

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50247054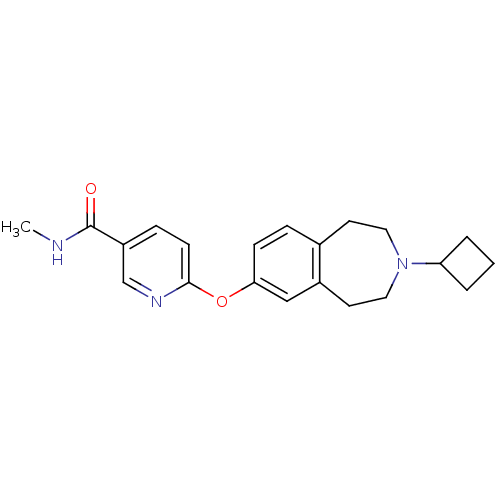 (6-(3-cyclobutyl-2,3,4,5-tetrahydro-1H-benzo[d]azep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Binding affinity to histamine H3 receptor | J Med Chem 52: 3855-68 (2009) Article DOI: 10.1021/jm900409x BindingDB Entry DOI: 10.7270/Q2SB46NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50247053 (1-(3-(3-(4-chlorophenyl)propoxy)propyl)piperidine ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in HEK293 cells by [35S]gammaS binding assay | J Med Chem 52: 3855-68 (2009) Article DOI: 10.1021/jm900409x BindingDB Entry DOI: 10.7270/Q2SB46NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50268293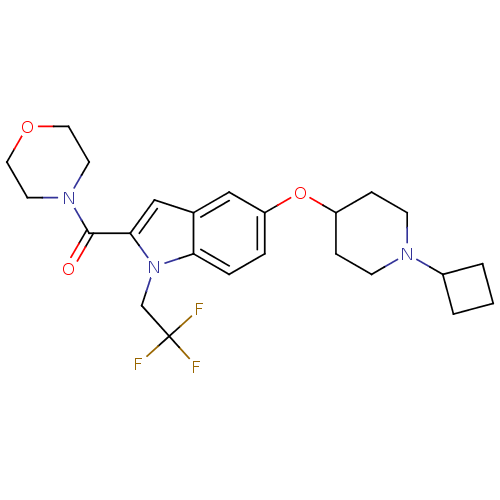 ((5-(1-cyclobutylpiperidin-4-yloxy)-1-(2,2,2-triflu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from rat recombinant histamine H3 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 52: 3855-68 (2009) Article DOI: 10.1021/jm900409x BindingDB Entry DOI: 10.7270/Q2SB46NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50268291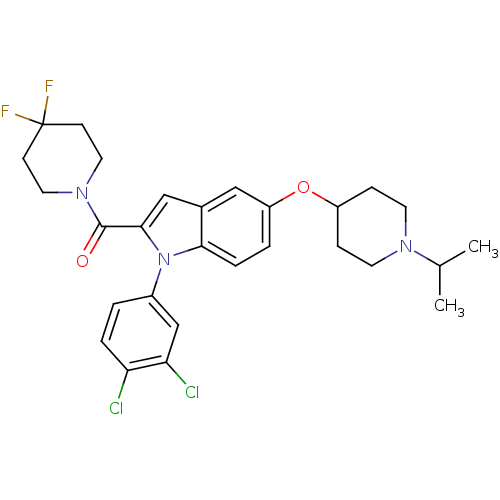 ((1-(3,4-dichlorophenyl)-5-(1-isopropylpiperidin-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from rat recombinant histamine H3 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 52: 3855-68 (2009) Article DOI: 10.1021/jm900409x BindingDB Entry DOI: 10.7270/Q2SB46NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50212733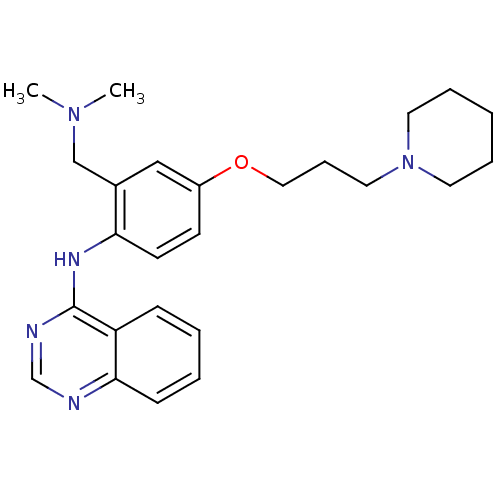 (CHEMBL425994 | N-(2-((dimethylamino)methyl)-4-(3-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Binding affinity to human histamine H3 receptor | Bioorg Med Chem Lett 17: 3670-5 (2007) Article DOI: 10.1016/j.bmcl.2007.04.056 BindingDB Entry DOI: 10.7270/Q2N879GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50268323 ((5-(1-cyclopropylpiperidin-4-yloxy)-1-(2,2,2-trifl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from rat recombinant histamine H3 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 52: 3855-68 (2009) Article DOI: 10.1021/jm900409x BindingDB Entry DOI: 10.7270/Q2SB46NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139391 ((R)-4-(2-(2-(2-methylpyrrolidin-1-yl)ethyl)benzofu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Binding affinity to histamine H3 receptor | J Med Chem 52: 3855-68 (2009) Article DOI: 10.1021/jm900409x BindingDB Entry DOI: 10.7270/Q2SB46NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50110288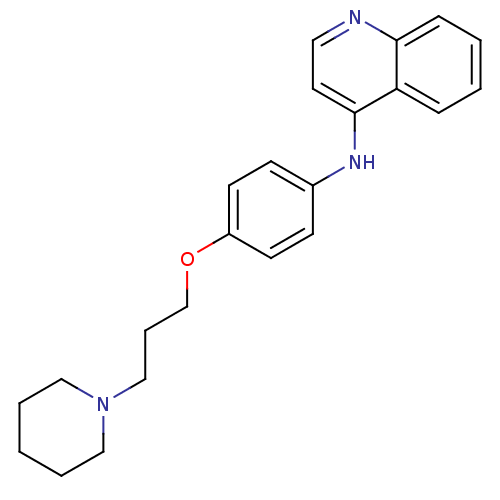 (CHEMBL15153 | N-(4-(3-(piperidin-1-yl)propoxy)phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]RAMH from human histamine H3 receptor expressed in CHO cells | Bioorg Med Chem Lett 18: 4377-9 (2008) Article DOI: 10.1016/j.bmcl.2008.06.062 BindingDB Entry DOI: 10.7270/Q2V40W49 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50159110 (1-(3-(4-(piperidin-1-ylmethyl)phenoxy)propyl)piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Binding affinity to histamine H3 receptor | J Med Chem 52: 3855-68 (2009) Article DOI: 10.1021/jm900409x BindingDB Entry DOI: 10.7270/Q2SB46NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM141768 (US8921397, 10) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description liquots of membrane preparations were thawed at RT, resuspended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KC1... | US Patent US8921397 (2014) BindingDB Entry DOI: 10.7270/Q2VQ31C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM141781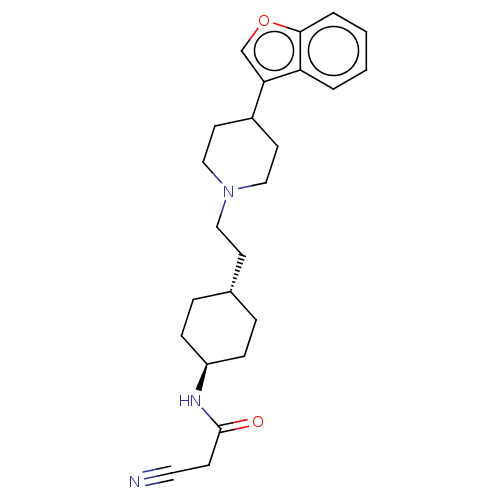 (US8921397, 23) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description liquots of membrane preparations were thawed at RT, resuspended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KC1... | US Patent US8921397 (2014) BindingDB Entry DOI: 10.7270/Q2VQ31C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM121503 (US8722683, 50) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc. US Patent | Assay Description Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... | US Patent US8722683 (2014) BindingDB Entry DOI: 10.7270/Q2J101VJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM121467 (US8722683, 14) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc. US Patent | Assay Description Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... | US