 Found 100 hits with Last Name = 'richert' and Initial = 'p'
Found 100 hits with Last Name = 'richert' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314628
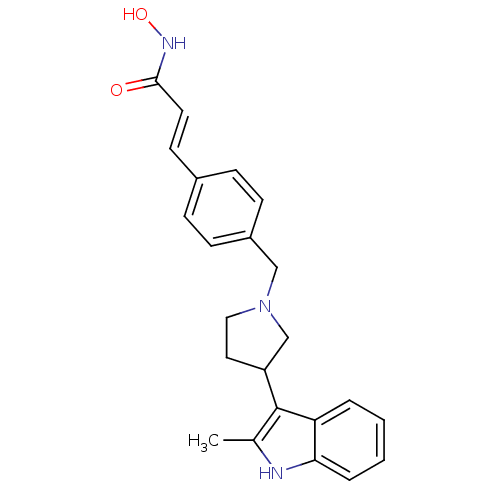
((E)-N-Hydroxy-3-{4-[3-(2-methyl-1H-indol-3-yl)pyrr...)Show SMILES Cc1[nH]c2ccccc2c1C1CCN(Cc2ccc(\C=C\C(=O)NO)cc2)C1 Show InChI InChI=1S/C23H25N3O2/c1-16-23(20-4-2-3-5-21(20)24-16)19-12-13-26(15-19)14-18-8-6-17(7-9-18)10-11-22(27)25-28/h2-11,19,24,28H,12-15H2,1H3,(H,25,27)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314637

((E)-3-{4-[3-(2-tert-Butyl-1H-indol-3-yl)piperidin-...)Show SMILES CC(C)(C)c1[nH]c2ccccc2c1C1CCCN(Cc2ccc(\C=C\C(=O)NO)cc2)C1 Show InChI InChI=1S/C27H33N3O2/c1-27(2,3)26-25(22-8-4-5-9-23(22)28-26)21-7-6-16-30(18-21)17-20-12-10-19(11-13-20)14-15-24(31)29-32/h4-5,8-15,21,28,32H,6-7,16-18H2,1-3H3,(H,29,31)/b15-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314630

((E)-N-Hydroxy-3-(4-{1-[2-(2-methyl-1H-indol-3-yl)e...)Show SMILES Cc1[nH]c2ccccc2c1CCN1CCCC1c1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C24H27N3O2/c1-17-20(21-5-2-3-6-22(21)25-17)14-16-27-15-4-7-23(27)19-11-8-18(9-12-19)10-13-24(28)26-29/h2-3,5-6,8-13,23,25,29H,4,7,14-16H2,1H3,(H,26,28)/b13-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50427040

(CHEMBL2322207)Show SMILES COCCCCC1(CNC(=O)[C@@H]2CNC[C@@H](C2)NS(=O)(=O)c2ccc(C)cc2)c2ccccc2Oc2ccccc12 |r| Show InChI InChI=1S/C32H39N3O5S/c1-23-13-15-26(16-14-23)41(37,38)35-25-19-24(20-33-21-25)31(36)34-22-32(17-7-8-18-39-2)27-9-3-5-11-29(27)40-30-12-6-4-10-28(30)32/h3-6,9-16,24-25,33,35H,7-8,17-22H2,1-2H3,(H,34,36)/t24-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin using fluorescence-quenched (RE(EDANS)IHPFHLVIHTK(Dabcyl)R as substrate incubated for 1 hr prior to substrate a... |
J Med Chem 56: 2196-206 (2013)
Article DOI: 10.1021/jm301706j
BindingDB Entry DOI: 10.7270/Q25X2B8Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM50427040

(CHEMBL2322207)Show SMILES COCCCCC1(CNC(=O)[C@@H]2CNC[C@@H](C2)NS(=O)(=O)c2ccc(C)cc2)c2ccccc2Oc2ccccc12 |r| Show InChI InChI=1S/C32H39N3O5S/c1-23-13-15-26(16-14-23)41(37,38)35-25-19-24(20-33-21-25)31(36)34-22-32(17-7-8-18-39-2)27-9-3-5-11-29(27)40-30-12-6-4-10-28(30)32/h3-6,9-16,24-25,33,35H,7-8,17-22H2,1-2H3,(H,34,36)/t24-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 56: 2196-206 (2013)
Article DOI: 10.1021/jm301706j
BindingDB Entry DOI: 10.7270/Q25X2B8Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314627
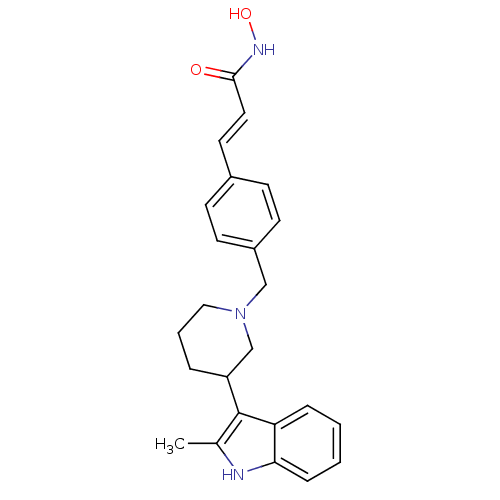
((E)-N-Hydroxy-3-{4-[3-(2-methyl-1H-indol-3-yl)pipe...)Show SMILES Cc1[nH]c2ccccc2c1C1CCCN(Cc2ccc(\C=C\C(=O)NO)cc2)C1 Show InChI InChI=1S/C24H27N3O2/c1-17-24(21-6-2-3-7-22(21)25-17)20-5-4-14-27(16-20)15-19-10-8-18(9-11-19)12-13-23(28)26-29/h2-3,6-13,20,25,29H,4-5,14-16H2,1H3,(H,26,28)/b13-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314642

