 Found 3635 hits with Last Name = 'queener' and Initial = 's'
Found 3635 hits with Last Name = 'queener' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dihydrofolate reductase
(Rattus norvegicus (rat)) | BDBM50059955

(4-({[4-(2,4-Diamino-6-ethyl-pyrimidin-5-yl)-2-nitr...)Show SMILES CCc1nc(N)nc(N)c1-c1ccc(N(C)Cc2ccc(cc2)C(=O)NC)c(c1)[N+]([O-])=O Show InChI InChI=1S/C22H25N7O3/c1-4-16-19(20(23)27-22(24)26-16)15-9-10-17(18(11-15)29(31)32)28(3)12-13-5-7-14(8-6-13)21(30)25-2/h5-11H,4,12H2,1-3H3,(H,25,30)(H4,23,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.000350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against rat liver Dihydrofolate reductase |
J Med Chem 40: 3040-8 (1997)
Article DOI: 10.1021/jm970055k
BindingDB Entry DOI: 10.7270/Q2C53JZ2 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Rattus norvegicus (rat)) | BDBM50059948

(4-({[4-(2,4-Diamino-6-ethyl-pyrimidin-5-yl)-2-nitr...)Show SMILES CCc1nc(N)nc(N)c1-c1ccc(N(C)Cc2ccc(cc2)C(O)=O)c(c1)[N+]([O-])=O Show InChI InChI=1S/C21H22N6O4/c1-3-15-18(19(22)25-21(23)24-15)14-8-9-16(17(10-14)27(30)31)26(2)11-12-4-6-13(7-5-12)20(28)29/h4-10H,3,11H2,1-2H3,(H,28,29)(H4,22,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.000400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against rat liver Dihydrofolate reductase |
J Med Chem 40: 3040-8 (1997)
Article DOI: 10.1021/jm970055k
BindingDB Entry DOI: 10.7270/Q2C53JZ2 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
PubMed
| 0.00120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Wild-type human DHFR |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Pneumocystis carinii) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity of the compound was reported with purified recombinant P. carnii Dihydrofolate reductase |
J Med Chem 38: 4739-59 (1996)
BindingDB Entry DOI: 10.7270/Q26D5S01 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Rattus norvegicus (rat)) | BDBM50058420
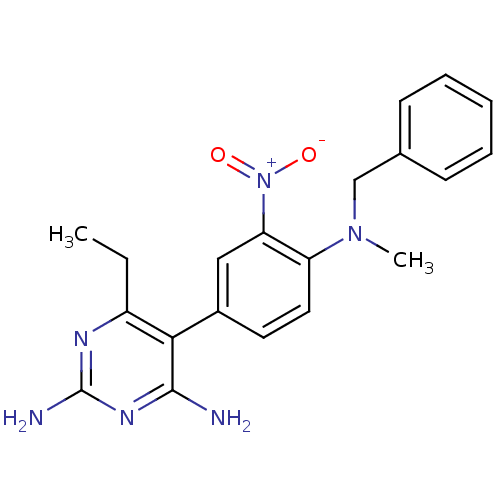
((methylbenzoprim, MBP) 5-[4-(Benzyl-methyl-amino)-...)Show SMILES CCc1nc(N)nc(N)c1-c1ccc(N(C)Cc2ccccc2)c(c1)[N+]([O-])=O Show InChI InChI=1S/C20H22N6O2/c1-3-15-18(19(21)24-20(22)23-15)14-9-10-16(17(11-14)26(27)28)25(2)12-13-7-5-4-6-8-13/h4-11H,3,12H2,1-2H3,(H4,21,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against rat liver Dihydrofolate reductase |
J Med Chem 40: 3040-8 (1997)
Article DOI: 10.1021/jm970055k
BindingDB Entry DOI: 10.7270/Q2C53JZ2 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50026273
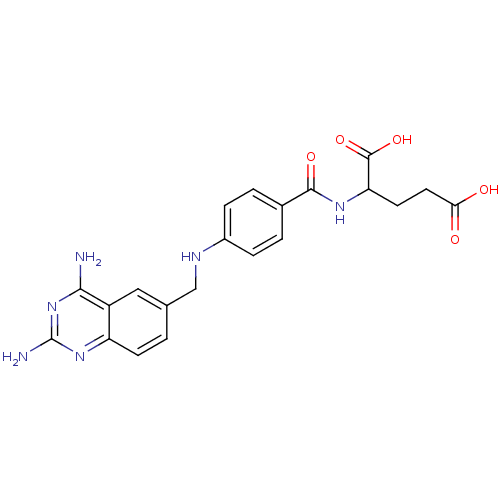
(2-{4-[(2,4-Diamino-quinazolin-6-ylmethyl)-amino]-b...)Show SMILES Nc1nc(N)c2cc(CNc3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)ccc2n1 Show InChI InChI=1S/C21H22N6O5/c22-18-14-9-11(1-6-15(14)26-21(23)27-18)10-24-13-4-2-12(3-5-13)19(30)25-16(20(31)32)7-8-17(28)29/h1-6,9,16,24H,7-8,10H2,(H,25,30)(H,28,29)(H,31,32)(H4,22,23,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50026273

