

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM520 (1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of Wild-type protease | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of Wild-type protease | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50366785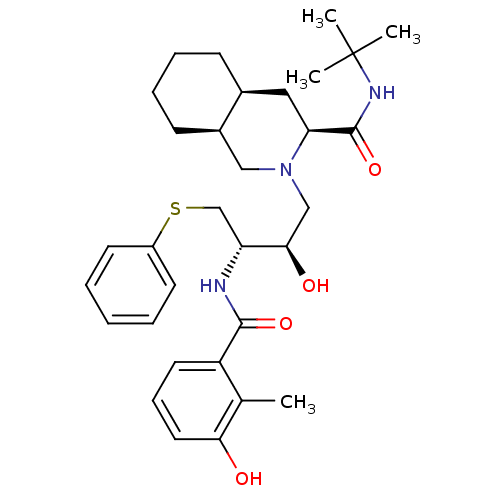 (NELFINAVIR) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of Wild-type protease | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM517 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of Wild-type protease | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of mutant HIV-protease (K-60) | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM517 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of mutant HIV-protease (A-44) | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of mutant HIV-protease (A-44) | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50366785 (NELFINAVIR) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of mutant HIV-protease (A-44) | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM520 (1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of mutant HIV-protease (V-18) | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50366785 (NELFINAVIR) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of mutant HIV-protease (V-18) | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50366785 (NELFINAVIR) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of mutant HIV-protease (K-60) | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM517 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of