

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM111568 (US8618158, NCL-00016149) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | 25 |
Cancer Research Technology Limited US Patent | Assay Description Streptavidin-coated 96-well plates are used to immobilise a biotin-tagged IP3 p53-derived peptide (MPRFMDYWEGLN). This is a peptide analogue derived ... | US Patent US8618158 (2013) BindingDB Entry DOI: 10.7270/Q27D2STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM111547 (US8618158, NCL-00013774 | US8618158, NCL-00013775 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | 25 |
Cancer Research Technology Limited US Patent | Assay Description Streptavidin-coated 96-well plates are used to immobilise a biotin-tagged IP3 p53-derived peptide (MPRFMDYWEGLN). This is a peptide analogue derived ... | US Patent US8618158 (2013) BindingDB Entry DOI: 10.7270/Q27D2STZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50229787 ((4S,5R)-Nutlin-3 | (rac)-(4,5-bis(4-chlorophenyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Mdm2 -p53 protein interaction by ELISA | J Med Chem 54: 1233-43 (2011) Article DOI: 10.1021/jm1011929 BindingDB Entry DOI: 10.7270/Q29K4BHN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM111569 (US8618158, NCL-00016659) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | 25 |
Cancer Research Technology Limited US Patent | Assay Description Streptavidin-coated 96-well plates are used to immobilise a biotin-tagged IP3 p53-derived peptide (MPRFMDYWEGLN). This is a peptide analogue derived ... | US Patent US8618158 (2013) BindingDB Entry DOI: 10.7270/Q27D2STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM111570 (US8618158, NCL-00016653) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | 25 |
Cancer Research Technology Limited US Patent | Assay Description Streptavidin-coated 96-well plates are used to immobilise a biotin-tagged IP3 p53-derived peptide (MPRFMDYWEGLN). This is a peptide analogue derived ... | US Patent US8618158 (2013) BindingDB Entry DOI: 10.7270/Q27D2STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM111547 (US8618158, NCL-00013774 | US8618158, NCL-00013775 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 143 | n/a | n/a | n/a | n/a | n/a | 25 |
Cancer Research Technology Limited US Patent | Assay Description Streptavidin-coated 96-well plates are used to immobilise a biotin-tagged IP3 p53-derived peptide (MPRFMDYWEGLN). This is a peptide analogue derived ... | US Patent US8618158 (2013) BindingDB Entry DOI: 10.7270/Q27D2STZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM111532 (US8618158, NU8399) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 152 | n/a | n/a | n/a | n/a | n/a | 25 |
