

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343634 (4-((E)-4-((S)-1-((1R,2S,5S)-2-((S)-5-amino-1-(benz...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343641 (CHEMBL1774964 | cis-4-((E)-4-((S)-4-methyl-1-oxo-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343632 (4-((E)-4-((S)-1-((1R,2S,5S)-2-((S)-5-amino-1-(benz...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343644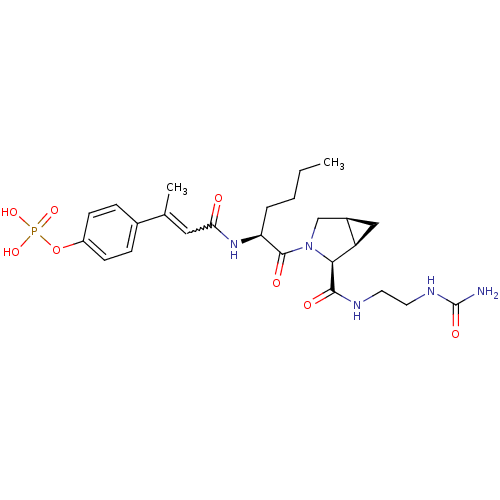 (CHEMBL1774967 | cis-4-((E)-4-oxo-4-((S)-1-oxo-1-((...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343633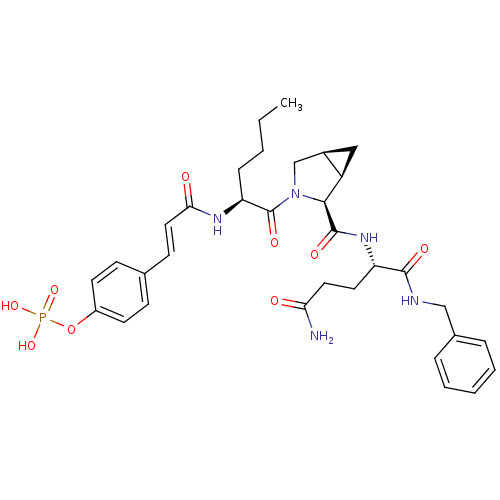 (4-((E)-3-((S)-1-((1R,2S,5S)-2-((S)-5-amino-1-(benz...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343635 (4-((E)-4-((3S,6S)-6-((S)-5-amino-1-(benzylamino)-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343631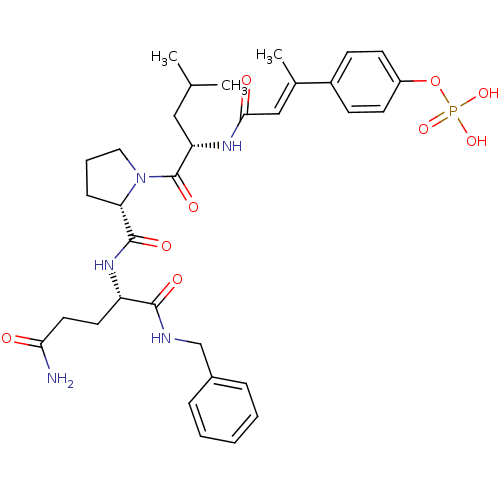 (4-((E)-4-((S)-1-((S)-2-((S)-5-amino-1-(benzylamino...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343642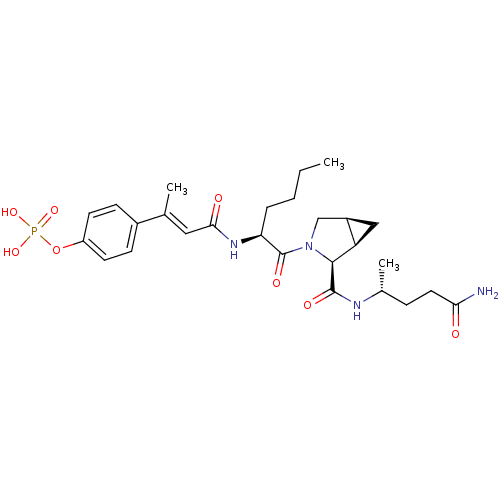 (CHEMBL1774965 | cis-4-((E)-4-((S)-1-((1R,2S,5S)-2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343639 (CHEMBL1774962 | cis-4-((E)-4-((S)-1-((1R,2S,5S)-2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343638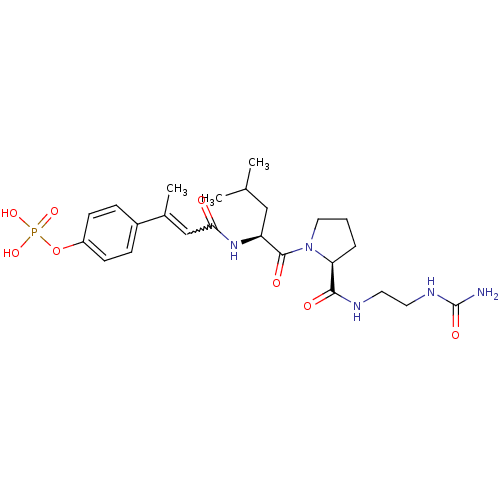 (4-((E)-4-((S)-4-methyl-1-oxo-1-((S)-2-(2-ureidoeth...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343645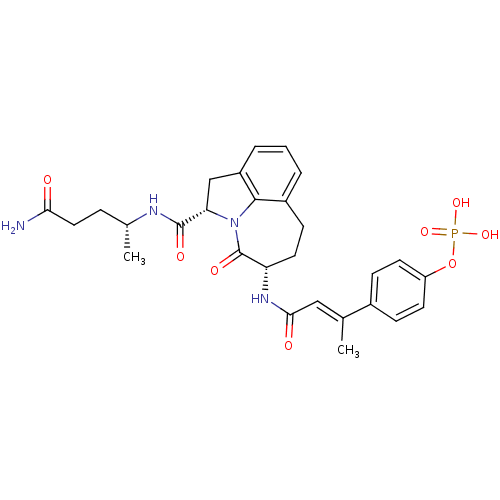 (4-((E)-4-((3S,6S)-6-((R)-5-amino-5-oxopentan-2-ylc...