Patent US8722683 (2014) BindingDB Entry DOI: 10.7270/Q2J101VJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM141770 (US8921397, 12) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description liquots of membrane preparations were thawed at RT, resuspended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KC1... | US Patent US8921397 (2014) BindingDB Entry DOI: 10.7270/Q2VQ31C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50269054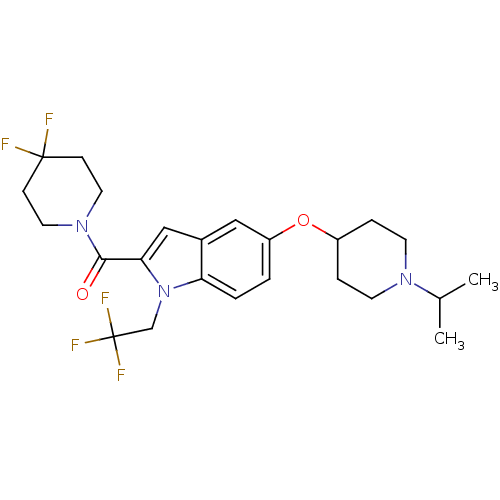 ((4,4-difluoropiperidin-1-yl)(5-(1-isopropylpiperid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from rat recombinant histamine H3 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 52: 3855-68 (2009) Article DOI: 10.1021/jm900409x BindingDB Entry DOI: 10.7270/Q2SB46NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50269053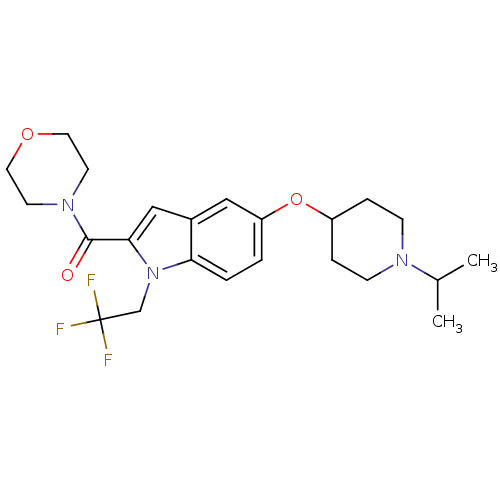 ((5-(1-isopropylpiperidin-4-yloxy)-1-(2,2,2-trifluo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from rat recombinant histamine H3 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 52: 3855-68 (2009) Article DOI: 10.1021/jm900409x BindingDB Entry DOI: 10.7270/Q2SB46NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50268293 ((5-(1-cyclobutylpiperidin-4-yloxy)-1-(2,2,2-triflu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human recombinant histamine H3 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 52: 3855-68 (2009) Article DOI: 10.1021/jm900409x BindingDB Entry DOI: 10.7270/Q2SB46NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50268291 ((1-(3,4-dichlorophenyl)-5-(1-isopropylpiperidin-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human recombinant histamine H3 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 52: 3855-68 (2009) Article DOI: 10.1021/jm900409x BindingDB Entry DOI: 10.7270/Q2SB46NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM121528 (US8722683, 75) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.04 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc. US Patent | Assay Description Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... | US Patent US8722683 (2014) BindingDB