 Found 195 hits with Last Name = 'shen' and Initial = 'mw'
Found 195 hits with Last Name = 'shen' and Initial = 'mw' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239500
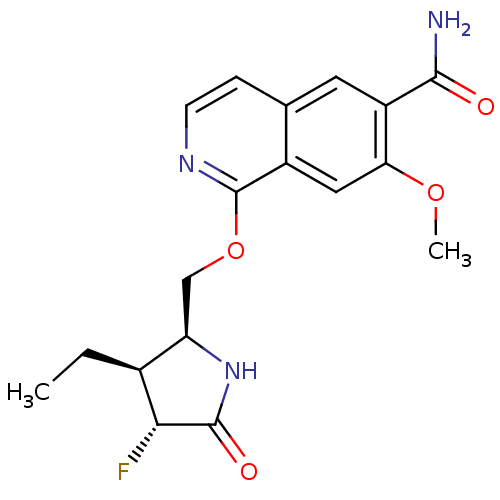
(CHEMBL4066705 | US10329302, Example 337 | US107935...)Show SMILES CC[C@H]1[C@@H](COc2nccc3cc(C(N)=O)c(OC)cc23)NC(=O)[C@@H]1F |r| Show InChI InChI=1S/C18H20FN3O4/c1-3-10-13(22-17(24)15(10)19)8-26-18-11-7-14(25-2)12(16(20)23)6-9(11)4-5-21-18/h4-7,10,13,15H,3,8H2,1-2H3,(H2,20,23)(H,22,24)/t10-,13+,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239499

(CHEMBL4081711 | US10329302, Example 344 | US107935...)Show SMILES CC[C@H]1[C@@H](COc2nccc3cc(C(N)=O)c(OC)cc23)NC(=O)[C@H]1F |r| Show InChI InChI=1S/C18H20FN3O4/c1-3-10-13(22-17(24)15(10)19)8-26-18-11-7-14(25-2)12(16(20)23)6-9(11)4-5-21-18/h4-7,10,13,15H,3,8H2,1-2H3,(H2,20,23)(H,22,24)/t10-,13+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239498
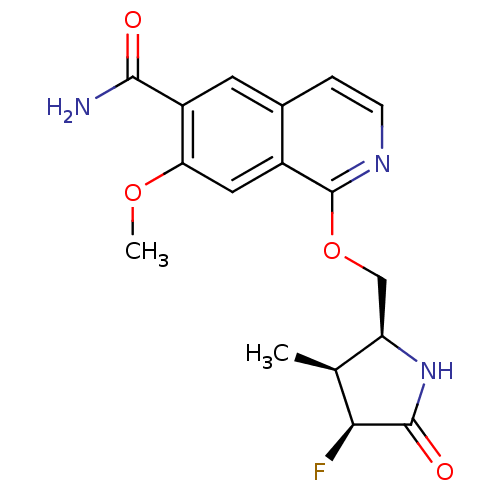
(CHEMBL4093120 | US10329302, Example 189 | US107935...)Show SMILES COc1cc2c(OC[C@H]3NC(=O)[C@@H](F)[C@H]3C)nccc2cc1C(N)=O |r| Show InChI InChI=1S/C17H18FN3O4/c1-8-12(21-16(23)14(8)18)7-25-17-10-6-13(24-2)11(15(19)22)5-9(10)3-4-20-17/h3-6,8,12,14H,7H2,1-2H3,(H2,19,22)(H,21,23)/t8-,12+,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239507
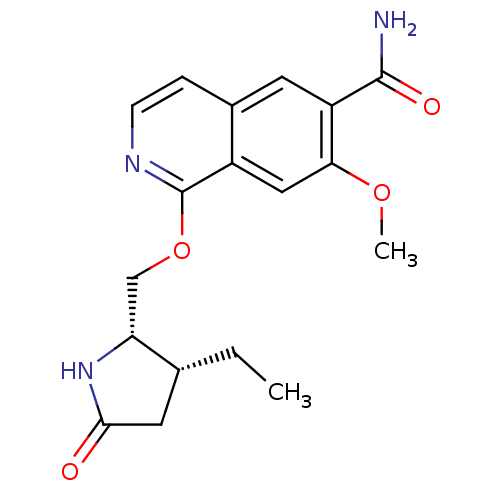
(CHEMBL4091434 | US10329302, Example 246 | US107935...)Show SMILES CC[C@@H]1CC(=O)N[C@@H]1COc1nccc2cc(C(N)=O)c(OC)cc12 |r| Show InChI InChI=1S/C18H21N3O4/c1-3-10-7-16(22)21-14(10)9-25-18-12-8-15(24-2)13(17(19)23)6-11(12)4-5-20-18/h4-6,8,10,14H,3,7,9H2,1-2H3,(H2,19,23)(H,21,22)/t10-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239508
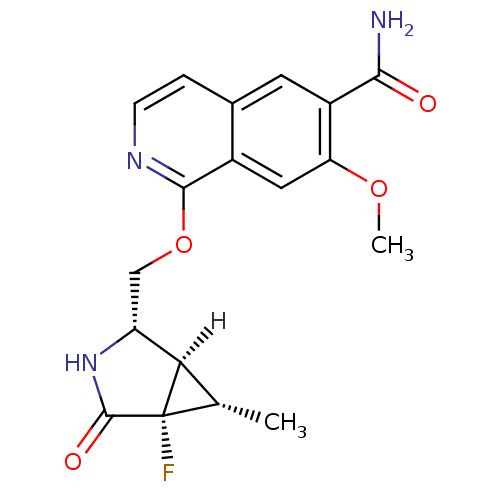
(CHEMBL4085199 | US10329302, Example 309 | US107935...)Show SMILES [H][C@]12[C@H](C)[C@@]1(F)C(=O)N[C@@H]2COc1nccc2cc(C(N)=O)c(OC)cc12 |r| Show InChI InChI=1S/C18H18FN3O4/c1-8-14-12(22-17(24)18(8,14)19)7-26-16-10-6-13(25-2)11(15(20)23)5-9(10)3-4-21-16/h3-6,8,12,14H,7H2,1-2H3,(H2,20,23)(H,22,24)/t8-,12+,14+,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239493
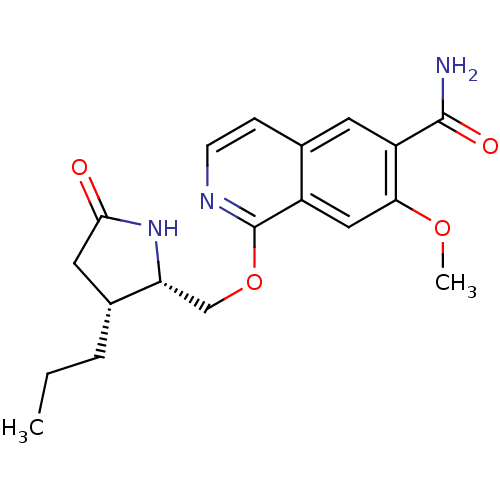
(CHEMBL4103497 | US10329302, Example 312 | US107935...)Show SMILES CCC[C@@H]1CC(=O)N[C@@H]1COc1nccc2cc(C(N)=O)c(OC)cc12 |r| Show InChI InChI=1S/C19H23N3O4/c1-3-4-12-8-17(23)22-15(12)10-26-19-13-9-16(25-2)14(18(20)24)7-11(13)5-6-21-19/h5-7,9,12,15H,3-4,8,10H2,1-2H3,(H2,20,24)(H,22,23)/t12-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239491
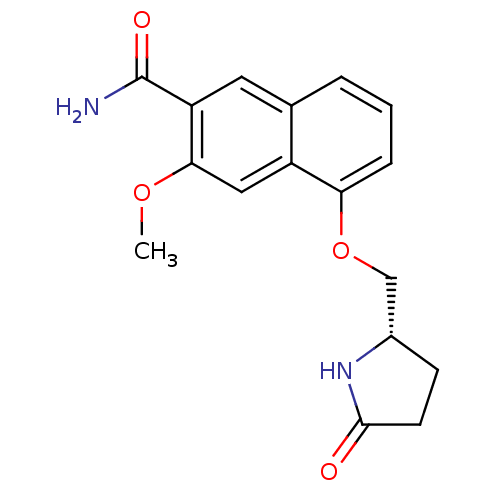
(CHEMBL4083655 | US10329302, Example 173 | US107935...)Show SMILES COc1cc2c(OC[C@@H]3CCC(=O)N3)cccc2cc1C(N)=O |r| Show InChI InChI=1S/C17H18N2O4/c1-22-15-8-12-10(7-13(15)17(18)21)3-2-4-14(12)23-9-11-5-6-16(20)19-11/h2-4,7-8,11H,5-6,9H2,1H3,(H2,18,21)(H,19,20)/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239506

