 Found 137 hits with Last Name = 'rovati' and Initial = 'ge'
Found 137 hits with Last Name = 'rovati' and Initial = 'ge' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neuronal acetylcholine receptor subunit alpha-6
(Chick) | BDBM50004108

((+-)-nicotine | (R,S)-nicotine | (RS)-nicotine | 3...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan
Curated by PDSP Ki Database
| |
Mol Pharmacol 63: 1329-37 (2003)
Article DOI: 10.1124/mol.63.6.1329
BindingDB Entry DOI: 10.7270/Q29W0D27 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-6
(Chick) | BDBM86240
(Alpha-Conotoxin MII | Gly-L-Cys(1)-L-Cys(2)-L-Ser-...)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@@H]2CSSC[C@H](NC(=O)CN)C(=O)N[C@@H](CSSC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](Cc3cnc[nH]3)NC(=O)[C@H](CCC(O)=O)NC1=O)C(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)N2 Show InChI InChI=1S/C67H103N23O22S4/c1-29(2)12-35-55(100)77-34(9-10-51(96)97)54(99)80-38(15-33-20-73-28-75-33)58(103)84-41(21-91)60(105)82-39(16-48(69)93)59(104)79-36(13-30(3)4)56(101)86-43(53(71)98)23-113-115-25-45-64(109)85-42(22-92)61(106)83-40(17-49(70)94)67(112)90-11-7-8-47(90)65(110)89-52(31(5)6)66(111)88-46(26-116-114-24-44(62(107)87-45)76-50(95)18-68)63(108)81-37(57(102)78-35)14-32-19-72-27-74-32/h19-20,27-31,34-47,52,91-92H,7-18,21-26,68H2,1-6H3,(H2,69,93)(H2,70,94)(H2,71,98)(H,72,74)(H,73,75)(H,76,95)(H,77,100)(H,78,102)(H,79,104)(H,80,99)(H,81,108)(H,82,105)(H,83,106)(H,84,103)(H,85,109)(H,86,101)(H,87,107)(H,88,111)(H,89,110)(H,96,97)/t34-,35-,36-,37-,38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,52-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan
Curated by PDSP Ki Database
| |
Mol Pharmacol 63: 1329-37 (2003)
Article DOI: 10.1124/mol.63.6.1329
BindingDB Entry DOI: 10.7270/Q29W0D27 |
More data for this
Ligand-Target Pair | |
Uncharacterized protein
(Chick) | BDBM10759

(2-acetoxyethyl(trimethyl)ammonium;bromide | 2-acet...)Show InChI InChI=1S/C7H16NO2/c1-7(9)10-6-5-8(2,3)4/h5-6H2,1-4H3/q+1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan
Curated by PDSP Ki Database
| |
Mol Pharmacol 63: 1329-37 (2003)
Article DOI: 10.1124/mol.63.6.1329
BindingDB Entry DOI: 10.7270/Q29W0D27 |
More data for this
Ligand-Target Pair | |
Uncharacterized protein
(Chick) | BDBM50004108

((+-)-nicotine | (R,S)-nicotine | (RS)-nicotine | 3...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan
Curated by PDSP Ki Database
| |
Mol Pharmacol 63: 1329-37 (2003)
Article DOI: 10.1124/mol.63.6.1329
BindingDB Entry DOI: 10.7270/Q29W0D27 |
More data for this
Ligand-Target Pair | |
Cholinergic receptor nicotinic alpha 6 subunit
(Chick) | BDBM10759

(2-acetoxyethyl(trimethyl)ammonium;bromide | 2-acet...)Show InChI InChI=1S/C7H16NO2/c1-7(9)10-6-5-8(2,3)4/h5-6H2,1-4H3/q+1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 123 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan
Curated by PDSP Ki Database
| |
Mol Pharmacol 63: 1329-37 (2003)
Article DOI: 10.1124/mol.63.6.1329
BindingDB Entry DOI: 10.7270/Q29W0D27 |
More data for this
Ligand-Target Pair | |
Cholinergic receptor nicotinic alpha 6 subunit
(Chick) | BDBM50004108

((+-)-nicotine | (R,S)-nicotine | (RS)-nicotine | 3...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 131 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan
Curated by PDSP Ki Database
| |
Mol Pharmacol 63: 1329-37 (2003)
Article DOI: 10.1124/mol.63.6.1329
BindingDB Entry DOI: 10.7270/Q29W0D27 |
More data for this
Ligand-Target Pair | |
Uncharacterized protein
(Chick) | BDBM86241
(CAS_23255-54-1 | Dihydro-Beta-erythroidine | NSC_3...)Show SMILES COC1CC=C2CCN3CCC4=C(CC(=O)OC4)C23C1 |t:4,11| Show InChI InChI=1S/C16H21NO3/c1-19-13-3-2-12-5-7-17-6-4-11-10-20-15(18)8-14(11)16(12,17)9-13/h2,13H,3-10H2,1H3 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 201 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan
Curated by PDSP Ki Database
| |
Mol Pharmacol 63: 1329-37 (2003)
Article DOI: 10.1124/mol.63.6.1329
BindingDB Entry DOI: 10.7270/Q29W0D27 |
More data for this
Ligand-Target Pair | |
Cholinergic receptor nicotinic alpha 6 subunit
(Chick) | BDBM86241