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343643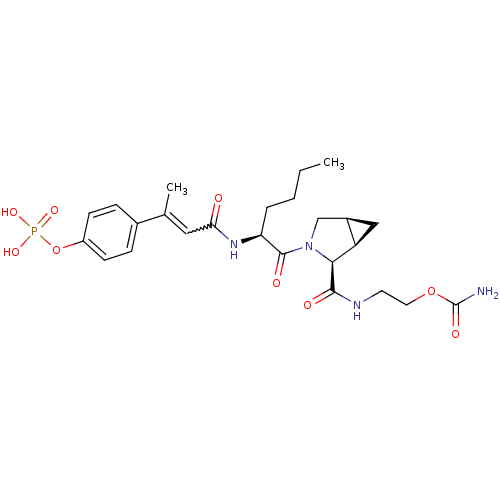 (CHEMBL1774966 | cis-2-((1R,2S,5S)-3-((S)-2-((E)-3-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 114 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343636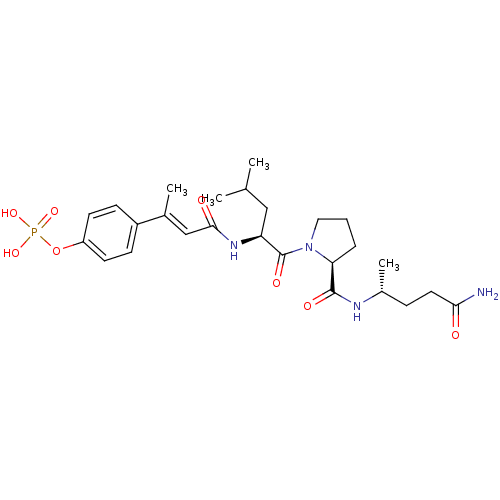 (4-((E)-4-((S)-1-((S)-2-((R)-5-amino-5-oxopentan-2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 144 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343646 (2-((3S,6S)-4-oxo-3-((E)-3-(4-(phosphonooxy)phenyl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 188 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343640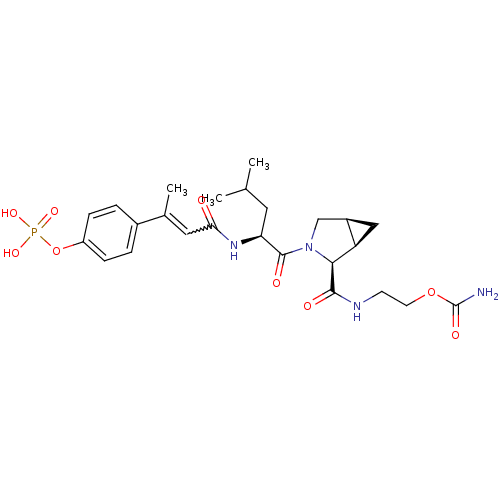 (CHEMBL1774963 | cis-2-((1R,2S,5S)-3-((S)-4-methyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 193 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343637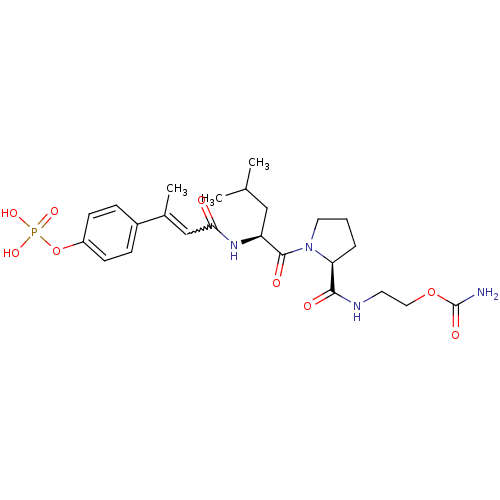 (2-((S)-1-((S)-4-methyl-2-((E)-3-(4-(phosphonooxy)p...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 203 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343647 (4-((E)-4-oxo-4-((3S,6S)-4-oxo-6-(2-ureidoethylcarb...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 386 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Rattus norvegicus) | BDBM50434850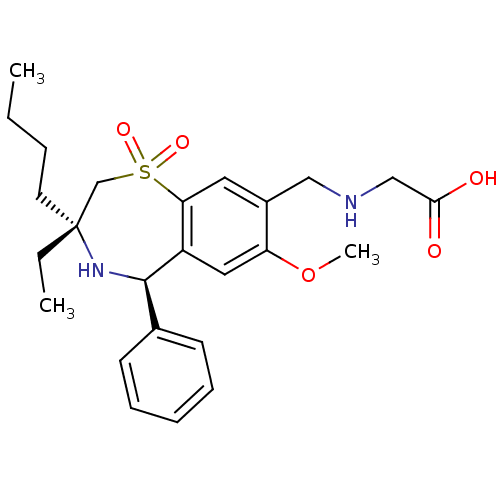 (CHEMBL2387397) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of rat ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysis | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Mus