((E)-3-{4-[(R)-3-(2-tert-Butyl-1H-indol-3-yl)piperi...)Show SMILES CC(C)(C)c1[nH]c2ccccc2c1[C@H]1CCCN(Cc2ccc(\C=C\C(=O)NO)cc2F)C1 |r| Show InChI InChI=1S/C27H32FN3O2/c1-27(2,3)26-25(21-8-4-5-9-23(21)29-26)20-7-6-14-31(17-20)16-19-12-10-18(15-22(19)28)11-13-24(32)30-33/h4-5,8-13,15,20,29,33H,6-7,14,16-17H2,1-3H3,(H,30,32)/b13-11+/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM18012
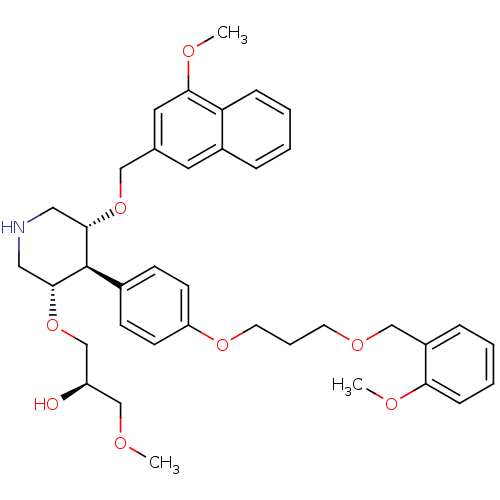
(trans,trans-4-arylpiperidine-based compound, 1)Show SMILES COC[C@@H](O)CO[C@@H]1CNC[C@H](OCc2cc(OC)c3ccccc3c2)[C@H]1c1ccc(OCCCOCc2ccccc2OC)cc1 |r| Show InChI InChI=1S/C38H47NO8/c1-41-25-31(40)26-47-37-22-39-21-36(46-23-27-19-29-9-4-6-11-33(29)35(20-27)43-3)38(37)28-13-15-32(16-14-28)45-18-8-17-44-24-30-10-5-7-12-34(30)42-2/h4-7,9-16,19-20,31,36-40H,8,17-18,21-26H2,1-3H3/t31-,36+,37-,38-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of plasma renin (unknown origin) |
J Med Chem 56: 2196-206 (2013)
Article DOI: 10.1021/jm301706j
BindingDB Entry DOI: 10.7270/Q25X2B8Z |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50314642

((E)-3-{4-[(R)-3-(2-tert-Butyl-1H-indol-3-yl)piperi...)Show SMILES CC(C)(C)c1[nH]c2ccccc2c1[C@H]1CCCN(Cc2ccc(\C=C\C(=O)NO)cc2F)C1 |r| Show InChI InChI=1S/C27H32FN3O2/c1-27(2,3)26-25(21-8-4-5-9-23(21)29-26)20-7-6-14-31(17-20)16-19-12-10-18(15-22(19)28)11-13-24(32)30-33/h4-5,8-13,15,20,29,33H,6-7,14,16-17H2,1-3H3,(H,30,32)/b13-11+/t20-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC3 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19428
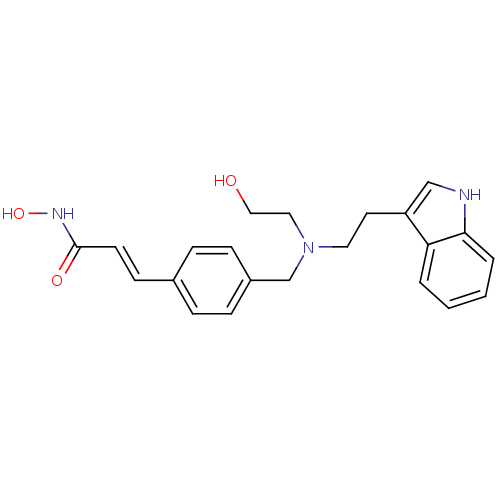
((2E)-N-hydroxy-3-(4-{[(2-hydroxyethyl)[2-(1H-indol...)Show SMILES OCCN(CCc1c[nH]c2ccccc12)Cc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C22H25N3O3/c26-14-13-25(12-11-19-15-23-21-4-2-1-3-20(19)21)16-18-7-5-17(6-8-18)9-10-22(27)24-28/h1-10,15,23,26,28H,11-14,16H2,(H,24,27)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314640
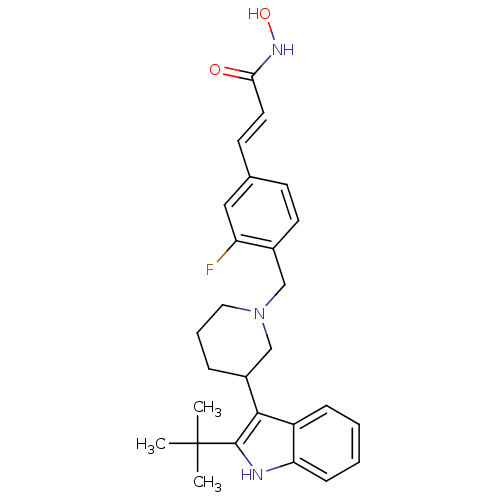
((E)-3-{4-[3-(2-tert-Butyl-1H-indol-3-yl)piperidin-...)Show SMILES CC(C)(C)c1[nH]c2ccccc2c1C1CCCN(Cc2ccc(\C=C\C(=O)NO)cc2F)C1 Show InChI InChI=1S/C27H32FN3O2/c1-27(2,3)26-25(21-8-4-5-9-23(21)29-26)20-7-6-14-31(17-20)16-19-12-10-18(15-22(19)28)11-13-24(32)30-33/h4-5,8-13,15,20,29,33H,6-7,14,16-17H2,1-3H3,(H,30,32)/b13-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314636
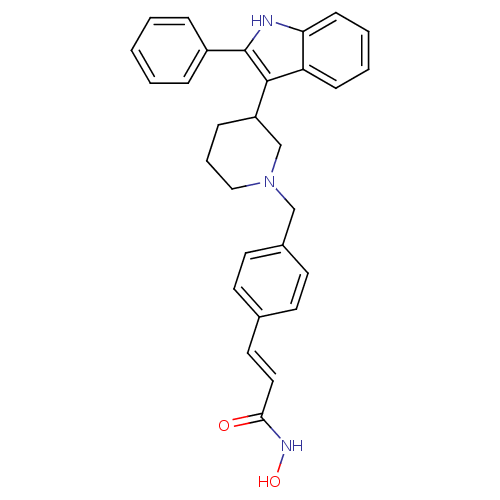
((E)-N-Hydroxy-3-{4-[3-(2-phenyl-1H-indol-3-yl)pipe...)Show SMILES ONC(=O)\C=C\c1ccc(CN2CCCC(C2)c2c([nH]c3ccccc23)-c2ccccc2)cc1 Show InChI InChI=1S/C29H29N3O2/c33-27(31-34)17-16-21-12-14-22(15-13-21)19-32-18-6-9-24(20-32)28-25-10-4-5-11-26(25)30-29(28)23-7-2-1-3-8-23/h1-5,7-8,10-17,24,30,34H,6,9,18-20H2,(H,31,33)/b17-16+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314635

