-4-6-16/h7-10,14,16,18H,3-6,11-13H2,1-2H3,(H,25,26,29)/t14-,18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE9A expressed in Sf9 cells by SPA |
J Med Chem 52: 7946-9 (2009)
Article DOI: 10.1021/jm9015334
BindingDB Entry DOI: 10.7270/Q2RV0NRS |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM31589
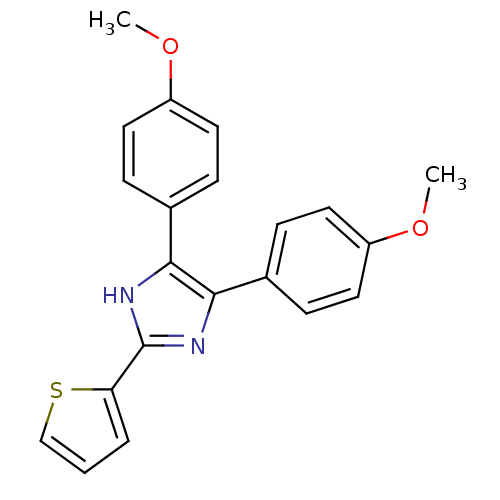
(triaryl imidazole, 1)Show SMILES COc1ccc(cc1)-c1nc([nH]c1-c1ccc(OC)cc1)-c1cccs1 Show InChI InChI=1S/C21H18N2O2S/c1-24-16-9-5-14(6-10-16)19-20(15-7-11-17(25-2)12-8-15)23-21(22-19)18-4-3-13-26-18/h3-13H,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
| MMDB
PDB
Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Pfizer
| Assay Description
PDE activity was measured using a plate-based Scintillation Proximity Assay (SPA). For competitive enzyme inhibition assays, the substrate [3H]cAMP c... |
J Med Chem 52: 5188-96 (2009)
Article DOI: 10.1021/jm900521k
BindingDB Entry DOI: 10.7270/Q290223B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50300103
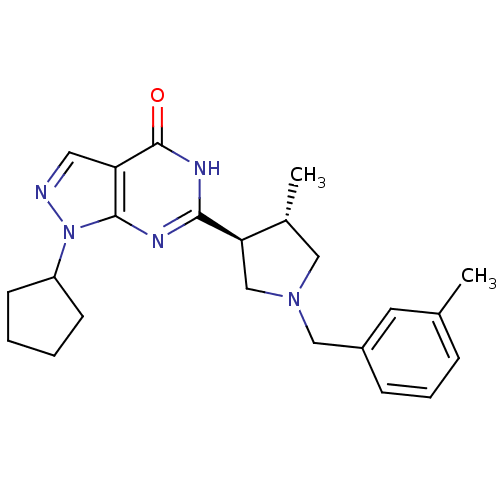
(CHEMBL575745 | trans-1-cyclopentyl-6-(4-methyl-1-(...)Show SMILES C[C@@H]1CN(Cc2cccc(C)c2)C[C@H]1c1nc2n(ncc2c(=O)[nH]1)C1CCCC1 |r| Show InChI InChI=1S/C23H29N5O/c1-15-6-5-7-17(10-15)13-27-12-16(2)20(14-27)21-25-22-19(23(29)26-21)11-24-28(22)18-8-3-4-9-18/h5-7,10-11,16,18,20H,3-4,8-9,12-14H2,1-2H3,(H,25,26,29)/t16-,20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE9A expressed in Sf9 cells by SPA |
J Med Chem 52: 7946-9 (2009)
Article DOI: 10.1021/jm9015334
BindingDB Entry DOI: 10.7270/Q2RV0NRS |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data