(2-{4-[(2,4-Diamino-quinazolin-6-ylmethyl)-amino]-b...)Show SMILES Nc1nc(N)c2cc(CNc3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)ccc2n1 Show InChI InChI=1S/C21H22N6O5/c22-18-14-9-11(1-6-15(14)26-21(23)27-18)10-24-13-4-2-12(3-5-13)19(30)25-16(20(31)32)7-8-17(28)29/h1-6,9,16,24H,7-8,10H2,(H,25,30)(H,28,29)(H,31,32)(H4,22,23,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Rattus norvegicus (rat)) | BDBM50059957

(4-({[4-(2,4-Diamino-6-ethyl-pyrimidin-5-yl)-2-nitr...)Show SMILES CCc1nc(N)nc(N)c1-c1ccc(N(C)Cc2ccc(cc2)C(=O)OC)c(c1)[N+]([O-])=O Show InChI InChI=1S/C22H24N6O4/c1-4-16-19(20(23)26-22(24)25-16)15-9-10-17(18(11-15)28(30)31)27(2)12-13-5-7-14(8-6-13)21(29)32-3/h5-11H,4,12H2,1-3H3,(H4,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against rat liver Dihydrofolate reductase |
J Med Chem 40: 3040-8 (1997)
Article DOI: 10.1021/jm970055k
BindingDB Entry DOI: 10.7270/Q2C53JZ2 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Rattus norvegicus (rat)) | BDBM50059956
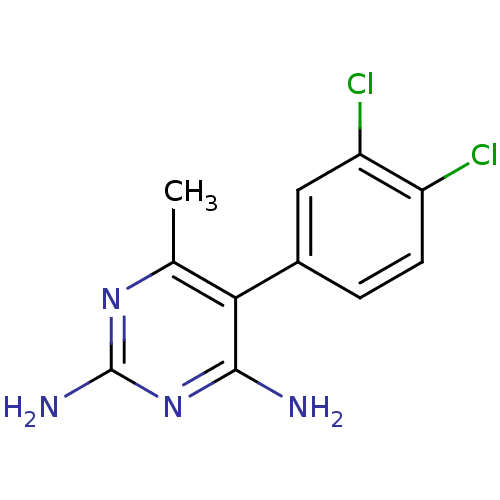
(5-(3,4-DICHLOROPHENYL)-6-METHYLPYRIMIDINE-2,4-DIAM...)Show InChI InChI=1S/C11H10Cl2N4/c1-5-9(10(14)17-11(15)16-5)6-2-3-7(12)8(13)4-6/h2-4H,1H3,(H4,14,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against rat liver Dihydrofolate reductase |
J Med Chem 40: 3040-8 (1997)
Article DOI: 10.1021/jm970055k
BindingDB Entry DOI: 10.7270/Q2C53JZ2 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Pneumocystis carinii) | BDBM18224

(6-[(2,5-dimethoxyphenyl)methyl]-5-methylpyrido[2,3...)Show InChI InChI=1S/C17H19N5O2/c1-9-11(6-10-7-12(23-2)4-5-13(10)24-3)8-20-16-14(9)15(18)21-17(19)22-16/h4-5,7-8H,6H2,1-3H3,(H4,18,19,20,21,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity of the compound was reported with purified recombinant P. carnii Dihydrofolate reductase |
J Med Chem 38: 4739-59 (1996)
BindingDB Entry DOI: 10.7270/Q26D5S01 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50367055

(4-Aminofolic acid | 4-Aminopteroic acid | AMINOPTE...)Show SMILES Nc1nc(N)c2nc(CNc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cnc2n1 |r| Show InChI InChI=1S/C19H20N8O5/c20-15-14-16(27-19(21)26-15)23-8-11(24-14)7-22-10-3-1-9(2-4-10)17(30)25-12(18(31)32)5-6-13(28)29/h1-4,8,12,22H,5-7H2,(H,25,30)(H,28,29)(H,31,32)(H4,20,21,23,26,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50367055

(4-Aminofolic acid | 4-Aminopteroic acid | AMINOPTE...)Show SMILES Nc1nc(N)c2nc(CNc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cnc2n1 |r| Show InChI InChI=1S/C19H20N8O5/c20-15-14-16(27-19(21)26-15)23-8-11(24-14)7-22-10-3-1-9(2-4-10)17(30)25-12(18(31)32)5-6-13(28)29/h1-4,8,12,22H,5-7H2,(H,25,30)(H,28,29)(H,31,32)(H4,20,21,23,26,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036495
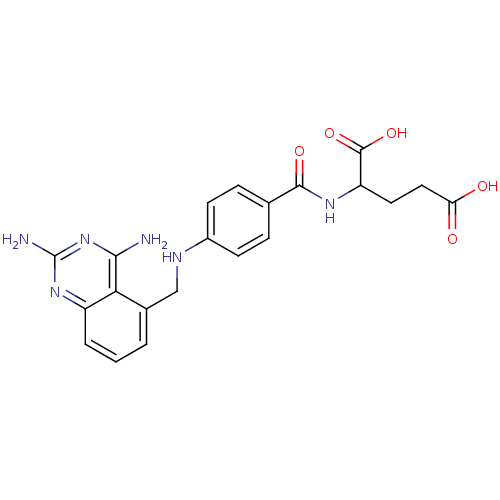
(2-{4-[(2,4-Diamino-quinazolin-5-ylmethyl)-amino]-b...)Show SMILES Nc1nc(N)c2c(CNc3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)cccc2n1 Show InChI InChI=1S/C21H22N6O5/c22-18-17-12(2-1-3-14(17)26-21(23)27-18)10-24-13-6-4-11(5-7-13)19(30)25-15(20(31)32)8-9-16(28)29/h1-7,15,24H,8-10H2,(H,25,30)(H,28,29)(H,31,32)(H4,22,23,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Wild-type human DHFR |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Pneumocystis carinii) | BDBM18268