((E)-N-Hydroxy-3-{4-[3-(1H-indol-3-yl)piperidin-1-y...)Show SMILES ONC(=O)\C=C\c1ccc(CN2CCCC(C2)c2c[nH]c3ccccc23)cc1 Show InChI InChI=1S/C23H25N3O2/c27-23(25-28)12-11-17-7-9-18(10-8-17)15-26-13-3-4-19(16-26)21-14-24-22-6-2-1-5-20(21)22/h1-2,5-12,14,19,24,28H,3-4,13,15-16H2,(H,25,27)/b12-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314633

(CHEMBL1093362 | N-hydroxy-3-(2-(2-(2-methyl-1H-ind...)Show SMILES Cc1[nH]c2ccccc2c1CCN1CCc2ccc(\C=C\C(=O)NO)cc2C1 Show InChI InChI=1S/C23H25N3O2/c1-16-20(21-4-2-3-5-22(21)24-16)11-13-26-12-10-18-8-6-17(14-19(18)15-26)7-9-23(27)25-28/h2-9,14,24,28H,10-13,15H2,1H3,(H,25,27)/b9-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314643
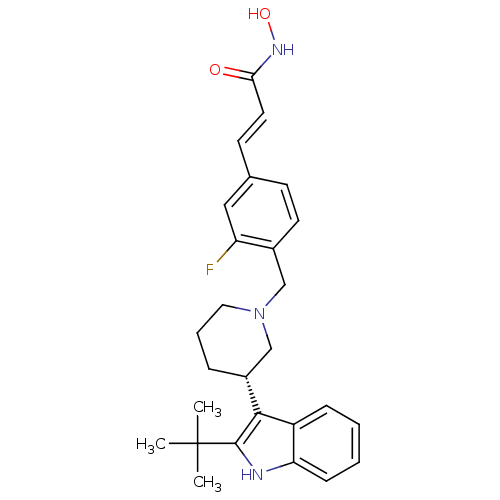
((E)-3-{4-[(S)-3-(2-tert-Butyl-1H-indol-3-yl)piperi...)Show SMILES CC(C)(C)c1[nH]c2ccccc2c1[C@@H]1CCCN(Cc2ccc(\C=C\C(=O)NO)cc2F)C1 |r| Show InChI InChI=1S/C27H32FN3O2/c1-27(2,3)26-25(21-8-4-5-9-23(21)29-26)20-7-6-14-31(17-20)16-19-12-10-18(15-22(19)28)11-13-24(32)30-33/h4-5,8-13,15,20,29,33H,6-7,14,16-17H2,1-3H3,(H,30,32)/b13-11+/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314639

((E)-3-{3-Fluoro-4-[3-(2-phenyl-1H-indol-3-yl)piper...)Show SMILES ONC(=O)\C=C\c1ccc(CN2CCCC(C2)c2c([nH]c3ccccc23)-c2ccccc2)c(F)c1 Show InChI InChI=1S/C29H28FN3O2/c30-25-17-20(13-15-27(34)32-35)12-14-22(25)18-33-16-6-9-23(19-33)28-24-10-4-5-11-26(24)31-29(28)21-7-2-1-3-8-21/h1-5,7-8,10-15,17,23,31,35H,6,9,16,18-19H2,(H,32,34)/b15-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314641
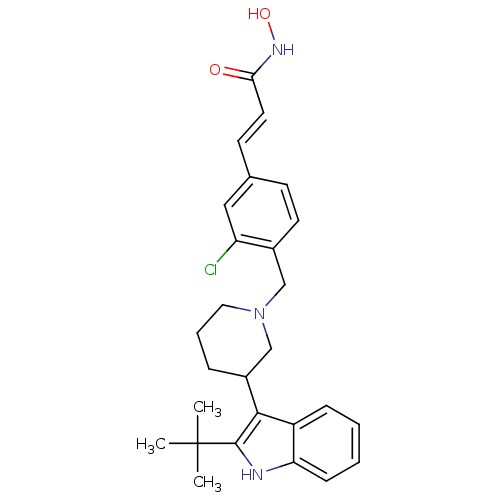
((E)-3-{4-[3-(2-tert-Butyl-1H-indol-3-yl)piperidin-...)Show SMILES CC(C)(C)c1[nH]c2ccccc2c1C1CCCN(Cc2ccc(\C=C\C(=O)NO)cc2Cl)C1 Show InChI InChI=1S/C27H32ClN3O2/c1-27(2,3)26-25(21-8-4-5-9-23(21)29-26)20-7-6-14-31(17-20)16-19-12-10-18(15-22(19)28)11-13-24(32)30-33/h4-5,8-13,15,20,29,33H,6-7,14,16-17H2,1-3H3,(H,30,32)/b13-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50314643

