 Found 119 hits with Last Name = 'shelton' and Initial = 'je'
Found 119 hits with Last Name = 'shelton' and Initial = 'je' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50028059
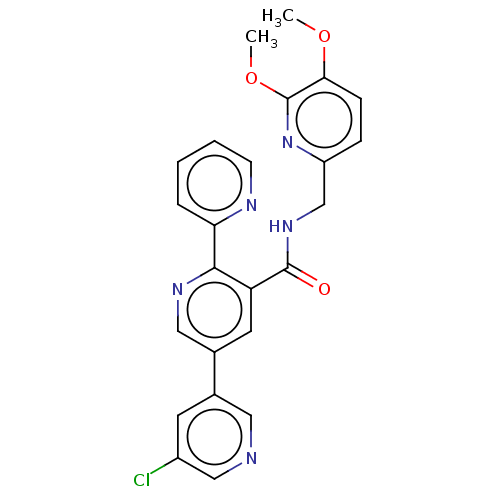
(CHEMBL3338866)Show SMILES COc1ccc(CNC(=O)c2cc(cnc2-c2ccccn2)-c2cncc(Cl)c2)nc1OC Show InChI InChI=1S/C24H20ClN5O3/c1-32-21-7-6-18(30-24(21)33-2)14-29-23(31)19-10-16(15-9-17(25)13-26-11-15)12-28-22(19)20-5-3-4-8-27-20/h3-13H,14H2,1-2H3,(H,29,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of (S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-((3H)-1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2 rec... |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50089369
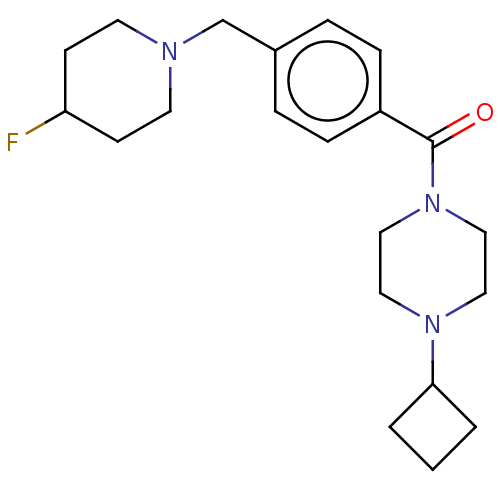
(CHEMBL3577959)Show SMILES FC1CCN(Cc2ccc(cc2)C(=O)N2CCN(CC2)C2CCC2)CC1 Show InChI InChI=1S/C21H30FN3O/c22-19-8-10-23(11-9-19)16-17-4-6-18(7-5-17)21(26)25-14-12-24(13-15-25)20-2-1-3-20/h4-7,19-20H,1-3,8-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 mins |
ACS Med Chem Lett 6: 450-4 (2015)
Article DOI: 10.1021/ml5005156
BindingDB Entry DOI: 10.7270/Q22F7Q59 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50089375
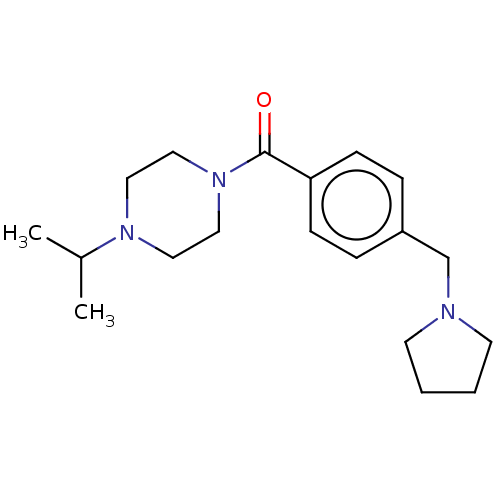
(CHEMBL3577953)Show InChI InChI=1S/C19H29N3O/c1-16(2)21-11-13-22(14-12-21)19(23)18-7-5-17(6-8-18)15-20-9-3-4-10-20/h5-8,16H,3-4,9-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 mins |
ACS Med Chem Lett 6: 450-4 (2015)
Article DOI: 10.1021/ml5005156
BindingDB Entry DOI: 10.7270/Q22F7Q59 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50089374
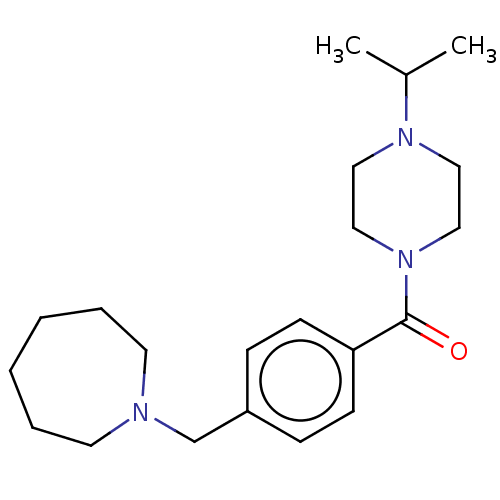
(CHEMBL3577954)Show InChI InChI=1S/C21H33N3O/c1-18(2)23-13-15-24(16-14-23)21(25)20-9-7-19(8-10-20)17-22-11-5-3-4-6-12-22/h7-10,18H,3-6,11-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 mins |
ACS Med Chem Lett 6: 450-4 (2015)
Article DOI: 10.1021/ml5005156
BindingDB Entry DOI: 10.7270/Q22F7Q59 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50089372
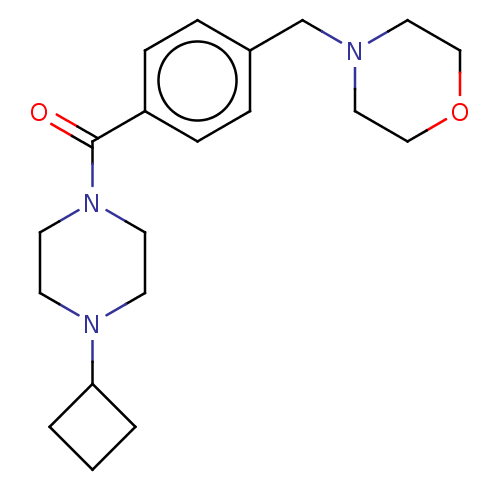
(CHEMBL3577956)Show InChI InChI=1S/C20H29N3O2/c24-20(23-10-8-22(9-11-23)19-2-1-3-19)18-6-4-17(5-7-18)16-21-12-14-25-15-13-21/h4-7,19H,1-3,8-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 mins |
ACS Med Chem Lett 6: 450-4 (2015)
Article DOI: 10.1021/ml5005156
BindingDB Entry DOI: 10.7270/Q22F7Q59 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50346208
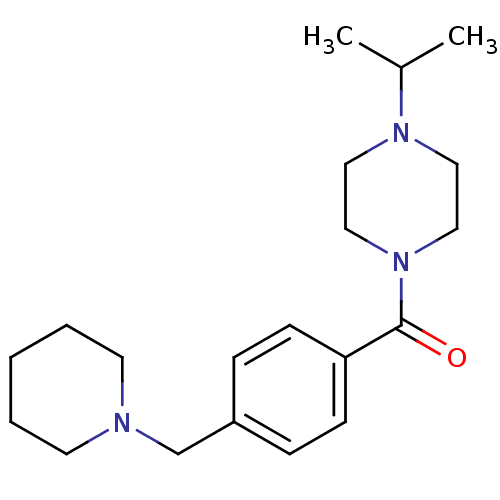
((1-isopropylpiperidin-4-yl)(4-(piperidin-1-ylmethy...)Show InChI InChI=1S/C20H31N3O/c1-17(2)22-12-14-23(15-13-22)20(24)19-8-6-18(7-9-19)16-21-10-4-3-5-11-21/h6-9,17H,3-5,10-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 mins |
ACS Med Chem Lett 6: 450-4 (2015)
Article DOI: 10.1021/ml5005156
BindingDB Entry DOI: 10.7270/Q22F7Q59 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50089370