mutant HIV-protease (V-18) | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM520 (1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of mutant HIV-protease (K-60) | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM517 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of mutant HIV-protease (K-60) | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM520 (1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of mutant HIV-protease (A-44) | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of mutant HIV-protease (V-18) | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9113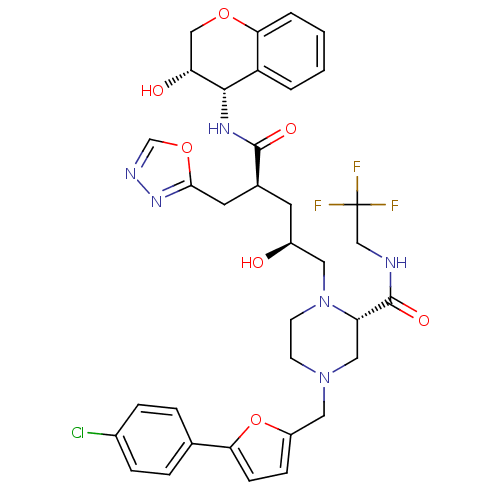 ((2S)-4-{[5-(4-chlorophenyl)furan-2-yl]methyl}-1-[(...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0150 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 14: 4651-4 (2004) Article DOI: 10.1016/j.bmcl.2004.06.092 BindingDB Entry DOI: 10.7270/Q22Z13QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9114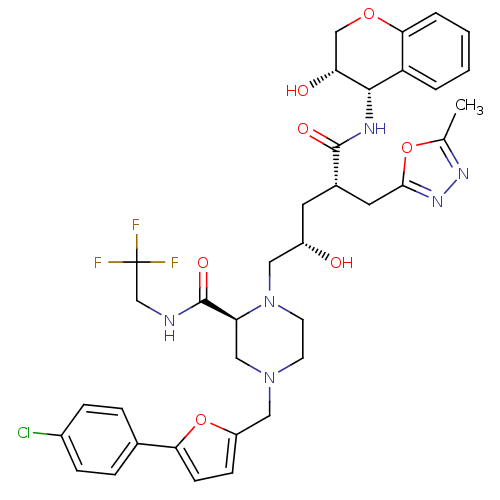 ((2S)-4-{[5-(4-chlorophenyl)furan-2-yl]methyl}-1-[(...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.0150 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 14: 4651-4 (2004) Article DOI: 10.1016/j.bmcl.2004.06.092 BindingDB Entry DOI: 10.7270/Q22Z13QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9112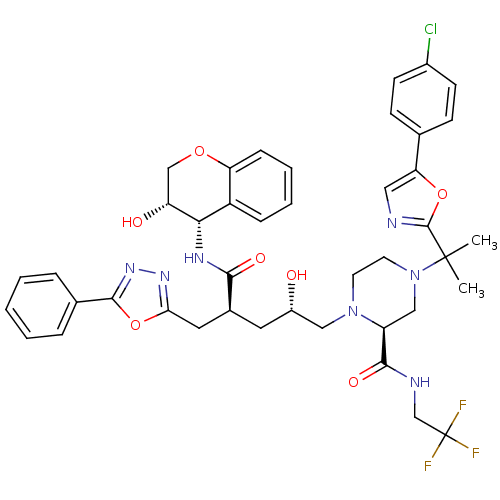 ((2S)-4-{2-[5-(4-chlorophenyl)-1,3-oxazol-2-yl]prop...