 Found 710 hits with Last Name = 'schadt' and Initial = 'o'
Found 710 hits with Last Name = 'schadt' and Initial = 'o' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50065458
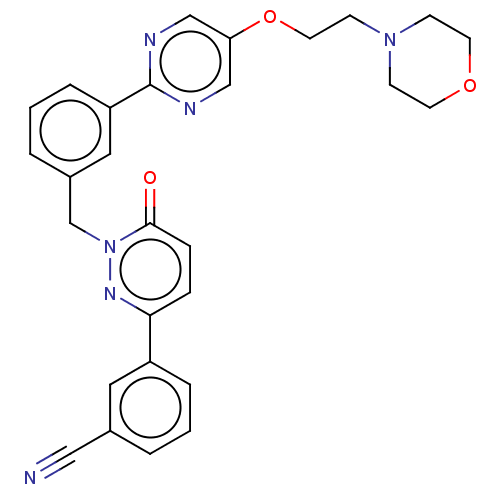
(CHEMBL3402761)Show SMILES O=c1ccc(nn1Cc1cccc(c1)-c1ncc(OCCN2CCOCC2)cn1)-c1cccc(c1)C#N Show InChI InChI=1S/C28H26N6O3/c29-17-21-3-1-5-23(15-21)26-7-8-27(35)34(32-26)20-22-4-2-6-24(16-22)28-30-18-25(19-31-28)37-14-11-33-9-12-36-13-10-33/h1-8,15-16,18-19H,9-14,20H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of GST-His6-tagged recombinant human c-Met kinase domain (956 to 1390) after 60 mins by anoff-chip mobility shift assay |
Bioorg Med Chem Lett 25: 1597-602 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.002
BindingDB Entry DOI: 10.7270/Q2HX1FBT |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50065485
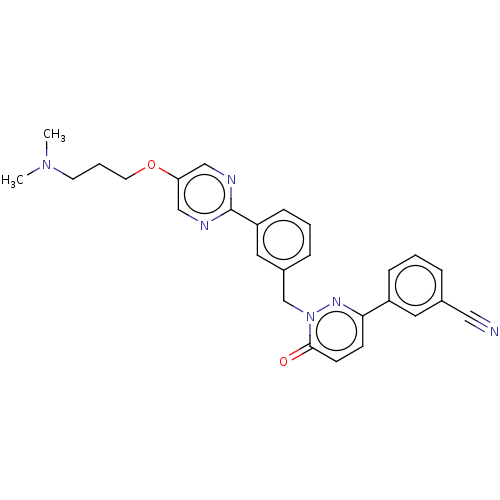
(CHEMBL3402760)Show SMILES CN(C)CCCOc1cnc(nc1)-c1cccc(Cn2nc(ccc2=O)-c2cccc(c2)C#N)c1 Show InChI InChI=1S/C27H26N6O2/c1-32(2)12-5-13-35-24-17-29-27(30-18-24)23-9-4-7-21(15-23)19-33-26(34)11-10-25(31-33)22-8-3-6-20(14-22)16-28/h3-4,6-11,14-15,17-18H,5,12-13,19H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of GST-His6-tagged recombinant human c-Met kinase domain (956 to 1390) after 60 mins by anoff-chip mobility shift assay |
Bioorg Med Chem Lett 25: 1597-602 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.002
BindingDB Entry DOI: 10.7270/Q2HX1FBT |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50065490
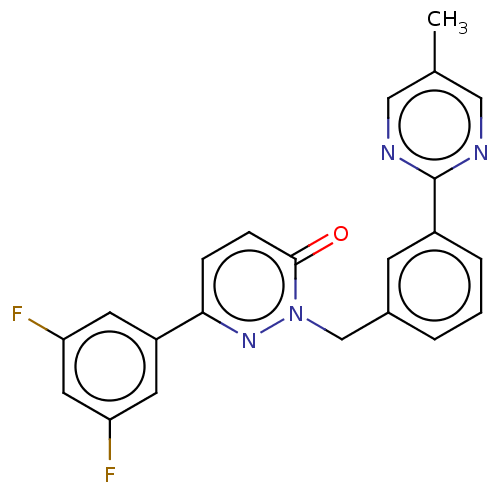
(CHEMBL3402754)Show SMILES Cc1cnc(nc1)-c1cccc(Cn2nc(ccc2=O)-c2cc(F)cc(F)c2)c1 Show InChI InChI=1S/C22H16F2N4O/c1-14-11-25-22(26-12-14)16-4-2-3-15(7-16)13-28-21(29)6-5-20(27-28)17-8-18(23)10-19(24)9-17/h2-12H,13H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of c-Met kinase in human A549 cells assessed as inhibition of phosphorylation after 45 mins by electrochemiluminescence assay |
Bioorg Med Chem Lett 25: 1597-602 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.002
BindingDB Entry DOI: 10.7270/Q2HX1FBT |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50065457
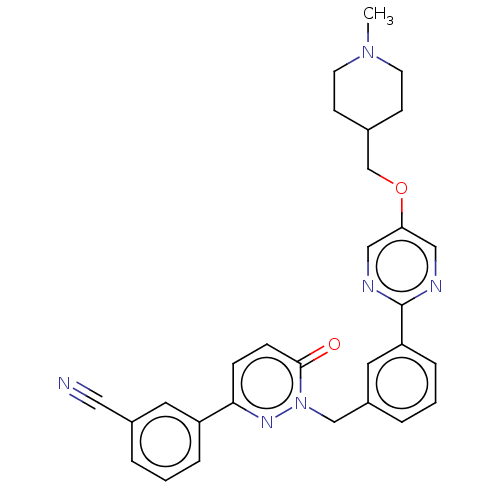
(EMD-1214063 | MSC-2156119 | MSC-2156119J | Tepotin...)Show SMILES CN1CCC(COc2cnc(nc2)-c2cccc(Cn3nc(ccc3=O)-c3cccc(c3)C#N)c2)CC1 Show InChI InChI=1S/C29H28N6O2/c1-34-12-10-21(11-13-34)20-37-26-17-31-29(32-18-26)25-7-3-5-23(15-25)19-35-28(36)9-8-27(33-35)24-6-2-4-22(14-24)16-30/h2-9,14-15,17-18,21H,10-13,19-20H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of GST-His6-tagged recombinant human c-Met kinase domain (956 to 1390) after 60 mins by anoff-chip mobility shift assay |
Bioorg Med Chem Lett 25: 1597-602 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.002
BindingDB Entry DOI: 10.7270/Q2HX1FBT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50069989