(CHEMBL3577958)Show InChI InChI=1S/C20H30FN3O/c1-16(2)23-11-13-24(14-12-23)20(25)18-5-3-17(4-6-18)15-22-9-7-19(21)8-10-22/h3-6,16,19H,7-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 mins |
ACS Med Chem Lett 6: 450-4 (2015)
Article DOI: 10.1021/ml5005156
BindingDB Entry DOI: 10.7270/Q22F7Q59 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50089368
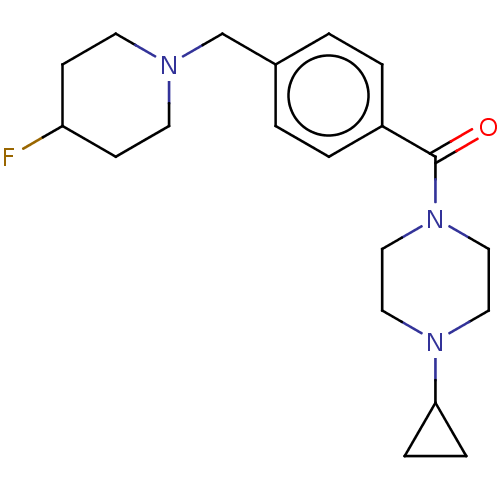
(CHEMBL3577960)Show InChI InChI=1S/C20H28FN3O/c21-18-7-9-22(10-8-18)15-16-1-3-17(4-2-16)20(25)24-13-11-23(12-14-24)19-5-6-19/h1-4,18-19H,5-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 mins |
ACS Med Chem Lett 6: 450-4 (2015)
Article DOI: 10.1021/ml5005156
BindingDB Entry DOI: 10.7270/Q22F7Q59 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Rattus norvegicus (Rat)) | BDBM50097380
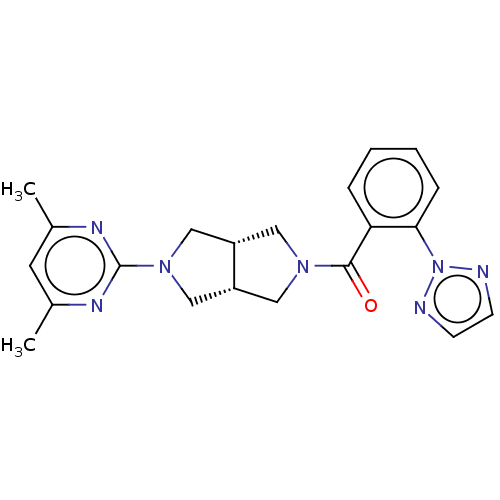
(CHEMBL3586432)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1ccccc1-n1nccn1 |r| Show InChI InChI=1S/C21H23N7O/c1-14-9-15(2)25-21(24-14)27-12-16-10-26(11-17(16)13-27)20(29)18-5-3-4-6-19(18)28-22-7-8-23-28/h3-9,16-17H,10-13H2,1-2H3/t16-,17+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to rat OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50089373