(CHEMBL4071526 | US10329302, Example 188 | US107935...)Show SMILES COc1cc2c(OC[C@H]3NC(=O)[C@H](F)[C@H]3C)nccc2cc1C(N)=O |r| Show InChI InChI=1S/C17H18FN3O4/c1-8-12(21-16(23)14(8)18)7-25-17-10-6-13(24-2)11(15(19)22)5-9(10)3-4-20-17/h3-6,8,12,14H,7H2,1-2H3,(H2,19,22)(H,21,23)/t8-,12+,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239499

(CHEMBL4081711 | US10329302, Example 344 | US107935...)Show SMILES CC[C@H]1[C@@H](COc2nccc3cc(C(N)=O)c(OC)cc23)NC(=O)[C@H]1F |r| Show InChI InChI=1S/C18H20FN3O4/c1-3-10-13(22-17(24)15(10)19)8-26-18-11-7-14(25-2)12(16(20)23)6-9(11)4-5-21-18/h4-7,10,13,15H,3,8H2,1-2H3,(H2,20,23)(H,22,24)/t10-,13+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 in human PBMC assessed as reduction in R848-stimulated TNF alpha production after 3 hrs |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239499

(CHEMBL4081711 | US10329302, Example 344 | US107935...)Show SMILES CC[C@H]1[C@@H](COc2nccc3cc(C(N)=O)c(OC)cc23)NC(=O)[C@H]1F |r| Show InChI InChI=1S/C18H20FN3O4/c1-3-10-13(22-17(24)15(10)19)8-26-18-11-7-14(25-2)12(16(20)23)6-9(11)4-5-21-18/h4-7,10,13,15H,3,8H2,1-2H3,(H2,20,23)(H,22,24)/t10-,13+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 in human whole blood assessed as reduction R848-induced IL-6 secretion by measuring plasma protein binding corrected IC50 preincu... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239499

(CHEMBL4081711 | US10329302, Example 344 | US107935...)Show SMILES CC[C@H]1[C@@H](COc2nccc3cc(C(N)=O)c(OC)cc23)NC(=O)[C@H]1F |r| Show InChI InChI=1S/C18H20FN3O4/c1-3-10-13(22-17(24)15(10)19)8-26-18-11-7-14(25-2)12(16(20)23)6-9(11)4-5-21-18/h4-7,10,13,15H,3,8H2,1-2H3,(H2,20,23)(H,22,24)/t10-,13+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 in human PBMC assessed as reduction in R848-stimulated TNF alpha production by measuring plasma protein binding corrected IC50 af... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239497
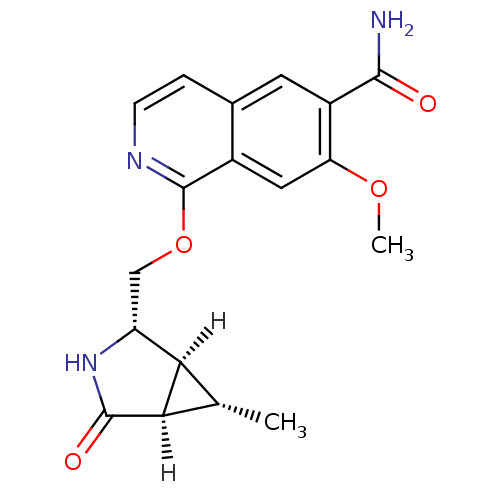
(CHEMBL4084228 | US10329302, Example 271 | US107935...)Show SMILES [H][C@]12[C@H](C)[C@@]1([H])C(=O)N[C@@H]2COc1nccc2cc(C(N)=O)c(OC)cc12 |r| Show InChI InChI=1S/C18H19N3O4/c1-8-14-12(21-17(23)15(8)14)7-25-18-10-6-13(24-2)11(16(19)22)5-9(10)3-4-20-18/h3-6,8,12,14-15H,7H2,1-2H3,(H2,19,22)(H,21,23)/t8-,12+,14+,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239492