(CAS_23255-54-1 | Dihydro-Beta-erythroidine | NSC_3...)Show SMILES COC1CC=C2CCN3CCC4=C(CC(=O)OC4)C23C1 |t:4,11| Show InChI InChI=1S/C16H21NO3/c1-19-13-3-2-12-5-7-17-6-4-11-10-20-15(18)8-14(11)16(12,17)9-13/h2,13H,3-10H2,1H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 502 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan
Curated by PDSP Ki Database
| |
Mol Pharmacol 63: 1329-37 (2003)
Article DOI: 10.1124/mol.63.6.1329
BindingDB Entry DOI: 10.7270/Q29W0D27 |
More data for this
Ligand-Target Pair | |
Cholinergic receptor nicotinic alpha 6 subunit
(Chick) | BDBM86240

(Alpha-Conotoxin MII | Gly-L-Cys(1)-L-Cys(2)-L-Ser-...)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@@H]2CSSC[C@H](NC(=O)CN)C(=O)N[C@@H](CSSC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](Cc3cnc[nH]3)NC(=O)[C@H](CCC(O)=O)NC1=O)C(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)N2 Show InChI InChI=1S/C67H103N23O22S4/c1-29(2)12-35-55(100)77-34(9-10-51(96)97)54(99)80-38(15-33-20-73-28-75-33)58(103)84-41(21-91)60(105)82-39(16-48(69)93)59(104)79-36(13-30(3)4)56(101)86-43(53(71)98)23-113-115-25-45-64(109)85-42(22-92)61(106)83-40(17-49(70)94)67(112)90-11-7-8-47(90)65(110)89-52(31(5)6)66(111)88-46(26-116-114-24-44(62(107)87-45)76-50(95)18-68)63(108)81-37(57(102)78-35)14-32-19-72-27-74-32/h19-20,27-31,34-47,52,91-92H,7-18,21-26,68H2,1-6H3,(H2,69,93)(H2,70,94)(H2,71,98)(H,72,74)(H,73,75)(H,76,95)(H,77,100)(H,78,102)(H,79,104)(H,80,99)(H,81,108)(H,82,105)(H,83,106)(H,84,103)(H,85,109)(H,86,101)(H,87,107)(H,88,111)(H,89,110)(H,96,97)/t34-,35-,36-,37-,38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,52-/m0/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan
Curated by PDSP Ki Database
| |
Mol Pharmacol 63: 1329-37 (2003)
Article DOI: 10.1124/mol.63.6.1329
BindingDB Entry DOI: 10.7270/Q29W0D27 |
More data for this
Ligand-Target Pair | |
Uncharacterized protein
(Chick) | BDBM86240

(Alpha-Conotoxin MII | Gly-L-Cys(1)-L-Cys(2)-L-Ser-...)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@@H]2CSSC[C@H](NC(=O)CN)C(=O)N[C@@H](CSSC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](Cc3cnc[nH]3)NC(=O)[C@H](CCC(O)=O)NC1=O)C(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)N2 Show InChI InChI=1S/C67H103N23O22S4/c1-29(2)12-35-55(100)77-34(9-10-51(96)97)54(99)80-38(15-33-20-73-28-75-33)58(103)84-41(21-91)60(105)82-39(16-48(69)93)59(104)79-36(13-30(3)4)56(101)86-43(53(71)98)23-113-115-25-45-64(109)85-42(22-92)61(106)83-40(17-49(70)94)67(112)90-11-7-8-47(90)65(110)89-52(31(5)6)66(111)88-46(26-116-114-24-44(62(107)87-45)76-50(95)18-68)63(108)81-37(57(102)78-35)14-32-19-72-27-74-32/h19-20,27-31,34-47,52,91-92H,7-18,21-26,68H2,1-6H3,(H2,69,93)(H2,70,94)(H2,71,98)(H,72,74)(H,73,75)(H,76,95)(H,77,100)(H,78,102)(H,79,104)(H,80,99)(H,81,108)(H,82,105)(H,83,106)(H,84,103)(H,85,109)(H,86,101)(H,87,107)(H,88,111)(H,89,110)(H,96,97)/t34-,35-,36-,37-,38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,52-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan
Curated by PDSP Ki Database
| |
Mol Pharmacol 63: 1329-37 (2003)
Article DOI: 10.1124/mol.63.6.1329
BindingDB Entry DOI: 10.7270/Q29W0D27 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-6
(Chick) | BDBM86241