(CHEMBL3577955)Show SMILES OC(=O)\C=C/C(O)=O.CC(C)N1CCN(CC1)C(=O)c1ccc(CN2CCOCC2)cc1 Show InChI InChI=1S/C19H29N3O2.C4H4O4/c1-16(2)21-7-9-22(10-8-21)19(23)18-5-3-17(4-6-18)15-20-11-13-24-14-12-20;5-3(6)1-2-4(7)8/h3-6,16H,7-15H2,1-2H3;1-2H,(H,5,6)(H,7,8)/b;2-1- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 mins |
ACS Med Chem Lett 6: 450-4 (2015)
Article DOI: 10.1021/ml5005156
BindingDB Entry DOI: 10.7270/Q22F7Q59 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50412863
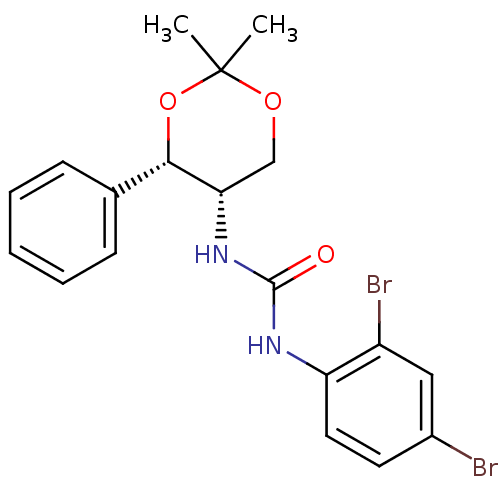
(CHEMBL359632 | JNJ-10397049)Show SMILES CC1(C)OC[C@H](NC(=O)Nc2ccc(Br)cc2Br)[C@@H](O1)c1ccccc1 |r| Show InChI InChI=1S/C19H20Br2N2O3/c1-19(2)25-11-16(17(26-19)12-6-4-3-5-7-12)23-18(24)22-15-9-8-13(20)10-14(15)21/h3-10,16-17H,11H2,1-2H3,(H2,22,23,24)/t16-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate by LC-MS/MS analysis |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50089378

(CHEMBL3577951)Show InChI InChI=1S/C17H27N3O/c1-14(2)19-9-11-20(12-10-19)17(21)16-7-5-15(6-8-16)13-18(3)4/h5-8,14H,9-13H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 mins |
ACS Med Chem Lett 6: 450-4 (2015)
Article DOI: 10.1021/ml5005156
BindingDB Entry DOI: 10.7270/Q22F7Q59 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50089371

(CHEMBL3577957)Show InChI InChI=1S/C19H27N3O2.ClH/c23-19(22-9-7-21(8-10-22)18-5-6-18)17-3-1-16(2-4-17)15-20-11-13-24-14-12-20;/h1-4,18H,5-15H2;1H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 mins |
ACS Med Chem Lett 6: 450-4 (2015)
Article DOI: 10.1021/ml5005156
BindingDB Entry DOI: 10.7270/Q22F7Q59 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50089377
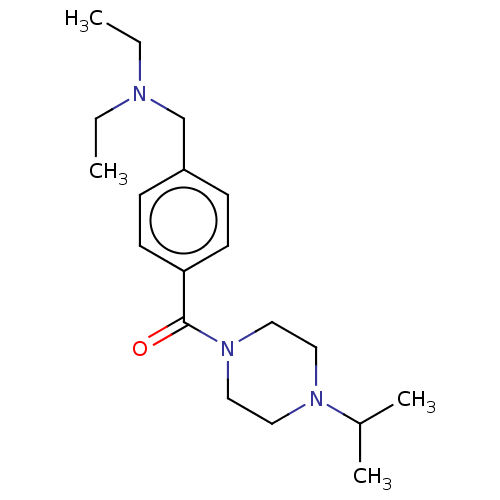
(CHEMBL3577952)Show InChI InChI=1S/C19H31N3O/c1-5-20(6-2)15-17-7-9-18(10-8-17)19(23)22-13-11-21(12-14-22)16(3)4/h7-10,16H,5-6,11-15H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 mins |
ACS Med Chem Lett 6: 450-4 (2015)
Article DOI: 10.1021/ml5005156
BindingDB Entry DOI: 10.7270/Q22F7Q59 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Rattus norvegicus (Rat)) | BDBM50097388
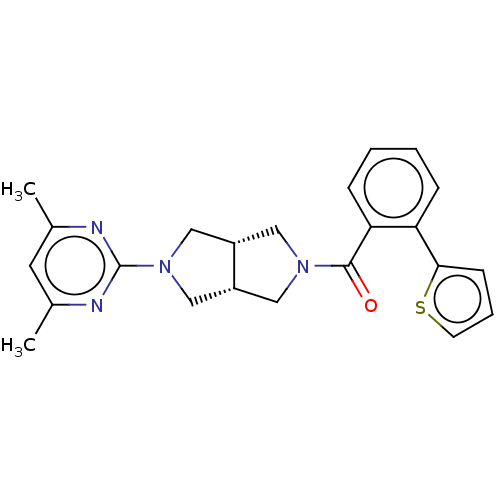
(CHEMBL3586426)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1ccccc1-c1cccs1 |r| Show InChI InChI=1S/C23H24N4OS/c1-15-10-16(2)25-23(24-15)27-13-17-11-26(12-18(17)14-27)22(28)20-7-4-3-6-19(20)21-8-5-9-29-21/h3-10,17-18H,11-14H2,1-2H3/t17-,18+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to rat OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50097360
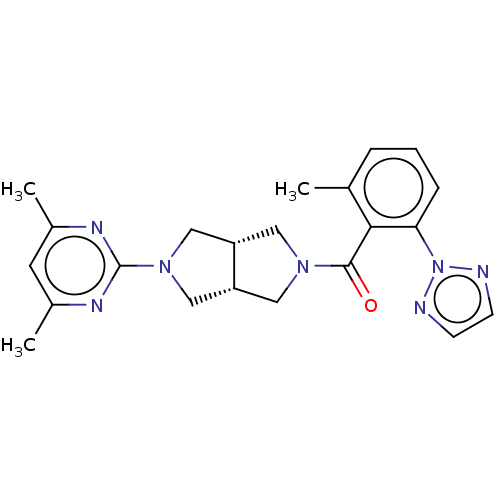
(CHEMBL3586440)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1c(C)cccc1-n1nccn1 |r| Show InChI InChI=1S/C22H25N7O/c1-14-5-4-6-19(29-23-7-8-24-29)20(14)21(30)27-10-17-12-28(13-18(17)11-27)22-25-15(2)9-16(3)26-22/h4-9,17-18H,10-13H2,1-3H3/t17-,18+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50097388