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.0150 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 14: 4651-4 (2004) Article DOI: 10.1016/j.bmcl.2004.06.092 BindingDB Entry DOI: 10.7270/Q22Z13QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9115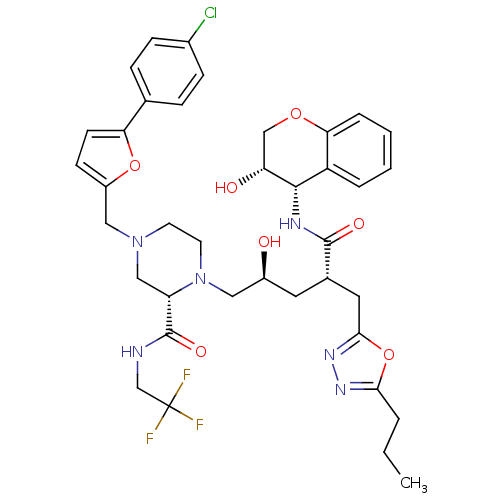 ((2S)-4-{[5-(4-chlorophenyl)furan-2-yl]methyl}-1-[(...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.0150 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 14: 4651-4 (2004) Article DOI: 10.1016/j.bmcl.2004.06.092 BindingDB Entry DOI: 10.7270/Q22Z13QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9109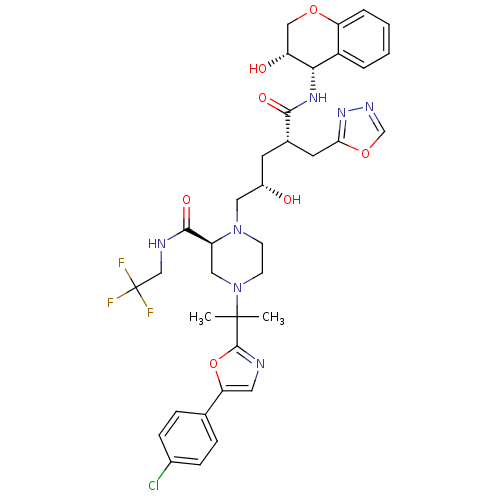 ((2S)-4-{2-[5-(4-chlorophenyl)-1,3-oxazol-2-yl]prop...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.0150 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 14: 4651-4 (2004) Article DOI: 10.1016/j.bmcl.2004.06.092 BindingDB Entry DOI: 10.7270/Q22Z13QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9111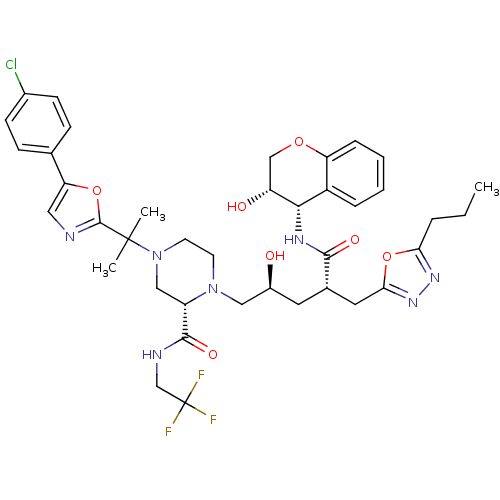 ((2S)-4-{2-[5-(4-chlorophenyl)-1,3-oxazol-2-yl]prop...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.0150 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 14: 4651-4 (2004) Article DOI: 10.1016/j.bmcl.2004.06.092 BindingDB Entry DOI: 10.7270/Q22Z13QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9107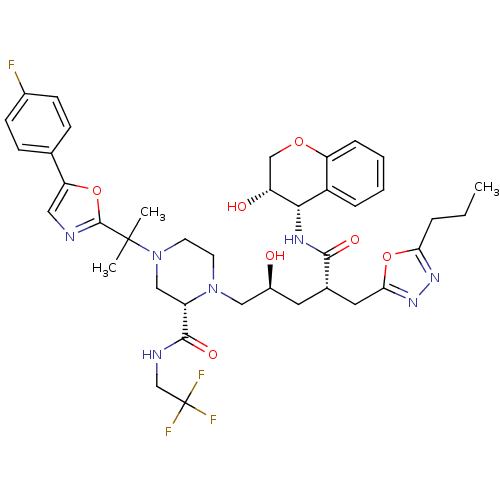 ((2S)-4-{2-[5-(4-fluorophenyl)-1,3-oxazol-2-yl]prop...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0160 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 14: 4651-4 (2004) Article DOI: 10.1016/j.bmcl.2004.06.092 BindingDB Entry DOI: 10.7270/Q22Z13QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9110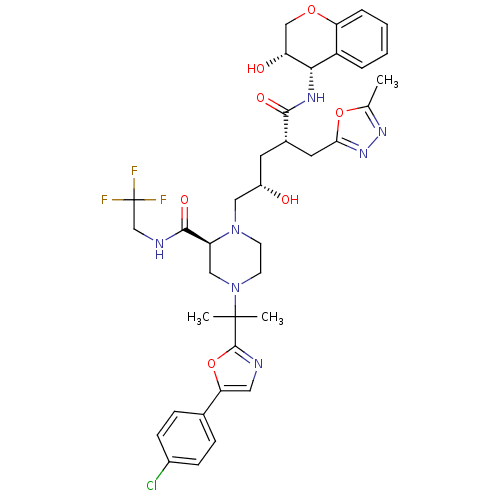 ((2S)-4-{2-[5-(4-chlorophenyl)-1,3-oxazol-2-yl]prop...