(CAS_23255-54-1 | Dihydro-Beta-erythroidine | NSC_3...)Show SMILES COC1CC=C2CCN3CCC4=C(CC(=O)OC4)C23C1 |t:4,11| Show InChI InChI=1S/C16H21NO3/c1-19-13-3-2-12-5-7-17-6-4-11-10-20-15(18)8-14(11)16(12,17)9-13/h2,13H,3-10H2,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan
Curated by PDSP Ki Database
| |
Mol Pharmacol 63: 1329-37 (2003)
Article DOI: 10.1124/mol.63.6.1329
BindingDB Entry DOI: 10.7270/Q29W0D27 |
More data for this
Ligand-Target Pair | |
Cholinergic receptor nicotinic alpha 6 subunit
(Chick) | BDBM86242
(CAS_64645 | NSC_64645 | Tubocurarine,d)Show SMILES COc1cc2CC[N+](C)(C)C3Cc4ccc(O)c(Oc5cc6C(Cc7ccc(Oc(c1O)c23)cc7)[NH+](C)CCc6cc5OC)c4 Show InChI InChI=1S/C37H40N2O6/c1-38-14-12-24-19-32(42-4)33-21-27(24)28(38)16-22-6-9-26(10-7-22)44-37-35-25(20-34(43-5)36(37)41)13-15-39(2,3)29(35)17-23-8-11-30(40)31(18-23)45-33/h6-11,18-21,28-29H,12-17H2,1-5H3,(H-,40,41)/p+2 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 3.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan
Curated by PDSP Ki Database
| |
Mol Pharmacol 63: 1329-37 (2003)
Article DOI: 10.1124/mol.63.6.1329
BindingDB Entry DOI: 10.7270/Q29W0D27 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-6
(Chick) | BDBM86243
(CAS_17360 | MG624 | NSC_17360)Show SMILES CC[N+](CC)(CC)CCOc1ccc(C=Cc2ccccc2)cc1 |w:14.13| Show InChI InChI=1S/C22H30NO/c1-4-23(5-2,6-3)18-19-24-22-16-14-21(15-17-22)13-12-20-10-8-7-9-11-20/h7-17H,4-6,18-19H2,1-3H3/q+1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan
Curated by PDSP Ki Database
| |
Mol Pharmacol 63: 1329-37 (2003)
Article DOI: 10.1124/mol.63.6.1329
BindingDB Entry DOI: 10.7270/Q29W0D27 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50530480

(CHEMBL4445524)Show SMILES OC(=O)CCCc1nc(c(o1)-c1ccccc1)-c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C19H15Cl2NO3/c20-14-10-9-13(11-15(14)21)18-19(12-5-2-1-3-6-12)25-16(22-18)7-4-8-17(23)24/h1-3,5-6,9-11H,4,7-8H2,(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Competitive inhibition of full length human soluble epoxide hydrolase pre-incubated for 30 mins before DiFMUP substrate addition by fluorescence base... |
J Med Chem 62: 8443-8460 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00445
BindingDB Entry DOI: 10.7270/Q2V98CJ7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50530480

(CHEMBL4445524)Show SMILES OC(=O)CCCc1nc(c(o1)-c1ccccc1)-c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C19H15Cl2NO3/c20-14-10-9-13(11-15(14)21)18-19(12-5-2-1-3-6-12)25-16(22-18)7-4-8-17(23)24/h1-3,5-6,9-11H,4,7-8H2,(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Competitive inhibition of full length human soluble epoxide hydrolase pre-incubated for 30 mins before DiFMUP substrate addition by fluorescence base... |
J Med Chem 62: 8443-8460 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00445
BindingDB Entry DOI: 10.7270/Q2V98CJ7 |
More data for this
Ligand-Target Pair | |
Uncharacterized protein
(Chick) | BDBM86242

(CAS_64645 | NSC_64645 | Tubocurarine,d)Show SMILES COc1cc2CC[N+](C)(C)C3Cc4ccc(O)c(Oc5cc6C(Cc7ccc(Oc(c1O)c23)cc7)[NH+](C)CCc6cc5OC)c4 Show InChI InChI=1S/C37H40N2O6/c1-38-14-12-24-19-32(42-4)33-21-27(24)28(38)16-22-6-9-26(10-7-22)44-37-35-25(20-34(43-5)36(37)41)13-15-39(2,3)29(35)17-23-8-11-30(40)31(18-23)45-33/h6-11,18-21,28-29H,12-17H2,1-5H3,(H-,40,41)/p+2 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan
Curated by PDSP Ki Database
| |
Mol Pharmacol 63: 1329-37 (2003)
Article DOI: 10.1124/mol.63.6.1329
BindingDB Entry DOI: 10.7270/Q29W0D27 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-6
(Chick) | BDBM86242

(CAS_64645 | NSC_64645 | Tubocurarine,d)Show SMILES COc1cc2CC[N+](C)(C)C3Cc4ccc(O)c(Oc5cc6C(Cc7ccc(Oc(c1O)c23)cc7)[NH+](C)CCc6cc5OC)c4 Show InChI InChI=1S/C37H40N2O6/c1-38-14-12-24-19-32(42-4)33-21-27(24)28(38)16-22-6-9-26(10-7-22)44-37-35-25(20-34(43-5)36(37)41)13-15-39(2,3)29(35)17-23-8-11-30(40)31(18-23)45-33/h6-11,18-21,28-29H,12-17H2,1-5H3,(H-,40,41)/p+2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan
Curated by PDSP Ki Database
| |
Mol Pharmacol 63: 1329-37 (2003)
Article DOI: 10.1124/mol.63.6.1329
BindingDB Entry DOI: 10.7270/Q29W0D27 |
More data for this
Ligand-Target Pair | |
Uncharacterized protein
(Chick) | BDBM86243