(5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...)Show InChI InChI=1S/C19H23N5O3/c1-10-11(5-6-13-16(10)18(20)24-19(21)23-13)9-22-12-7-14(25-2)17(27-4)15(8-12)26-3/h5-8,22H,9H2,1-4H3,(H4,20,21,23,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity of the compound was reported with purified recombinant P. carnii Dihydrofolate reductase |
J Med Chem 38: 4739-59 (1996)
BindingDB Entry DOI: 10.7270/Q26D5S01 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036484
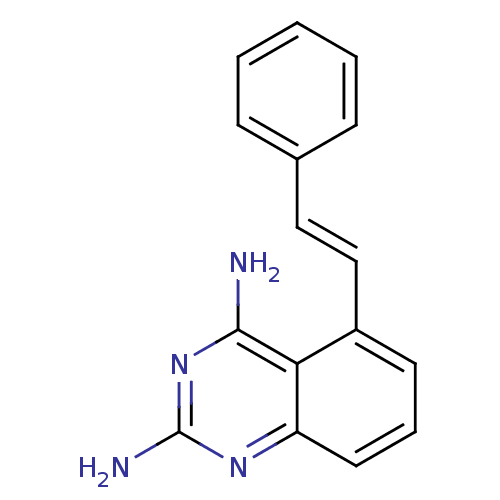
(5-((E)-Styryl)-quinazoline-2,4-diamine | CHEMBL164...)Show InChI InChI=1S/C16H14N4/c17-15-14-12(10-9-11-5-2-1-3-6-11)7-4-8-13(14)19-16(18)20-15/h1-10H,(H4,17,18,19,20)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036495

(2-{4-[(2,4-Diamino-quinazolin-5-ylmethyl)-amino]-b...)Show SMILES Nc1nc(N)c2c(CNc3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)cccc2n1 Show InChI InChI=1S/C21H22N6O5/c22-18-17-12(2-1-3-14(17)26-21(23)27-18)10-24-13-6-4-11(5-7-13)19(30)25-15(20(31)32)8-9-16(28)29/h1-7,15,24H,8-10H2,(H,25,30)(H,28,29)(H,31,32)(H4,22,23,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036483
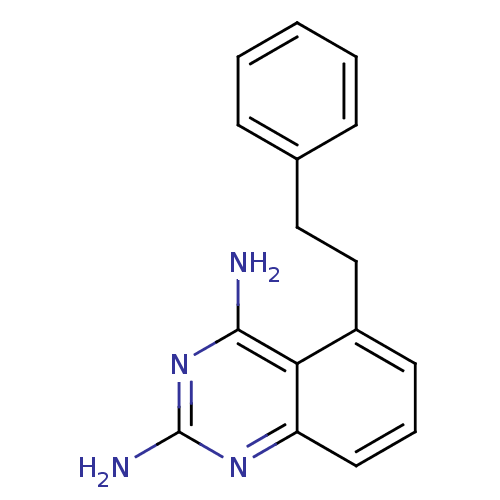