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0160 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 14: 4651-4 (2004) Article DOI: 10.1016/j.bmcl.2004.06.092 BindingDB Entry DOI: 10.7270/Q22Z13QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9116 ((2S)-4-{[5-(4-chlorophenyl)furan-2-yl]methyl}-1-[(...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0190 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 14: 4651-4 (2004) Article DOI: 10.1016/j.bmcl.2004.06.092 BindingDB Entry DOI: 10.7270/Q22Z13QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9159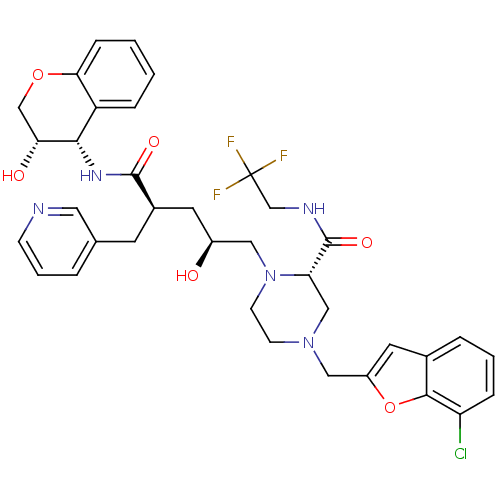 ((2S)-4-[(7-chloro-1-benzofuran-2-yl)methyl]-1-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 13: 3323-6 (2003) Article DOI: 10.1016/s0960-894x(03)00680-2 BindingDB Entry DOI: 10.7270/Q2K072GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9152 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(3S,4S)-3-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 13: 2573-6 (2003) Article DOI: 10.1016/s0960-894x(03)00474-8 BindingDB Entry DOI: 10.7270/Q2TD9VJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9151 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(3S,4S)-3-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 13: 2573-6 (2003) Article DOI: 10.1016/s0960-894x(03)00474-8 BindingDB Entry DOI: 10.7270/Q2TD9VJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9139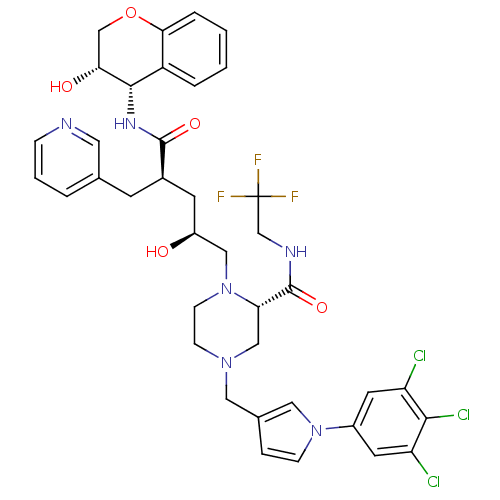 ((2S)-1-[(2S,4R)-2-hydroxy-4-{[(3S,4S)-3-hydroxy-3,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 13: 4027-30 (2003) Article DOI: 10.1016/j.bmcl.2003.08.049 BindingDB Entry DOI: 10.7270/Q2Z60M7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9138 ((2S)-4-{[1-(3,4-dichlorophenyl)-1H-pyrrol-3-yl]met...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 13: 4027-30 (2003) Article DOI: 10.1016/j.bmcl.2003.08.049 BindingDB Entry DOI: 10.7270/Q2Z60M7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9108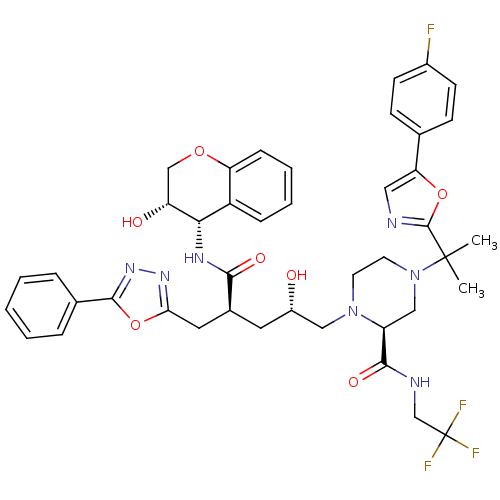 ((2S)-4-{2-[5-(4-fluorophenyl)-1,3-oxazol-2-yl]prop...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 14: 4651-4 (2004) Article DOI: 10.1016/j.bmcl.2004.06.092 BindingDB Entry DOI: 10.7270/Q22Z13QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9099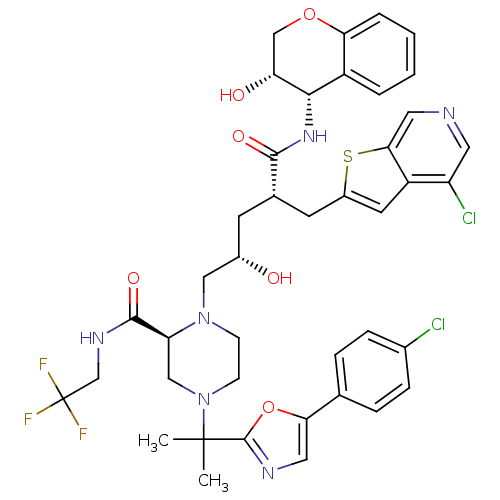 ((2S)-4-{2-[5-(4-chlorophenyl)-1,3-oxazol-2-yl]prop...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.0200 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 15: 5311-4 (2005) Article DOI: 10.1016/j.bmcl.2005.08.072 BindingDB Entry DOI: 10.7270/Q26Q1VF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9098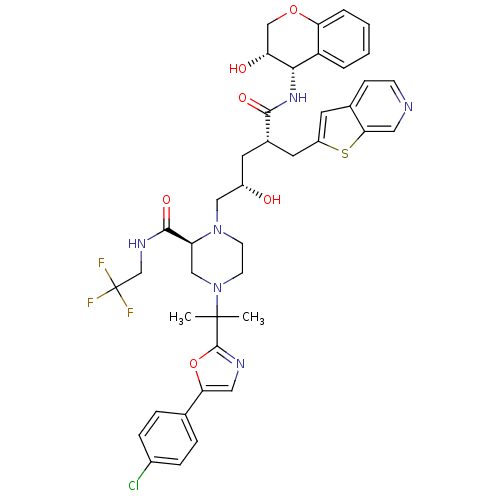 ((2S)-4-{2-[5-(4-chlorophenyl)-1,3-oxazol-2-yl]prop...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.0200 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 15: 5311-4 (2005) Article DOI: 10.1016/j.bmcl.2005.08.072 BindingDB Entry DOI: 10.7270/Q26Q1VF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9121 ((2S)-1-[(2S,4R)-2-hydroxy-4-{[(3S,4S)-3-hydroxy-3,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 