Entry DOI: 10.7270/Q2J101VJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM141759 (US8921397, 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.08 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description liquots of membrane preparations were thawed at RT, resuspended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KC1... | US Patent US8921397 (2014) BindingDB Entry DOI: 10.7270/Q2VQ31C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM121454 (US8722683, 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc. US Patent | Assay Description Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... | US Patent US8722683 (2014) BindingDB Entry DOI: 10.7270/Q2J101VJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM107516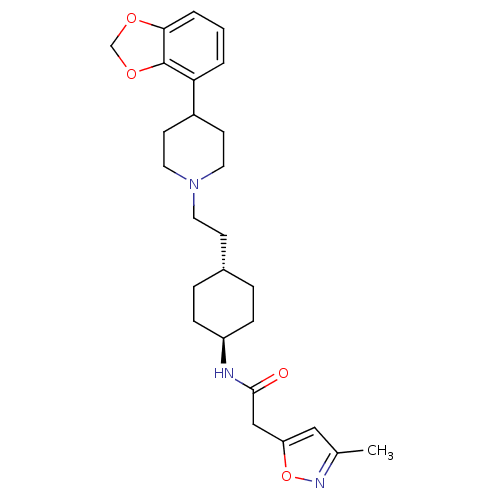 (US8598357, 19) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The ability of the compounds to bind to the 5-HT2A, D3 and D2 receptors was determined using radioligand binding to cloned receptors selectively expr... | US Patent US8598357 (2013) BindingDB Entry DOI: 10.7270/Q27P8X2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM141760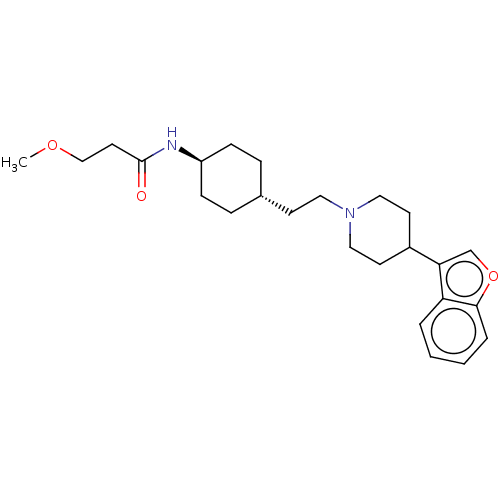 (US8921397, 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description liquots of membrane preparations were thawed at RT, resuspended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KC1... | US Patent US8921397 (2014) BindingDB Entry DOI: 10.7270/Q2VQ31C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM121461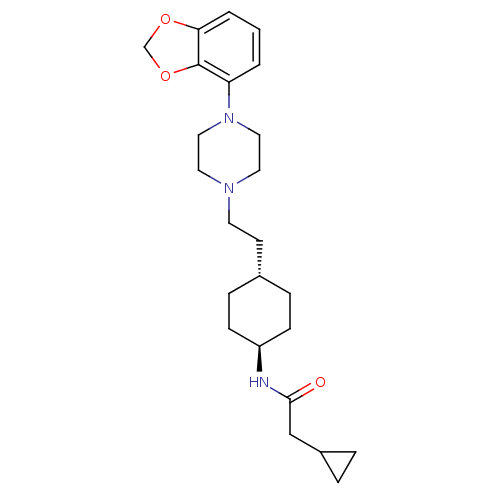 (US8722683, 8) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc. US Patent | Assay Description Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... | US Patent US8722683 (2014) BindingDB Entry DOI: 10.7270/Q2J101VJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM141763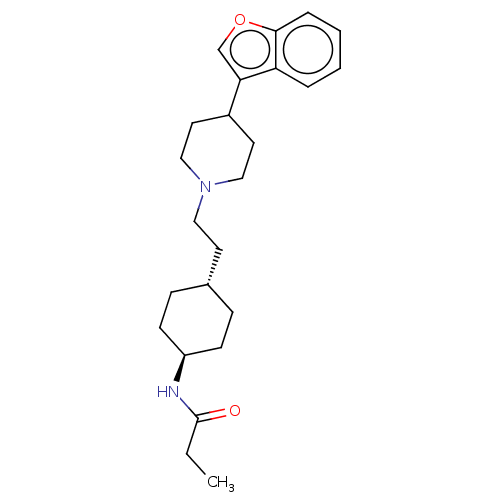 (US8921397, 5) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description liquots of membrane preparations were thawed at RT, resuspended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KC1... | US Patent US8921397 (2014) BindingDB Entry DOI: 