(CHEMBL4070515 | US10329302, Example 211 | US107935...)Show SMILES COc1cc2c(OC[C@H]3NC(=O)C[C@H]3C)nccc2cc1C(N)=O |r| Show InChI InChI=1S/C17H19N3O4/c1-9-5-15(21)20-13(9)8-24-17-11-7-14(23-2)12(16(18)22)6-10(11)3-4-19-17/h3-4,6-7,9,13H,5,8H2,1-2H3,(H2,18,22)(H,20,21)/t9-,13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239505
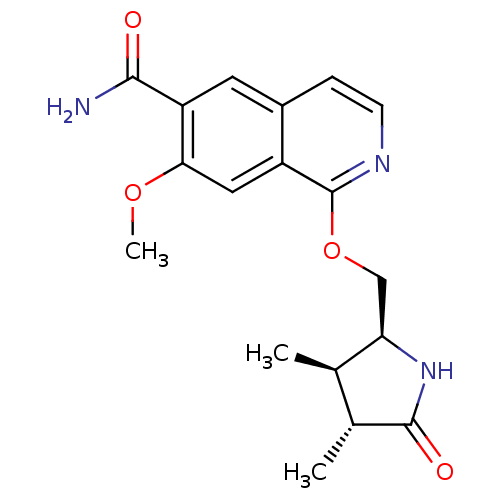
(CHEMBL4061801 | US10329302, Example 248 | US107935...)Show SMILES COc1cc2c(OC[C@H]3NC(=O)[C@H](C)[C@H]3C)nccc2cc1C(N)=O |r| Show InChI InChI=1S/C18H21N3O4/c1-9-10(2)17(23)21-14(9)8-25-18-12-7-15(24-3)13(16(19)22)6-11(12)4-5-20-18/h4-7,9-10,14H,8H2,1-3H3,(H2,19,22)(H,21,23)/t9-,10-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239488
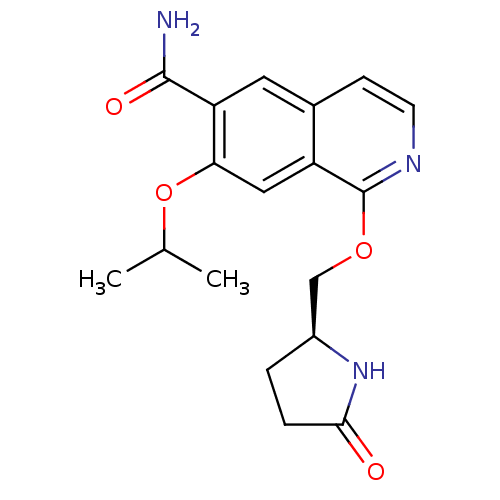
(CHEMBL4092338 | US10329302, Example 26 | US1079357...)Show SMILES CC(C)Oc1cc2c(OC[C@@H]3CCC(=O)N3)nccc2cc1C(N)=O |r| Show InChI InChI=1S/C18H21N3O4/c1-10(2)25-15-8-13-11(7-14(15)17(19)23)5-6-20-18(13)24-9-12-3-4-16(22)21-12/h5-8,10,12H,3-4,9H2,1-2H3,(H2,19,23)(H,21,22)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239496

(CHEMBL4075552 | US10329302, Example 264 | US107935...)Show SMILES [H][C@]12C[C@@]1([H])C(=O)N[C@@H]2COc1nccc2cc(C(N)=O)c(OC)cc12 |r| Show InChI InChI=1S/C17H17N3O4/c1-23-14-6-9-8(4-12(14)15(18)21)2-3-19-17(9)24-7-13-10-5-11(10)16(22)20-13/h2-4,6,10-11,13H,5,7H2,1H3,(H2,18,21)(H,20,22)/t10-,11+,13+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239504

(CHEMBL4061890 | US10329302, Example 262 | US107935...)Show SMILES COc1cc2c(OC[C@@H]3CCC(=O)N3)ccnc2cc1C(N)=O |r| Show InChI InChI=1S/C16H17N3O4/c1-22-14-7-10-12(6-11(14)16(17)21)18-5-4-13(10)23-8-9-2-3-15(20)19-9/h4-7,9H,2-3,8H2,1H3,(H2,17,21)(H,19,20)/t9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Cytosolic phospholipase A2
(Homo sapiens (Human)) | BDBM50292791

(4-{3-[2-{2-[({2-[(4-Acetylpiperazin-1-yl)methyl]be...)Show SMILES CC(=O)N1CCN(Cc2ccccc2CS(=O)(=O)NCCc2c(CCCc3ccc(cc3)C(O)=O)c3cc(Cl)ccc3n2C(c2ccccc2)c2ccccc2)CC1 Show InChI InChI=1S/C47H49ClN4O5S/c1-34(53)51-29-27-50(28-30-51)32-39-16-8-9-17-40(39)33-58(56,57)49-26-25-45-42(18-10-11-35-19-21-38(22-20-35)47(54)55)43-31-41(48)23-24-44(43)52(45)46(36-12-4-2-5-13-36)37-14-6-3-7-15-37/h2-9,12-17,19-24,31,46,49H,10-11,18,25-30,32-33H2,1H3,(H,54,55) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human cytosolic PLA2alpha by GLU micelle assay |
J Med Chem 52: 1156-71 (2009)
Article DOI: 10.1021/jm8009876
BindingDB Entry DOI: 10.7270/Q20C4VSK |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239500