musculus) | BDBM50434850 (CHEMBL2387397) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of mouse ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysi... | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Mus musculus) | BDBM50434849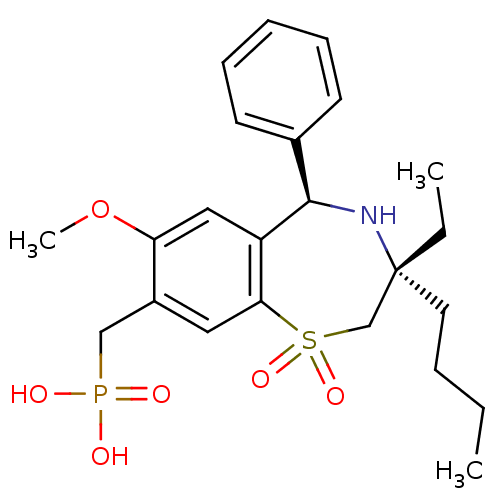 (CHEMBL2385105 | US9040518, 6) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of mouse ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysi... | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Mus musculus) | BDBM50434846 (CHEMBL2387399 | US9040518, 35) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of mouse ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysi... | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Rattus norvegicus) | BDBM50434849 (CHEMBL2385105 | US9040518, 6) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of rat ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysis | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Rattus norvegicus) | BDBM47370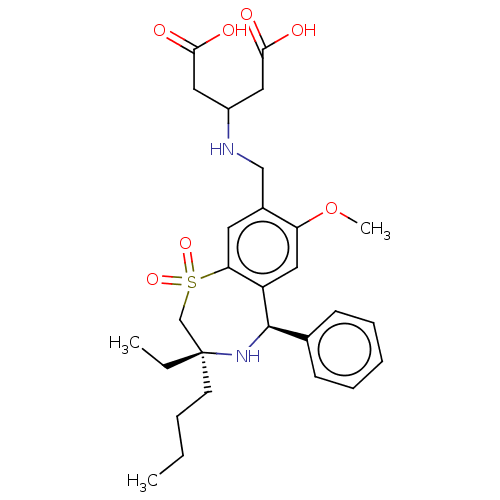 (BDBM50434858 | US9040518, 26) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of rat ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysis | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Homo sapiens (Human)) | BDBM50434848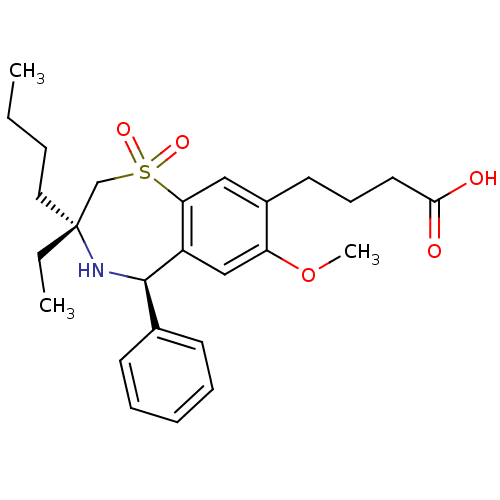 (CHEMBL2387520) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of human ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysi... | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Homo