(CAS_17360 | MG624 | NSC_17360)Show SMILES CC[N+](CC)(CC)CCOc1ccc(C=Cc2ccccc2)cc1 |w:14.13| Show InChI InChI=1S/C22H30NO/c1-4-23(5-2,6-3)18-19-24-22-16-14-21(15-17-22)13-12-20-10-8-7-9-11-20/h7-17H,4-6,18-19H2,1-3H3/q+1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan
Curated by PDSP Ki Database
| |
Mol Pharmacol 63: 1329-37 (2003)
Article DOI: 10.1124/mol.63.6.1329
BindingDB Entry DOI: 10.7270/Q29W0D27 |
More data for this
Ligand-Target Pair | |
Uncharacterized protein
(Chick) | BDBM50038416
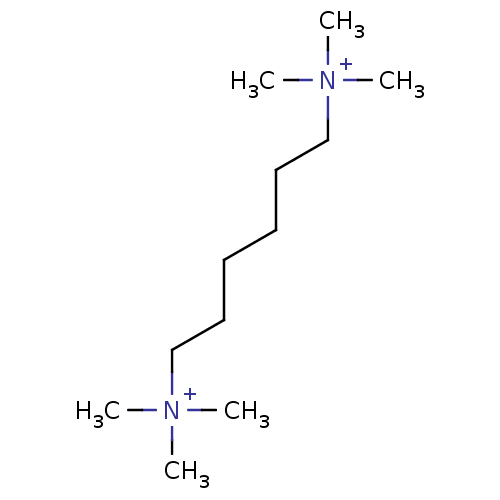
((1,6-trimethyl ammonium)hexane; dibromide | CHEMBL...)Show InChI InChI=1S/C12H30N2/c1-13(2,3)11-9-7-8-10-12-14(4,5)6/h7-12H2,1-6H3/q+2 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan
Curated by PDSP Ki Database
| |
Mol Pharmacol 63: 1329-37 (2003)
Article DOI: 10.1124/mol.63.6.1329
BindingDB Entry DOI: 10.7270/Q29W0D27 |
More data for this
Ligand-Target Pair | |
Uncharacterized protein
(Chick) | BDBM50060582
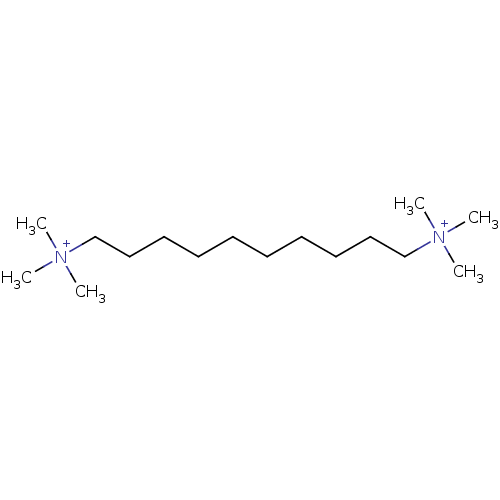
(1,10-Bis(trimethyl ammonium)decane dibromide | 1,1...)Show InChI InChI=1S/C16H38N2/c1-17(2,3)15-13-11-9-7-8-10-12-14-16-18(4,5)6/h7-16H2,1-6H3/q+2 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan
Curated by PDSP Ki Database
| |
Mol Pharmacol 63: 1329-37 (2003)
Article DOI: 10.1124/mol.63.6.1329
BindingDB Entry DOI: 10.7270/Q29W0D27 |
More data for this
Ligand-Target Pair | |
Cholinergic receptor nicotinic alpha 6 subunit
(Chick) | BDBM86243

(CAS_17360 | MG624 | NSC_17360)Show SMILES CC[N+](CC)(CC)CCOc1ccc(C=Cc2ccccc2)cc1 |w:14.13| Show InChI InChI=1S/C22H30NO/c1-4-23(5-2,6-3)18-19-24-22-16-14-21(15-17-22)13-12-20-10-8-7-9-11-20/h7-17H,4-6,18-19H2,1-3H3/q+1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan
Curated by PDSP Ki Database
| |
Mol Pharmacol 63: 1329-37 (2003)
Article DOI: 10.1124/mol.63.6.1329
BindingDB Entry DOI: 10.7270/Q29W0D27 |
More data for this
Ligand-Target Pair | |
Cholinergic receptor nicotinic alpha 6 subunit
(Chick) | BDBM50060582

(1,10-Bis(trimethyl ammonium)decane dibromide | 1,1...)Show InChI InChI=1S/C16H38N2/c1-17(2,3)15-13-11-9-7-8-10-12-14-16-18(4,5)6/h7-16H2,1-6H3/q+2 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan
Curated by PDSP Ki Database
| |
Mol Pharmacol 63: 1329-37 (2003)
Article DOI: 10.1124/mol.63.6.1329
BindingDB Entry DOI: 10.7270/Q29W0D27 |
More data for this
Ligand-Target Pair | |
Cholinergic receptor nicotinic alpha 6 subunit
(Chick) | BDBM50038416