(CHEMBL4066705 | US10329302, Example 337 | US107935...)Show SMILES CC[C@H]1[C@@H](COc2nccc3cc(C(N)=O)c(OC)cc23)NC(=O)[C@@H]1F |r| Show InChI InChI=1S/C18H20FN3O4/c1-3-10-13(22-17(24)15(10)19)8-26-18-11-7-14(25-2)12(16(20)23)6-9(11)4-5-21-18/h4-7,10,13,15H,3,8H2,1-2H3,(H2,20,23)(H,22,24)/t10-,13+,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 in human PBMC assessed as reduction in R848-stimulated TNF alpha production after 3 hrs |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239489
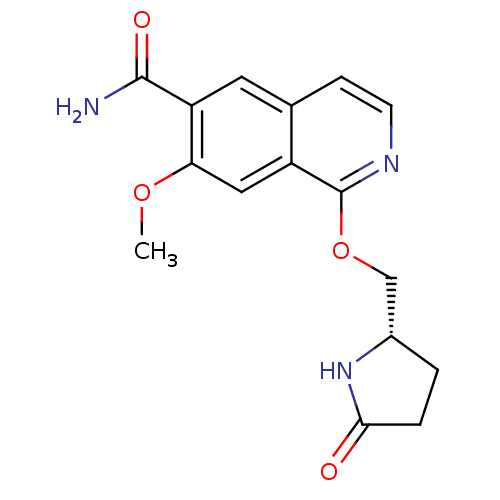
(CHEMBL4100091 | US10329302, Example 121 | US107935...)Show SMILES COc1cc2c(OC[C@@H]3CCC(=O)N3)nccc2cc1C(N)=O |r| Show InChI InChI=1S/C16H17N3O4/c1-22-13-7-11-9(6-12(13)15(17)21)4-5-18-16(11)23-8-10-2-3-14(20)19-10/h4-7,10H,2-3,8H2,1H3,(H2,17,21)(H,19,20)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239507

(CHEMBL4091434 | US10329302, Example 246 | US107935...)Show SMILES CC[C@@H]1CC(=O)N[C@@H]1COc1nccc2cc(C(N)=O)c(OC)cc12 |r| Show InChI InChI=1S/C18H21N3O4/c1-3-10-7-16(22)21-14(10)9-25-18-12-8-15(24-2)13(17(19)23)6-11(12)4-5-20-18/h4-6,8,10,14H,3,7,9H2,1-2H3,(H2,19,23)(H,21,22)/t10-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 in human PBMC assessed as reduction in R848-stimulated TNF alpha production after 3 hrs |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Cytosolic phospholipase A2
(Homo sapiens (Human)) | BDBM50292792
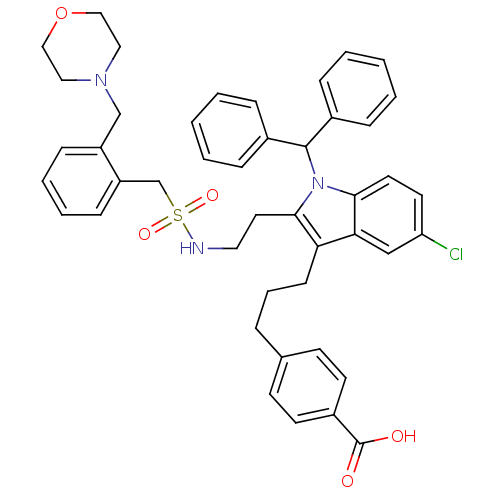
(4-(3-{5-Chloro-1-(diphenylmethyl)-2-[2-({[2-(morph...)Show SMILES OC(=O)c1ccc(CCCc2c(CCNS(=O)(=O)Cc3ccccc3CN3CCOCC3)n(C(c3ccccc3)c3ccccc3)c3ccc(Cl)cc23)cc1 Show InChI InChI=1S/C45H46ClN3O5S/c46-39-22-23-42-41(30-39)40(17-9-10-33-18-20-36(21-19-33)45(50)51)43(49(42)44(34-11-3-1-4-12-34)35-13-5-2-6-14-35)24-25-47-55(52,53)32-38-16-8-7-15-37(38)31-48-26-28-54-29-27-48/h1-8,11-16,18-23,30,44,47H,9-10,17,24-29,31-32H2,(H,50,51) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human cytosolic PLA2alpha by GLU micelle assay |
J Med Chem 52: 1156-71 (2009)
Article DOI: 10.1021/jm8009876
BindingDB Entry DOI: 10.7270/Q20C4VSK |
More data for this
Ligand-Target Pair | |
Cytosolic phospholipase A2
(Homo sapiens (Human)) | BDBM50226793

(4-(3-(1-benzhydryl-5-chloro-2-(2-((2,6-dimethylphe...)Show SMILES Cc1cccc(C)c1CS(=O)(=O)NCCc1c(CCCc2ccc(cc2)C(O)=O)c2cc(Cl)ccc2n1C(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C42H41ClN2O4S/c1-29-11-9-12-30(2)38(29)28-50(48,49)44-26-25-40-36(18-10-13-31-19-21-34(22-20-31)42(46)47)37-27-35(43)23-24-39(37)45(40)41(32-14-5-3-6-15-32)33-16-7-4-8-17-33/h3-9,11-12,14-17,19-24,27,41,44H,10,13,18,25-26,28H2,1-2H3,(H,46,47) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human cytosolic PLA2alpha by GLU micelle assay |
J Med Chem 52: 1156-71 (2009)
Article DOI: 10.1021/jm8009876
BindingDB Entry DOI: 10.7270/Q20C4VSK |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239494