((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)c1cnccn1)B(O)O |r| Show InChI InChI=1S/C19H25BN4O4/c1-13(2)10-17(20(27)28)24-18(25)15(11-14-6-4-3-5-7-14)23-19(26)16-12-21-8-9-22-16/h3-9,12-13,15,17,27-28H,10-11H2,1-2H3,(H,23,26)(H,24,25)/t15-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00604
BindingDB Entry DOI: 10.7270/Q200064W |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50601642
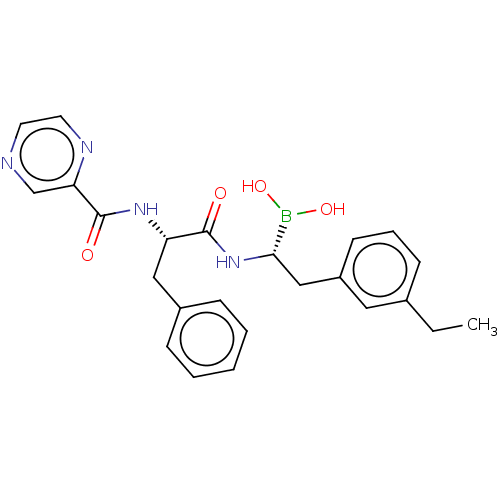
(CHEMBL5188533)Show SMILES CCc1cccc(C[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)c2cnccn2)B(O)O)c1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00604
BindingDB Entry DOI: 10.7270/Q200064W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM388435
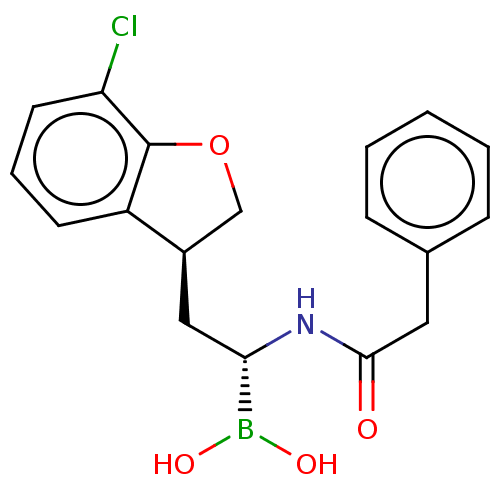
(US10294246, Compound No. 132)Show SMILES OB(O)[C@H](C[C@@H]1COc2c1cccc2Cl)NC(=O)Cc1ccccc1 |r| Show InChI InChI=1S/C18H19BClNO4/c20-15-8-4-7-14-13(11-25-18(14)15)10-16(19(23)24)21-17(22)9-12-5-2-1-3-6-12/h1-8,13,16,23-24H,9-11H2,(H,21,22)/t13-,16+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00604
BindingDB Entry DOI: 10.7270/Q200064W |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM388321
(US10294246, Compound No. 18)Show SMILES OB(O)[C@H](Cc1coc2ccccc12)NC(=O)Cc1cnccn1 |r| Show InChI InChI=1S/C16H16BN3O4/c21-16(8-12-9-18-5-6-19-12)20-15(17(22)23)7-11-10-24-14-4-2-1-3-13(11)14/h1-6,9-10,15,22-23H,7-8H2,(H,20,21)/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00604
BindingDB Entry DOI: 10.7270/Q200064W |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50601652
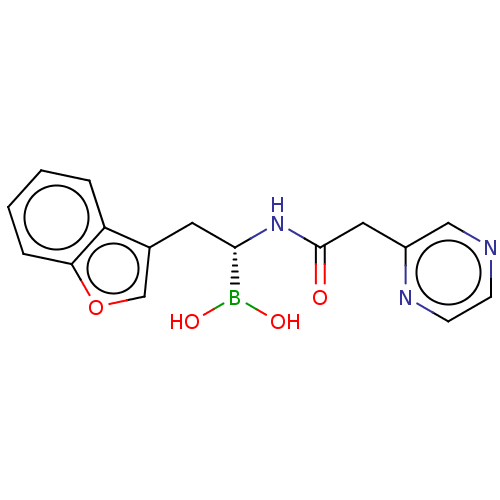
(CHEMBL5191857) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00604
BindingDB Entry DOI: 10.7270/Q200064W |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50277889
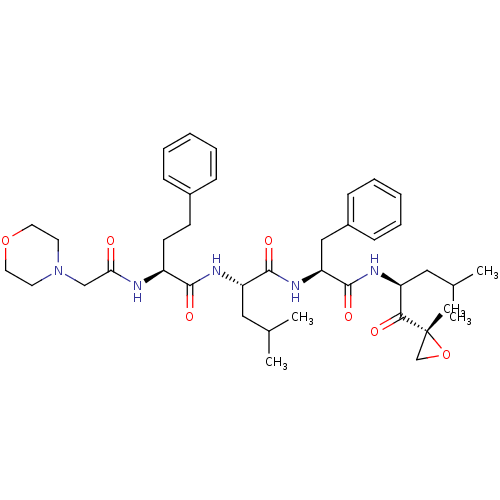
(CARFILZOMIB | CHEMBL451887)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)NC(=O)CN1CCOCC1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)[C@@]1(C)CO1 |r| Show InChI InChI=1S/C40H57N5O7/c1-27(2)22-32(36(47)40(5)26-52-40)42-39(50)34(24-30-14-10-7-11-15-30)44-38(49)33(23-28(3)4)43-37(48)31(17-16-29-12-8-6-9-13-29)41-35(46)25-45-18-20-51-21-19-45/h6-15,27-28,31-34H,16-26H2,1-5H3,(H,41,46)(H,42,50)(H,43,48)(H,44,49)/t31-,32-,33-,34-,40+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00604
BindingDB Entry DOI: 10.7270/Q200064W |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM388309
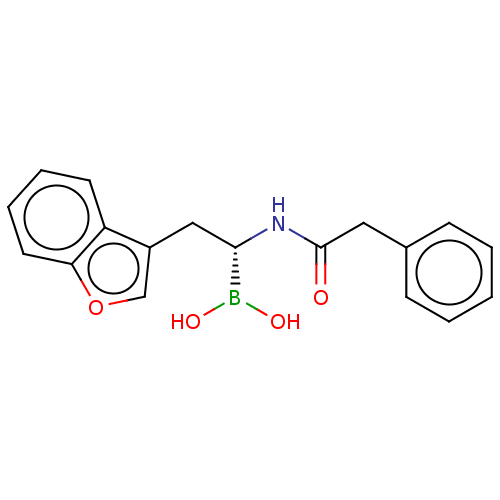
(US10294246, Compound No. 6)Show SMILES OB(O)[C@H](Cc1coc2ccccc12)NC(=O)Cc1ccccc1 |r| Show InChI InChI=1S/C18H18BNO4/c21-18(10-13-6-2-1-3-7-13)20-17(19(22)23)11-14-12-24-16-9-5-4-8-15(14)16/h1-9,12,17,22-23H,10-11H2,(H,20,21)/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00604
BindingDB Entry DOI: 10.7270/Q200064W |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM388395
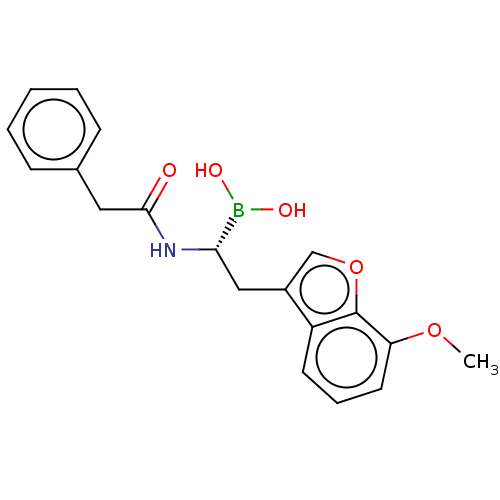