((E)-3-{4-[(S)-3-(2-tert-Butyl-1H-indol-3-yl)piperi...)Show SMILES CC(C)(C)c1[nH]c2ccccc2c1[C@@H]1CCCN(Cc2ccc(\C=C\C(=O)NO)cc2F)C1 |r| Show InChI InChI=1S/C27H32FN3O2/c1-27(2,3)26-25(21-8-4-5-9-23(21)29-26)20-7-6-14-31(17-20)16-19-12-10-18(15-22(19)28)11-13-24(32)30-33/h4-5,8-13,15,20,29,33H,6-7,14,16-17H2,1-3H3,(H,30,32)/b13-11+/t20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC3 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314629
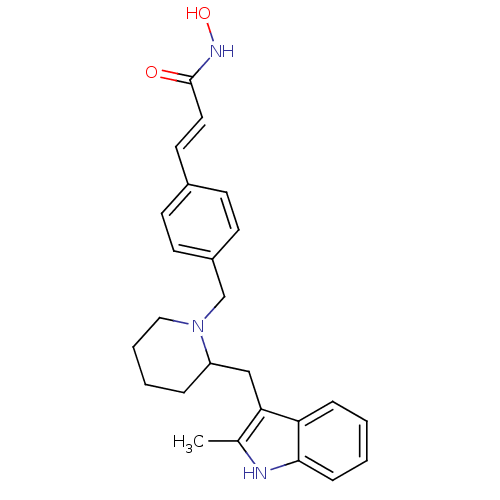
((E)-N-Hydroxy-3-{4-[2-(2-methyl-1H-indol-3-ylmethy...)Show SMILES Cc1[nH]c2ccccc2c1CC1CCCCN1Cc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C25H29N3O2/c1-18-23(22-7-2-3-8-24(22)26-18)16-21-6-4-5-15-28(21)17-20-11-9-19(10-12-20)13-14-25(29)27-30/h2-3,7-14,21,26,30H,4-6,15-17H2,1H3,(H,27,29)/b14-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50427040

(CHEMBL2322207)Show SMILES COCCCCC1(CNC(=O)[C@@H]2CNC[C@@H](C2)NS(=O)(=O)c2ccc(C)cc2)c2ccccc2Oc2ccccc12 |r| Show InChI InChI=1S/C32H39N3O5S/c1-23-13-15-26(16-14-23)41(37,38)35-25-19-24(20-33-21-25)31(36)34-22-32(17-7-8-18-39-2)27-9-3-5-11-29(27)40-30-12-6-4-10-28(30)32/h3-6,9-16,24-25,33,35H,7-8,17-22H2,1-2H3,(H,34,36)/t24-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 56: 2196-206 (2013)
Article DOI: 10.1021/jm301706j
BindingDB Entry DOI: 10.7270/Q25X2B8Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314631

(CHEMBL1088949 | N-hydroxy-3-(2-(2-(2-methyl-1H-ind...)Show SMILES Cc1[nH]c2ccccc2c1CCN1Cc2ccc(\C=C\C(=O)NO)cc2C1 Show InChI InChI=1S/C22H23N3O2/c1-15-19(20-4-2-3-5-21(20)23-15)10-11-25-13-17-8-6-16(12-18(17)14-25)7-9-22(26)24-27/h2-9,12,23,27H,10-11,13-14H2,1H3,(H,24,26)/b9-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50314642

((E)-3-{4-[(R)-3-(2-tert-Butyl-1H-indol-3-yl)piperi...)Show SMILES CC(C)(C)c1[nH]c2ccccc2c1[C@H]1CCCN(Cc2ccc(\C=C\C(=O)NO)cc2F)C1 |r| Show InChI InChI=1S/C27H32FN3O2/c1-27(2,3)26-25(21-8-4-5-9-23(21)29-26)20-7-6-14-31(17-20)16-19-12-10-18(15-22(19)28)11-13-24(32)30-33/h4-5,8-13,15,20,29,33H,6-7,14,16-17H2,1-3H3,(H,30,32)/b13-11+/t20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC4 expressed in baculovirus system by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50314642

((E)-3-{4-[(R)-3-(2-tert-Butyl-1H-indol-3-yl)piperi...)Show SMILES CC(C)(C)c1[nH]c2ccccc2c1[C@H]1CCCN(Cc2ccc(\C=C\C(=O)NO)cc2F)C1 |r| Show InChI InChI=1S/C27H32FN3O2/c1-27(2,3)26-25(21-8-4-5-9-23(21)29-26)20-7-6-14-31(17-20)16-19-12-10-18(15-22(19)28)11-13-24(32)30-33/h4-5,8-13,15,20,29,33H,6-7,14,16-17H2,1-3H3,(H,30,32)/b13-11+/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 expressed in baculovirus system by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50314643

((E)-3-{4-[(S)-3-(2-tert-Butyl-1H-indol-3-yl)piperi...)Show SMILES CC(C)(C)c1[nH]c2ccccc2c1[C@@H]1CCCN(Cc2ccc(\C=C\C(=O)NO)cc2F)C1 |r| Show InChI InChI=1S/C27H32FN3O2/c1-27(2,3)26-25(21-8-4-5-9-23(21)29-26)20-7-6-14-31(17-20)16-19-12-10-18(15-22(19)28)11-13-24(32)30-33/h4-5,8-13,15,20,29,33H,6-7,14,16-17H2,1-3H3,(H,30,32)/b13-11+/t20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC4 expressed in baculovirus system by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314632
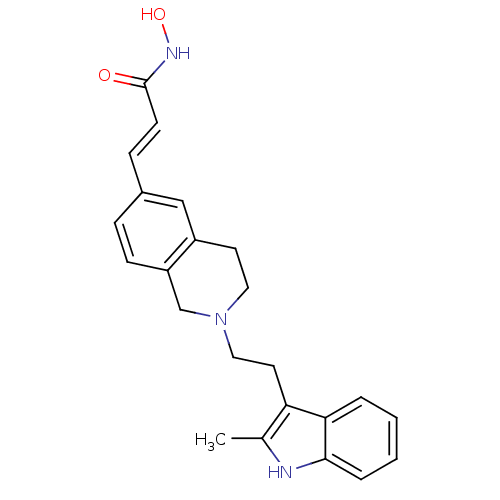
(CHEMBL1089701 | N-hydroxy-3-(2-(2-(2-methyl-1H-ind...)Show SMILES Cc1[nH]c2ccccc2c1CCN1CCc2cc(\C=C\C(=O)NO)ccc2C1 Show InChI InChI=1S/C23H25N3O2/c1-16-20(21-4-2-3-5-22(21)24-16)11-13-26-12-10-18-14-17(6-8-19(18)15-26)7-9-23(27)25-28/h2-9,14,24,28H,10-13,15H2,1H3,(H,25,27)/b9-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50314643