sapiens (Human)) | BDBM50434847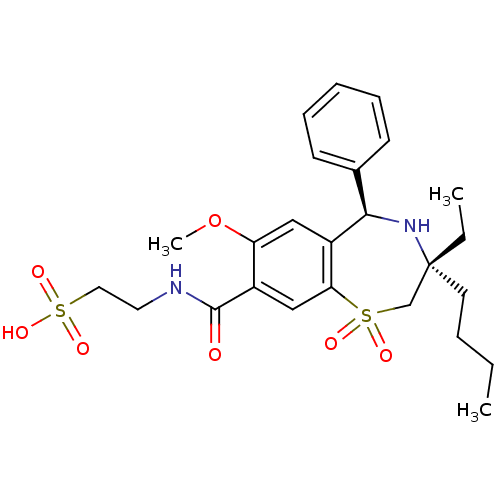 (CHEMBL2387421 | US9040518, 3) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of human ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysi... | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Mus musculus) | BDBM47370 (BDBM50434858 | US9040518, 26) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of mouse ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysi... | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Rattus norvegicus) | BDBM50434846 (CHEMBL2387399 | US9040518, 35) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of rat ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysis | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Mus musculus) | BDBM50134411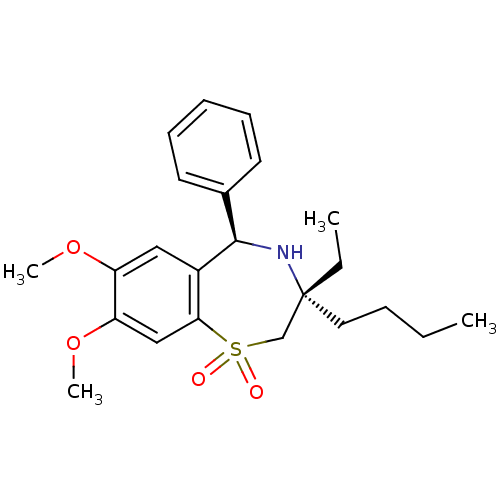 ((7R,9R)-7-Butyl-7-ethyl-2,3-dimethoxy-9-phenyl-6,7...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of mouse ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysi... | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Homo sapiens (Human)) | BDBM50434869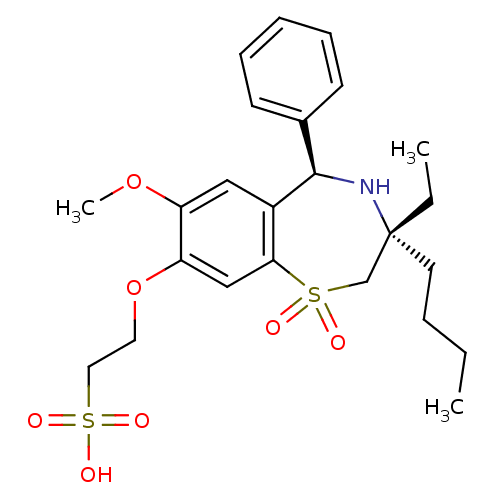 (CHEMBL2387527) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of human ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysi... | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Homo sapiens (Human)) | BDBM50434867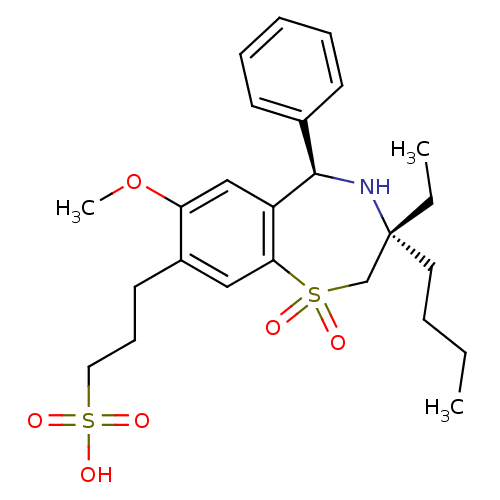 (CHEMBL2387522 | US9040518, 20) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of human ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysi... | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Homo sapiens (Human)) | BDBM50434868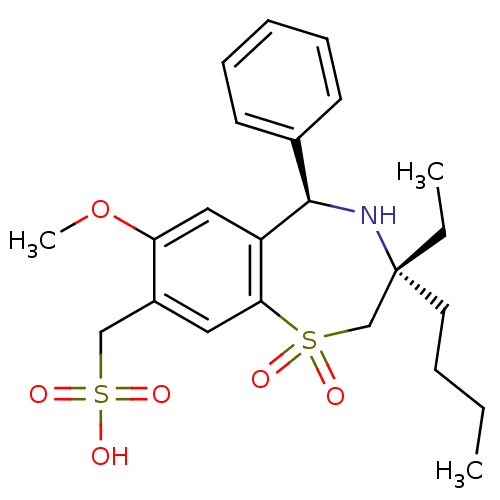 (CHEMBL2387521) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of human ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysi... | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Homo sapiens (Human)) | BDBM50434850 (CHEMBL2387397) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of human ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysi... | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Homo sapiens (Human)) | BDBM50434866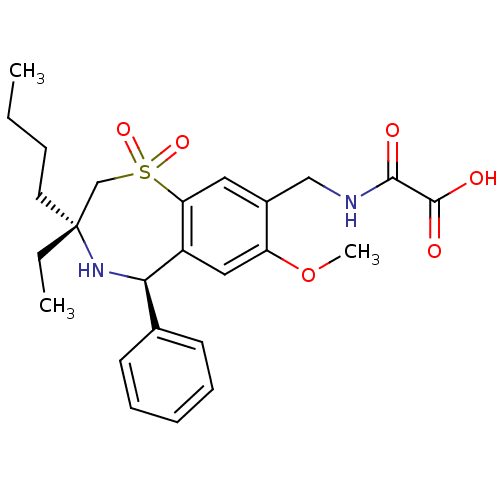 (CHEMBL2387510 | US9040518, 63) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of human ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysi... | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Homo sapiens (Human)) | BDBM50434865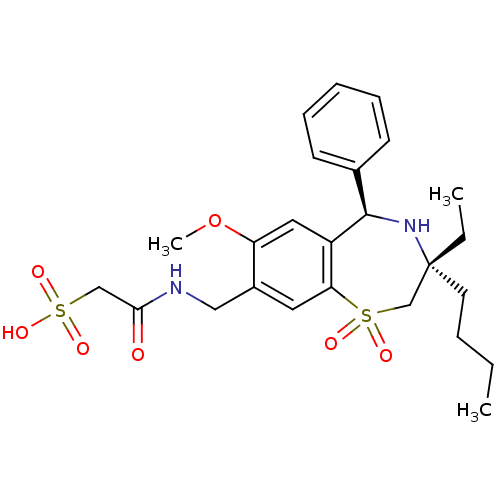 (CHEMBL2387511 | US9040518, 37) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of human ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysi... | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Homo sapiens (Human)) | BDBM50434864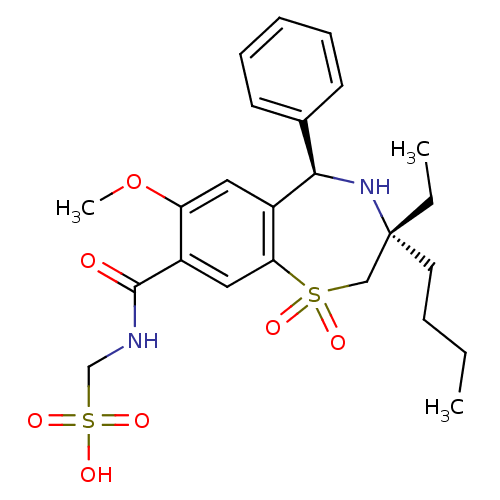 (CHEMBL2387420 | US9040518, 2) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of human ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysi... | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Homo