(5-Phenethyl-quinazoline-2,4-diamine | CHEMBL341703)Show InChI InChI=1S/C16H16N4/c17-15-14-12(10-9-11-5-2-1-3-6-11)7-4-8-13(14)19-16(18)20-15/h1-8H,9-10H2,(H4,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Pneumocystis carinii) | BDBM50029763
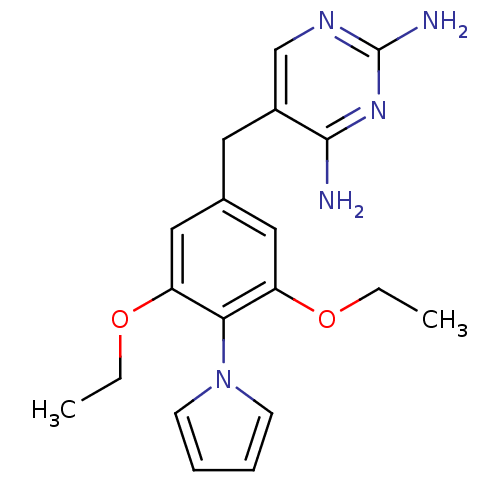
(5-(3,5-Diethoxy-4-pyrrol-1-yl-benzyl)-pyrimidine-2...)Show InChI InChI=1S/C19H23N5O2/c1-3-25-15-10-13(9-14-12-22-19(21)23-18(14)20)11-16(26-4-2)17(15)24-7-5-6-8-24/h5-8,10-12H,3-4,9H2,1-2H3,(H4,20,21,22,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity of the compound was reported with purified recombinant P. carnii Dihydrofolate reductase |
J Med Chem 38: 4739-59 (1996)
BindingDB Entry DOI: 10.7270/Q26D5S01 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036491
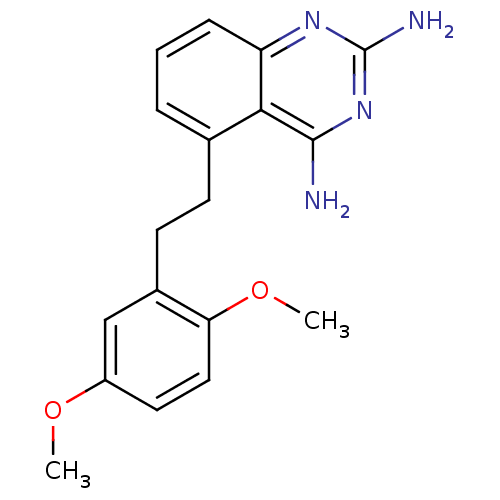
(5-[2-(2,5-Dimethoxy-phenyl)-ethyl]-quinazoline-2,4...)Show InChI InChI=1S/C18H20N4O2/c1-23-13-8-9-15(24-2)12(10-13)7-6-11-4-3-5-14-16(11)17(19)22-18(20)21-14/h3-5,8-10H,6-7H2,1-2H3,(H4,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036486
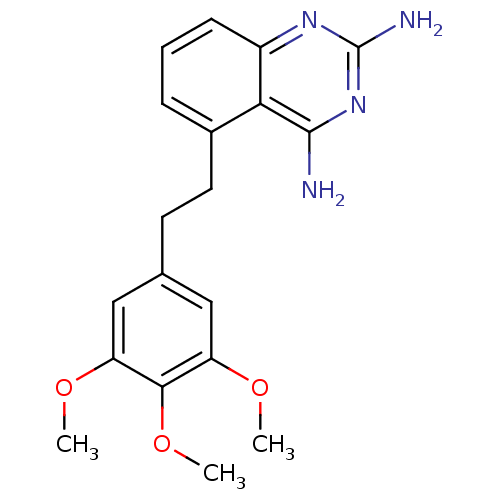
(5-[2-(3,4,5-Trimethoxy-phenyl)-ethyl]-quinazoline-...)Show InChI InChI=1S/C19H22N4O3/c1-24-14-9-11(10-15(25-2)17(14)26-3)7-8-12-5-4-6-13-16(12)18(20)23-19(21)22-13/h4-6,9-10H,7-8H2,1-3H3,(H4,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036484