10.7270/Q2VQ31C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM141779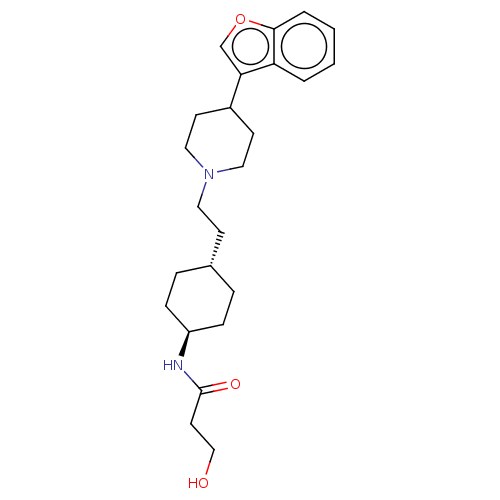 (US8921397, 21) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description liquots of membrane preparations were thawed at RT, resuspended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KC1... | US Patent US8921397 (2014) BindingDB Entry DOI: 10.7270/Q2VQ31C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM121460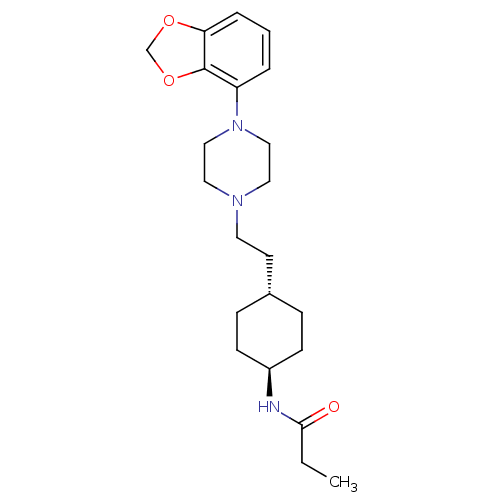 (US8722683, 7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc. US Patent | Assay Description Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... | US Patent US8722683 (2014) BindingDB Entry DOI: 10.7270/Q2J101VJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM121530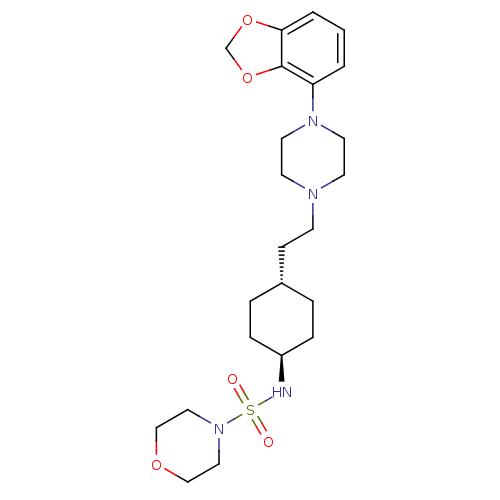 (US8722683, 77) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc. US Patent | Assay Description Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... | US Patent US8722683 (2014) BindingDB Entry DOI: 10.7270/Q2J101VJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM121465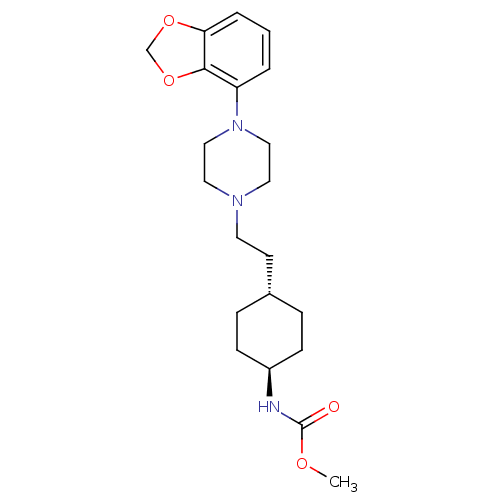 (US8722683, 12) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc. US Patent | Assay Description Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... | US Patent US8722683 (2014) BindingDB Entry DOI: 10.7270/Q2J101VJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM141839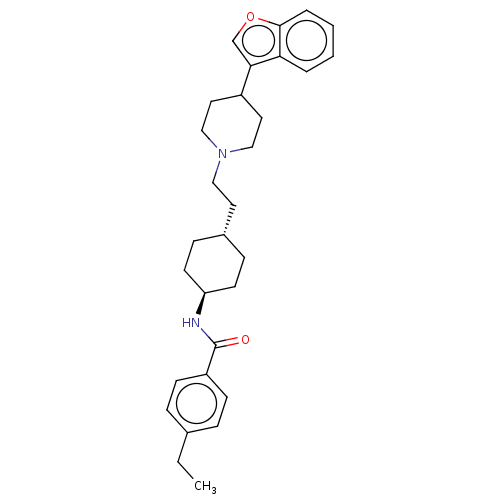 (US8921397, 81) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description liquots of membrane preparations were thawed at RT, resuspended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KC1... | US Patent US8921397 (2014) BindingDB Entry DOI: 10.7270/Q2VQ31C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM121466 (US8722683, 13) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc. US Patent | Assay Description Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... | US Patent US8722683 (2014) BindingDB Entry DOI: 10.7270/Q2J101VJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM141767 (US8921397, 9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description liquots of membrane preparations were thawed