(US10294246, Compound No. 92)Show SMILES COc1cccc2c(C[C@H](NC(=O)Cc3ccccc3)B(O)O)coc12 |r| Show InChI InChI=1S/C19H20BNO5/c1-25-16-9-5-8-15-14(12-26-19(15)16)11-17(20(23)24)21-18(22)10-13-6-3-2-4-7-13/h2-9,12,17,23-24H,10-11H2,1H3,(H,21,22)/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00604
BindingDB Entry DOI: 10.7270/Q200064W |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM388323
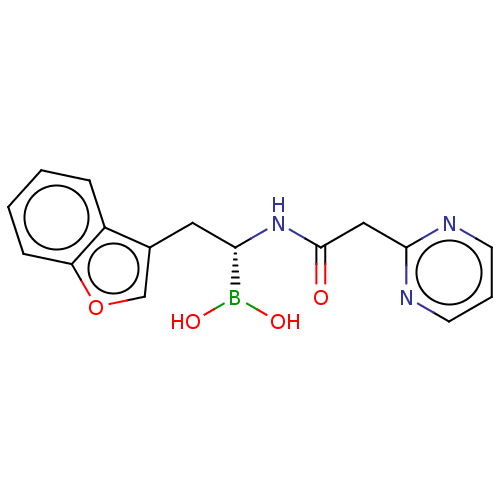
(US10294246, Compound No. 20)Show SMILES OB(O)[C@H](Cc1coc2ccccc12)NC(=O)Cc1ncccn1 |r| Show InChI InChI=1S/C16H16BN3O4/c21-16(9-15-18-6-3-7-19-15)20-14(17(22)23)8-11-10-24-13-5-2-1-4-12(11)13/h1-7,10,14,22-23H,8-9H2,(H,20,21)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00604
BindingDB Entry DOI: 10.7270/Q200064W |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM388311
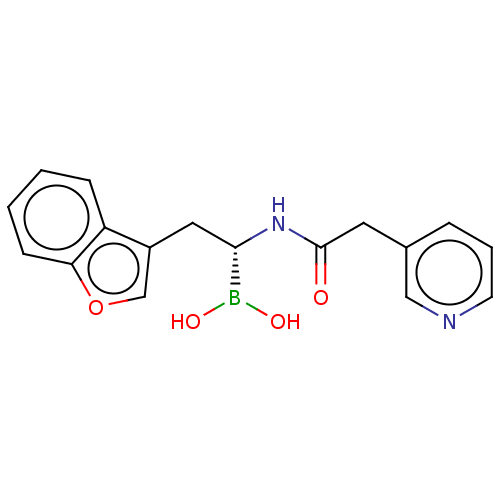
(US10294246, Compound No. 8)Show SMILES OB(O)[C@H](Cc1coc2ccccc12)NC(=O)Cc1cccnc1 |r| Show InChI InChI=1S/C17H17BN2O4/c21-17(8-12-4-3-7-19-10-12)20-16(18(22)23)9-13-11-24-15-6-2-1-5-14(13)15/h1-7,10-11,16,22-23H,8-9H2,(H,20,21)/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00604
BindingDB Entry DOI: 10.7270/Q200064W |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-9
(Homo sapiens (Human)) | BDBM50398609
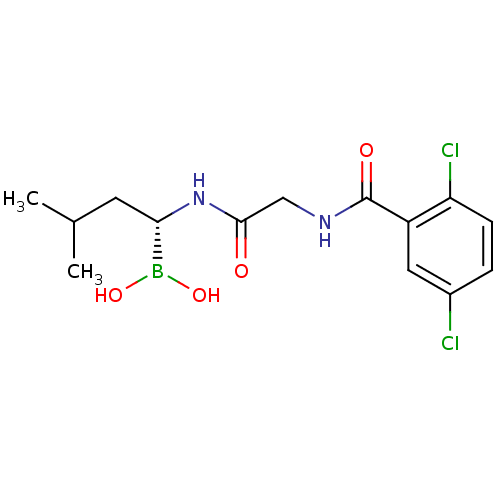
(CHEMBL2141296 | IXAZOMIB CITRATE | Ixazomib | MLN2...)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)c1cc(Cl)ccc1Cl)B(O)O Show InChI InChI=1S/C14H19BCl2N2O4/c1-8(2)5-12(15(22)23)19-13(20)7-18-14(21)10-6-9(16)3-4-11(10)17/h3-4,6,8,12,22-23H,5,7H2,1-2H3,(H,18,21)(H,19,20)/t12-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00604
BindingDB Entry DOI: 10.7270/Q200064W |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50065489
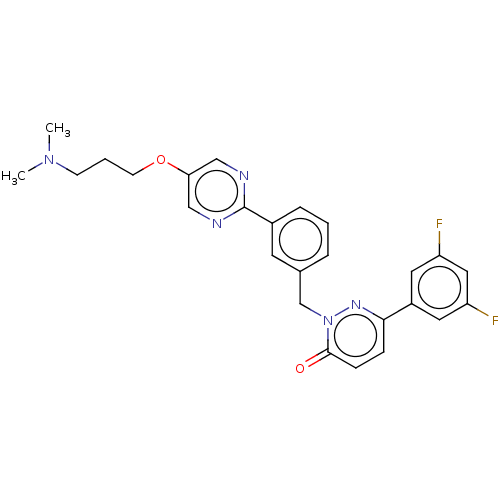
(CHEMBL3402756)Show SMILES CN(C)CCCOc1cnc(nc1)-c1cccc(Cn2nc(ccc2=O)-c2cc(F)cc(F)c2)c1 Show InChI InChI=1S/C26H25F2N5O2/c1-32(2)9-4-10-35-23-15-29-26(30-16-23)19-6-3-5-18(11-19)17-33-25(34)8-7-24(31-33)20-12-21(27)14-22(28)13-20/h3,5-8,11-16H,4,9-10,17H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of GST-His6-tagged recombinant human c-Met kinase domain (956 to 1390) after 60 mins by anoff-chip mobility shift assay |
Bioorg Med Chem Lett 25: 1597-602 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.002
BindingDB Entry DOI: 10.7270/Q2HX1FBT |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM388437
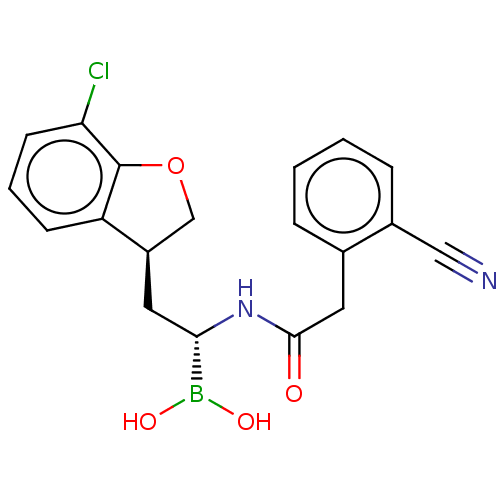
(US10294246, Compound No. 134)Show SMILES OB(O)[C@H](C[C@@H]1COc2c1cccc2Cl)NC(=O)Cc1ccccc1C#N |r| Show InChI InChI=1S/C19H18BClN2O4/c21-16-7-3-6-15-14(11-27-19(15)16)8-17(20(25)26)23-18(24)9-12-4-1-2-5-13(12)10-22/h1-7,14,17,25-26H,8-9,11H2,(H,23,24)/t14-,17+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00604
BindingDB Entry DOI: 10.7270/Q200064W |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50398609