(5-((E)-Styryl)-quinazoline-2,4-diamine | CHEMBL164...)Show InChI InChI=1S/C16H14N4/c17-15-14-12(10-9-11-5-2-1-3-6-11)7-4-8-13(14)19-16(18)20-15/h1-10H,(H4,17,18,19,20)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory compound against Wild-type human DHFR. |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Pneumocystis carinii) | BDBM18512

(5-(4-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |...)Show InChI InChI=1S/C12H13ClN4/c1-2-9-10(11(14)17-12(15)16-9)7-3-5-8(13)6-4-7/h3-6H,2H2,1H3,(H4,14,15,16,17) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 9.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity of the compound was reported with purified recombinant P. carnii Dihydrofolate reductase |
J Med Chem 38: 4739-59 (1996)
BindingDB Entry DOI: 10.7270/Q26D5S01 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18268

(5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...)Show InChI InChI=1S/C19H23N5O3/c1-10-11(5-6-13-16(10)18(20)24-19(21)23-13)9-22-12-7-14(25-2)17(27-4)15(8-12)26-3/h5-8,22H,9H2,1-4H3,(H4,20,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory compound against Wild-type human DHFR. |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036483

(5-Phenethyl-quinazoline-2,4-diamine | CHEMBL341703)Show InChI InChI=1S/C16H16N4/c17-15-14-12(10-9-11-5-2-1-3-6-11)7-4-8-13(14)19-16(18)20-15/h1-8H,9-10H2,(H4,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory compound against Wild-type human DHFR. |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Pneumocystis carinii) | BDBM18792
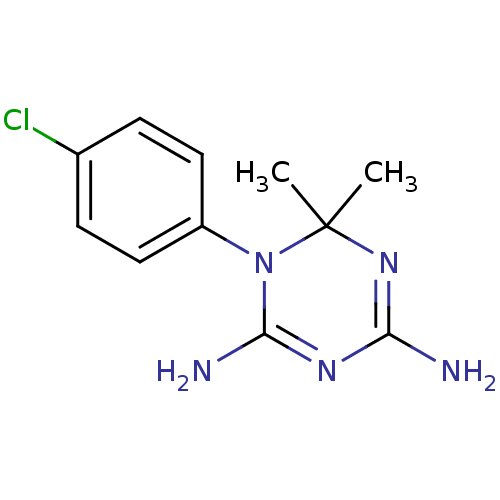
(1-(4-chlorophenyl)-6,6-dimethyl-1,6-dihydro-1,3,5-...)Show InChI InChI=1S/C11H14ClN5/c1-11(2)16-9(13)15-10(14)17(11)8-5-3-7(12)4-6-8/h3-6H,1-2H3,(H4,13,14,15,16) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity of the compound was reported with purified recombinant P. carnii Dihydrofolate reductase |
J Med Chem 38: 4739-59 (1996)
BindingDB Entry DOI: 10.7270/Q26D5S01 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036488
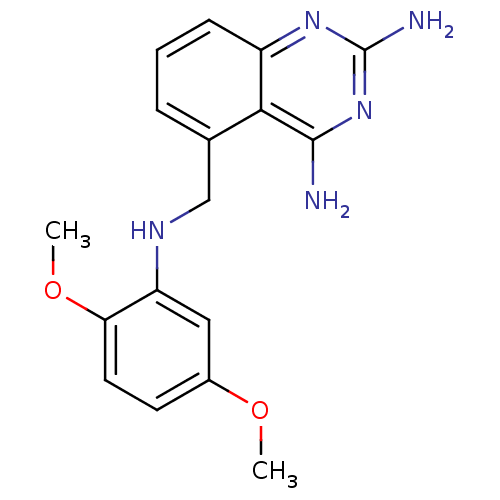
(5-[(2,5-Dimethoxy-phenylamino)-methyl]-quinazoline...)Show InChI InChI=1S/C17H19N5O2/c1-23-11-6-7-14(24-2)13(8-11)20-9-10-4-3-5-12-15(10)16(18)22-17(19)21-12/h3-8,20H,9H2,1-2H3,(H4,18,19,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18224

(6-[(2,5-dimethoxyphenyl)methyl]-5-methylpyrido[2,3...)Show InChI InChI=1S/C17H19N5O2/c1-9-11(6-10-7-12(23-2)4-5-13(10)24-3)8-20-16-14(9)15(18)21-17(19)22-16/h4-5,7-8H,6H2,1-3H3,(H4,18,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Wild-type human DHFR |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18224

(6-[(2,5-dimethoxyphenyl)methyl]-5-methylpyrido[2,3...)Show InChI InChI=1S/C17H19N5O2/c1-9-11(6-10-7-12(23-2)4-5-13(10)24-3)8-20-16-14(9)15(18)21-17(19)22-16/h4-5,7-8H,6H2,1-3H3,(H4,18,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036491

(5-[2-(2,5-Dimethoxy-phenyl)-ethyl]-quinazoline-2,4...)Show InChI InChI=1S/C18H20N4O2/c1-23-13-8-9-15(24-2)12(10-13)7-6-11-4-3-5-14-16(11)17(19)22-18(20)21-14/h3-5,8-10H,6-7H2,1-2H3,(H4,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory compound against Wild-type human DHFR. |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036487
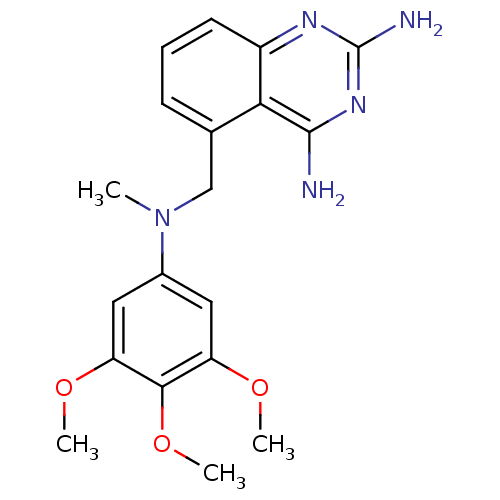
(5-{[Methyl-(3,4,5-trimethoxy-phenyl)-amino]-methyl...)Show SMILES COc1cc(cc(OC)c1OC)N(C)Cc1cccc2nc(N)nc(N)c12 Show InChI InChI=1S/C19H23N5O3/c1-24(12-8-14(25-2)17(27-4)15(9-12)26-3)10-11-6-5-7-13-16(11)18(20)23-19(21)22-13/h5-9H,10H2,1-4H3,(H4,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036486

(5-[2-(3,4,5-Trimethoxy-phenyl)-ethyl]-quinazoline-...)Show InChI InChI=1S/C19H22N4O3/c1-24-14-9-11(10-15(25-2)17(14)26-3)7-8-12-5-4-6-13-16(12)18(20)23-19(21)22-13/h4-6,9-10H,7-8H2,1-3H3,(H4,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory compound against Wild-type human DHFR. |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18268