((1,6-trimethyl ammonium)hexane; dibromide | CHEMBL...)Show InChI InChI=1S/C12H30N2/c1-13(2,3)11-9-7-8-10-12-14(4,5)6/h7-12H2,1-6H3/q+2 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan
Curated by PDSP Ki Database
| |
Mol Pharmacol 63: 1329-37 (2003)
Article DOI: 10.1124/mol.63.6.1329
BindingDB Entry DOI: 10.7270/Q29W0D27 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-6
(Chick) | BDBM50060582

(1,10-Bis(trimethyl ammonium)decane dibromide | 1,1...)Show InChI InChI=1S/C16H38N2/c1-17(2,3)15-13-11-9-7-8-10-12-14-16-18(4,5)6/h7-16H2,1-6H3/q+2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan
Curated by PDSP Ki Database
| |
Mol Pharmacol 63: 1329-37 (2003)
Article DOI: 10.1124/mol.63.6.1329
BindingDB Entry DOI: 10.7270/Q29W0D27 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-6
(Chick) | BDBM50038416

((1,6-trimethyl ammonium)hexane; dibromide | CHEMBL...)Show InChI InChI=1S/C12H30N2/c1-13(2,3)11-9-7-8-10-12-14(4,5)6/h7-12H2,1-6H3/q+2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan
Curated by PDSP Ki Database
| |
Mol Pharmacol 63: 1329-37 (2003)
Article DOI: 10.1124/mol.63.6.1329
BindingDB Entry DOI: 10.7270/Q29W0D27 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50465529

(CHEMBL4279086)Show SMILES COc1cc(ccc1Cc1cn(C)c2ccc(NC(=O)NC3CCCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1 Show InChI InChI=1S/C31H34N4O5S/c1-35-20-23(27-19-25(15-16-28(27)35)33-31(37)32-24-9-5-3-6-10-24)17-21-13-14-22(18-29(21)40-2)30(36)34-41(38,39)26-11-7-4-8-12-26/h4,7-8,11-16,18-20,24H,3,5-6,9-10,17H2,1-2H3,(H,34,36)(H2,32,33,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of human sEH in human HepG2 cells using (+/-)14(15)-EET-d11 as substrate assessed as formation of (+/-)14(15)-DHET-d11 preincubated for 15... |
J Med Chem 61: 5758-5764 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00458
BindingDB Entry DOI: 10.7270/Q2TX3J21 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50465529

(CHEMBL4279086)Show SMILES COc1cc(ccc1Cc1cn(C)c2ccc(NC(=O)NC3CCCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1 Show InChI InChI=1S/C31H34N4O5S/c1-35-20-23(27-19-25(15-16-28(27)35)33-31(37)32-24-9-5-3-6-10-24)17-21-13-14-22(18-29(21)40-2)30(36)34-41(38,39)26-11-7-4-8-12-26/h4,7-8,11-16,18-20,24H,3,5-6,9-10,17H2,1-2H3,(H,34,36)(H2,32,33,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human sEH using PHOME as substrate preincubated for 30 mins followed by substrate addition and measured after 30 mins by sp... |
J Med Chem 61: 5758-5764 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00458
BindingDB Entry DOI: 10.7270/Q2TX3J21 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50002861
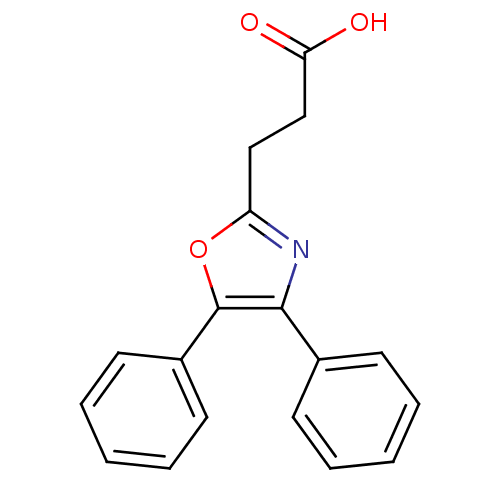
(3-(4,5-Diphenyl-oxazol-2-yl)-propionic acid | CHEM...)Show InChI InChI=1S/C18H15NO3/c20-16(21)12-11-15-19-17(13-7-3-1-4-8-13)18(22-15)14-9-5-2-6-10-14/h1-10H,11-12H2,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in LPS-stimulated human monocytes assessed as reduction in PGE2 production by LC-tandem MIS analysis |
J Med Chem 62: 8443-8460 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00445
BindingDB Entry DOI: 10.7270/Q2V98CJ7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM34233

(2-Phenyl-benzo[d]isoselenazol-3-one | 2-Phenyl-ben...)Show InChI InChI=1S/C13H9NOSe/c15-13-11-8-4-5-9-12(11)16-14(13)10-6-2-1-3-7-10/h1-9H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of hexa-His-tagged human soluble epoxide hydrolase C-terminal hydrolase domain expressed in Escherichia coli BL21(DE3) pre-incubated for 3... |
J Med Chem 62: 8443-8460 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00445
BindingDB Entry DOI: 10.7270/Q2V98CJ7 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50002861