(CHEMBL2141296 | IXAZOMIB CITRATE | Ixazomib | MLN2...)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)c1cc(Cl)ccc1Cl)B(O)O Show InChI InChI=1S/C14H19BCl2N2O4/c1-8(2)5-12(15(22)23)19-13(20)7-18-14(21)10-6-9(16)3-4-11(10)17/h3-4,6,8,12,22-23H,5,7H2,1-2H3,(H,18,21)(H,19,20)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00604
BindingDB Entry DOI: 10.7270/Q200064W |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-9
(Homo sapiens (Human)) | BDBM50069989

((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)c1cnccn1)B(O)O |r| Show InChI InChI=1S/C19H25BN4O4/c1-13(2)10-17(20(27)28)24-18(25)15(11-14-6-4-3-5-7-14)23-19(26)16-12-21-8-9-22-16/h3-9,12-13,15,17,27-28H,10-11H2,1-2H3,(H,23,26)(H,24,25)/t15-,17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00604
BindingDB Entry DOI: 10.7270/Q200064W |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM388427
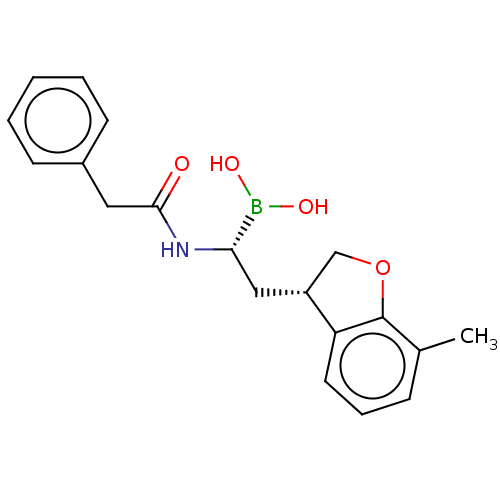
(US10294246, Compound No. 124)Show SMILES Cc1cccc2[C@H](C[C@H](NC(=O)Cc3ccccc3)B(O)O)COc12 |r| Show InChI InChI=1S/C19H22BNO4/c1-13-6-5-9-16-15(12-25-19(13)16)11-17(20(23)24)21-18(22)10-14-7-3-2-4-8-14/h2-9,15,17,23-24H,10-12H2,1H3,(H,21,22)/t15-,17+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00604
BindingDB Entry DOI: 10.7270/Q200064W |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM388408
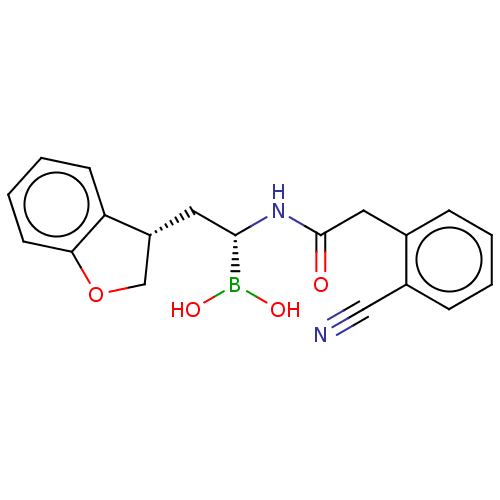
(US10294246, Compound No. 105)Show SMILES OB(O)[C@H](C[C@@H]1COc2ccccc12)NC(=O)Cc1ccccc1C#N |r| Show InChI InChI=1S/C19H19BN2O4/c21-11-14-6-2-1-5-13(14)10-19(23)22-18(20(24)25)9-15-12-26-17-8-4-3-7-16(15)17/h1-8,15,18,24-25H,9-10,12H2,(H,22,23)/t15-,18+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00604
BindingDB Entry DOI: 10.7270/Q200064W |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM388428
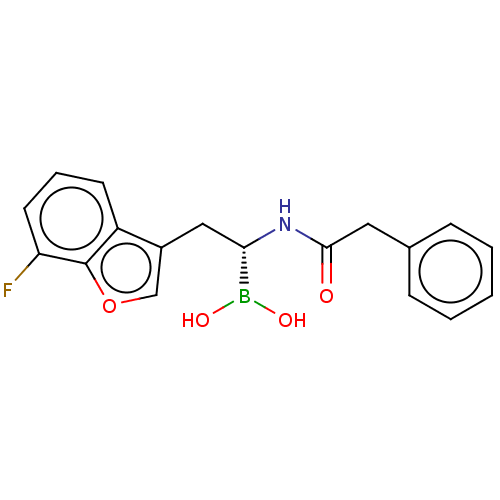
(US10294246, Compound No. 125)Show SMILES OB(O)[C@H](Cc1coc2c(F)cccc12)NC(=O)Cc1ccccc1 |r| Show InChI InChI=1S/C18H17BFNO4/c20-15-8-4-7-14-13(11-25-18(14)15)10-16(19(23)24)21-17(22)9-12-5-2-1-3-6-12/h1-8,11,16,23-24H,9-10H2,(H,21,22)/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00604
BindingDB Entry DOI: 10.7270/Q200064W |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50069989