((E)-3-{4-[(S)-3-(2-tert-Butyl-1H-indol-3-yl)piperi...)Show SMILES CC(C)(C)c1[nH]c2ccccc2c1[C@@H]1CCCN(Cc2ccc(\C=C\C(=O)NO)cc2F)C1 |r| Show InChI InChI=1S/C27H32FN3O2/c1-27(2,3)26-25(21-8-4-5-9-23(21)29-26)20-7-6-14-31(17-20)16-19-12-10-18(15-22(19)28)11-13-24(32)30-33/h4-5,8-13,15,20,29,33H,6-7,14,16-17H2,1-3H3,(H,30,32)/b13-11+/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 expressed in baculovirus system by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314634
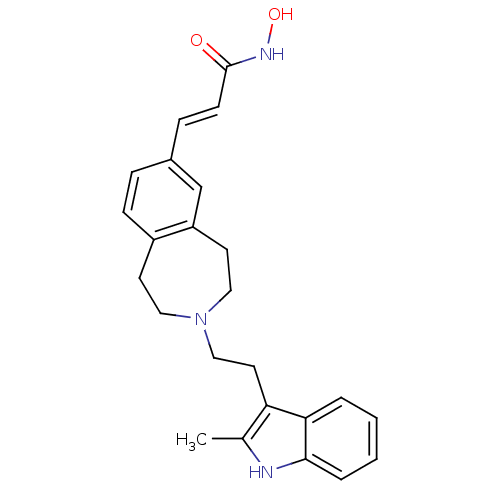
(CHEMBL1089301 | N-hydroxy-3-(3-(2-(2-methyl-1H-ind...)Show SMILES Cc1[nH]c2ccccc2c1CCN1CCc2ccc(\C=C\C(=O)NO)cc2CC1 Show InChI InChI=1S/C24H27N3O2/c1-17-21(22-4-2-3-5-23(22)25-17)12-15-27-13-10-19-8-6-18(7-9-24(28)26-29)16-20(19)11-14-27/h2-9,16,25,29H,10-15H2,1H3,(H,26,28)/b9-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50427039
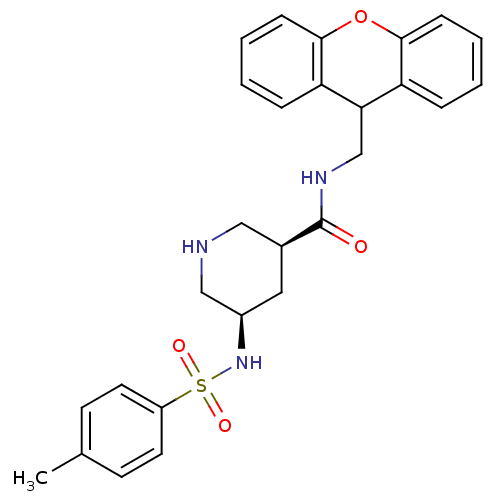
(CHEMBL2322208)Show SMILES Cc1ccc(cc1)S(=O)(=O)N[C@H]1CNC[C@H](C1)C(=O)NCC1c2ccccc2Oc2ccccc12 |r| Show InChI InChI=1S/C27H29N3O4S/c1-18-10-12-21(13-11-18)35(32,33)30-20-14-19(15-28-16-20)27(31)29-17-24-22-6-2-4-8-25(22)34-26-9-5-3-7-23(24)26/h2-13,19-20,24,28,30H,14-17H2,1H3,(H,29,31)/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 56: 2196-206 (2013)
Article DOI: 10.1021/jm301706j
BindingDB Entry DOI: 10.7270/Q25X2B8Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM50427041
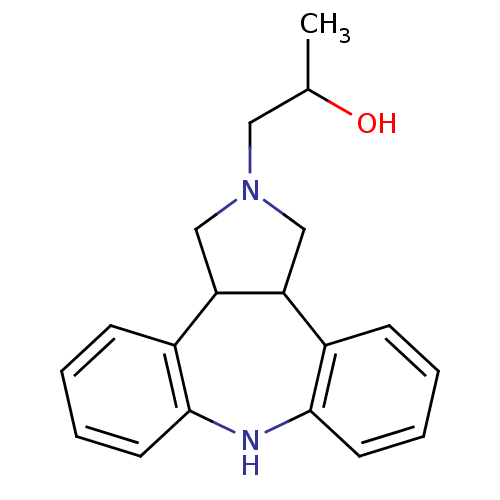
(CHEMBL2322210)Show InChI InChI=1S/C19H22N2O/c1-13(22)10-21-11-16-14-6-2-4-8-18(14)20-19-9-5-3-7-15(19)17(16)12-21/h2-9,13,16-17,20,22H,10-12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 56: 2196-206 (2013)
Article DOI: 10.1021/jm301706j
BindingDB Entry DOI: 10.7270/Q25X2B8Z |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50427042
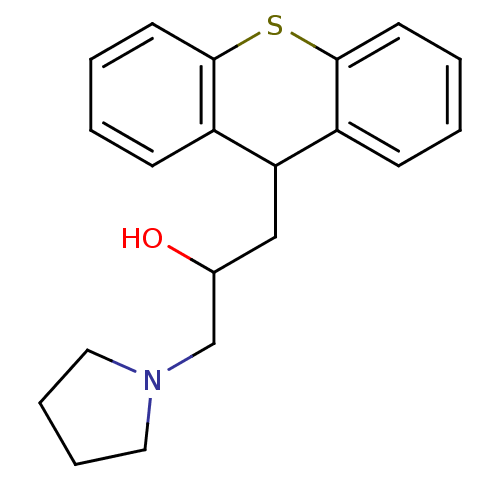
(CHEMBL2322211)Show InChI InChI=1S/C20H23NOS/c22-15(14-21-11-5-6-12-21)13-18-16-7-1-3-9-19(16)23-20-10-4-2-8-17(18)20/h1-4,7-10,15,18,22H,5-6,11-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin using fluorescence-quenched (RE(EDANS)IHPFHLVIHTK(Dabcyl)R as substrate incubated for 1 hr prior to substrate a... |
J Med Chem 56: 2196-206 (2013)
Article DOI: 10.1021/jm301706j
BindingDB Entry DOI: 10.7270/Q25X2B8Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50314643