(3-(4,5-Diphenyl-oxazol-2-yl)-propionic acid | CHEM...)Show InChI InChI=1S/C18H15NO3/c20-16(21)12-11-15-19-17(13-7-3-1-4-8-13)18(22-15)14-9-5-2-6-10-14/h1-10H,11-12H2,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in LPS-stimulated human monocytes assessed as reduction in PGE2 production by LC-tandem MIS analysis |
J Med Chem 62: 8443-8460 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00445
BindingDB Entry DOI: 10.7270/Q2V98CJ7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM34233

(2-Phenyl-benzo[d]isoselenazol-3-one | 2-Phenyl-ben...)Show InChI InChI=1S/C13H9NOSe/c15-13-11-8-4-5-9-12(11)16-14(13)10-6-2-1-3-7-10/h1-9H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of hexa-His-tagged human soluble epoxide hydrolase C-terminal hydrolase domain expressed in Escherichia coli BL21(DE3) pre-incubated for 3... |
J Med Chem 62: 8443-8460 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00445
BindingDB Entry DOI: 10.7270/Q2V98CJ7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50465527

(CHEMBL4288574)Show SMILES COc1cc(ccc1Cc1cn(C)c2ccc(NC(=O)NC3CCCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1C Show InChI InChI=1S/C32H36N4O5S/c1-21-9-7-8-12-30(21)42(39,40)35-31(37)23-14-13-22(29(18-23)41-3)17-24-20-36(2)28-16-15-26(19-27(24)28)34-32(38)33-25-10-5-4-6-11-25/h7-9,12-16,18-20,25H,4-6,10-11,17H2,1-3H3,(H,35,37)(H2,33,34,38) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human sEH using PHOME as substrate preincubated for 30 mins followed by substrate addition and measured after 30 mins by sp... |
J Med Chem 61: 5758-5764 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00458
BindingDB Entry DOI: 10.7270/Q2TX3J21 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM34233

(2-Phenyl-benzo[d]isoselenazol-3-one | 2-Phenyl-ben...)Show InChI InChI=1S/C13H9NOSe/c15-13-11-8-4-5-9-12(11)16-14(13)10-6-2-1-3-7-10/h1-9H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of hydrolase activity of full length human soluble epoxide hydrolase pre-incubated for 30 mins before PHOME substrate addition by fluoresc... |
J Med Chem 62: 8443-8460 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00445
BindingDB Entry DOI: 10.7270/Q2V98CJ7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM34233

(2-Phenyl-benzo[d]isoselenazol-3-one | 2-Phenyl-ben...)Show InChI InChI=1S/C13H9NOSe/c15-13-11-8-4-5-9-12(11)16-14(13)10-6-2-1-3-7-10/h1-9H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of hydrolase activity of full length human soluble epoxide hydrolase pre-incubated for 30 mins before PHOME substrate addition by fluoresc... |
J Med Chem 62: 8443-8460 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00445
BindingDB Entry DOI: 10.7270/Q2V98CJ7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50465530

(CHEMBL4289768)Show SMILES COc1cc(ccc1Cc1cn(C)c2ccc(NC(=O)NC3CCCCC3)cc12)C(=O)NS(=O)(=O)c1cccc(F)c1 Show InChI InChI=1S/C31H33FN4O5S/c1-36-19-22(27-18-25(13-14-28(27)36)34-31(38)33-24-8-4-3-5-9-24)15-20-11-12-21(16-29(20)41-2)30(37)35-42(39,40)26-10-6-7-23(32)17-26/h6-7,10-14,16-19,24H,3-5,8-9,15H2,1-2H3,(H,35,37)(H2,33,34,38) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human sEH using PHOME as substrate preincubated for 30 mins followed by substrate addition and measured after 30 mins by sp... |
J Med Chem 61: 5758-5764 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00458
BindingDB Entry DOI: 10.7270/Q2TX3J21 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50530480

(CHEMBL4445524)Show SMILES OC(=O)CCCc1nc(c(o1)-c1ccccc1)-c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C19H15Cl2NO3/c20-14-10-9-13(11-15(14)21)18-19(12-5-2-1-3-6-12)25-16(22-18)7-4-8-17(23)24/h1-3,5-6,9-11H,4,7-8H2,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in LPS-stimulated human monocytes assessed as reduction in PGE2 production by LC-tandem MIS analysis |
J Med Chem 62: 8443-8460 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00445
BindingDB Entry DOI: 10.7270/Q2V98CJ7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM34233

(2-Phenyl-benzo[d]isoselenazol-3-one | 2-Phenyl-ben...)Show InChI InChI=1S/C13H9NOSe/c15-13-11-8-4-5-9-12(11)16-14(13)10-6-2-1-3-7-10/h1-9H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of phosphatase activity of full length human soluble epoxide hydrolase pre-incubated for 30 mins before FDP substrate addition by fluoresc... |
J Med Chem 62: 8443-8460 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00445
BindingDB Entry DOI: 10.7270/Q2V98CJ7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM34233