sapiens (Human)) | BDBM50434863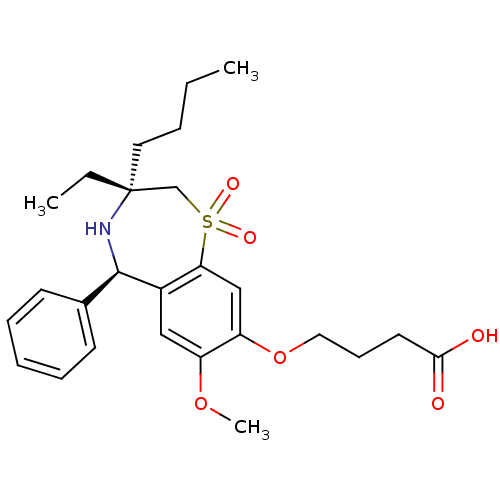 (CHEMBL2387526) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of human ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysi... | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Homo sapiens (Human)) | BDBM50434862 (CHEMBL2387519) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of human ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysi... | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Rattus norvegicus) | BDBM50134411 ((7R,9R)-7-Butyl-7-ethyl-2,3-dimethoxy-9-phenyl-6,7...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of rat ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysis | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Homo sapiens (Human)) | BDBM50434849 (CHEMBL2385105 | US9040518, 6) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of human ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysi... | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 6 (Homo sapiens (Human)) | BDBM50499614 (CHEMBL3741938) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Rice University Curated by ChEMBL | Assay Description Inhibition of STAT6 (unknown origin) expressed in Escherichia coli using FAM-Ala-pTyr-Lys-ProPhe-Gln-Asp-Leu-Ile-NH2 as substrate by fluorescence pol... | J Med Chem 58: 8970-84 (2015) Article DOI: 10.1021/acs.jmedchem.5b01321 BindingDB Entry DOI: 