13: 4027-30 (2003) Article DOI: 10.1016/j.bmcl.2003.08.049 BindingDB Entry DOI: 10.7270/Q2Z60M7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9091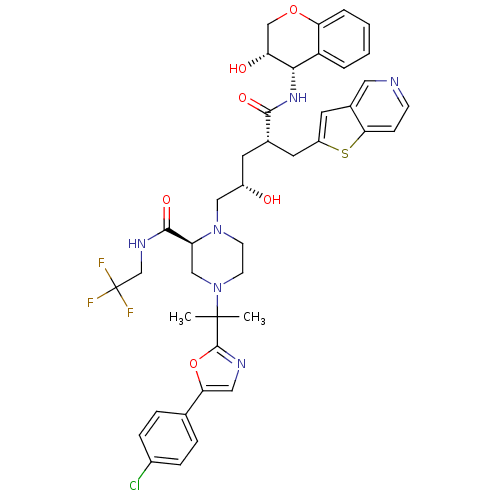 ((2S)-4-{2-[5-(4-chlorophenyl)-1,3-oxazol-2-yl]prop...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 15: 5311-4 (2005) Article DOI: 10.1016/j.bmcl.2005.08.072 BindingDB Entry DOI: 10.7270/Q26Q1VF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9097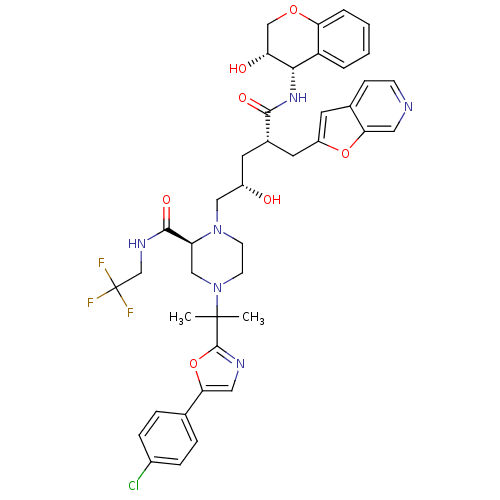 ((2S)-4-{2-[5-(4-chlorophenyl)-1,3-oxazol-2-yl]prop...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 15: 5311-4 (2005) Article DOI: 10.1016/j.bmcl.2005.08.072 BindingDB Entry DOI: 10.7270/Q26Q1VF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9105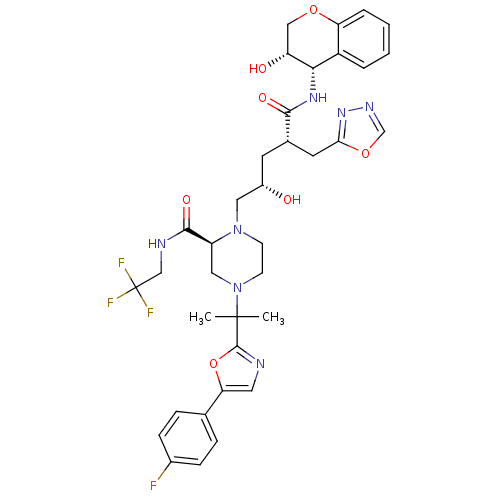 ((2S)-4-{2-[5-(4-fluorophenyl)-1,3-oxazol-2-yl]prop...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0240 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 14: 4651-4 (2004) Article DOI: 10.1016/j.bmcl.2004.06.092 BindingDB Entry DOI: 10.7270/Q22Z13QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9106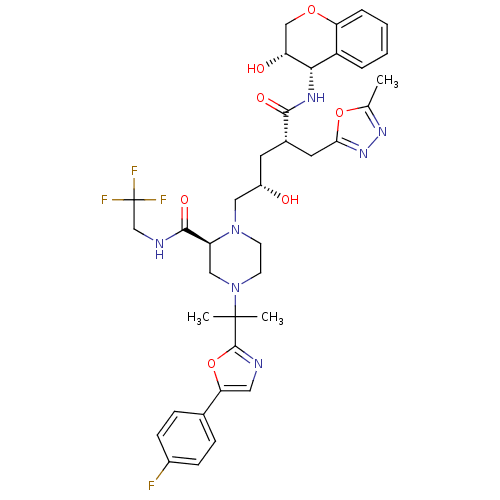 ((2S)-4-{2-[5-(4-fluorophenyl)-1,3-oxazol-2-yl]prop...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0270 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 14: 4651-4 (2004) Article DOI: 10.1016/j.bmcl.2004.06.092 BindingDB Entry DOI: 10.7270/Q22Z13QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9093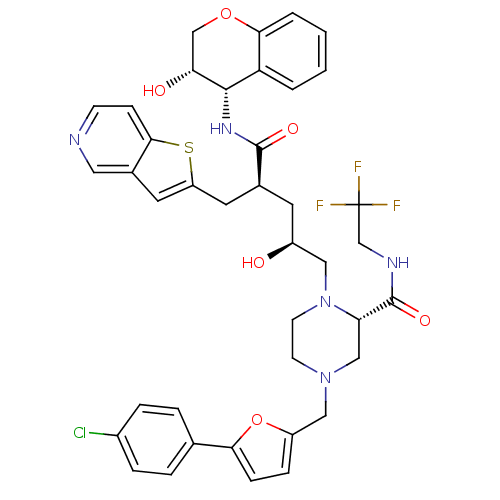 ((2S)-4-{[5-(4-chlorophenyl)furan-2-yl]methyl}-1-[(...