(2-Phenyl-benzo[d]isoselenazol-3-one | 2-Phenyl-ben...)Show InChI InChI=1S/C13H9NOSe/c15-13-11-8-4-5-9-12(11)16-14(13)10-6-2-1-3-7-10/h1-9H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of phosphatase activity of full length human soluble epoxide hydrolase pre-incubated for 30 mins before FDP substrate addition by fluoresc... |
J Med Chem 62: 8443-8460 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00445
BindingDB Entry DOI: 10.7270/Q2V98CJ7 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50530480

(CHEMBL4445524)Show SMILES OC(=O)CCCc1nc(c(o1)-c1ccccc1)-c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C19H15Cl2NO3/c20-14-10-9-13(11-15(14)21)18-19(12-5-2-1-3-6-12)25-16(22-18)7-4-8-17(23)24/h1-3,5-6,9-11H,4,7-8H2,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in LPS-stimulated human monocytes assessed as reduction in PGE2 production by LC-tandem MIS analysis |
J Med Chem 62: 8443-8460 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00445
BindingDB Entry DOI: 10.7270/Q2V98CJ7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
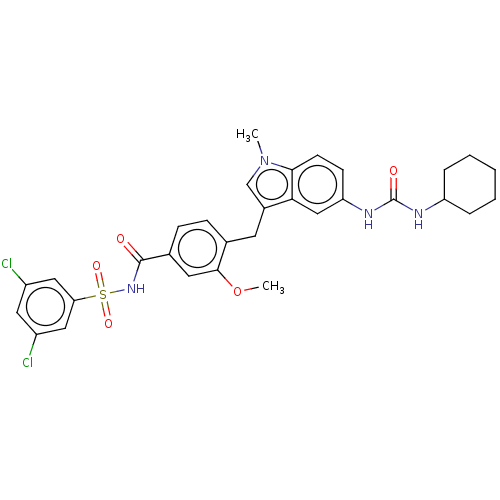
(Homo sapiens (Human)) | BDBM50465532
(CHEMBL4286347)Show SMILES COc1cc(ccc1Cc1cn(C)c2ccc(NC(=O)NC3CCCCC3)cc12)C(=O)NS(=O)(=O)c1cc(Cl)cc(Cl)c1 Show InChI InChI=1S/C31H32Cl2N4O5S/c1-37-18-21(27-17-25(10-11-28(27)37)35-31(39)34-24-6-4-3-5-7-24)12-19-8-9-20(13-29(19)42-2)30(38)36-43(40,41)26-15-22(32)14-23(33)16-26/h8-11,13-18,24H,3-7,12H2,1-2H3,(H,36,38)(H2,34,35,39) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human sEH using PHOME as substrate preincubated for 30 mins followed by substrate addition and measured after 30 mins by sp... |
J Med Chem 61: 5758-5764 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00458
BindingDB Entry DOI: 10.7270/Q2TX3J21 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50015540

(CHEMBL431348 | N-{4-[5-(3-Cyclopentyl-ureido)-1-me...)Show SMILES COc1cc(ccc1Cc1cn(C)c2ccc(NC(=O)NC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1C Show InChI InChI=1S/C31H34N4O5S/c1-20-8-4-7-11-29(20)41(38,39)34-30(36)22-13-12-21(28(17-22)40-3)16-23-19-35(2)27-15-14-25(18-26(23)27)33-31(37)32-24-9-5-6-10-24/h4,7-8,11-15,17-19,24H,5-6,9-10,16H2,1-3H3,(H,34,36)(H2,32,33,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human sEH using PHOME as substrate preincubated for 30 mins followed by substrate addition and measured after 30 mins by sp... |
J Med Chem 61: 5758-5764 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00458
BindingDB Entry DOI: 10.7270/Q2TX3J21 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50465528

(CHEMBL4290919)Show SMILES COc1cc(ccc1Cc1cn(C)c2ccc(NC(=O)NC3CCCCC3)cc12)C(=O)NS(=O)(=O)c1cccc(Cl)c1 Show InChI InChI=1S/C31H33ClN4O5S/c1-36-19-22(27-18-25(13-14-28(27)36)34-31(38)33-24-8-4-3-5-9-24)15-20-11-12-21(16-29(20)41-2)30(37)35-42(39,40)26-10-6-7-23(32)17-26/h6-7,10-14,16-19,24H,3-5,8-9,15H2,1-2H3,(H,35,37)(H2,33,34,38) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human sEH using PHOME as substrate preincubated for 30 mins followed by substrate addition and measured after 30 mins by sp... |
J Med Chem 61: 5758-5764 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00458
BindingDB Entry DOI: 10.7270/Q2TX3J21 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50002861

(3-(4,5-Diphenyl-oxazol-2-yl)-propionic acid | CHEM...)Show InChI InChI=1S/C18H15NO3/c20-16(21)12-11-15-19-17(13-7-3-1-4-8-13)18(22-15)14-9-5-2-6-10-14/h1-10H,11-12H2,(H,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of COX1 in A23187-stimulated human platelets assessed as reduction in TXA2 production |
J Med Chem 62: 8443-8460 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00445
BindingDB Entry DOI: 10.7270/Q2V98CJ7 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50002861