(CHEMBL3586426)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1ccccc1-c1cccs1 |r| Show InChI InChI=1S/C23H24N4OS/c1-15-10-16(2)25-23(24-15)27-13-17-11-26(12-18(17)14-27)22(28)20-7-4-3-6-19(20)21-8-5-9-29-21/h3-10,17-18H,11-14H2,1-2H3/t17-,18+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50092813
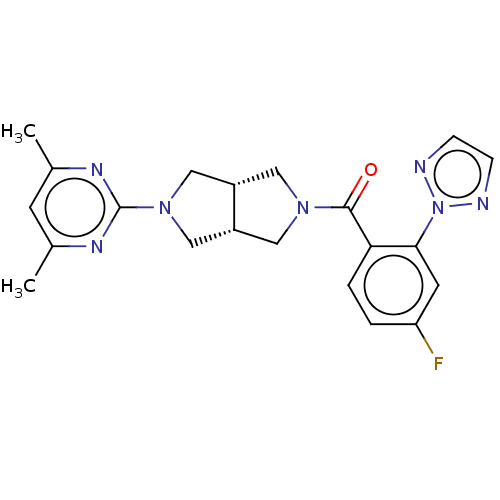
(CHEMBL3586434)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1ccc(F)cc1-n1nccn1 |r| Show InChI InChI=1S/C21H22FN7O/c1-13-7-14(2)26-21(25-13)28-11-15-9-27(10-16(15)12-28)20(30)18-4-3-17(22)8-19(18)29-23-5-6-24-29/h3-8,15-16H,9-12H2,1-2H3/t15-,16+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50092814
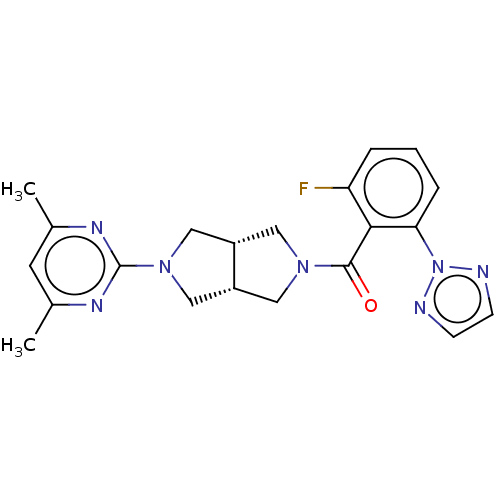
(CHEMBL3586436)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1c(F)cccc1-n1nccn1 |r| Show InChI InChI=1S/C21H22FN7O/c1-13-8-14(2)26-21(25-13)28-11-15-9-27(10-16(15)12-28)20(30)19-17(22)4-3-5-18(19)29-23-6-7-24-29/h3-8,15-16H,9-12H2,1-2H3/t15-,16+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Rattus norvegicus (Rat)) | BDBM50092812
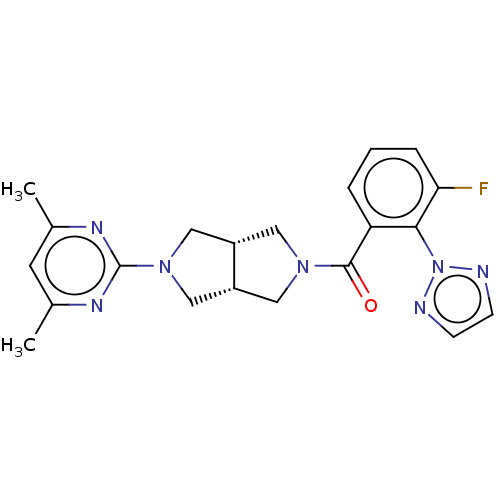
(CHEMBL3586433)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1cccc(F)c1-n1nccn1 |r| Show InChI InChI=1S/C21H22FN7O/c1-13-8-14(2)26-21(25-13)28-11-15-9-27(10-16(15)12-28)20(30)17-4-3-5-18(22)19(17)29-23-6-7-24-29/h3-8,15-16H,9-12H2,1-2H3/t15-,16+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to rat OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Rattus norvegicus (Rat)) | BDBM50092814

(CHEMBL3586436)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1c(F)cccc1-n1nccn1 |r| Show InChI InChI=1S/C21H22FN7O/c1-13-8-14(2)26-21(25-13)28-11-15-9-27(10-16(15)12-28)20(30)19-17(22)4-3-5-18(19)29-23-6-7-24-29/h3-8,15-16H,9-12H2,1-2H3/t15-,16+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to rat OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50097380

(CHEMBL3586432)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1ccccc1-n1nccn1 |r| Show InChI InChI=1S/C21H23N7O/c1-14-9-15(2)25-21(24-14)27-12-16-10-26(11-17(16)13-27)20(29)18-5-3-4-6-19(18)28-22-7-8-23-28/h3-9,16-17H,10-13H2,1-2H3/t16-,17+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Rattus norvegicus (Rat)) | BDBM50097360