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 15: 5311-4 (2005) Article DOI: 10.1016/j.bmcl.2005.08.072 BindingDB Entry DOI: 10.7270/Q26Q1VF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9102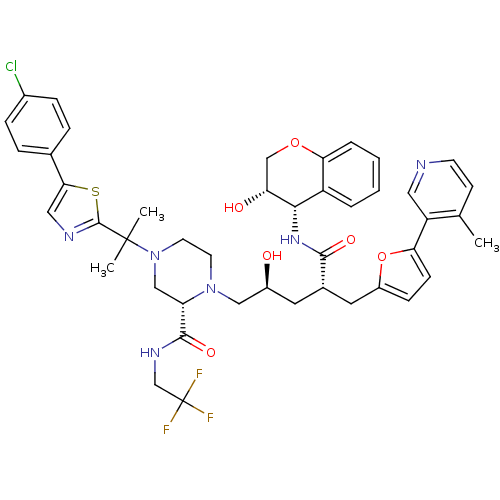 ((2S)-4-{2-[5-(4-chlorophenyl)-1,3-thiazol-2-yl]pro...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 15: 5311-4 (2005) Article DOI: 10.1016/j.bmcl.2005.08.072 BindingDB Entry DOI: 10.7270/Q26Q1VF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9104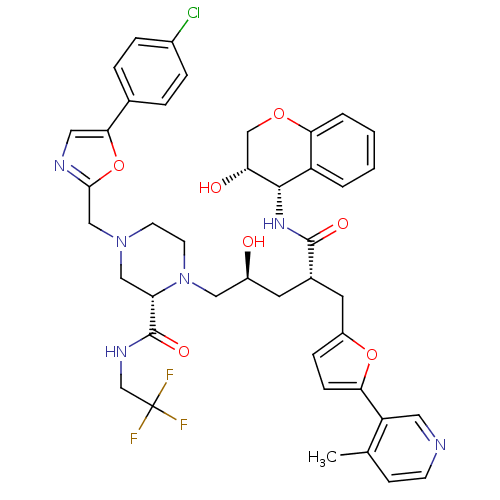 ((2S)-4-{[5-(4-chlorophenyl)-1,3-oxazol-2-yl]methyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 15: 5311-4 (2005) Article DOI: 10.1016/j.bmcl.2005.08.072 BindingDB Entry DOI: 10.7270/Q26Q1VF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9137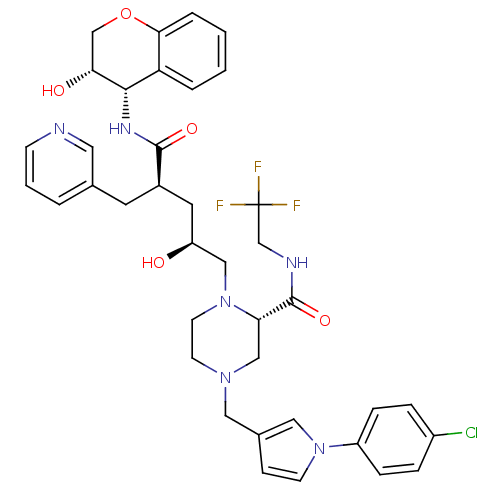 ((2S)-4-{[1-(4-chlorophenyl)-1H-pyrrol-3-yl]methyl}...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 13: 4027-30 (2003) Article DOI: 10.1016/j.bmcl.2003.08.049 BindingDB Entry DOI: 10.7270/Q2Z60M7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9144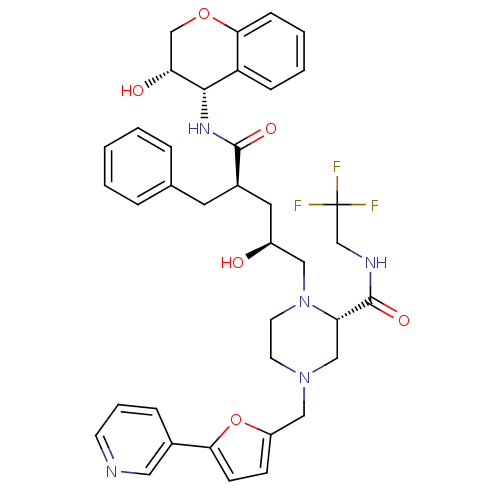 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(3S,4S)-3-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 13: 2573-6 (2003) Article DOI: 10.1016/s0960-894x(03)00474-8 BindingDB Entry DOI: 10.7270/Q2TD9VJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9155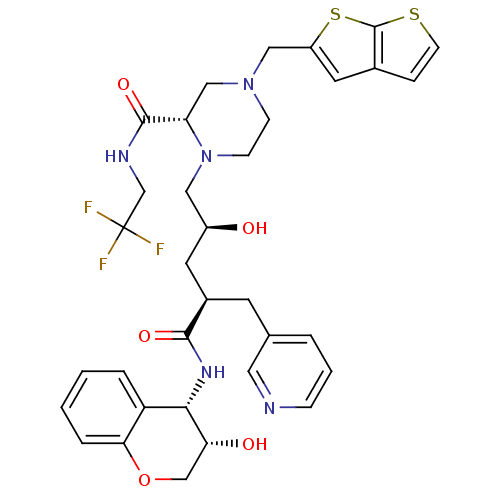 ((2S)-1-[(2S,4R)-2-hydroxy-4-{[(3S,4S)-3-hydroxy-3,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 13: 