(3-(4,5-Diphenyl-oxazol-2-yl)-propionic acid | CHEM...)Show InChI InChI=1S/C18H15NO3/c20-16(21)12-11-15-19-17(13-7-3-1-4-8-13)18(22-15)14-9-5-2-6-10-14/h1-10H,11-12H2,(H,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of COX1 in A23187-stimulated human platelets assessed as reduction in TXA2 production |
J Med Chem 62: 8443-8460 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00445
BindingDB Entry DOI: 10.7270/Q2V98CJ7 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50530480

(CHEMBL4445524)Show SMILES OC(=O)CCCc1nc(c(o1)-c1ccccc1)-c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C19H15Cl2NO3/c20-14-10-9-13(11-15(14)21)18-19(12-5-2-1-3-6-12)25-16(22-18)7-4-8-17(23)24/h1-3,5-6,9-11H,4,7-8H2,(H,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of COX1 in A23187-stimulated human platelets assessed as reduction in TXA2 production |
J Med Chem 62: 8443-8460 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00445
BindingDB Entry DOI: 10.7270/Q2V98CJ7 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50530480

(CHEMBL4445524)Show SMILES OC(=O)CCCc1nc(c(o1)-c1ccccc1)-c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C19H15Cl2NO3/c20-14-10-9-13(11-15(14)21)18-19(12-5-2-1-3-6-12)25-16(22-18)7-4-8-17(23)24/h1-3,5-6,9-11H,4,7-8H2,(H,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of COX1 in A23187-stimulated human platelets assessed as reduction in TXA2 production |
J Med Chem 62: 8443-8460 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00445
BindingDB Entry DOI: 10.7270/Q2V98CJ7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50465526
(CHEMBL4294239)Show SMILES COc1cc(ccc1Cc1cn(C)c2ccc(NC(=O)NC3CCCCC3)cc12)C(=O)NS(=O)(=O)c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C32H33F3N4O5S/c1-39-19-22(27-18-25(13-14-28(27)39)37-31(41)36-24-8-4-3-5-9-24)15-20-11-12-21(16-29(20)44-2)30(40)38-45(42,43)26-10-6-7-23(17-26)32(33,34)35/h6-7,10-14,16-19,24H,3-5,8-9,15H2,1-2H3,(H,38,40)(H2,36,37,41) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human sEH using PHOME as substrate preincubated for 30 mins followed by substrate addition and measured after 30 mins by sp... |
J Med Chem 61: 5758-5764 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00458
BindingDB Entry DOI: 10.7270/Q2TX3J21 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50009073

(4-(5-cyclopentyloxycarbonylamino-1-methyl-1H-indol...)Show SMILES COc1cc(ccc1Cc1cn(C)c2ccc(NC(=O)OC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1C Show InChI InChI=1S/C31H33N3O6S/c1-20-8-4-7-11-29(20)41(37,38)33-30(35)22-13-12-21(28(17-22)39-3)16-23-19-34(2)27-15-14-24(18-26(23)27)32-31(36)40-25-9-5-6-10-25/h4,7-8,11-15,17-19,25H,5-6,9-10,16H2,1-3H3,(H,32,36)(H,33,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human sEH using PHOME as substrate preincubated for 30 mins followed by substrate addition and measured after 30 mins by sp... |
J Med Chem 61: 5758-5764 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00458
BindingDB Entry DOI: 10.7270/Q2TX3J21 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50530481

(CHEMBL1555989)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#16]-[#6]-[#6@H](-[#7]-[#6](-[#6])=O)-[#6](-[#8])=O Show InChI InChI=1S/C20H33NO3S/c1-15(2)8-6-9-16(3)10-7-11-17(4)12-13-25-14-19(20(23)24)21-18(5)22/h8,10,12,19H,6-7,9,11,13-14H2,1-5H3,(H,21,22)(H,23,24)/b16-10+,17-12+/t19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of phosphatase activity of full length human soluble epoxide hydrolase pre-incubated for 30 mins before FDP substrate addition by fluoresc... |
J Med Chem 62: 8443-8460 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00445
BindingDB Entry DOI: 10.7270/Q2V98CJ7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50530481

(CHEMBL1555989)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#16]-[#6]-[#6@H](-[#7]-[#6](-[#6])=O)-[#6](-[#8])=O Show InChI InChI=1S/C20H33NO3S/c1-15(2)8-6-9-16(3)10-7-11-17(4)12-13-25-14-19(20(23)24)21-18(5)22/h8,10,12,19H,6-7,9,11,13-14H2,1-5H3,(H,21,22)(H,23,24)/b16-10+,17-12+/t19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of phosphatase activity of full length human soluble epoxide hydrolase pre-incubated for 30 mins before FDP substrate addition by fluoresc... |
J Med Chem 62: 8443-8460 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00445
BindingDB Entry DOI: 10.7270/Q2V98CJ7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data