((E)-3-{4-[(S)-3-(2-tert-Butyl-1H-indol-3-yl)piperi...)Show SMILES CC(C)(C)c1[nH]c2ccccc2c1[C@@H]1CCCN(Cc2ccc(\C=C\C(=O)NO)cc2F)C1 |r| Show InChI InChI=1S/C27H32FN3O2/c1-27(2,3)26-25(21-8-4-5-9-23(21)29-26)20-7-6-14-31(17-20)16-19-12-10-18(15-22(19)28)11-13-24(32)30-33/h4-5,8-13,15,20,29,33H,6-7,14,16-17H2,1-3H3,(H,30,32)/b13-11+/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC6 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50314642

((E)-3-{4-[(R)-3-(2-tert-Butyl-1H-indol-3-yl)piperi...)Show SMILES CC(C)(C)c1[nH]c2ccccc2c1[C@H]1CCCN(Cc2ccc(\C=C\C(=O)NO)cc2F)C1 |r| Show InChI InChI=1S/C27H32FN3O2/c1-27(2,3)26-25(21-8-4-5-9-23(21)29-26)20-7-6-14-31(17-20)16-19-12-10-18(15-22(19)28)11-13-24(32)30-33/h4-5,8-13,15,20,29,33H,6-7,14,16-17H2,1-3H3,(H,30,32)/b13-11+/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 118 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC6 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314638

((E)-3-{3-Fluoro-4-[3-(1H-indol-3-yl)piperidin-1-yl...)Show SMILES ONC(=O)\C=C\c1ccc(CN2CCCC(C2)c2c[nH]c3ccccc23)c(F)c1 Show InChI InChI=1S/C23H24FN3O2/c24-21-12-16(8-10-23(28)26-29)7-9-18(21)15-27-11-3-4-17(14-27)20-13-25-22-6-2-1-5-19(20)22/h1-2,5-10,12-13,17,25,29H,3-4,11,14-15H2,(H,26,28)/b10-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 136 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50427564

(CHEMBL2322611)Show SMILES COCCCOc1cc(ccc1OC)C(=O)N(C[C@@H]1CNC[C@H]1Cc1ccccc1)C(C)C |r| Show InChI InChI=1S/C27H38N2O4/c1-20(2)29(19-24-18-28-17-23(24)15-21-9-6-5-7-10-21)27(30)22-11-12-25(32-4)26(16-22)33-14-8-13-31-3/h5-7,9-12,16,20,23-24,28H,8,13-15,17-19H2,1-4H3/t23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin expressed in CHO cells using RE(EDANS)IHPFHLVIHTK(Dabcyl)R as substrate incubated for 1 hr prior to substrate a... |
J Med Chem 56: 2207-17 (2013)
Article DOI: 10.1021/jm3017078
BindingDB Entry DOI: 10.7270/Q2FB5487 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50314642

((E)-3-{4-[(R)-3-(2-tert-Butyl-1H-indol-3-yl)piperi...)Show SMILES CC(C)(C)c1[nH]c2ccccc2c1[C@H]1CCCN(Cc2ccc(\C=C\C(=O)NO)cc2F)C1 |r| Show InChI InChI=1S/C27H32FN3O2/c1-27(2,3)26-25(21-8-4-5-9-23(21)29-26)20-7-6-14-31(17-20)16-19-12-10-18(15-22(19)28)11-13-24(32)30-33/h4-5,8-13,15,20,29,33H,6-7,14,16-17H2,1-3H3,(H,30,32)/b13-11+/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 217 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC8 expressed in baculovirus system by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50427564

(CHEMBL2322611)Show SMILES COCCCOc1cc(ccc1OC)C(=O)N(C[C@@H]1CNC[C@H]1Cc1ccccc1)C(C)C |r| Show InChI InChI=1S/C27H38N2O4/c1-20(2)29(19-24-18-28-17-23(24)15-21-9-6-5-7-10-21)27(30)22-11-12-25(32-4)26(16-22)33-14-8-13-31-3/h5-7,9-12,16,20,23-24,28H,8,13-15,17-19H2,1-4H3/t23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of renin in human plasma using RE(EDANS)IHPFHLVIHTK(Dabcyl)R as substrate incubated for 1 hr prior to substrate addition measured after 2 ... |
J Med Chem 56: 2207-17 (2013)
Article DOI: 10.1021/jm3017078
BindingDB Entry DOI: 10.7270/Q2FB5487 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50314643

((E)-3-{4-[(S)-3-(2-tert-Butyl-1H-indol-3-yl)piperi...)Show SMILES CC(C)(C)c1[nH]c2ccccc2c1[C@@H]1CCCN(Cc2ccc(\C=C\C(=O)NO)cc2F)C1 |r| Show InChI InChI=1S/C27H32FN3O2/c1-27(2,3)26-25(21-8-4-5-9-23(21)29-26)20-7-6-14-31(17-20)16-19-12-10-18(15-22(19)28)11-13-24(32)30-33/h4-5,8-13,15,20,29,33H,6-7,14,16-17H2,1-3H3,(H,30,32)/b13-11+/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 364 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC8 expressed in baculovirus system by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM50409037
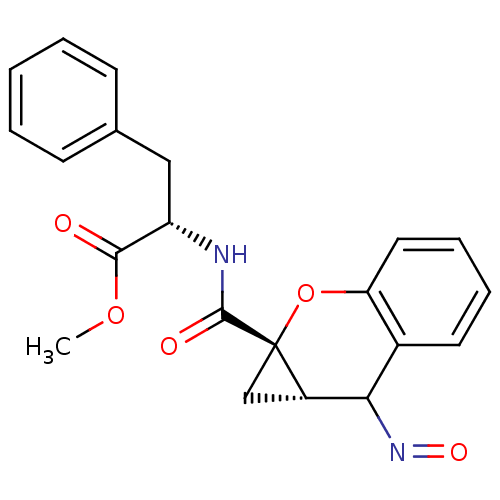
(CHEMBL2111944)Show SMILES COC(=O)[C@H](Cc1ccccc1)NC(=O)[C@]12C[C@H]1C(N=O)c1ccccc1O2 Show InChI InChI=1S/C21H20N2O5/c1-27-19(24)16(11-13-7-3-2-4-8-13)22-20(25)21-12-15(21)18(23-26)14-9-5-6-10-17(14)28-21/h2-10,15-16,18H,11-12H2,1H3,(H,22,25)/t15-,16-,18?,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of Quisqualate-Induced PI Hydrolysis measured in CHO Metabotropic glutamate receptor 1 Expressing Cells |
J Med Chem 43: 4428-36 (2000)
BindingDB Entry DOI: 10.7270/Q2988688 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50427564