((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)c1cnccn1)B(O)O |r| Show InChI InChI=1S/C19H25BN4O4/c1-13(2)10-17(20(27)28)24-18(25)15(11-14-6-4-3-5-7-14)23-19(26)16-12-21-8-9-22-16/h3-9,12-13,15,17,27-28H,10-11H2,1-2H3,(H,23,26)(H,24,25)/t15-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00604
BindingDB Entry DOI: 10.7270/Q200064W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50277889

(CARFILZOMIB | CHEMBL451887)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)NC(=O)CN1CCOCC1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)[C@@]1(C)CO1 |r| Show InChI InChI=1S/C40H57N5O7/c1-27(2)22-32(36(47)40(5)26-52-40)42-39(50)34(24-30-14-10-7-11-15-30)44-38(49)33(23-28(3)4)43-37(48)31(17-16-29-12-8-6-9-13-29)41-35(46)25-45-18-20-51-21-19-45/h6-15,27-28,31-34H,16-26H2,1-5H3,(H,41,46)(H,42,50)(H,43,48)(H,44,49)/t31-,32-,33-,34-,40+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00604
BindingDB Entry DOI: 10.7270/Q200064W |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50601643
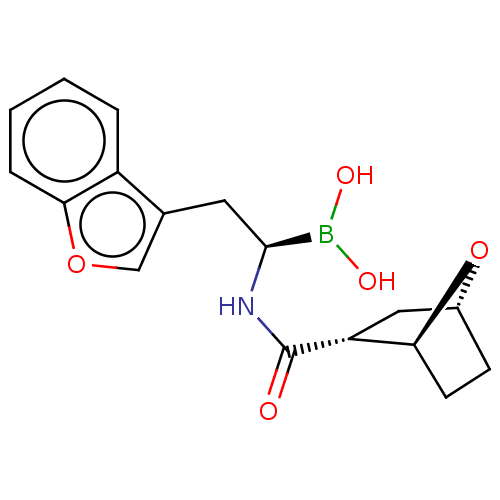
(CHEMBL5184466)Show SMILES [H][C@]12CC[C@]([H])(O1)[C@@H](C2)C(=O)N[C@@H](Cc1coc2ccccc12)B(O)O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00604
BindingDB Entry DOI: 10.7270/Q200064W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50601643

(CHEMBL5184466)Show SMILES [H][C@]12CC[C@]([H])(O1)[C@@H](C2)C(=O)N[C@@H](Cc1coc2ccccc12)B(O)O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00604
BindingDB Entry DOI: 10.7270/Q200064W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50601649
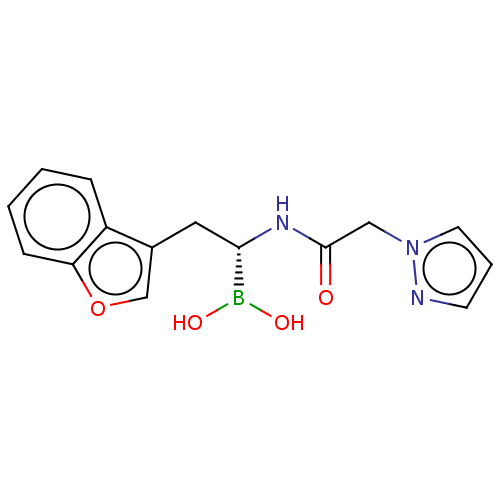
(CHEMBL5173345)Show SMILES OB(O)[C@H](Cc1coc2ccccc12)NC(=O)Cn1cccn1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00604
BindingDB Entry DOI: 10.7270/Q200064W |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM388388
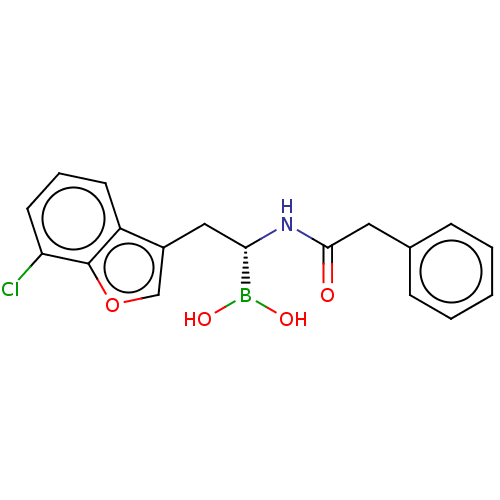
(US10294246, Compound No. 85)Show SMILES OB(O)[C@H](Cc1coc2c(Cl)cccc12)NC(=O)Cc1ccccc1 |r| Show InChI InChI=1S/C18H17BClNO4/c20-15-8-4-7-14-13(11-25-18(14)15)10-16(19(23)24)21-17(22)9-12-5-2-1-3-6-12/h1-8,11,16,23-24H,9-10H2,(H,21,22)/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00604
BindingDB Entry DOI: 10.7270/Q200064W |
More data for this
Ligand-Target Pair | |
Macrophage-stimulating protein receptor [983-1400,G1195S]
(Homo sapiens (Human)) | BDBM213708