Cancer Research Technology Limited US Patent | Assay Description Streptavidin-coated 96-well plates are used to immobilise a biotin-tagged IP3 p53-derived peptide (MPRFMDYWEGLN). This is a peptide analogue derived ... | US Patent US8618158 (2013) BindingDB Entry DOI: 10.7270/Q27D2STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM111540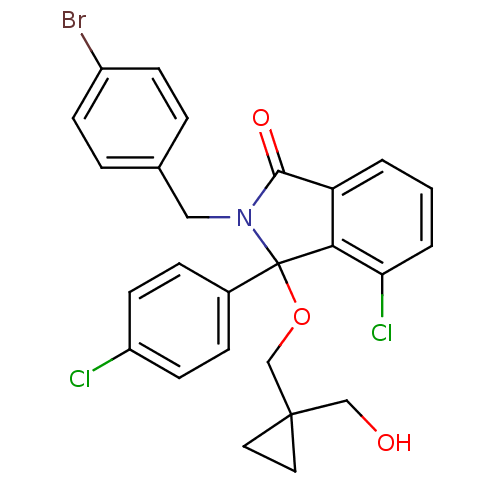 (US8618158, NCL-00010493) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 169 | n/a | n/a | n/a | n/a | n/a | 25 |
Cancer Research Technology Limited US Patent | Assay Description Streptavidin-coated 96-well plates are used to immobilise a biotin-tagged IP3 p53-derived peptide (MPRFMDYWEGLN). This is a peptide analogue derived ... | US Patent US8618158 (2013) BindingDB Entry DOI: 10.7270/Q27D2STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50339398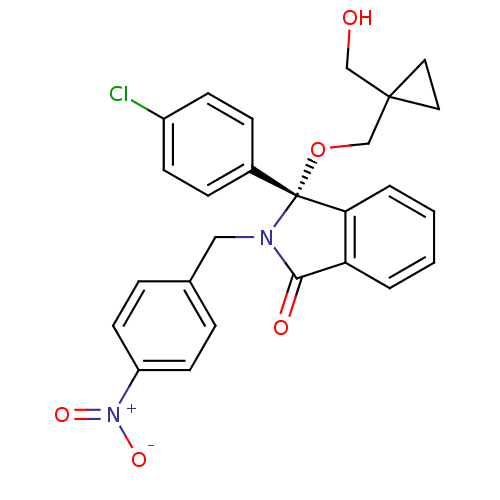 ((+)-R-3-(4-Chlorophenyl)-3-(1-hydroxymethylcyclopr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Mdm2 -p53 protein interaction by ELISA | J Med Chem 54: 1233-43 (2011) Article DOI: 10.1021/jm1011929 BindingDB Entry DOI: 10.7270/Q29K4BHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM111539 (US8618158, NCL-00010492) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 185 | n/a | n/a | n/a | n/a | n/a | 25 |
Cancer Research Technology Limited US Patent | Assay Description Streptavidin-coated 96-well plates are used to immobilise a biotin-tagged IP3 p53-derived peptide (MPRFMDYWEGLN). This is a peptide analogue derived ... | US Patent US8618158 (2013) BindingDB Entry DOI: 10.7270/Q27D2STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50339369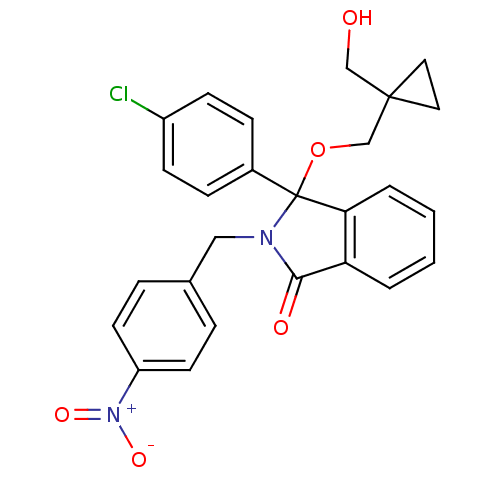 ((+/-)-3-(4-chlorophenyl)-3-((1-(hydroxymethyl)cycl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Mdm2 -p53 protein interaction by ELISA | J Med Chem 54: 1233-43 (2011) Article DOI: 10.1021/jm1011929 BindingDB Entry DOI: 10.7270/Q29K4BHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM111546 (US8618158, NU8405) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 274 | n/a | n/a | n/a | n/a | n/a | 25 |
Cancer Research Technology Limited US Patent | Assay Description Streptavidin-coated 96-well plates are used to immobilise a biotin-tagged IP3 p53-derived peptide (MPRFMDYWEGLN). This is a peptide analogue derived ... | US Patent US8618158 (2013) BindingDB Entry DOI: 10.7270/Q27D2STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM111542 (US8618158, NCL-00010495) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 295 | n/a | n/a | n/a | n/a | n/a | 25 |