at RT, resuspended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KC1... | US Patent US8921397 (2014) BindingDB Entry DOI: 10.7270/Q2VQ31C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM121481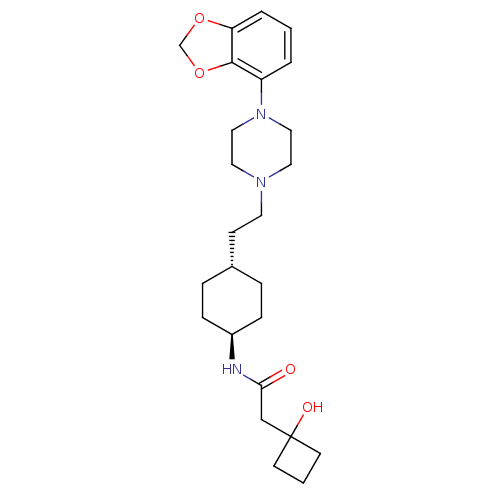 (US8722683, 28) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc. US Patent | Assay Description Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... | US Patent US8722683 (2014) BindingDB Entry DOI: 10.7270/Q2J101VJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50159110 (1-(3-(4-(piperidin-1-ylmethyl)phenoxy)propyl)piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Binding affinity to human histamine H3 receptor | Bioorg Med Chem Lett 17: 3670-5 (2007) Article DOI: 10.1016/j.bmcl.2007.04.056 BindingDB Entry DOI: 10.7270/Q2N879GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM106438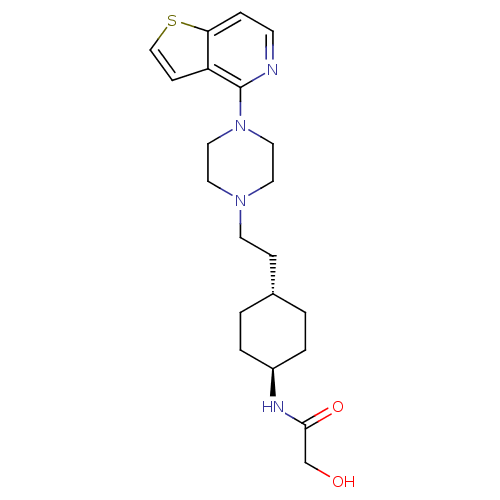 (US8586579, 27) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The ability of the compounds to bind to the 5-HT2A, D3 and D2 receptors was determined using radioligand binding to cloned receptors selectively expr... | US Patent US8586579 (2013) BindingDB Entry DOI: 10.7270/Q2QV3K55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM106438 (US8586579, 27) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The ability of the compounds to bind to the 5-HT2A, D3 and D2 receptors was determined using radioligand binding to cloned receptors selectively expr... | US Patent US8586579 (2013) BindingDB Entry DOI: 10.7270/Q2QV3K55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM180718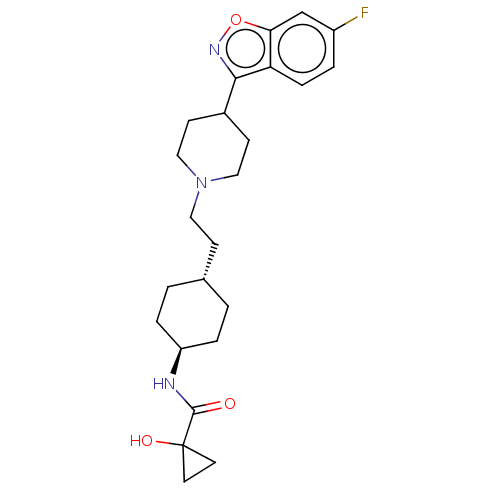 (US8829029, 18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Aliquots of membrane preparations were thawed at RT, resuspended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KC... | US Patent US8829029 (2014) BindingDB Entry DOI: 10.7270/Q22B8WSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM141765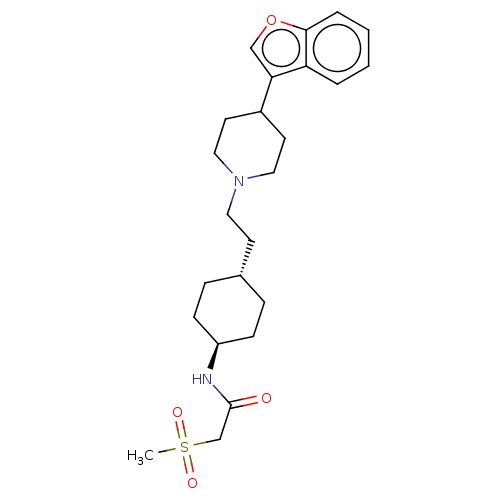 (US8921397, 7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description liquots of membrane preparations were thawed at