(US9283225, A1)Show SMILES Fc1cc(NC(=O)c2cnn(c2C(F)(F)F)-c2ccccc2)ccc1Oc1ccnc2NC(=O)NCc12 Show InChI InChI=1S/C24H16F4N6O3/c25-17-10-13(6-7-19(17)37-18-8-9-29-21-15(18)11-30-23(36)33-21)32-22(35)16-12-31-34(20(16)24(26,27)28)14-4-2-1-3-5-14/h1-10,12H,11H2,(H,32,35)(H2,29,30,33,36) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Patent GmbH
US Patent
| Assay Description
The kinase assay was carried out as 384-well FlashPlate assay. As test plates 384-well streptavidine coated FlashPlate microtitre plates from Perkin ... |
US Patent US9283225 (2016)
BindingDB Entry DOI: 10.7270/Q2959GC8 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM388433
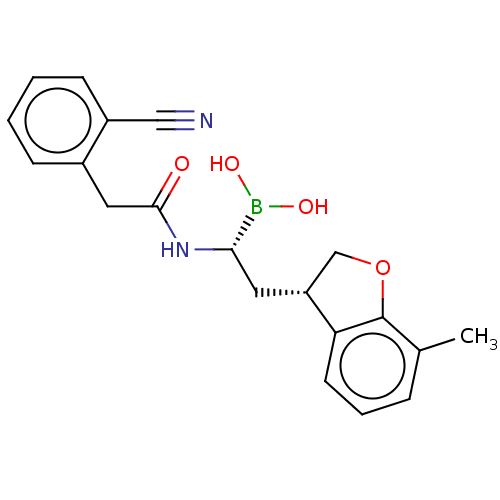
(US10294246, Compound No. 130)Show SMILES Cc1cccc2[C@H](C[C@H](NC(=O)Cc3ccccc3C#N)B(O)O)COc12 |r| Show InChI InChI=1S/C20H21BN2O4/c1-13-5-4-8-17-16(12-27-20(13)17)9-18(21(25)26)23-19(24)10-14-6-2-3-7-15(14)11-22/h2-8,16,18,25-26H,9-10,12H2,1H3,(H,23,24)/t16-,18+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00604
BindingDB Entry DOI: 10.7270/Q200064W |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM388378
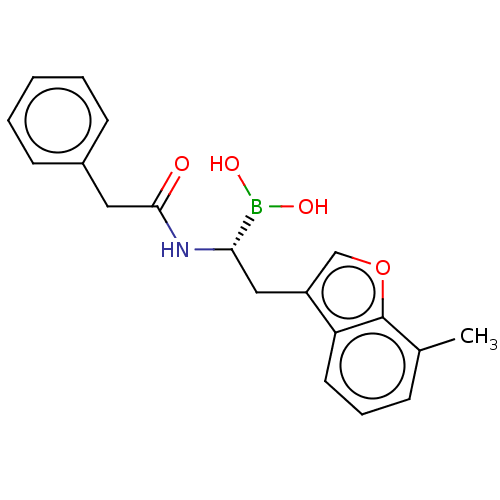
(US10294246, Compound No. 75)Show SMILES Cc1cccc2c(C[C@H](NC(=O)Cc3ccccc3)B(O)O)coc12 |r| Show InChI InChI=1S/C19H20BNO4/c1-13-6-5-9-16-15(12-25-19(13)16)11-17(20(23)24)21-18(22)10-14-7-3-2-4-8-14/h2-9,12,17,23-24H,10-11H2,1H3,(H,21,22)/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00604
BindingDB Entry DOI: 10.7270/Q200064W |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50398609

(CHEMBL2141296 | IXAZOMIB CITRATE | Ixazomib | MLN2...)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)c1cc(Cl)ccc1Cl)B(O)O Show InChI InChI=1S/C14H19BCl2N2O4/c1-8(2)5-12(15(22)23)19-13(20)7-18-14(21)10-6-9(16)3-4-11(10)17/h3-4,6,8,12,22-23H,5,7H2,1-2H3,(H,18,21)(H,19,20)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00604
BindingDB Entry DOI: 10.7270/Q200064W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM388393
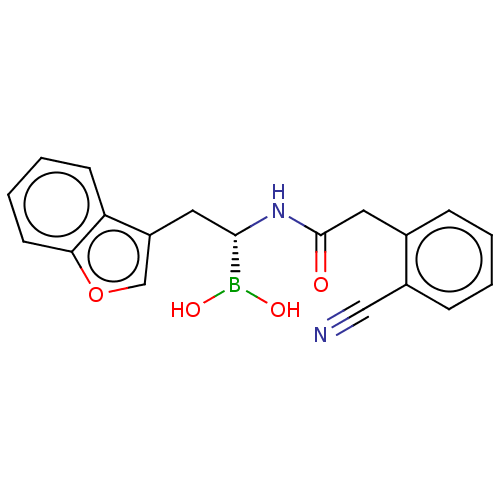
(US10294246, Compound No. 90)Show SMILES OB(O)[C@H](Cc1coc2ccccc12)NC(=O)Cc1ccccc1C#N |r| Show InChI InChI=1S/C19H17BN2O4/c21-11-14-6-2-1-5-13(14)10-19(23)22-18(20(24)25)9-15-12-26-17-8-4-3-7-16(15)17/h1-8,12,18,24-25H,9-10H2,(H,22,23)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00604
BindingDB Entry DOI: 10.7270/Q200064W |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50065486