Cancer Research Technology Limited US Patent | Assay Description Streptavidin-coated 96-well plates are used to immobilise a biotin-tagged IP3 p53-derived peptide (MPRFMDYWEGLN). This is a peptide analogue derived ... | US Patent US8618158 (2013) BindingDB Entry DOI: 10.7270/Q27D2STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM111513 (US8618158, NU8354) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 298 | n/a | n/a | n/a | n/a | n/a | 25 |
Cancer Research Technology Limited US Patent | Assay Description Streptavidin-coated 96-well plates are used to immobilise a biotin-tagged IP3 p53-derived peptide (MPRFMDYWEGLN). This is a peptide analogue derived ... | US Patent US8618158 (2013) BindingDB Entry DOI: 10.7270/Q27D2STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM111518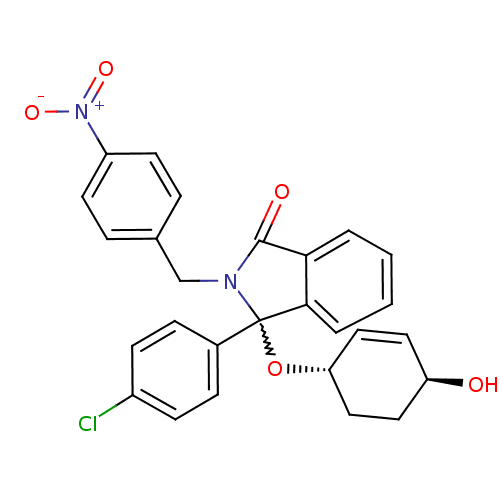 (US8618158, NU8361) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 306 | n/a | n/a | n/a | n/a | n/a | 25 |
Cancer Research Technology Limited US Patent | Assay Description Streptavidin-coated 96-well plates are used to immobilise a biotin-tagged IP3 p53-derived peptide (MPRFMDYWEGLN). This is a peptide analogue derived ... | US Patent US8618158 (2013) BindingDB Entry DOI: 10.7270/Q27D2STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50339371 ((+/-)-trans-3-(4-Chlorophenyl)-3-(4-hydroxycyclohe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Mdm2 -p53 protein interaction by ELISA | J Med Chem 54: 1233-43 (2011) Article DOI: 10.1021/jm1011929 BindingDB Entry DOI: 10.7270/Q29K4BHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50339355 ((+/-)-3-(4-Chlorophenyl)-3-(4-hydroxybutoxy)-2-(4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Mdm2 -p53 protein interaction by ELISA | J Med Chem 54: 1233-43 (2011) Article DOI: 10.1021/jm1011929 BindingDB Entry DOI: 10.7270/Q29K4BHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM111524 (US8618158, NU8391) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 368 | n/a | n/a | n/a | n/a | n/a | 25 |
Cancer Research Technology Limited US Patent | Assay Description Streptavidin-coated 96-well plates are used to immobilise a biotin-tagged IP3 p53-derived peptide (MPRFMDYWEGLN). This is a peptide analogue derived ... | US Patent US8618158 (2013) BindingDB Entry DOI: 10.7270/Q27D2STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM111511 (US8618158, NU8352) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 375 | n/a | n/a | n/a | n/a | n/a | 25 |