(CHEMBL2322611)Show SMILES COCCCOc1cc(ccc1OC)C(=O)N(C[C@@H]1CNC[C@H]1Cc1ccccc1)C(C)C |r| Show InChI InChI=1S/C27H38N2O4/c1-20(2)29(19-24-18-28-17-23(24)15-21-9-6-5-7-10-21)27(30)22-11-12-25(32-4)26(16-22)33-14-8-13-31-3/h5-7,9-12,16,20,23-24,28H,8,13-15,17-19H2,1-4H3/t23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin expressed in CHO cells using RE(EDANS)IHPFHLVIHTK(Dabcyl)R as substrate incubated for 1 hr prior to substrate a... |
J Med Chem 56: 2207-17 (2013)
Article DOI: 10.1021/jm3017078
BindingDB Entry DOI: 10.7270/Q2FB5487 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM50427038
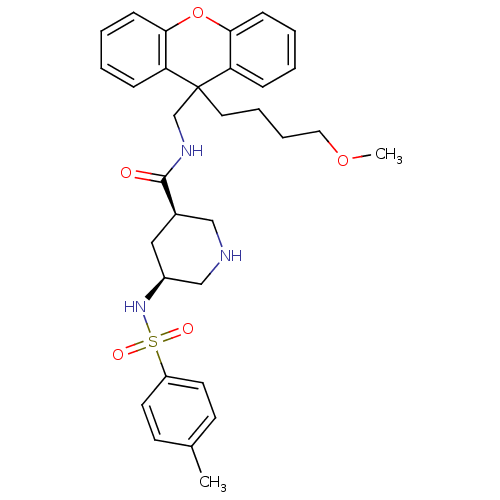
(CHEMBL2322206)Show SMILES COCCCCC1(CNC(=O)[C@H]2CNC[C@H](C2)NS(=O)(=O)c2ccc(C)cc2)c2ccccc2Oc2ccccc12 |r| Show InChI InChI=1S/C32H39N3O5S/c1-23-13-15-26(16-14-23)41(37,38)35-25-19-24(20-33-21-25)31(36)34-22-32(17-7-8-18-39-2)27-9-3-5-11-29(27)40-30-12-6-4-10-28(30)32/h3-6,9-16,24-25,33,35H,7-8,17-22H2,1-2H3,(H,34,36)/t24-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 56: 2196-206 (2013)
Article DOI: 10.1021/jm301706j
BindingDB Entry DOI: 10.7270/Q25X2B8Z |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM50409038

(CHEMBL2111943)Show SMILES COC(=O)[C@@H](Cc1ccccc1)NC(=O)[C@@]12C[C@@H]1C(N=O)c1ccccc1O2 Show InChI InChI=1S/C21H20N2O5/c1-27-19(24)16(11-13-7-3-2-4-8-13)22-20(25)21-12-15(21)18(23-26)14-9-5-6-10-17(14)28-21/h2-10,15-16,18H,11-12H2,1H3,(H,22,25)/t15-,16-,18?,21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of Quisqualate-Induced PI Hydrolysis Measured in CHO Metabotropic glutamate receptor 1 Expressing Cells |
J Med Chem 43: 4428-36 (2000)
BindingDB Entry DOI: 10.7270/Q2988688 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50427564

(CHEMBL2322611)Show SMILES COCCCOc1cc(ccc1OC)C(=O)N(C[C@@H]1CNC[C@H]1Cc1ccccc1)C(C)C |r| Show InChI InChI=1S/C27H38N2O4/c1-20(2)29(19-24-18-28-17-23(24)15-21-9-6-5-7-10-21)27(30)22-11-12-25(32-4)26(16-22)33-14-8-13-31-3/h5-7,9-12,16,20,23-24,28H,8,13-15,17-19H2,1-4H3/t23-,24+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of 5-HT1A (unknown origin) |
J Med Chem 56: 2207-17 (2013)
Article DOI: 10.1021/jm3017078
BindingDB Entry DOI: 10.7270/Q2FB5487 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50427564

(CHEMBL2322611)Show SMILES COCCCOc1cc(ccc1OC)C(=O)N(C[C@@H]1CNC[C@H]1Cc1ccccc1)C(C)C |r| Show InChI InChI=1S/C27H38N2O4/c1-20(2)29(19-24-18-28-17-23(24)15-21-9-6-5-7-10-21)27(30)22-11-12-25(32-4)26(16-22)33-14-8-13-31-3/h5-7,9-12,16,20,23-24,28H,8,13-15,17-19H2,1-4H3/t23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of 5-HT2A (unknown origin) |
J Med Chem 56: 2207-17 (2013)
Article DOI: 10.1021/jm3017078
BindingDB Entry DOI: 10.7270/Q2FB5487 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50427564

(CHEMBL2322611)Show SMILES COCCCOc1cc(ccc1OC)C(=O)N(C[C@@H]1CNC[C@H]1Cc1ccccc1)C(C)C |r| Show InChI InChI=1S/C27H38N2O4/c1-20(2)29(19-24-18-28-17-23(24)15-21-9-6-5-7-10-21)27(30)22-11-12-25(32-4)26(16-22)33-14-8-13-31-3/h5-7,9-12,16,20,23-24,28H,8,13-15,17-19H2,1-4H3/t23-,24+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of 5-HT2B (unknown origin) |
J Med Chem 56: 2207-17 (2013)
Article DOI: 10.1021/jm3017078
BindingDB Entry DOI: 10.7270/Q2FB5487 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM50094187
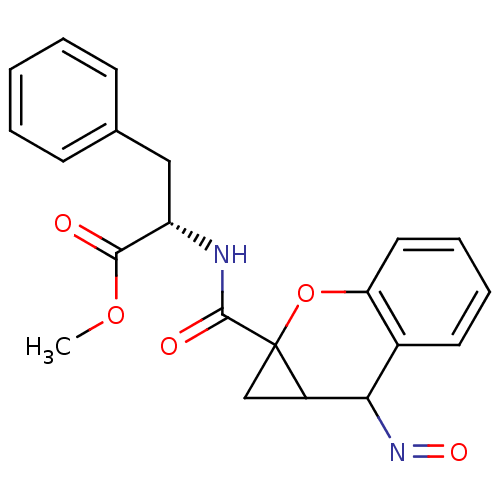
((+)1aR,7aR -2-[(2-Hydroxyimino-1a,2-dihydro-1H-7-o...)Show SMILES COC(=O)[C@H](Cc1ccccc1)NC(=O)C12CC1C(N=O)c1ccccc1O2 Show InChI InChI=1S/C21H20N2O5/c1-27-19(24)16(11-13-7-3-2-4-8-13)22-20(25)21-12-15(21)18(23-26)14-9-5-6-10-17(14)28-21/h2-10,15-16,18H,11-12H2,1H3,(H,22,25)/t15?,16-,18?,21?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of Quisqualate-Induced PI Hydrolysis measured in CHO Metabotropic glutamate receptor 1 Expressing Cells |
J Med Chem 43: 4428-36 (2000)
BindingDB Entry DOI: 10.7270/Q2988688 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50427037