(CHEMBL3402759)Show SMILES COc1cccc(c1)-c1ccc(=O)n(Cc2cccc(c2)-c2ncc(OCCCN(C)C)cn2)n1 Show InChI InChI=1S/C27H29N5O3/c1-31(2)13-6-14-35-24-17-28-27(29-18-24)22-9-4-7-20(15-22)19-32-26(33)12-11-25(30-32)21-8-5-10-23(16-21)34-3/h4-5,7-12,15-18H,6,13-14,19H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of GST-His6-tagged recombinant human c-Met kinase domain (956 to 1390) after 60 mins by anoff-chip mobility shift assay |
Bioorg Med Chem Lett 25: 1597-602 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.002
BindingDB Entry DOI: 10.7270/Q2HX1FBT |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50601653
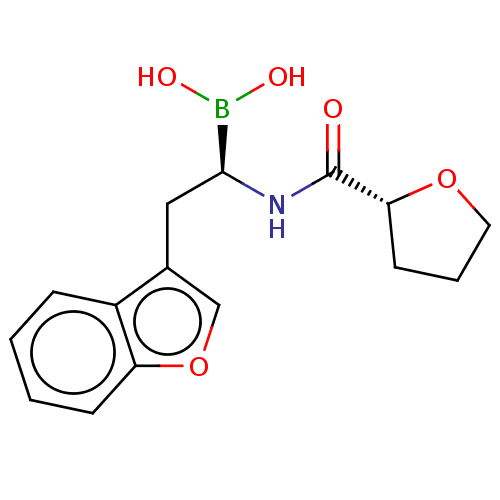
(CHEMBL5200546)Show SMILES OB(O)[C@H](Cc1coc2ccccc12)NC(=O)[C@H]1CCCO1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00604
BindingDB Entry DOI: 10.7270/Q200064W |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50601651
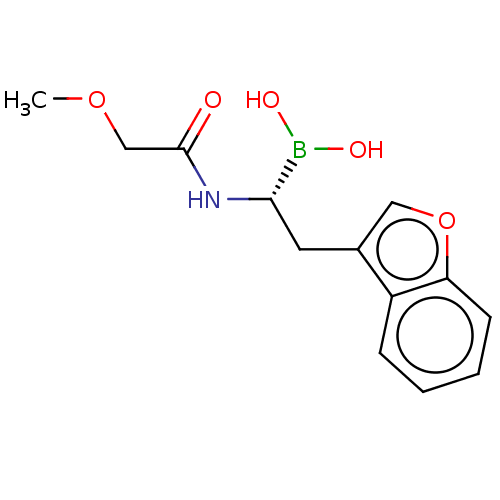
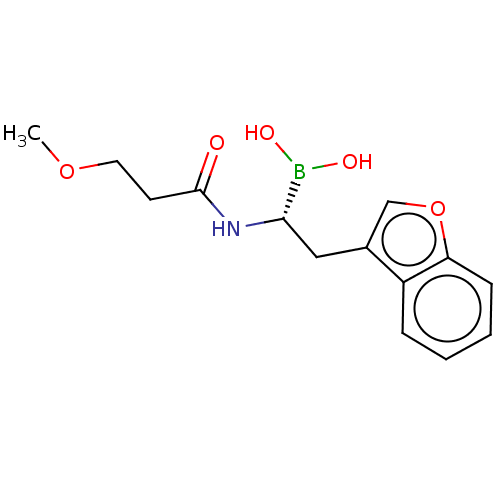
(CHEMBL5175203) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00604
BindingDB Entry DOI: 10.7270/Q200064W |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM388429
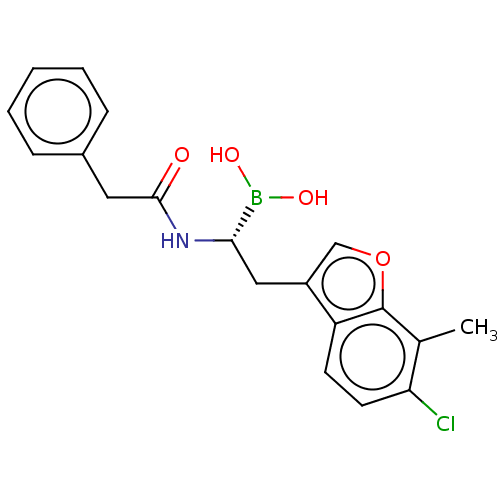
(US10294246, Compound No. 126)Show SMILES Cc1c(Cl)ccc2c(C[C@H](NC(=O)Cc3ccccc3)B(O)O)coc12 |r| Show InChI InChI=1S/C19H19BClNO4/c1-12-16(21)8-7-15-14(11-26-19(12)15)10-17(20(24)25)22-18(23)9-13-5-3-2-4-6-13/h2-8,11,17,24-25H,9-10H2,1H3,(H,22,23)/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00604
BindingDB Entry DOI: 10.7270/Q200064W |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50065488

(CHEMBL3402757)Show SMILES CN(C)CCCOc1cnc(nc1)-c1cccc(Cn2nc(ccc2=O)-c2cccc(F)c2)c1 Show InChI InChI=1S/C26H26FN5O2/c1-31(2)12-5-13-34-23-16-28-26(29-17-23)21-8-3-6-19(14-21)18-32-25(33)11-10-24(30-32)20-7-4-9-22(27)15-20/h3-4,6-11,14-17H,5,12-13,18H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of GST-His6-tagged recombinant human c-Met kinase domain (956 to 1390) after 60 mins by anoff-chip mobility shift assay |
Bioorg Med Chem Lett 25: 1597-602 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.002
BindingDB Entry DOI: 10.7270/Q2HX1FBT |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM388395

(US10294246, Compound No. 92)Show SMILES COc1cccc2c(C[C@H](NC(=O)Cc3ccccc3)B(O)O)coc12 |r| Show InChI InChI=1S/C19H20BNO5/c1-25-16-9-5-8-15-14(12-26-19(15)16)11-17(20(23)24)21-18(22)10-13-6-3-2-4-7-13/h2-9,12,17,23-24H,10-11H2,1H3,(H,21,22)/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00604
BindingDB Entry DOI: 10.7270/Q200064W |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50601656
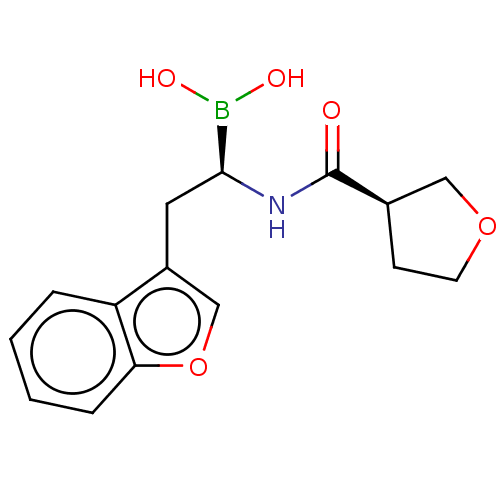
(CHEMBL5201811)Show SMILES OB(O)[C@H](Cc1coc2ccccc12)NC(=O)[C@@H]1CCOC1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00604
BindingDB Entry DOI: 10.7270/Q200064W |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50065487
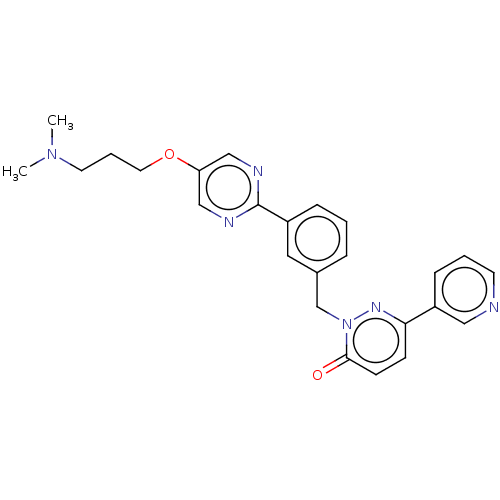
(CHEMBL3402758)Show SMILES CN(C)CCCOc1cnc(nc1)-c1cccc(Cn2nc(ccc2=O)-c2cccnc2)c1 Show InChI InChI=1S/C25H26N6O2/c1-30(2)12-5-13-33-22-16-27-25(28-17-22)20-7-3-6-19(14-20)18-31-24(32)10-9-23(29-31)21-8-4-11-26-15-21/h3-4,6-11,14-17H,5,12-13,18H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of GST-His6-tagged recombinant human c-Met kinase domain (956 to 1390) after 60 mins by anoff-chip mobility shift assay |
Bioorg Med Chem Lett 25: 1597-602 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.002
BindingDB Entry DOI: 10.7270/Q2HX1FBT |
More data for this
Ligand-Target Pair | |
Macrophage-stimulating protein receptor [983-1400,G1195S]
(Homo sapiens (Human)) | BDBM213709