10.7270/Q2TQ64KV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Homo sapiens (Human)) | BDBM50434861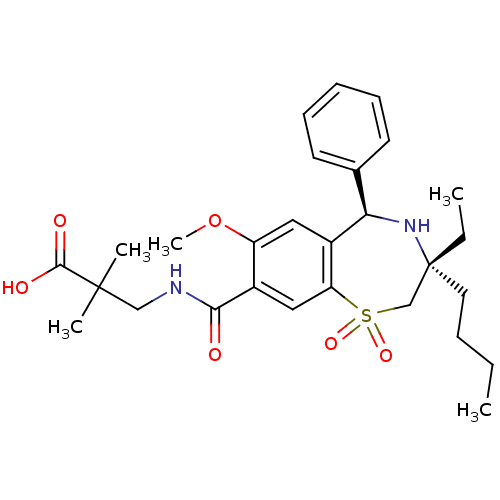 (CHEMBL2387525 | US9040518, 1) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of human ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysi... | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Homo sapiens (Human)) | BDBM50434860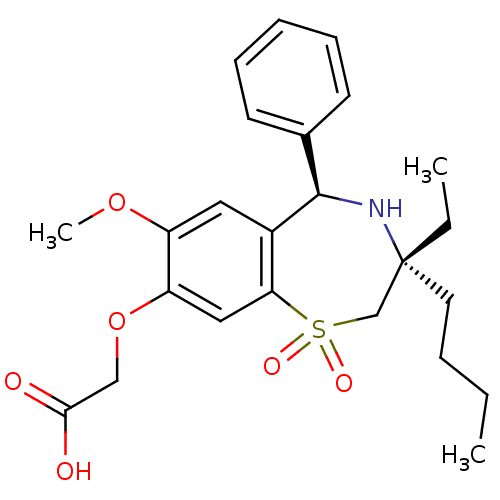 (CHEMBL2387529) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of human ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysi... | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Homo sapiens (Human)) | BDBM50434859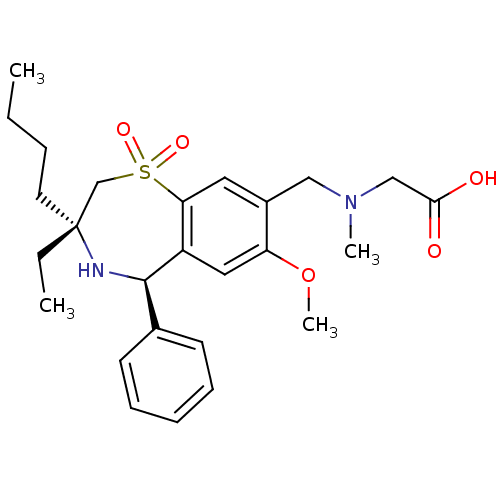 (CHEMBL2387398 | US9040518, 49) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of human ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysi... | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Homo sapiens (Human)) | BDBM47370 (BDBM50434858 | US9040518, 26) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of human ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysi... | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 6 (Homo sapiens (Human)) | BDBM50499605 (CHEMBL3741162) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Rice University Curated by ChEMBL | Assay Description Inhibition of STAT6 (unknown origin) expressed in Escherichia coli using FAM-Ala-pTyr-Lys-ProPhe-Gln-Asp-Leu-Ile-NH2 as substrate by fluorescence pol... | J Med Chem 58: 8970-84 (2015) Article DOI: 