3323-6 (2003) Article DOI: 10.1016/s0960-894x(03)00680-2 BindingDB Entry DOI: 10.7270/Q2K072GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127974 ((R)-3-[(2S,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild type HIV protease | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127969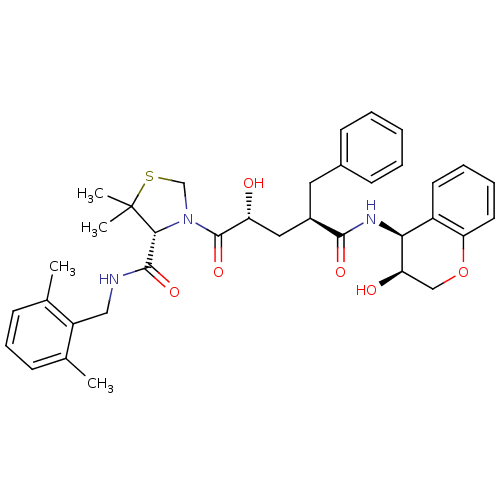 ((R)-3-[(2R,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild type HIV protease | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50127975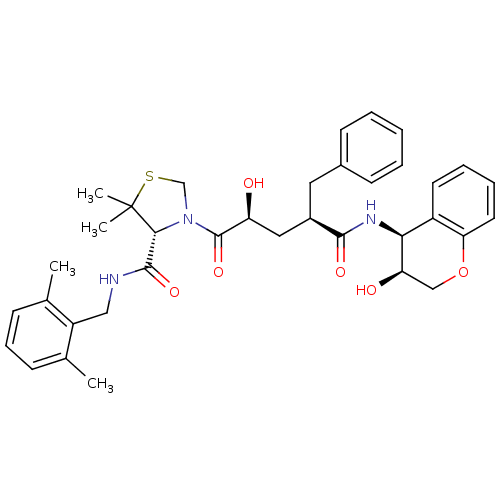 ((R)-3-[(2S,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 NL4-3 protease | Bioorg Med Chem Lett 17: 5432-6 (2007) Article DOI: 10.1016/j.bmcl.2007.07.040 BindingDB Entry DOI: 10.7270/Q2DF6V0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9160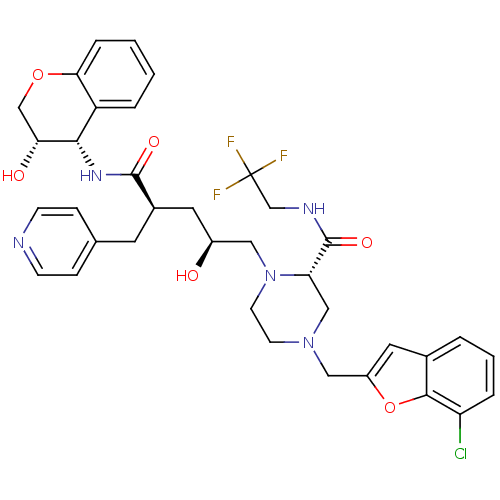 ((2S)-4-[(7-chloro-1-benzofuran-2-yl)methyl]-1-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 13: 3323-6 (2003) Article DOI: 10.1016/s0960-894x(03)00680-2 BindingDB Entry DOI: 10.7270/Q2K072GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9157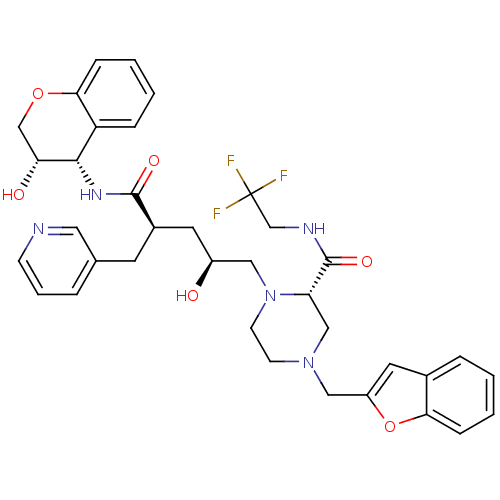 ((2S)-4-(1-benzofuran-2-ylmethyl)-1-[(2S,4R)-2-hydr...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 13: 3323-6 (2003) Article DOI: 10.1016/s0960-894x(03)00680-2 BindingDB Entry DOI: 10.7270/Q2K072GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127976 ((R)-3-[(2S,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV protease of HIV K-60C mutant strain | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 243 total ) | Next | Last >> |