(5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...)Show InChI InChI=1S/C19H23N5O3/c1-10-11(5-6-13-16(10)18(20)24-19(21)23-13)9-22-12-7-14(25-2)17(27-4)15(8-12)26-3/h5-8,22H,9H2,1-4H3,(H4,20,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036493
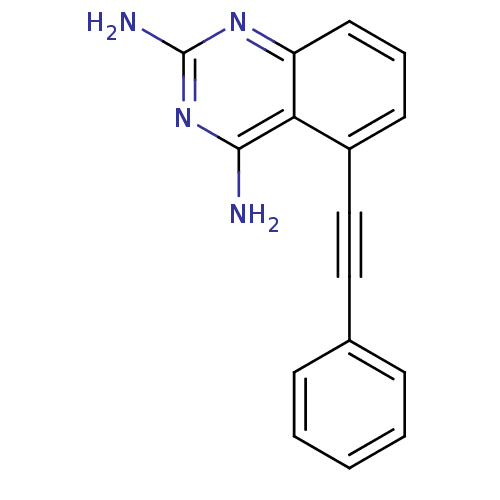
(5-Phenylethynyl-quinazoline-2,4-diamine | CHEMBL35...)Show InChI InChI=1S/C16H12N4/c17-15-14-12(10-9-11-5-2-1-3-6-11)7-4-8-13(14)19-16(18)20-15/h1-8H,(H4,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036489
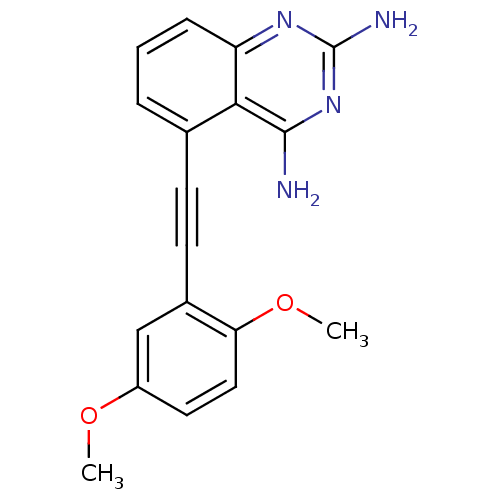
(5-(2,5-Dimethoxy-phenylethynyl)-quinazoline-2,4-di...)Show InChI InChI=1S/C18H16N4O2/c1-23-13-8-9-15(24-2)12(10-13)7-6-11-4-3-5-14-16(11)17(19)22-18(20)21-14/h3-5,8-10H,1-2H3,(H4,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Pneumocystis carinii) | BDBM18069

(5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-d...)Show InChI InChI=1S/C14H18N4O3/c1-19-10-5-8(6-11(20-2)12(10)21-3)4-9-7-17-14(16)18-13(9)15/h5-7H,4H2,1-3H3,(H4,15,16,17,18) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 152 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity of the compound was reported with purified recombinant P. carnii Dihydrofolate reductase |
J Med Chem 38: 4739-59 (1996)
BindingDB Entry DOI: 10.7270/Q26D5S01 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036488

(5-[(2,5-Dimethoxy-phenylamino)-methyl]-quinazoline...)Show InChI InChI=1S/C17H19N5O2/c1-23-11-6-7-14(24-2)13(8-11)20-9-10-4-3-5-12-15(10)16(18)22-17(19)21-12/h3-8,20H,9H2,1-2H3,(H4,18,19,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory compound against Wild-type human DHFR. |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036492
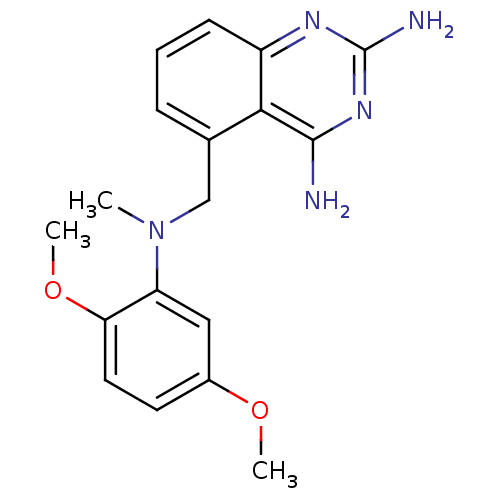
(5-{[(2,5-Dimethoxy-phenyl)-methyl-amino]-methyl}-q...)Show InChI InChI=1S/C18H21N5O2/c1-23(14-9-12(24-2)7-8-15(14)25-3)10-11-5-4-6-13-16(11)17(19)22-18(20)21-13/h4-9H,10H2,1-3H3,(H4,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036485
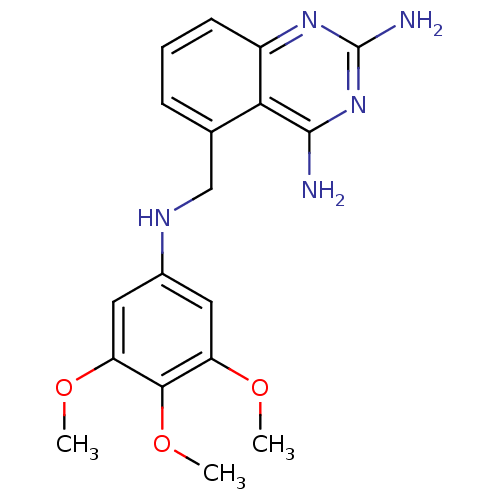
(5-[(3,4,5-Trimethoxy-phenylamino)-methyl]-quinazol...)Show InChI InChI=1S/C18H21N5O3/c1-24-13-7-11(8-14(25-2)16(13)26-3)21-9-10-5-4-6-12-15(10)17(19)23-18(20)22-12/h4-8,21H,9H2,1-3H3,(H4,19,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036487