(CHEMBL4079243 | US10329302, Example 340 | US107935...)Show SMILES COC[C@@H]1CC(=O)N[C@@H]1COc1nccc2cc(C(N)=O)c(OC)cc12 |r| Show InChI InChI=1S/C18H21N3O5/c1-24-8-11-6-16(22)21-14(11)9-26-18-12-7-15(25-2)13(17(19)23)5-10(12)3-4-20-18/h3-5,7,11,14H,6,8-9H2,1-2H3,(H2,19,23)(H,21,22)/t11-,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Cytosolic phospholipase A2
(Homo sapiens (Human)) | BDBM50292790
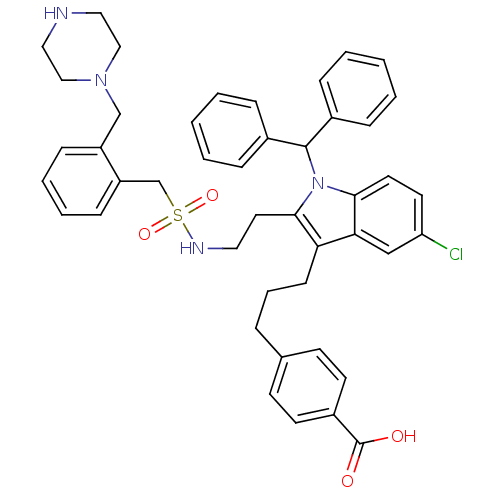
(4-(3-{5-Chloro-1-(diphenylmethyl)-2-[2-({[2-(piper...)Show SMILES OC(=O)c1ccc(CCCc2c(CCNS(=O)(=O)Cc3ccccc3CN3CCNCC3)n(C(c3ccccc3)c3ccccc3)c3ccc(Cl)cc23)cc1 Show InChI InChI=1S/C45H47ClN4O4S/c46-39-22-23-42-41(30-39)40(17-9-10-33-18-20-36(21-19-33)45(51)52)43(50(42)44(34-11-3-1-4-12-34)35-13-5-2-6-14-35)24-25-48-55(53,54)32-38-16-8-7-15-37(38)31-49-28-26-47-27-29-49/h1-8,11-16,18-23,30,44,47-48H,9-10,17,24-29,31-32H2,(H,51,52) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human cytosolic PLA2alpha by GLU micelle assay |
J Med Chem 52: 1156-71 (2009)
Article DOI: 10.1021/jm8009876
BindingDB Entry DOI: 10.7270/Q20C4VSK |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239498

(CHEMBL4093120 | US10329302, Example 189 | US107935...)Show SMILES COc1cc2c(OC[C@H]3NC(=O)[C@@H](F)[C@H]3C)nccc2cc1C(N)=O |r| Show InChI InChI=1S/C17H18FN3O4/c1-8-12(21-16(23)14(8)18)7-25-17-10-6-13(24-2)11(15(19)22)5-9(10)3-4-20-17/h3-6,8,12,14H,7H2,1-2H3,(H2,19,22)(H,21,23)/t8-,12+,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 in human PBMC assessed as reduction in R848-stimulated TNF alpha production after 3 hrs |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239508

(CHEMBL4085199 | US10329302, Example 309 | US107935...)Show SMILES [H][C@]12[C@H](C)[C@@]1(F)C(=O)N[C@@H]2COc1nccc2cc(C(N)=O)c(OC)cc12 |r| Show InChI InChI=1S/C18H18FN3O4/c1-8-14-12(22-17(24)18(8,14)19)7-26-16-10-6-13(25-2)11(15(20)23)5-9(10)3-4-21-16/h3-6,8,12,14H,7H2,1-2H3,(H2,20,23)(H,22,24)/t8-,12+,14+,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 in human PBMC assessed as reduction in R848-stimulated TNF alpha production after 3 hrs |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Cytosolic phospholipase A2
(Homo sapiens (Human)) | BDBM50292789