Cancer Research Technology Limited US Patent | Assay Description Streptavidin-coated 96-well plates are used to immobilise a biotin-tagged IP3 p53-derived peptide (MPRFMDYWEGLN). This is a peptide analogue derived ... | US Patent US8618158 (2013) BindingDB Entry DOI: 10.7270/Q27D2STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50339365 ((+/-)-trans-3-(4-Chlorophenyl)-3-(5-hydroxycyclooc...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Mdm2 -p53 protein interaction by ELISA | J Med Chem 54: 1233-43 (2011) Article DOI: 10.1021/jm1011929 BindingDB Entry DOI: 10.7270/Q29K4BHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM111517 (US8618158, NU8360) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 388 | n/a | n/a | n/a | n/a | n/a | 25 |
Cancer Research Technology Limited US Patent | Assay Description Streptavidin-coated 96-well plates are used to immobilise a biotin-tagged IP3 p53-derived peptide (MPRFMDYWEGLN). This is a peptide analogue derived ... | US Patent US8618158 (2013) BindingDB Entry DOI: 10.7270/Q27D2STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50339370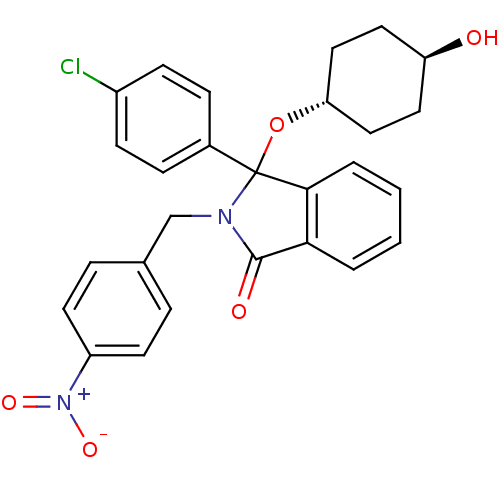 ((+/-)-trans-3-(4-Chlorophenyl)-3-(4-hydroxycyclohe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Mdm2 -p53 protein interaction by ELISA | J Med Chem 54: 1233-43 (2011) Article DOI: 10.1021/jm1011929 BindingDB Entry DOI: 10.7270/Q29K4BHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM111512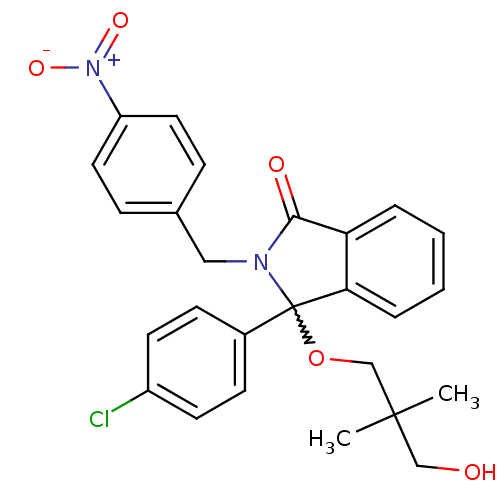 (US8618158, NU8353) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 395 | n/a | n/a | n/a | n/a | n/a | 25 |
Cancer Research Technology Limited US Patent | Assay Description Streptavidin-coated 96-well plates are used to immobilise a biotin-tagged IP3 p53-derived peptide (MPRFMDYWEGLN). This is a peptide analogue derived ... | US Patent US8618158 (2013) BindingDB Entry DOI: 10.7270/Q27D2STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50339353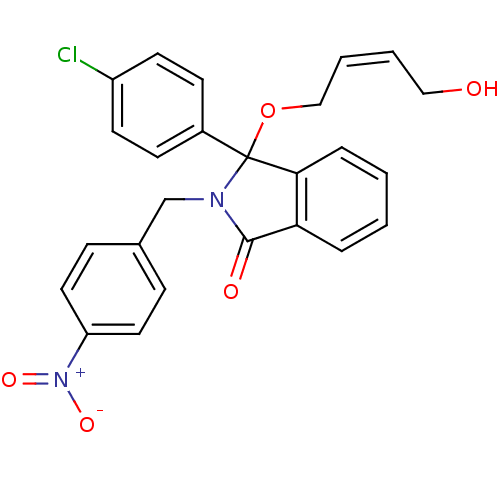 ((+/-)-(Z)-3-(4-Chlorophenyl)-3-(4-hydroxybut-2-eny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Mdm2 -p53 protein interaction by ELISA | J Med Chem 54: 1233-43 (2011) Article DOI: 10.1021/jm1011929 BindingDB Entry DOI: 10.7270/Q29K4BHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50339437 ((+/-)-3-(4-chlorophenyl)-3-((cis)-3-hydroxycyclope...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Mdm2 -p53 protein interaction by ELISA | J Med Chem 54: 1233-43 (2011) Article DOI: 10.1021/jm1011929 BindingDB Entry DOI: 10.7270/Q29K4BHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50339368 ((+/-)-3-(4-Chlorophenyl)-3-(3-hydroxy-2,2-dimethyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Mdm2 -p53 protein interaction by ELISA | J Med Chem 54: 1233-43 (2011) Article DOI: 10.1021/jm1011929 BindingDB Entry DOI: 10.7270/Q29K4BHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM111509 (US8618158, NU8350) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 402 | n/a | n/a | n/a | n/a | n/a | 25 |