(5-{[Methyl-(3,4,5-trimethoxy-phenyl)-amino]-methyl...)Show SMILES COc1cc(cc(OC)c1OC)N(C)Cc1cccc2nc(N)nc(N)c12 Show InChI InChI=1S/C19H23N5O3/c1-24(12-8-14(25-2)17(27-4)15(9-12)26-3)10-11-6-5-7-13-16(11)18(20)23-19(21)22-13/h5-9H,10H2,1-4H3,(H4,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory compound against Wild-type human DHFR. |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036492

(5-{[(2,5-Dimethoxy-phenyl)-methyl-amino]-methyl}-q...)Show InChI InChI=1S/C18H21N5O2/c1-23(14-9-12(24-2)7-8-15(14)25-3)10-11-5-4-6-13-16(11)17(19)22-18(20)21-13/h4-9H,10H2,1-3H3,(H4,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory compound against Wild-type human DHFR. |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036485

(5-[(3,4,5-Trimethoxy-phenylamino)-methyl]-quinazol...)Show InChI InChI=1S/C18H21N5O3/c1-24-13-7-11(8-14(25-2)16(13)26-3)21-9-10-5-4-6-12-15(10)17(19)23-18(20)22-12/h4-8,21H,9H2,1-3H3,(H4,19,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory compound against Wild-type human DHFR. |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036493

(5-Phenylethynyl-quinazoline-2,4-diamine | CHEMBL35...)Show InChI InChI=1S/C16H12N4/c17-15-14-12(10-9-11-5-2-1-3-6-11)7-4-8-13(14)19-16(18)20-15/h1-8H,(H4,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| >500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Wild-type human DHFR |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036489

(5-(2,5-Dimethoxy-phenylethynyl)-quinazoline-2,4-di...)Show InChI InChI=1S/C18H16N4O2/c1-23-13-8-9-15(24-2)12(10-13)7-6-11-4-3-5-14-16(11)17(19)22-18(20)21-14/h3-5,8-10H,1-2H3,(H4,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| >500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory compound against Wild-type human DHFR. |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mycobacterium avium) | BDBM18229
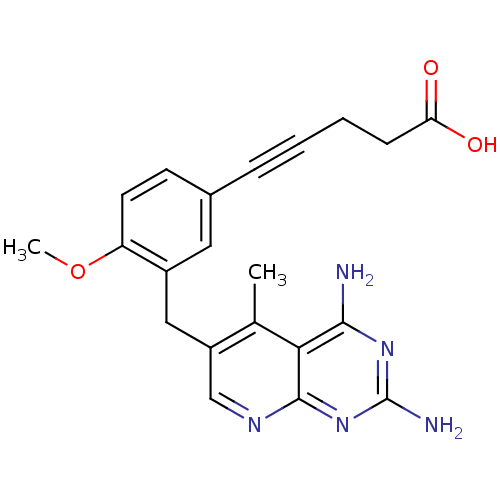
(5-[3-({2,4-diamino-5-methylpyrido[2,3-d]pyrimidin-...)Show SMILES COc1ccc(cc1Cc1cnc2nc(N)nc(N)c2c1C)C#CCCC(O)=O Show InChI InChI=1S/C21H21N5O3/c1-12-15(11-24-20-18(12)19(22)25-21(23)26-20)10-14-9-13(7-8-16(14)29-2)5-3-4-6-17(27)28/h7-9,11H,4,6,10H2,1-2H3,(H,27,28)(H4,22,23,24,25,26) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
| Assay Description
Dihydrofolate reductase was assayed with no inhibitor and with a series of concentrations of inhibitors to allow for a range of inhibition from 10 to... |
J Med Chem 48: 4420-31 (2005)
Article DOI: 10.1021/jm0581718
BindingDB Entry DOI: 10.7270/Q2W0946F |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Pneumocystis carinii) | BDBM18229