(4-[3-(5-Chloro-1-(diphenylmethyl)-2-{2-[({2-[(4-me...)Show SMILES CN1CCN(Cc2ccccc2CS(=O)(=O)NCCc2c(CCCc3ccc(cc3)C(O)=O)c3cc(Cl)ccc3n2C(c2ccccc2)c2ccccc2)CC1 Show InChI InChI=1S/C46H49ClN4O4S/c1-49-27-29-50(30-28-49)32-38-16-8-9-17-39(38)33-56(54,55)48-26-25-44-41(18-10-11-34-19-21-37(22-20-34)46(52)53)42-31-40(47)23-24-43(42)51(44)45(35-12-4-2-5-13-35)36-14-6-3-7-15-36/h2-9,12-17,19-24,31,45,48H,10-11,18,25-30,32-33H2,1H3,(H,52,53) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human cytosolic PLA2alpha by GLU micelle assay |
J Med Chem 52: 1156-71 (2009)
Article DOI: 10.1021/jm8009876
BindingDB Entry DOI: 10.7270/Q20C4VSK |
More data for this
Ligand-Target Pair | |
Cytosolic phospholipase A2
(Homo sapiens (Human)) | BDBM50292793
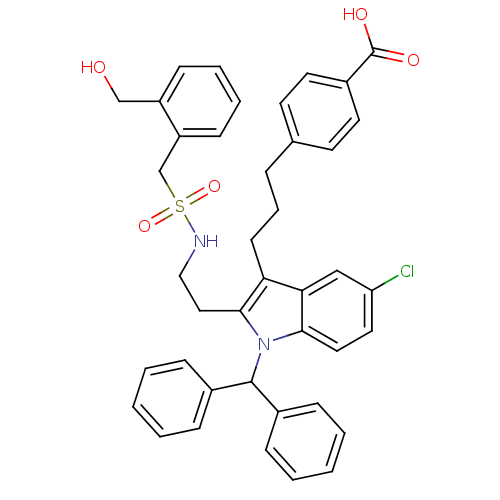
(4-(3-{5-Chloro-1-(diphenylmethyl)-2-[2-({[2-(hydro...)Show SMILES OCc1ccccc1CS(=O)(=O)NCCc1c(CCCc2ccc(cc2)C(O)=O)c2cc(Cl)ccc2n1C(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C41H39ClN2O5S/c42-35-22-23-38-37(26-35)36(17-9-10-29-18-20-32(21-19-29)41(46)47)39(24-25-43-50(48,49)28-34-16-8-7-15-33(34)27-45)44(38)40(30-11-3-1-4-12-30)31-13-5-2-6-14-31/h1-8,11-16,18-23,26,40,43,45H,9-10,17,24-25,27-28H2,(H,46,47) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human cytosolic PLA2alpha by GLU micelle assay |
J Med Chem 52: 1156-71 (2009)
Article DOI: 10.1021/jm8009876
BindingDB Entry DOI: 10.7270/Q20C4VSK |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239490
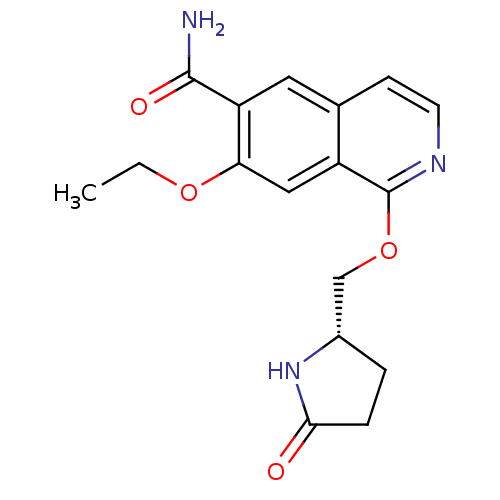
(CHEMBL4073250 | US10329302, Example 122 | US117024...)Show SMILES CCOc1cc2c(OC[C@@H]3CCC(=O)N3)nccc2cc1C(N)=O |r| Show InChI InChI=1S/C17H19N3O4/c1-2-23-14-8-12-10(7-13(14)16(18)22)5-6-19-17(12)24-9-11-3-4-15(21)20-11/h5-8,11H,2-4,9H2,1H3,(H2,18,22)(H,20,21)/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Cytosolic phospholipase A2
(Homo sapiens (Human)) | BDBM50247699

(4-(3-(1-benzhydryl-5-chloro-2-(2-(o-tolylmethylsul...)Show SMILES Cc1ccccc1CS(=O)(=O)NCCc1c(CCCc2ccc(cc2)C(O)=O)c2cc(Cl)ccc2n1C(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C41H39ClN2O4S/c1-29-11-8-9-17-34(29)28-49(47,48)43-26-25-39-36(18-10-12-30-19-21-33(22-20-30)41(45)46)37-27-35(42)23-24-38(37)44(39)40(31-13-4-2-5-14-31)32-15-6-3-7-16-32/h2-9,11,13-17,19-24,27,40,43H,10,12,18,25-26,28H2,1H3,(H,45,46) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human cytosolic PLA2alpha by GLU micelle assay |
J Med Chem 52: 1156-71 (2009)
Article DOI: 10.1021/jm8009876
BindingDB Entry DOI: 10.7270/Q20C4VSK |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239493

(CHEMBL4103497 | US10329302, Example 312 | US107935...)Show SMILES CCC[C@@H]1CC(=O)N[C@@H]1COc1nccc2cc(C(N)=O)c(OC)cc12 |r| Show InChI InChI=1S/C19H23N3O4/c1-3-4-12-8-17(23)22-15(12)10-26-19-13-9-16(25-2)14(18(20)24)7-11(13)5-6-21-19/h5-7,9,12,15H,3-4,8,10H2,1-2H3,(H2,20,24)(H,22,23)/t12-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 in human PBMC assessed as reduction in R848-stimulated TNF alpha production after 3 hrs |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239495

(CHEMBL4076912 | US10329302, Example 247 | US107935...)Show SMILES COc1cc2c(OC[C@H]3NC(=O)[C@@H](C)[C@H]3C)nccc2cc1C(N)=O |r| Show InChI InChI=1S/C18H21N3O4/c1-9-10(2)17(23)21-14(9)8-25-18-12-7-15(24-3)13(16(19)22)6-11(12)4-5-20-18/h4-7,9-10,14H,8H2,1-3H3,(H2,19,22)(H,21,23)/t9-,10+,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239506

(CHEMBL4071526 | US10329302, Example 188 | US107935...)Show SMILES COc1cc2c(OC[C@H]3NC(=O)[C@H](F)[C@H]3C)nccc2cc1C(N)=O |r| Show InChI InChI=1S/C17H18FN3O4/c1-8-12(21-16(23)14(8)18)7-25-17-10-6-13(24-2)11(15(19)22)5-9(10)3-4-20-17/h3-6,8,12,14H,7H2,1-2H3,(H2,19,22)(H,21,23)/t8-,12+,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 in human PBMC assessed as reduction in R848-stimulated TNF alpha production after 3 hrs |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Cytosolic phospholipase A2
(Homo sapiens (Human)) | BDBM50292794

(4-{3-[5-Chloro-2-{2-[({2-[(diethylamino)methyl]ben...)Show SMILES CCN(CC)Cc1ccccc1CS(=O)(=O)NCCc1c(CCCc2ccc(cc2)C(O)=O)c2cc(Cl)ccc2n1C(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C45H48ClN3O4S/c1-3-48(4-2)31-37-19-11-12-20-38(37)32-54(52,53)47-29-28-43-40(21-13-14-33-22-24-36(25-23-33)45(50)51)41-30-39(46)26-27-42(41)49(43)44(34-15-7-5-8-16-34)35-17-9-6-10-18-35/h5-12,15-20,22-27,30,44,47H,3-4,13-14,21,28-29,31-32H2,1-2H3,(H,50,51) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human cytosolic PLA2alpha by GLU micelle assay |
J Med Chem 52: 1156-71 (2009)
Article DOI: 10.1021/jm8009876
BindingDB Entry DOI: 10.7270/Q20C4VSK |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239491