(CHEMBL3586440)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1c(C)cccc1-n1nccn1 |r| Show InChI InChI=1S/C22H25N7O/c1-14-5-4-6-19(29-23-7-8-24-29)20(14)21(30)27-10-17-12-28(13-18(17)11-27)22-25-15(2)9-16(3)26-22/h4-9,17-18H,10-13H2,1-3H3/t17-,18+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to rat OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Rattus norvegicus (Rat)) | BDBM50092813

(CHEMBL3586434)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1ccc(F)cc1-n1nccn1 |r| Show InChI InChI=1S/C21H22FN7O/c1-13-7-14(2)26-21(25-13)28-11-15-9-27(10-16(15)12-28)20(30)18-4-3-17(22)8-19(18)29-23-5-6-24-29/h3-8,15-16H,9-12H2,1-2H3/t15-,16+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to rat OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50092812

(CHEMBL3586433)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1cccc(F)c1-n1nccn1 |r| Show InChI InChI=1S/C21H22FN7O/c1-13-8-14(2)26-21(25-13)28-11-15-9-27(10-16(15)12-28)20(30)17-4-3-5-18(22)19(17)29-23-6-7-24-29/h3-8,15-16H,9-12H2,1-2H3/t15-,16+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50089383
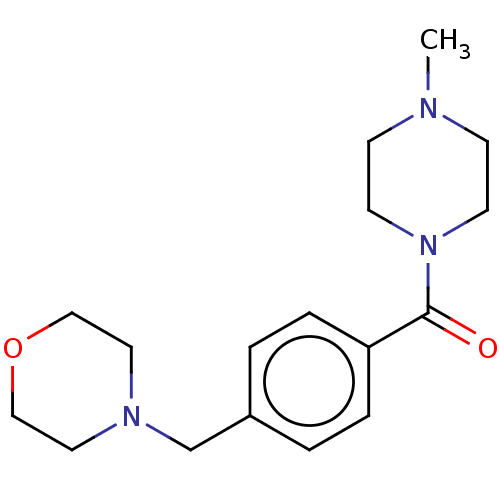
(CHEMBL1707983)Show InChI InChI=1S/C17H25N3O2/c1-18-6-8-20(9-7-18)17(21)16-4-2-15(3-5-16)14-19-10-12-22-13-11-19/h2-5H,6-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 mins |
ACS Med Chem Lett 6: 450-4 (2015)
Article DOI: 10.1021/ml5005156
BindingDB Entry DOI: 10.7270/Q22F7Q59 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50089384

(CHEMBL3577950)Show InChI InChI=1S/C18H27N3O/c1-19-11-13-21(14-12-19)18(22)17-7-5-16(6-8-17)15-20-9-3-2-4-10-20/h5-8H,2-4,9-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 mins |
ACS Med Chem Lett 6: 450-4 (2015)
Article DOI: 10.1021/ml5005156
BindingDB Entry DOI: 10.7270/Q22F7Q59 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50092810
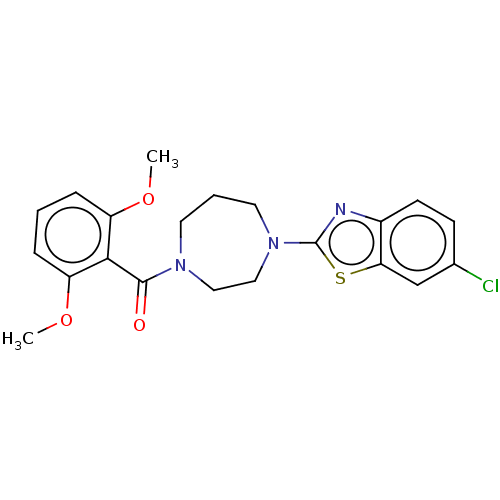
(CHEMBL3586412)Show SMILES COc1cccc(OC)c1C(=O)N1CCCN(CC1)c1nc2ccc(Cl)cc2s1 Show InChI InChI=1S/C21H22ClN3O3S/c1-27-16-5-3-6-17(28-2)19(16)20(26)24-9-4-10-25(12-11-24)21-23-15-8-7-14(22)13-18(15)29-21/h3,5-8,13H,4,9-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of (S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-((3H)-1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2 rec... |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50097384
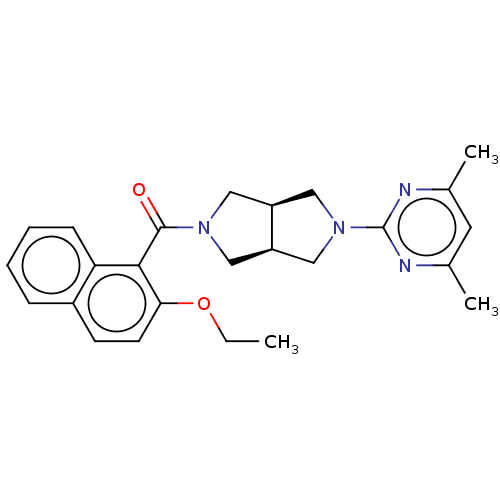
(CHEMBL3586430)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1c(OCC)ccc2ccccc12 |r| Show InChI InChI=1S/C25H28N4O2/c1-4-31-22-10-9-18-7-5-6-8-21(18)23(22)24(30)28-12-19-14-29(15-20(19)13-28)25-26-16(2)11-17(3)27-25/h5-11,19-20H,4,12-15H2,1-3H3/t19-,20+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50097376
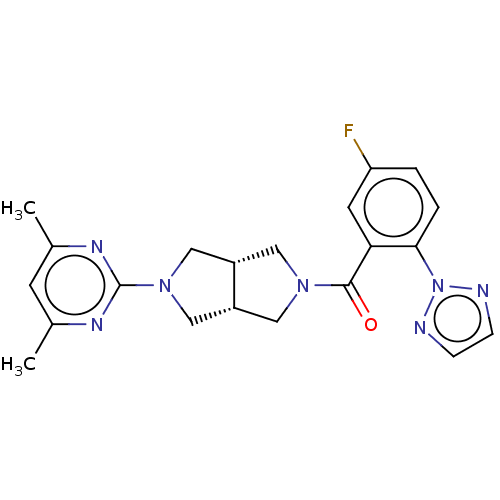
(CHEMBL3586435)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1cc(F)ccc1-n1nccn1 |r| Show InChI InChI=1S/C21H22FN7O/c1-13-7-14(2)26-21(25-13)28-11-15-9-27(10-16(15)12-28)20(30)18-8-17(22)3-4-19(18)29-23-5-6-24-29/h3-8,15-16H,9-12H2,1-2H3/t15-,16+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50097379
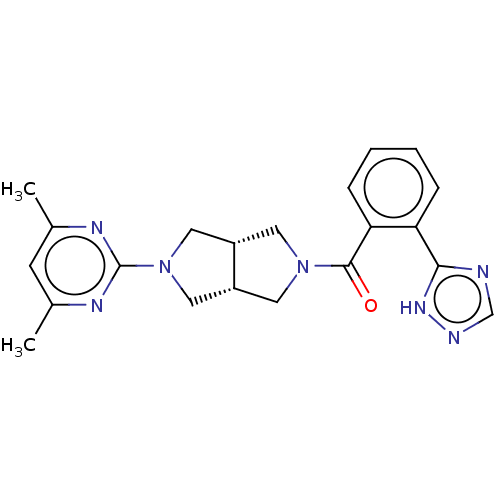
(CHEMBL3586437)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1ccccc1-c1ncn[nH]1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Rattus norvegicus (Rat)) | BDBM50097392