(5-[3-({2,4-diamino-5-methylpyrido[2,3-d]pyrimidin-...)Show SMILES COc1ccc(cc1Cc1cnc2nc(N)nc(N)c2c1C)C#CCCC(O)=O Show InChI InChI=1S/C21H21N5O3/c1-12-15(11-24-20-18(12)19(22)25-21(23)26-20)10-14-9-13(7-8-16(14)29-2)5-3-4-6-17(27)28/h7-9,11H,4,6,10H2,1-2H3,(H,27,28)(H4,22,23,24,25,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0970 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Harvard Medical School
| Assay Description
Dihydrofolate reductase was assayed with no inhibitor and with a series of concentrations of inhibitors to allow for a range of inhibition from 10 to... |
J Med Chem 48: 4420-31 (2005)
Article DOI: 10.1021/jm0581718
BindingDB Entry DOI: 10.7270/Q2W0946F |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Pneumocystis carinii) | BDBM18228
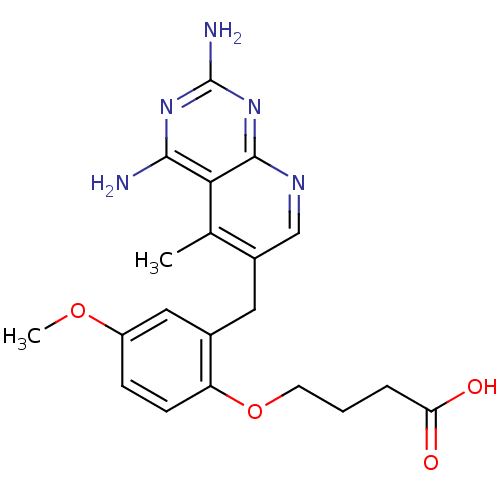
(4-[2-({2,4-diamino-5-methylpyrido[2,3-d]pyrimidin-...)Show SMILES COc1ccc(OCCCC(O)=O)c(Cc2cnc3nc(N)nc(N)c3c2C)c1 Show InChI InChI=1S/C20H23N5O4/c1-11-13(10-23-19-17(11)18(21)24-20(22)25-19)8-12-9-14(28-2)5-6-15(12)29-7-3-4-16(26)27/h5-6,9-10H,3-4,7-8H2,1-2H3,(H,26,27)(H4,21,22,23,24,25) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Harvard Medical School
| Assay Description
Dihydrofolate reductase was assayed with no inhibitor and with a series of concentrations of inhibitors to allow for a range of inhibition from 10 to... |
J Med Chem 48: 4420-31 (2005)
Article DOI: 10.1021/jm0581718
BindingDB Entry DOI: 10.7270/Q2W0946F |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50141010
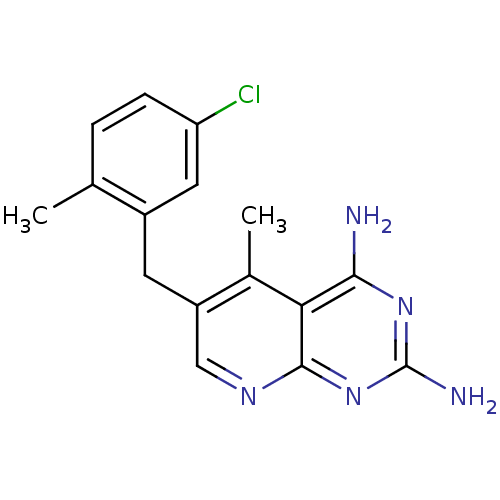
(6-(5-Chloro-2-methyl-benzyl)-5-methyl-pyrido[2,3-d...)Show InChI InChI=1S/C16H16ClN5/c1-8-3-4-12(17)6-10(8)5-11-7-20-15-13(9(11)2)14(18)21-16(19)22-15/h3-4,6-7H,5H2,1-2H3,(H4,18,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory concentration against dihydrofolate reductase of Mycobacterium avium |
J Med Chem 47: 1475-86 (2004)
Article DOI: 10.1021/jm030438k
BindingDB Entry DOI: 10.7270/Q2SJ1K14 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50145797

(6-[(3,5-Dipropoxy-phenylamino)-methyl]-5-methyl-py...)Show SMILES CCCOc1cc(NCc2cnc3nc(N)nc(N)c3c2C)cc(OCCC)c1 Show InChI InChI=1S/C21H28N6O2/c1-4-6-28-16-8-15(9-17(10-16)29-7-5-2)24-11-14-12-25-20-18(13(14)3)19(22)26-21(23)27-20/h8-10,12,24H,4-7,11H2,1-3H3,(H4,22,23,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the Mycobacterium avium Dihydrofolate reductase by 50% was determined |
J Med Chem 47: 2475-85 (2004)
Article DOI: 10.1021/jm030599o
BindingDB Entry DOI: 10.7270/Q2K64HHD |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mycobacterium avium) | BDBM18225
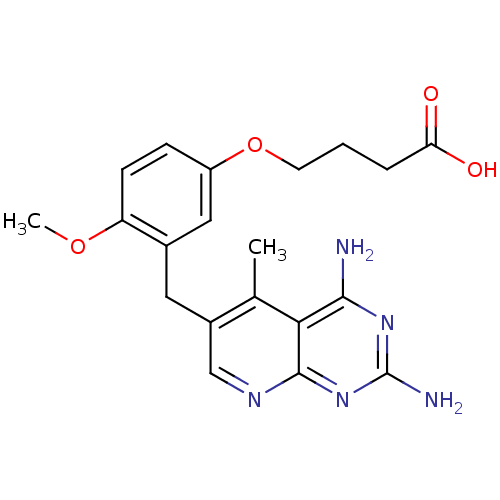
(2,4-diamino-5-deazapteridine, 4 | 4-[3-({2,4-diami...)Show SMILES COc1ccc(OCCCC(O)=O)cc1Cc1cnc2nc(N)nc(N)c2c1C Show InChI InChI=1S/C20H23N5O4/c1-11-13(10-23-19-17(11)18(21)24-20(22)25-19)8-12-9-14(5-6-15(12)28-2)29-7-3-4-16(26)27/h5-6,9-10H,3-4,7-8H2,1-2H3,(H,26,27)(H4,21,22,23,24,25) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
| Assay Description
Dihydrofolate reductase was assayed with no inhibitor and with a series of concentrations of inhibitors to allow for a range of inhibition from 10 to... |
J Med Chem 48: 4420-31 (2005)
Article DOI: 10.1021/jm0581718
BindingDB Entry DOI: 10.7270/Q2W0946F |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data