(CHEMBL4083655 | US10329302, Example 173 | US107935...)Show SMILES COc1cc2c(OC[C@@H]3CCC(=O)N3)cccc2cc1C(N)=O |r| Show InChI InChI=1S/C17H18N2O4/c1-22-15-8-12-10(7-13(15)17(18)21)3-2-4-14(12)23-9-11-5-6-16(20)19-11/h2-4,7-8,11H,5-6,9H2,1H3,(H2,18,21)(H,19,20)/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 in human PBMC assessed as reduction in R848-stimulated TNF alpha production after 3 hrs |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Cytosolic phospholipase A2
(Homo sapiens (Human)) | BDBM50226792

(4-(3-(1-benzhydryl-5-chloro-2-(2-((3,4-dichlorophe...)Show SMILES OC(=O)c1ccc(CCCc2c(CCNS(=O)(=O)Cc3ccc(Cl)c(Cl)c3)n(C(c3ccccc3)c3ccccc3)c3ccc(Cl)cc23)cc1 Show InChI InChI=1S/C40H35Cl3N2O4S/c41-32-19-21-37-34(25-32)33(13-7-8-27-14-17-31(18-15-27)40(46)47)38(22-23-44-50(48,49)26-28-16-20-35(42)36(43)24-28)45(37)39(29-9-3-1-4-10-29)30-11-5-2-6-12-30/h1-6,9-12,14-21,24-25,39,44H,7-8,13,22-23,26H2,(H,46,47) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human cytosolic PLA2alpha by GLU micelle assay |
J Med Chem 52: 1156-71 (2009)
Article DOI: 10.1021/jm8009876
BindingDB Entry DOI: 10.7270/Q20C4VSK |
More data for this
Ligand-Target Pair | |
Cytosolic phospholipase A2
(Homo sapiens (Human)) | BDBM50110881
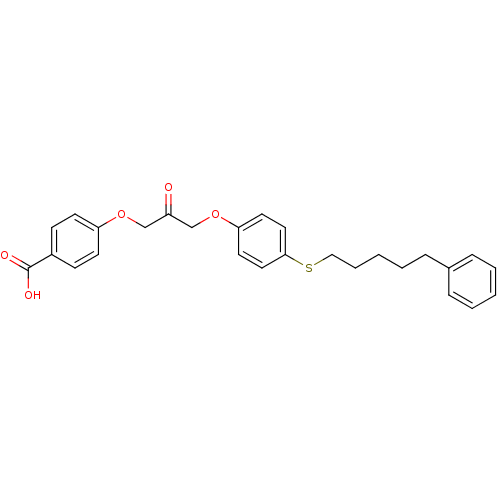
(4-(2-oxo-3-(4-(5-phenylpentylthio)phenoxy)propoxy)...)Show SMILES OC(=O)c1ccc(OCC(=O)COc2ccc(SCCCCCc3ccccc3)cc2)cc1 Show InChI InChI=1S/C27H28O5S/c28-23(19-31-24-12-10-22(11-13-24)27(29)30)20-32-25-14-16-26(17-15-25)33-18-6-2-5-9-21-7-3-1-4-8-21/h1,3-4,7-8,10-17H,2,5-6,9,18-20H2,(H,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of cytosolic phospholipase A2-alpha in GLU micelle assay |
J Med Chem 49: 135-58 (2006)
Article DOI: 10.1021/jm0507882
BindingDB Entry DOI: 10.7270/Q2MC8ZMP |
More data for this
Ligand-Target Pair | |
Cytosolic phospholipase A2
(Homo sapiens (Human)) | BDBM50205525
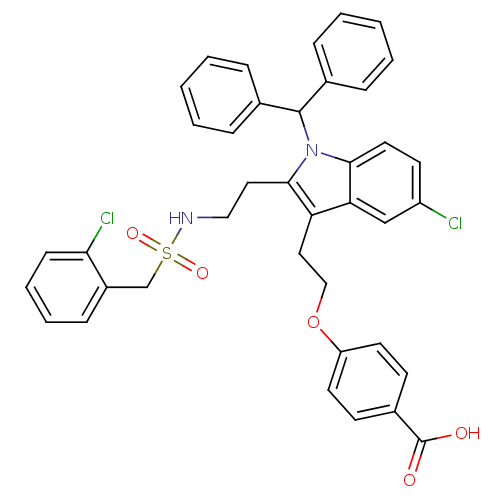
(4-{2-[5-Chloro-2-(2-{[(2-chlorobenzyl)sulfonyl]ami...)Show SMILES OC(=O)c1ccc(OCCc2c(CCNS(=O)(=O)Cc3ccccc3Cl)n(C(c3ccccc3)c3ccccc3)c3ccc(Cl)cc23)cc1 Show InChI InChI=1S/C39H34Cl2N2O5S/c40-31-17-20-36-34(25-31)33(22-24-48-32-18-15-29(16-19-32)39(44)45)37(21-23-42-49(46,47)26-30-13-7-8-14-35(30)41)43(36)38(27-9-3-1-4-10-27)28-11-5-2-6-12-28/h1-20,25,38,42H,21-24,26H2,(H,44,45) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human cPLA2 alpha by GLU micelle assay |
J Med Chem 50: 1380-400 (2007)
Article DOI: 10.1021/jm061131z
BindingDB Entry DOI: 10.7270/Q23F4PB9 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239492