(US9283225, A3)Show SMILES Cc1c(cnn1-c1ccccc1)C(=O)Nc1ccc(Oc2ccnc3NC(=O)NCc23)c(F)c1 Show InChI InChI=1S/C24H19FN6O3/c1-14-17(13-28-31(14)16-5-3-2-4-6-16)23(32)29-15-7-8-21(19(25)11-15)34-20-9-10-26-22-18(20)12-27-24(33)30-22/h2-11,13H,12H2,1H3,(H,29,32)(H2,26,27,30,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Patent GmbH
US Patent
| Assay Description
The kinase assay was carried out as 384-well FlashPlate assay. As test plates 384-well streptavidine coated FlashPlate microtitre plates from Perkin ... |
US Patent US9283225 (2016)
BindingDB Entry DOI: 10.7270/Q2959GC8 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM388322
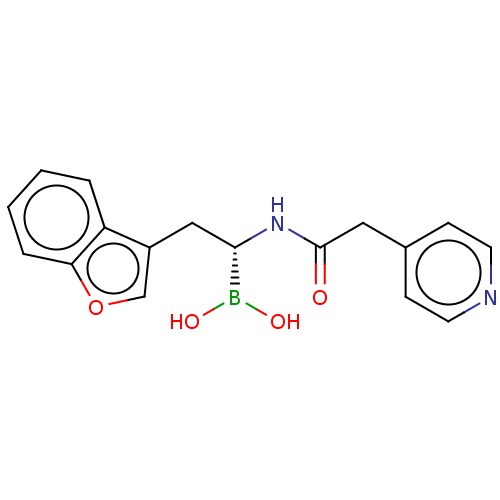
(US10294246, Compound No. 19)Show SMILES OB(O)[C@H](Cc1coc2ccccc12)NC(=O)Cc1ccncc1 |r| Show InChI InChI=1S/C17H17BN2O4/c21-17(9-12-5-7-19-8-6-12)20-16(18(22)23)10-13-11-24-15-4-2-1-3-14(13)15/h1-8,11,16,22-23H,9-10H2,(H,20,21)/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00604
BindingDB Entry DOI: 10.7270/Q200064W |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50065493
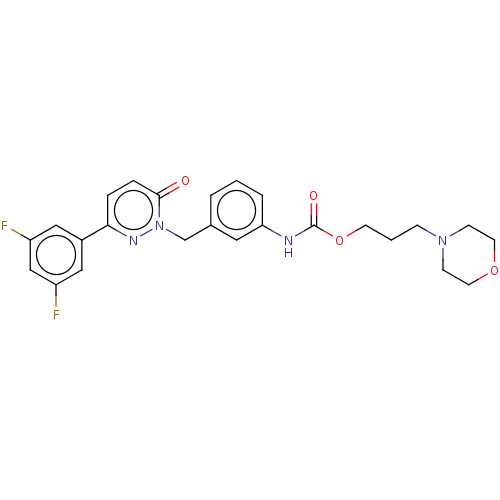
(CHEMBL3402765)Show SMILES Fc1cc(F)cc(c1)-c1ccc(=O)n(Cc2cccc(NC(=O)OCCCN3CCOCC3)c2)n1 Show InChI InChI=1S/C25H26F2N4O4/c26-20-14-19(15-21(27)16-20)23-5-6-24(32)31(29-23)17-18-3-1-4-22(13-18)28-25(33)35-10-2-7-30-8-11-34-12-9-30/h1,3-6,13-16H,2,7-12,17H2,(H,28,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of GST-His6-tagged recombinant human c-Met kinase domain (956 to 1390) after 60 mins by anoff-chip mobility shift assay |
Bioorg Med Chem Lett 25: 1597-602 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.002
BindingDB Entry DOI: 10.7270/Q2HX1FBT |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-9
(Homo sapiens (Human)) | BDBM50601642

(CHEMBL5188533)Show SMILES CCc1cccc(C[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)c2cnccn2)B(O)O)c1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00604
BindingDB Entry DOI: 10.7270/Q200064W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50601652

(CHEMBL5191857) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00604
BindingDB Entry DOI: 10.7270/Q200064W |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50065457

(EMD-1214063 | MSC-2156119 | MSC-2156119J | Tepotin...)Show SMILES CN1CCC(COc2cnc(nc2)-c2cccc(Cn3nc(ccc3=O)-c3cccc(c3)C#N)c2)CC1 Show InChI InChI=1S/C29H28N6O2/c1-34-12-10-21(11-13-34)20-37-26-17-31-29(32-18-26)25-7-3-5-23(15-25)19-35-28(36)9-8-27(33-35)24-6-2-4-22(14-24)16-30/h2-9,14-15,17-18,21H,10-13,19-20H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of c-Met kinase in human A549 cells assessed as inhibition of phosphorylation after 45 mins by electrochemiluminescence assay |
Bioorg Med Chem Lett 25: 1597-602 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.002
BindingDB Entry DOI: 10.7270/Q2HX1FBT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proteasome subunit beta type-10
(Homo sapiens (Human)) | BDBM50277889

(CARFILZOMIB | CHEMBL451887)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)NC(=O)CN1CCOCC1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)[C@@]1(C)CO1 |r| Show InChI InChI=1S/C40H57N5O7/c1-27(2)22-32(36(47)40(5)26-52-40)42-39(50)34(24-30-14-10-7-11-15-30)44-38(49)33(23-28(3)4)43-37(48)31(17-16-29-12-8-6-9-13-29)41-35(46)25-45-18-20-51-21-19-45/h6-15,27-28,31-34H,16-26H2,1-5H3,(H,41,46)(H,42,50)(H,43,48)(H,44,49)/t31-,32-,33-,34-,40+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00604
BindingDB Entry DOI: 10.7270/Q200064W |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50601656

(CHEMBL5201811)Show SMILES OB(O)[C@H](Cc1coc2ccccc12)NC(=O)[C@@H]1CCOC1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00604
BindingDB Entry DOI: 10.7270/Q200064W |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM388309

(US10294246, Compound No. 6)Show SMILES OB(O)[C@H](Cc1coc2ccccc12)NC(=O)Cc1ccccc1 |r| Show InChI InChI=1S/C18H18BNO4/c21-18(10-13-6-2-1-3-7-13)20-17(19(22)23)11-14-12-24-16-9-5-4-8-15(14)16/h1-9,12,17,22-23H,10-11H2,(H,20,21)/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00604
BindingDB Entry DOI: 10.7270/Q200064W |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data