(CHEMBL3586441)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1cc(OC)ccc1-n1nccn1 |r| Show InChI InChI=1S/C22H25N7O2/c1-14-8-15(2)26-22(25-14)28-12-16-10-27(11-17(16)13-28)21(30)19-9-18(31-3)4-5-20(19)29-23-6-7-24-29/h4-9,16-17H,10-13H2,1-3H3/t16-,17+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to rat OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Rattus norvegicus (Rat)) | BDBM50097379

(CHEMBL3586437)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1ccccc1-c1ncn[nH]1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to rat OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50097381
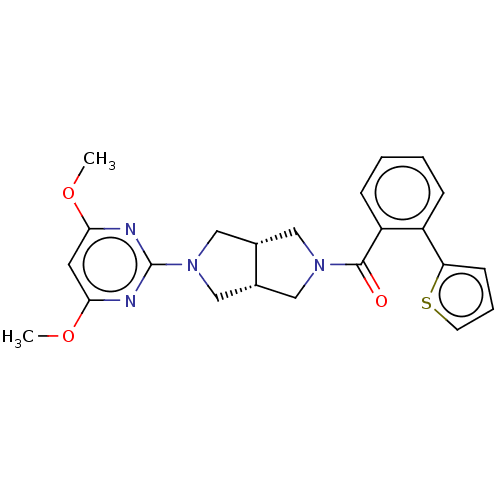
(CHEMBL3586431)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(OC)cc(OC)n1)C(=O)c1ccccc1-c1cccs1 |r| Show InChI InChI=1S/C23H24N4O3S/c1-29-20-10-21(30-2)25-23(24-20)27-13-15-11-26(12-16(15)14-27)22(28)18-7-4-3-6-17(18)19-8-5-9-31-19/h3-10,15-16H,11-14H2,1-2H3/t15-,16+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50097392

(CHEMBL3586441)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1cc(OC)ccc1-n1nccn1 |r| Show InChI InChI=1S/C22H25N7O2/c1-14-8-15(2)26-22(25-14)28-12-16-10-27(11-17(16)13-28)21(30)19-9-18(31-3)4-5-20(19)29-23-6-7-24-29/h4-9,16-17H,10-13H2,1-3H3/t16-,17+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Rattus norvegicus (Rat)) | BDBM50092811
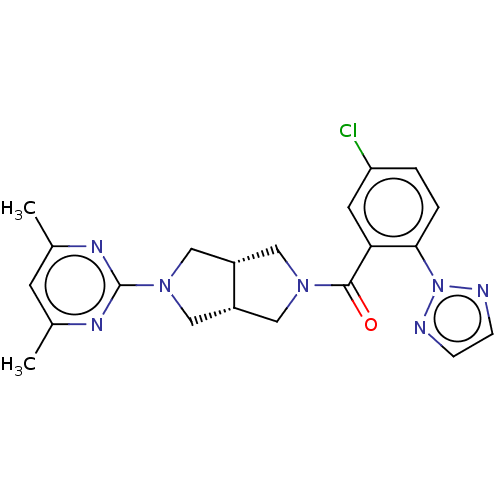
(CHEMBL3586442)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1cc(Cl)ccc1-n1nccn1 |r| Show InChI InChI=1S/C21H22ClN7O/c1-13-7-14(2)26-21(25-13)28-11-15-9-27(10-16(15)12-28)20(30)18-8-17(22)3-4-19(18)29-23-5-6-24-29/h3-8,15-16H,9-12H2,1-2H3/t15-,16+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to rat OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50097387
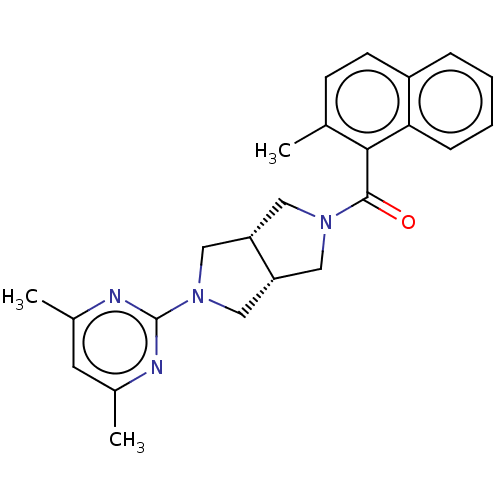
(CHEMBL3586427)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1c(C)ccc2ccccc12 |r| Show InChI InChI=1S/C24H26N4O/c1-15-8-9-18-6-4-5-7-21(18)22(15)23(29)27-11-19-13-28(14-20(19)12-27)24-25-16(2)10-17(3)26-24/h4-10,19-20H,11-14H2,1-3H3/t19-,20+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50097374
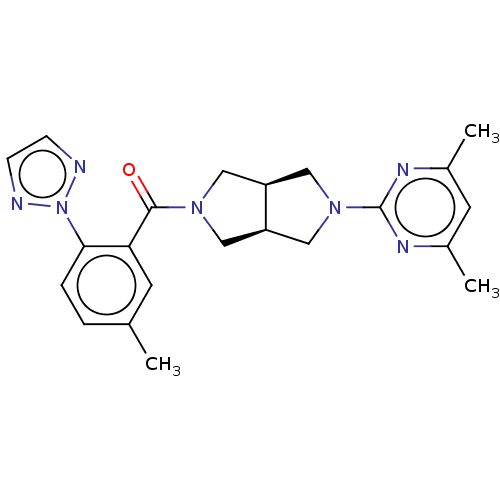
(CHEMBL3586439)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1cc(C)ccc1-n1nccn1 |r| Show InChI InChI=1S/C18H23N3O2/c1-21(2)14-7-12(8-14)16-9-19-17-4-3-11(6-15(16)17)5-13-10-23-18(22)20-13/h3-4,6,9,12-14,19H,5,7-8,10H2,1-2H3,(H,20,22)/t12?,13?,14- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50092811