Cancer Research Technology Limited US Patent | Assay Description Streptavidin-coated 96-well plates are used to immobilise a biotin-tagged IP3 p53-derived peptide (MPRFMDYWEGLN). This is a peptide analogue derived ... | US Patent US8618158 (2013) BindingDB Entry DOI: 10.7270/Q27D2STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM111510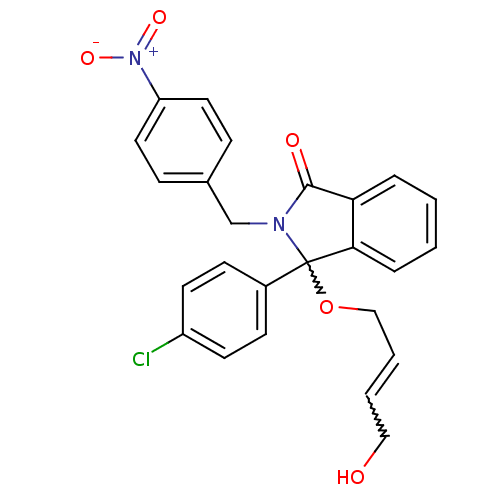 (US8618158, NU8351) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 405 | n/a | n/a | n/a | n/a | n/a | 25 |
Cancer Research Technology Limited US Patent | Assay Description Streptavidin-coated 96-well plates are used to immobilise a biotin-tagged IP3 p53-derived peptide (MPRFMDYWEGLN). This is a peptide analogue derived ... | US Patent US8618158 (2013) BindingDB Entry DOI: 10.7270/Q27D2STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50339354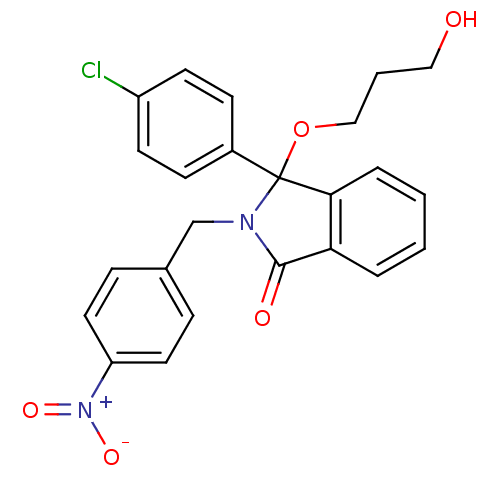 ((+/-)-3-(4-Chlorophenyl)-3-(3-hydroxypropoxy)-2-(4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Mdm2 -p53 protein interaction by ELISA | J Med Chem 54: 1233-43 (2011) Article DOI: 10.1021/jm1011929 BindingDB Entry DOI: 10.7270/Q29K4BHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM111545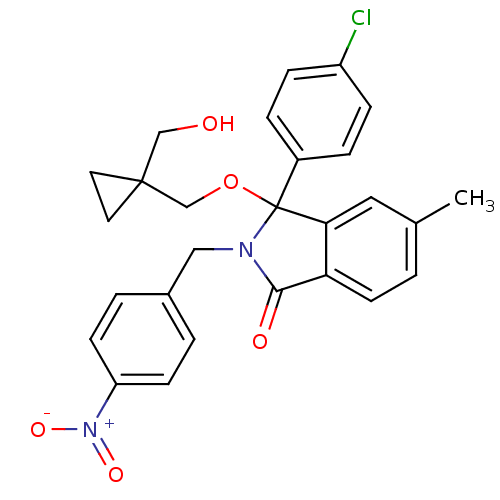 (US8618158, NU8401) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 492 | n/a | n/a | n/a | n/a | n/a | 25 |
Cancer Research Technology Limited US Patent | Assay Description Streptavidin-coated 96-well plates are used to immobilise a biotin-tagged IP3 p53-derived peptide (MPRFMDYWEGLN). This is a peptide analogue derived ... | US Patent US8618158 (2013) BindingDB Entry DOI: 10.7270/Q27D2STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM111554 (US8618158, NU8398) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | 25 |
Cancer Research Technology Limited US Patent | Assay Description Streptavidin-coated 96-well plates are used to immobilise a biotin-tagged IP3 p53-derived peptide (MPRFMDYWEGLN). This is a peptide analogue derived ... | US Patent US8618158 (2013) BindingDB Entry DOI: 10.7270/Q27D2STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM111531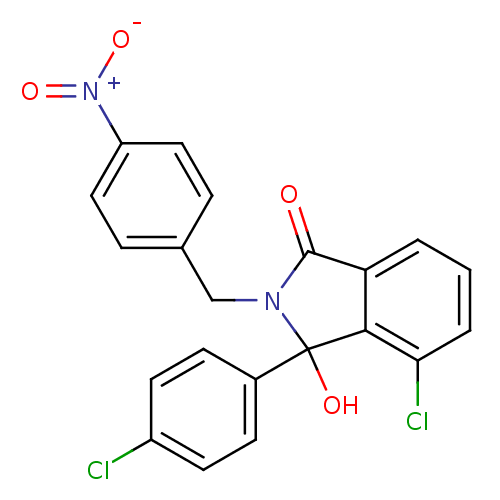 (US8618158, NU8398) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | 25 |