(CHEMBL2322204)Show SMILES Cc1ccc(cc1)S(=O)(=O)N[C@H]1CNC[C@H](C1)C(=O)NCC(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C27H31N3O3S/c1-20-12-14-25(15-13-20)34(32,33)30-24-16-23(17-28-18-24)27(31)29-19-26(21-8-4-2-5-9-21)22-10-6-3-7-11-22/h2-15,23-24,26,28,30H,16-19H2,1H3,(H,29,31)/t23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin using fluorescence-quenched (RE(EDANS)IHPFHLVIHTK(Dabcyl)R as substrate incubated for 1 hr prior to substrate a... |
J Med Chem 56: 2196-206 (2013)
Article DOI: 10.1021/jm301706j
BindingDB Entry DOI: 10.7270/Q25X2B8Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM50427037

(CHEMBL2322204)Show SMILES Cc1ccc(cc1)S(=O)(=O)N[C@H]1CNC[C@H](C1)C(=O)NCC(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C27H31N3O3S/c1-20-12-14-25(15-13-20)34(32,33)30-24-16-23(17-28-18-24)27(31)29-19-26(21-8-4-2-5-9-21)22-10-6-3-7-11-22/h2-15,23-24,26,28,30H,16-19H2,1H3,(H,29,31)/t23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 56: 2196-206 (2013)
Article DOI: 10.1021/jm301706j
BindingDB Entry DOI: 10.7270/Q25X2B8Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM50094188
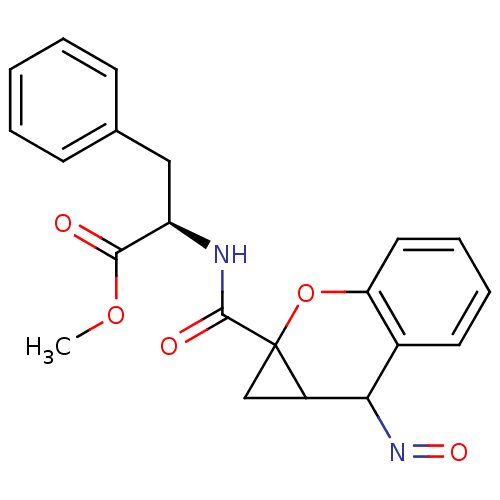
((+)1aR,7aR -2-[(2-Hydroxyimino-1a,2-dihydro-1H-7-o...)Show SMILES COC(=O)[C@@H](Cc1ccccc1)NC(=O)C12CC1C(N=O)c1ccccc1O2 Show InChI InChI=1S/C21H20N2O5/c1-27-19(24)16(11-13-7-3-2-4-8-13)22-20(25)21-12-15(21)18(23-26)14-9-5-6-10-17(14)28-21/h2-10,15-16,18H,11-12H2,1H3,(H,22,25)/t15?,16-,18?,21?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of Quisqualate-Induced PI Hydrolysis measured in CHO Metabotropic glutamate receptor 1 Expressing Cells |
J Med Chem 43: 4428-36 (2000)
BindingDB Entry DOI: 10.7270/Q2988688 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM50409036
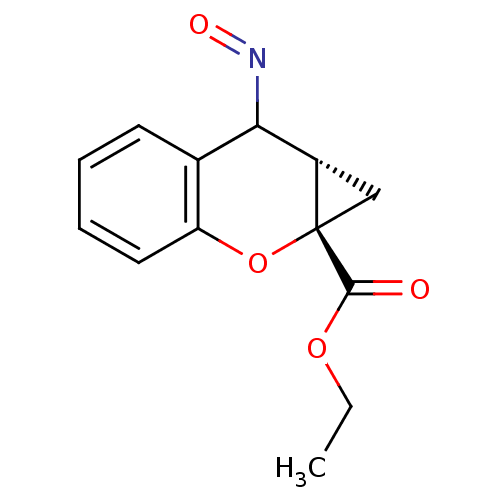
(CHEMBL2111945)Show InChI InChI=1S/C13H13NO4/c1-2-17-12(15)13-7-9(13)11(14-16)8-5-3-4-6-10(8)18-13/h3-6,9,11H,2,7H2,1H3/t9-,11?,13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of Quisqualate-Induced PI Hydrolysis measured in CHO Metabotropic glutamate receptor 1 Expressing Cells |
J Med Chem 43: 4428-36 (2000)
BindingDB Entry DOI: 10.7270/Q2988688 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50427568

(CHEMBL2322608)Show SMILES COCCCOc1cc(ccc1OC)C(=O)N(CC1CNCC1Cc1ccccc1)c1ccc(Cl)cc1 Show InChI InChI=1S/C30H35ClN2O4/c1-35-15-6-16-37-29-18-23(9-14-28(29)36-2)30(34)33(27-12-10-26(31)11-13-27)21-25-20-32-19-24(25)17-22-7-4-3-5-8-22/h3-5,7-14,18,24-25,32H,6,15-17,19-21H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin expressed in CHO cells using RE(EDANS)IHPFHLVIHTK(Dabcyl)R as substrate incubated for 1 hr prior to substrate a... |
J Med Chem 56: 2207-17 (2013)
Article DOI: 10.1021/jm3017078
BindingDB Entry DOI: 10.7270/Q2FB5487 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data