RT, resuspended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KC1... | US Patent US8921397 (2014) BindingDB Entry DOI: 10.7270/Q2VQ31C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM121508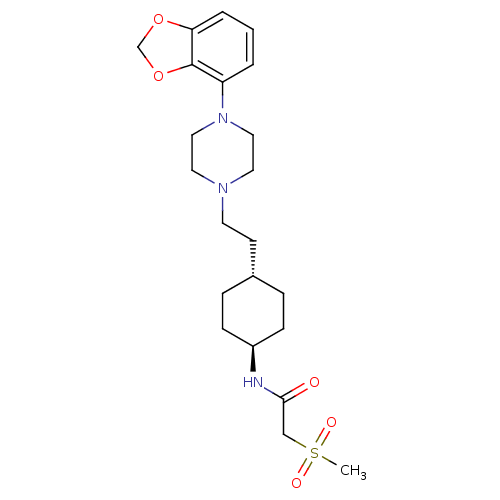 (US8722683, 55) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc. US Patent | Assay Description Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... | US Patent US8722683 (2014) BindingDB Entry DOI: 10.7270/Q2J101VJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM141810 (US8921397, 52) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description liquots of membrane preparations were thawed at RT, resuspended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KC1... | US Patent US8921397 (2014) BindingDB Entry DOI: 10.7270/Q2VQ31C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM141769 (US8921397, 11) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description liquots of membrane preparations were thawed at RT, resuspended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KC1... | US Patent US8921397 (2014) BindingDB Entry DOI: 10.7270/Q2VQ31C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM141780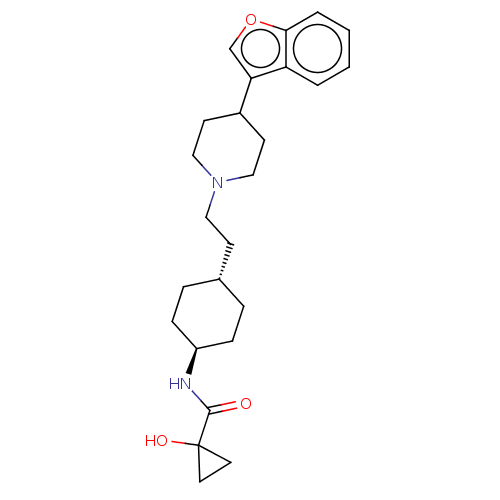 (US8921397, 22) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description liquots of membrane preparations were thawed at RT, resuspended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KC1... | US Patent US8921397 (2014) BindingDB Entry DOI: 10.7270/Q2VQ31C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM121480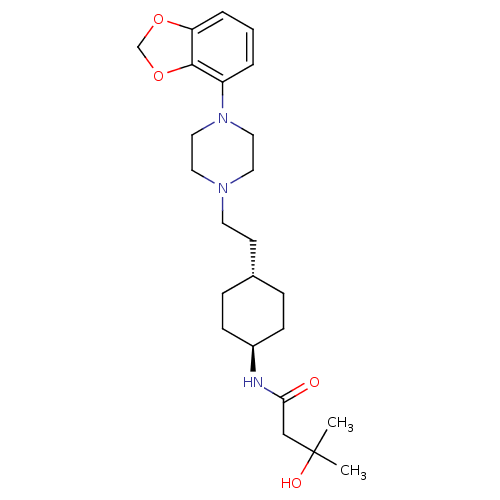 (US8722683, 27) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc. US Patent | Assay Description Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... | US Patent US8722683 (2014) BindingDB Entry DOI: 10.7270/Q2J101VJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM121469 (US8722683, 16) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc. US Patent | Assay Description Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... | US Patent US8722683 (2014) BindingDB Entry DOI: 10.7270/Q2J101VJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM121472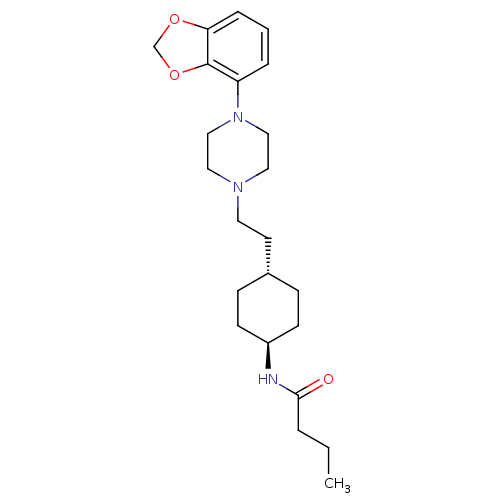 (US8722683, 19) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc. US Patent | Assay Description Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... | US Patent US8722683 (2014) BindingDB Entry DOI: 10.7270/Q2J101VJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM106429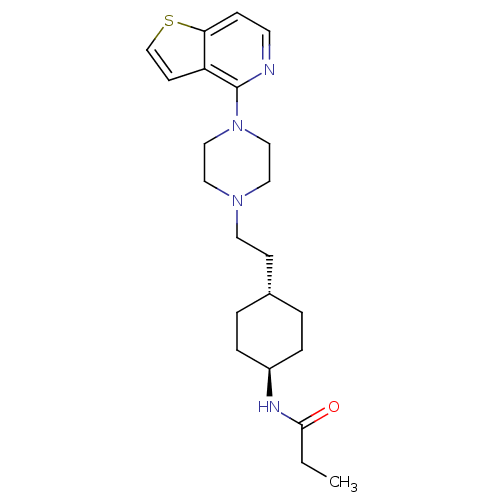 (US8586579, 18) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The ability of the compounds to bind to the 5-HT2A, D3 and D2 receptors was determined using radioligand binding to cloned receptors selectively expr... | US Patent US8586579 (2013) BindingDB Entry DOI: 10.7270/Q2QV3K55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM180713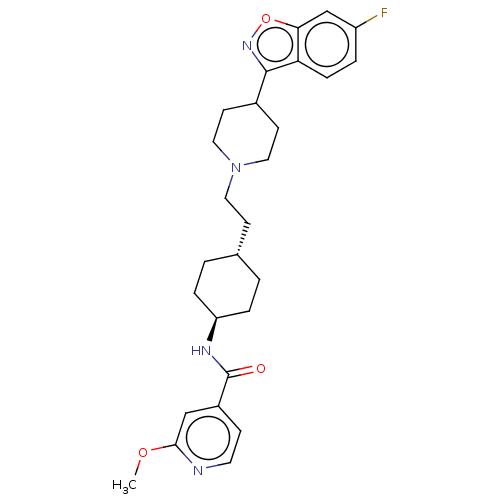 (US8829029, 13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Aliquots of membrane preparations were thawed at RT, resuspended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KC... | US Patent US8829029 (2014) BindingDB Entry DOI: 10.7270/Q22B8WSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM141834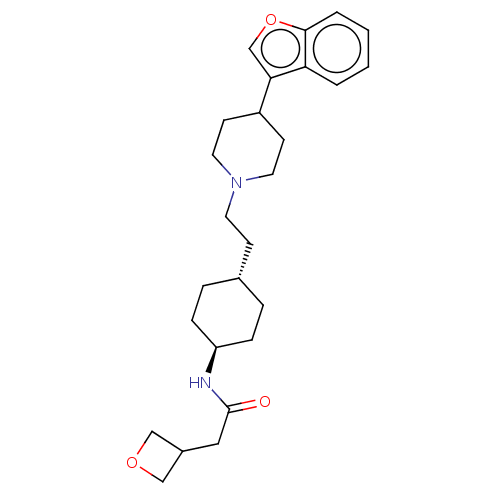 (US8921397, 76) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description liquots of membrane preparations were thawed at RT, resuspended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KC1... | US Patent US8921397 (2014) BindingDB Entry DOI: 10.7270/Q2VQ31C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM141832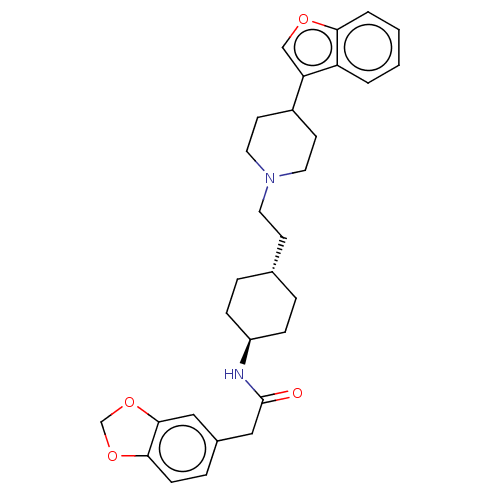 (US8921397, 74) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description liquots of membrane preparations were thawed at RT, resuspended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KC1... | US Patent US8921397 (2014) BindingDB Entry DOI: 10.7270/Q2VQ31C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM180752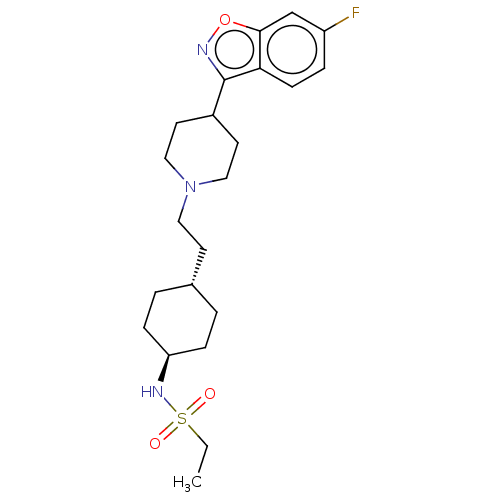 (US8829029, 47) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Aliquots of membrane preparations were thawed at RT, resuspended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KC... | US Patent US8829029 (2014) BindingDB Entry DOI: 10.7270/Q22B8WSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2298 total ) | Next | Last >> |