Cancer Research Technology Limited US Patent | Assay Description Streptavidin-coated 96-well plates are used to immobilise a biotin-tagged IP3 p53-derived peptide (MPRFMDYWEGLN). This is a peptide analogue derived ... | US Patent US8618158 (2013) BindingDB Entry DOI: 10.7270/Q27D2STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM111516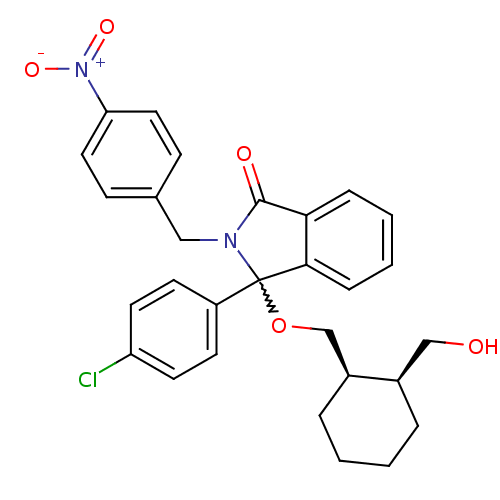 (US8618158, NU8359) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 569 | n/a | n/a | n/a | n/a | n/a | 25 |
Cancer Research Technology Limited US Patent | Assay Description Streptavidin-coated 96-well plates are used to immobilise a biotin-tagged IP3 p53-derived peptide (MPRFMDYWEGLN). This is a peptide analogue derived ... | US Patent US8618158 (2013) BindingDB Entry DOI: 10.7270/Q27D2STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50339361 ((+/-)-3-(4-Bromophenyl)-3-(4-hydroxybutoxy)-2-(4-n...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Mdm2 -p53 protein interaction by ELISA | J Med Chem 54: 1233-43 (2011) Article DOI: 10.1021/jm1011929 BindingDB Entry DOI: 10.7270/Q29K4BHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50339367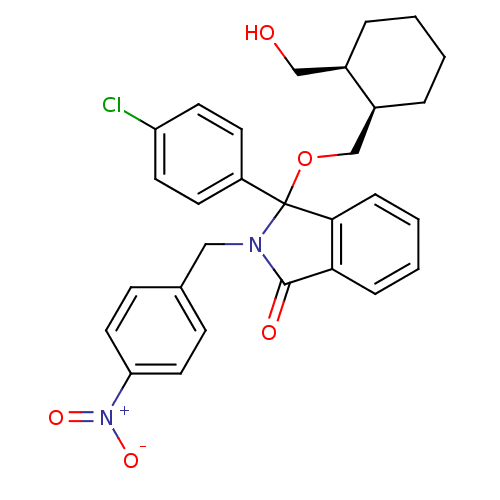 ((+/-)-cis-3-(4-Chlorophenyl)-3-(2-hydroxymethylcyc...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Mdm2 -p53 protein interaction by ELISA | J Med Chem 54: 1233-43 (2011) Article DOI: 10.1021/jm1011929 BindingDB Entry DOI: 10.7270/Q29K4BHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM111523 (US8618158, NU8390) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | 25 |
Cancer Research Technology Limited US Patent | Assay Description Streptavidin-coated 96-well plates are used to immobilise a biotin-tagged IP3 p53-derived peptide (MPRFMDYWEGLN). This is a peptide analogue derived ... | US Patent US8618158 (2013) BindingDB Entry DOI: 10.7270/Q27D2STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50339363 ((+/-)-trans-3-(4-Chlorophenyl)-3-(4-hydroxymethylc...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Mdm2 -p53 protein interaction by ELISA | J Med Chem 54: 1233-43 (2011) Article DOI: 10.1021/jm1011929 BindingDB Entry DOI: 10.7270/Q29K4BHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM111515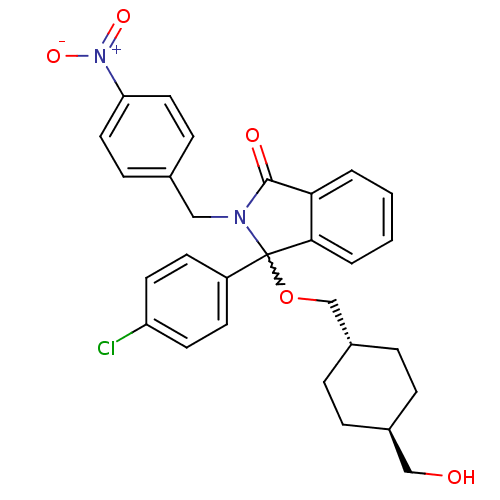 (US8618158, NU8358) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 582 | n/a | n/a | n/a | n/a | n/a | 25 |
Cancer Research Technology Limited US Patent | Assay Description Streptavidin-coated 96-well plates are used to immobilise a biotin-tagged IP3 p53-derived peptide (MPRFMDYWEGLN). This is a peptide analogue derived ... | US Patent US8618158 (2013) BindingDB Entry DOI: 10.7270/Q27D2STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM111514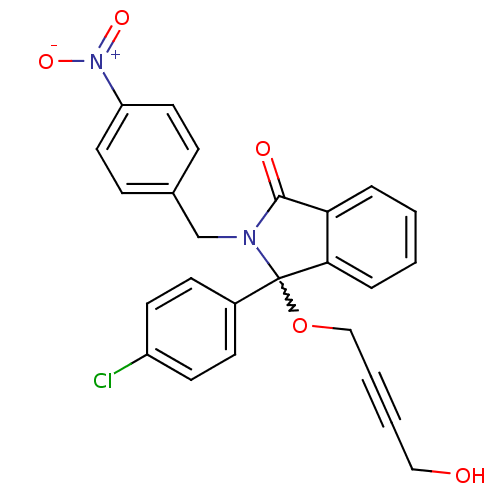 (US8618158, NU8357) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 656 | n/a | n/a | n/a | n/a | n/a | 25 |