(CHEMBL3586442)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1cc(Cl)ccc1-n1nccn1 |r| Show InChI InChI=1S/C21H22ClN7O/c1-13-7-14(2)26-21(25-13)28-11-15-9-27(10-16(15)12-28)20(30)18-8-17(22)3-4-19(18)29-23-5-6-24-29/h3-8,15-16H,9-12H2,1-2H3/t15-,16+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50097385
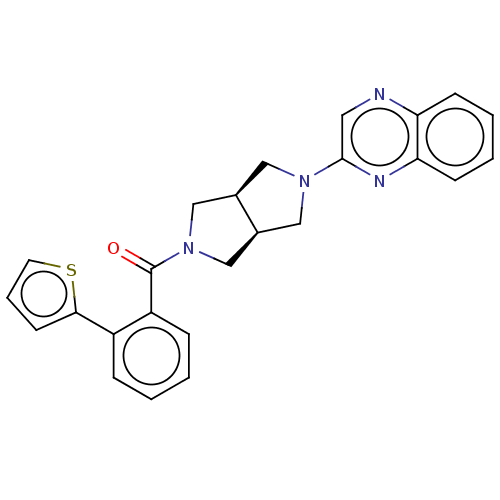
(CHEMBL3586425)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1cnc2ccccc2n1)C(=O)c1ccccc1-c1cccs1 |r| Show InChI InChI=1S/C25H22N4OS/c30-25(20-7-2-1-6-19(20)23-10-5-11-31-23)29-15-17-13-28(14-18(17)16-29)24-12-26-21-8-3-4-9-22(21)27-24/h1-12,17-18H,13-16H2/t17-,18+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50097377
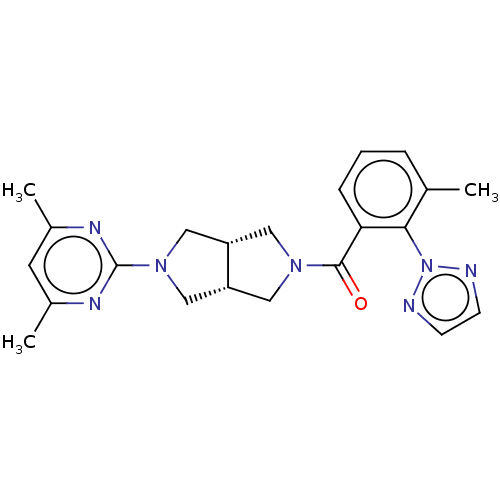
(CHEMBL3586438)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1cccc(C)c1-n1nccn1 |r| Show InChI InChI=1S/C22H25N7O/c1-14-5-4-6-19(20(14)29-23-7-8-24-29)21(30)27-10-17-12-28(13-18(17)11-27)22-25-15(2)9-16(3)26-22/h4-9,17-18H,10-13H2,1-3H3/t17-,18+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50092819
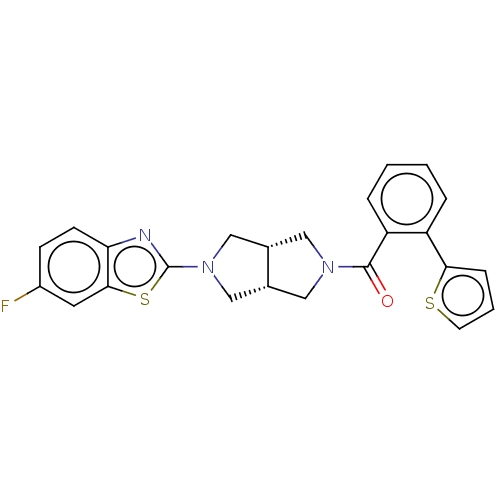
(CHEMBL3586420)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc2ccc(F)cc2s1)C(=O)c1ccccc1-c1cccs1 |r| Show InChI InChI=1S/C24H20FN3OS2/c25-17-7-8-20-22(10-17)31-24(26-20)28-13-15-11-27(12-16(15)14-28)23(29)19-5-2-1-4-18(19)21-6-3-9-30-21/h1-10,15-16H,11-14H2/t15-,16+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 137 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 in human liver microsomes using paclitaxel as substrate by LC-MS/MS analysis |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50097383