10.1021/acs.jmedchem.5b01321 BindingDB Entry DOI: 10.7270/Q2TQ64KV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Homo sapiens (Human)) | BDBM50434857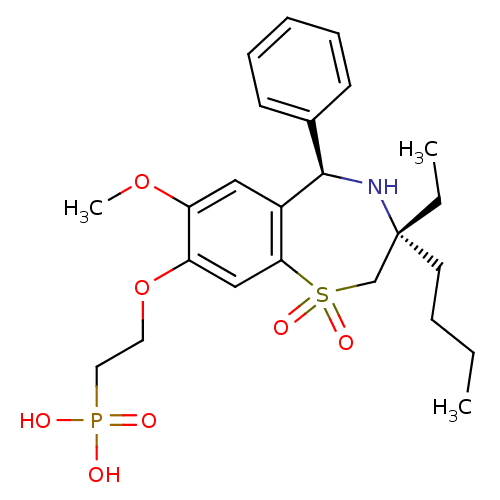 (CHEMBL2387517) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of human ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysi... | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Homo sapiens (Human)) | BDBM50434856 (CHEMBL2387403) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of human ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysi... | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Homo sapiens (Human)) | BDBM50434855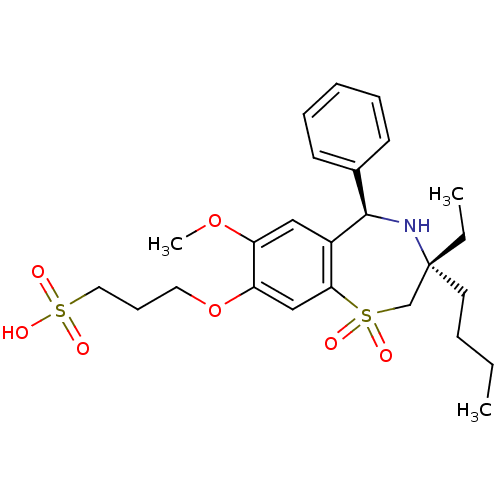 (CHEMBL2387528) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of human ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysi... | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Homo sapiens (Human)) | BDBM50434853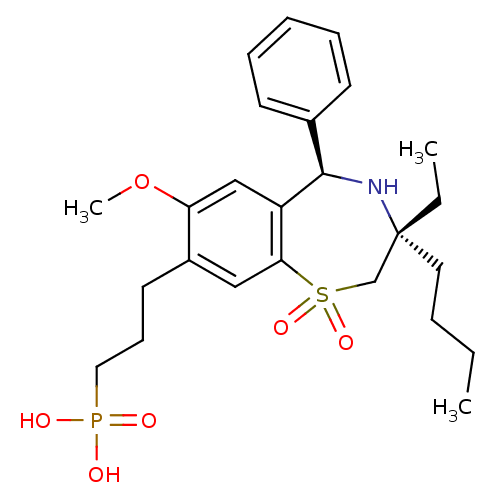 (CHEMBL2387524 | US9040518, 21) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of human ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysi... | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Homo sapiens (Human)) | BDBM50434854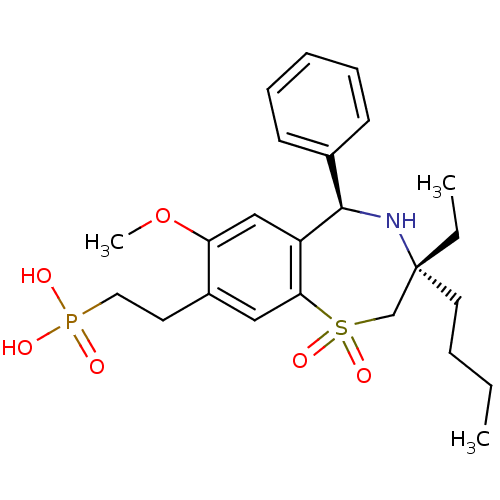 (CHEMBL2387523 | US9040518, 51) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of human ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysi... | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 281 total ) | Next | Last >> |