Cancer Research Technology Limited US Patent | Assay Description Streptavidin-coated 96-well plates are used to immobilise a biotin-tagged IP3 p53-derived peptide (MPRFMDYWEGLN). This is a peptide analogue derived ... | US Patent US8618158 (2013) BindingDB Entry DOI: 10.7270/Q27D2STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50339366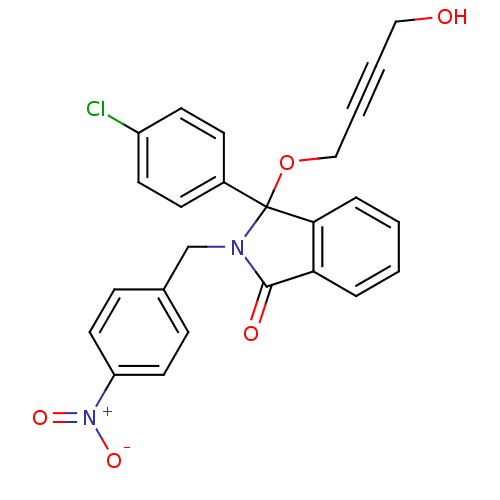 ((+/-)-3-(4-Chlorophenyl)-3-(4-hydroxybut-2-ynyloxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Mdm2 -p53 protein interaction by ELISA | J Med Chem 54: 1233-43 (2011) Article DOI: 10.1021/jm1011929 BindingDB Entry DOI: 10.7270/Q29K4BHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM111573 (US8618158, NCL-00016895) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | 25 |
Cancer Research Technology Limited US Patent | Assay Description Streptavidin-coated 96-well plates are used to immobilise a biotin-tagged IP3 p53-derived peptide (MPRFMDYWEGLN). This is a peptide analogue derived ... | US Patent US8618158 (2013) BindingDB Entry DOI: 10.7270/Q27D2STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50339436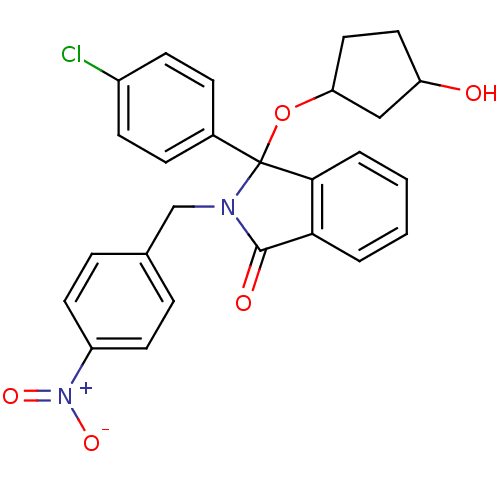 ((+/-)-3-(4-Chlorophenyl)-3-(3-hydroxycyclopentoxy)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Mdm2 -p53 protein interaction by ELISA | J Med Chem 54: 1233-43 (2011) Article DOI: 10.1021/jm1011929 BindingDB Entry DOI: 10.7270/Q29K4BHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50339364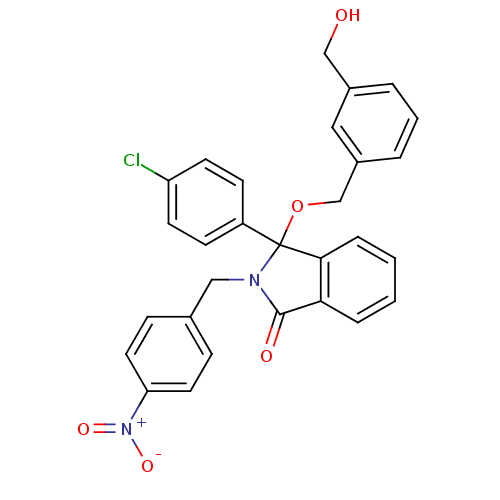 ((+/-)-3-(4-Chlorophenyl)-3-(3-hydroxymethylbenzylo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Mdm2 -p53 protein interaction by ELISA | J Med Chem 54: 1233-43 (2011) Article DOI: 10.1021/jm1011929 BindingDB Entry DOI: 10.7270/Q29K4BHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM111521 (US8618158, NU8367) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 732 | n/a | n/a | n/a | n/a | n/a | 25 |
Cancer Research Technology Limited US Patent | Assay Description Streptavidin-coated 96-well plates are used to immobilise a biotin-tagged IP3 p53-derived peptide (MPRFMDYWEGLN). This is a peptide analogue derived ... | US Patent US8618158 (2013) BindingDB Entry DOI: 10.7270/Q27D2STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM111544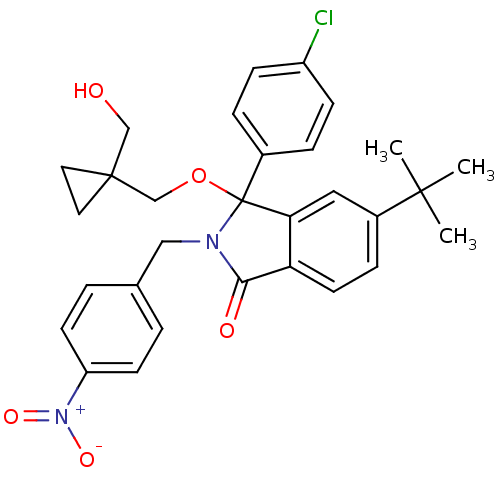 (US8618158, NU8400) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 733 | n/a | n/a | n/a | n/a | n/a | 25 |