(CHEMBL3586428)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nccc(OC)n1)C(=O)c1c(C)ccc2ccccc12 |r| Show InChI InChI=1S/C23H24N4O2/c1-15-7-8-16-5-3-4-6-19(16)21(15)22(28)26-11-17-13-27(14-18(17)12-26)23-24-10-9-20(25-23)29-2/h3-10,17-18H,11-14H2,1-2H3/t17-,18+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 141 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50097382
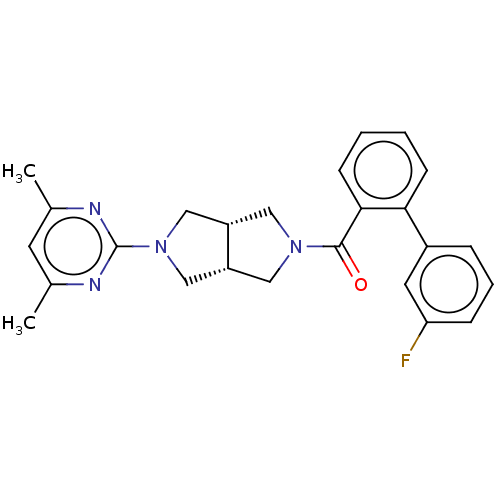
(CHEMBL3586429)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1ccccc1-c1cccc(F)c1 |r| Show InChI InChI=1S/C25H25FN4O/c1-16-10-17(2)28-25(27-16)30-14-19-12-29(13-20(19)15-30)24(31)23-9-4-3-8-22(23)18-6-5-7-21(26)11-18/h3-11,19-20H,12-15H2,1-2H3/t19-,20+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50092810

(CHEMBL3586412)Show SMILES COc1cccc(OC)c1C(=O)N1CCCN(CC1)c1nc2ccc(Cl)cc2s1 Show InChI InChI=1S/C21H22ClN3O3S/c1-27-16-5-3-6-17(28-2)19(16)20(26)24-9-4-10-25(12-11-24)21-23-15-8-7-14(22)13-18(15)29-21/h3,5-8,13H,4,9-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 216 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]EMPA from human OX2 receptor expressed in human PFSK-1 cells |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50097391
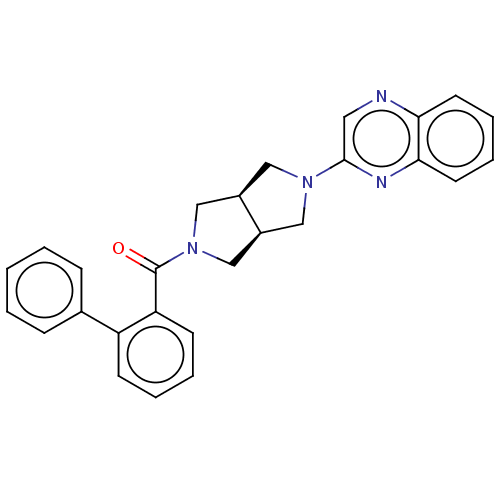
(CHEMBL3586422)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1cnc2ccccc2n1)C(=O)c1ccccc1-c1ccccc1 |r| Show InChI InChI=1S/C27H24N4O/c32-27(23-11-5-4-10-22(23)19-8-2-1-3-9-19)31-17-20-15-30(16-21(20)18-31)26-14-28-24-12-6-7-13-25(24)29-26/h1-14,20-21H,15-18H2/t20-,21+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50097381

(CHEMBL3586431)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(OC)cc(OC)n1)C(=O)c1ccccc1-c1cccs1 |r| Show InChI InChI=1S/C23H24N4O3S/c1-29-20-10-21(30-2)25-23(24-20)27-13-15-11-26(12-16(15)14-27)22(28)18-7-4-3-6-17(18)19-8-5-9-31-19/h3-10,15-16H,11-14H2,1-2H3/t15-,16+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 257 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of (S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-((3H)-1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2 rec... |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50092819

(CHEMBL3586420)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc2ccc(F)cc2s1)C(=O)c1ccccc1-c1cccs1 |r| Show InChI InChI=1S/C24H20FN3OS2/c25-17-7-8-20-22(10-17)31-24(26-20)28-13-15-11-27(12-16(15)14-28)23(29)19-5-2-1-4-18(19)21-6-3-9-30-21/h1-10,15-16H,11-14H2/t15-,16+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 267 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human OX1 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50092818

(CHEMBL3586419)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc2ccc(F)cc2s1)C(=O)c1ccccc1-c1ccccc1 |r| Show InChI InChI=1S/C26H22FN3OS/c27-20-10-11-23-24(12-20)32-26(28-23)30-15-18-13-29(14-19(18)16-30)25(31)22-9-5-4-8-21(22)17-6-2-1-3-7-17/h1-12,18-19H,13-16H2/t18-,19+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 294 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate by LC-MS/MS analysis |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50097360

(CHEMBL3586440)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1c(C)cccc1-n1nccn1 |r| Show InChI InChI=1S/C22H25N7O/c1-14-5-4-6-19(29-23-7-8-24-29)20(14)21(30)27-10-17-12-28(13-18(17)11-27)22-25-15(2)9-16(3)26-22/h4-9,17-18H,10-13H2,1-3H3/t17-,18+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 346 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human OX1 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data