Cancer Research Technology Limited US Patent | Assay Description Streptavidin-coated 96-well plates are used to immobilise a biotin-tagged IP3 p53-derived peptide (MPRFMDYWEGLN). This is a peptide analogue derived ... | US Patent US8618158 (2013) BindingDB Entry DOI: 10.7270/Q27D2STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM111530 (US8618158, NU8397) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 837 | n/a | n/a | n/a | n/a | n/a | 25 |
Cancer Research Technology Limited US Patent | Assay Description Streptavidin-coated 96-well plates are used to immobilise a biotin-tagged IP3 p53-derived peptide (MPRFMDYWEGLN). This is a peptide analogue derived ... | US Patent US8618158 (2013) BindingDB Entry DOI: 10.7270/Q27D2STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM111538 (US8618158, NCL-00010490) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 847 | n/a | n/a | n/a | n/a | n/a | 25 |
Cancer Research Technology Limited US Patent | Assay Description Streptavidin-coated 96-well plates are used to immobilise a biotin-tagged IP3 p53-derived peptide (MPRFMDYWEGLN). This is a peptide analogue derived ... | US Patent US8618158 (2013) BindingDB Entry DOI: 10.7270/Q27D2STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM111543 (US8618158, NCL-00010496) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 852 | n/a | n/a | n/a | n/a | n/a | 25 |
Cancer Research Technology Limited US Patent | Assay Description Streptavidin-coated 96-well plates are used to immobilise a biotin-tagged IP3 p53-derived peptide (MPRFMDYWEGLN). This is a peptide analogue derived ... | US Patent US8618158 (2013) BindingDB Entry DOI: 10.7270/Q27D2STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM111519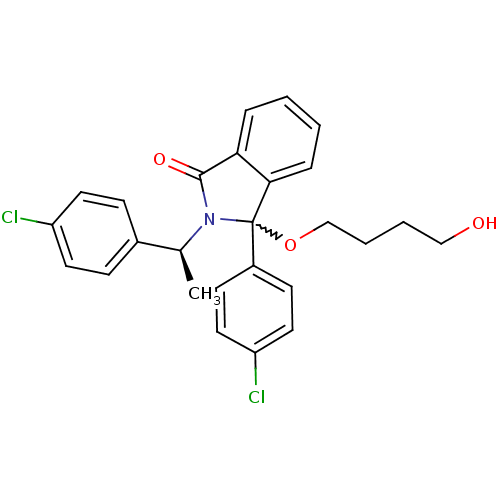 (US8618158, NU8365) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 869 | n/a | n/a | n/a | n/a | n/a | 25 |
Cancer Research Technology Limited US Patent | Assay Description Streptavidin-coated 96-well plates are used to immobilise a biotin-tagged IP3 p53-derived peptide (MPRFMDYWEGLN). This is a peptide analogue derived ... | US Patent US8618158 (2013) BindingDB Entry DOI: 10.7270/Q27D2STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50339357 ((+/-)-(S)-3-(4-Chlorophenyl)-2-[1-(4-chlorophenyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Mdm2 -p53 protein interaction by ELISA | J Med Chem 54: 1233-43 (2011) Article DOI: 10.1021/jm1011929 BindingDB Entry DOI: 10.7270/Q29K4BHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 171 total ) | Next | Last >> |