(CHEMBL4070515 | US10329302, Example 211 | US107935...)Show SMILES COc1cc2c(OC[C@H]3NC(=O)C[C@H]3C)nccc2cc1C(N)=O |r| Show InChI InChI=1S/C17H19N3O4/c1-9-5-15(21)20-13(9)8-24-17-11-7-14(23-2)12(16(18)22)6-10(11)3-4-19-17/h3-4,6-7,9,13H,5,8H2,1-2H3,(H2,18,22)(H,20,21)/t9-,13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 in human PBMC assessed as reduction in R848-stimulated TNF alpha production after 3 hrs |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239497

(CHEMBL4084228 | US10329302, Example 271 | US107935...)Show SMILES [H][C@]12[C@H](C)[C@@]1([H])C(=O)N[C@@H]2COc1nccc2cc(C(N)=O)c(OC)cc12 |r| Show InChI InChI=1S/C18H19N3O4/c1-8-14-12(21-17(23)15(8)14)7-25-18-10-6-13(24-2)11(16(19)22)5-9(10)3-4-20-18/h3-6,8,12,14-15H,7H2,1-2H3,(H2,19,22)(H,21,23)/t8-,12+,14+,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 in human PBMC assessed as reduction in R848-stimulated TNF alpha production after 3 hrs |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239509

(CHEMBL4086222)Show InChI InChI=1S/C21H18N2O2/c1-13(2)25-20-11-18-16(10-19(20)21(23)24)4-3-5-17(18)15-8-6-14(12-22)7-9-15/h3-11,13H,1-2H3,(H2,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239505

(CHEMBL4061801 | US10329302, Example 248 | US107935...)Show SMILES COc1cc2c(OC[C@H]3NC(=O)[C@H](C)[C@H]3C)nccc2cc1C(N)=O |r| Show InChI InChI=1S/C18H21N3O4/c1-9-10(2)17(23)21-14(9)8-25-18-12-7-15(24-3)13(16(19)22)6-11(12)4-5-20-18/h4-7,9-10,14H,8H2,1-3H3,(H2,19,22)(H,21,23)/t9-,10-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 in human PBMC assessed as reduction in R848-stimulated TNF alpha production after 3 hrs |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Cytosolic phospholipase A2
(Homo sapiens (Human)) | BDBM50226800
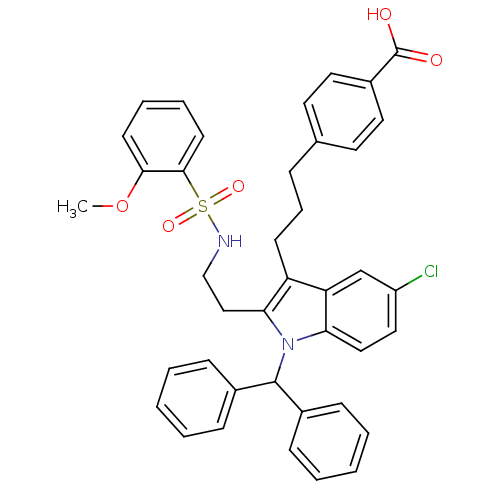
(4-(3-(1-benzhydryl-5-chloro-2-(2-(2-methoxyphenyls...)Show SMILES COc1ccccc1S(=O)(=O)NCCc1c(CCCc2ccc(cc2)C(O)=O)c2cc(Cl)ccc2n1C(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C40H37ClN2O5S/c1-48-37-17-8-9-18-38(37)49(46,47)42-26-25-36-33(16-10-11-28-19-21-31(22-20-28)40(44)45)34-27-32(41)23-24-35(34)43(36)39(29-12-4-2-5-13-29)30-14-6-3-7-15-30/h2-9,12-15,17-24,27,39,42H,10-11,16,25-26H2,1H3,(H,44,45) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human cytosolic PLA2alpha by GLU micelle assay |
J Med Chem 52: 1156-71 (2009)
Article DOI: 10.1021/jm8009876
BindingDB Entry DOI: 10.7270/Q20C4VSK |
More data for this
Ligand-Target Pair | |
Cytosolic phospholipase A2
(Homo sapiens (Human)) | BDBM50292796
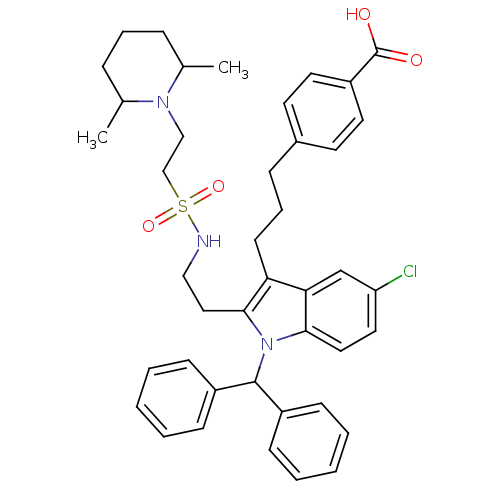
(4-(3-{1-Benzhydryl-5-chloro-2-[2-({[2-(2,6-dimethy...)Show SMILES CC1CCCC(C)N1CCS(=O)(=O)NCCc1c(CCCc2ccc(cc2)C(O)=O)c2cc(Cl)ccc2n1C(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C42H48ClN3O4S/c1-30-11-9-12-31(2)45(30)27-28-51(49,50)44-26-25-40-37(18-10-13-32-19-21-35(22-20-32)42(47)48)38-29-36(43)23-24-39(38)46(40)41(33-14-5-3-6-15-33)34-16-7-4-8-17-34/h3-8,14-17,19-24,29-31,41,44H,9-13,18,25-28H2,1-2H3,(H,47,48) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human cytosolic PLA2alpha by GLU micelle assay |
J Med Chem 52: 1156-71 (2009)
Article DOI: 10.1021/jm8009876
BindingDB Entry DOI: 10.7270/Q20C4VSK |
More data for this
Ligand-Target Pair | |
Cytosolic phospholipase A2
(Homo sapiens (Human)) | BDBM50205516
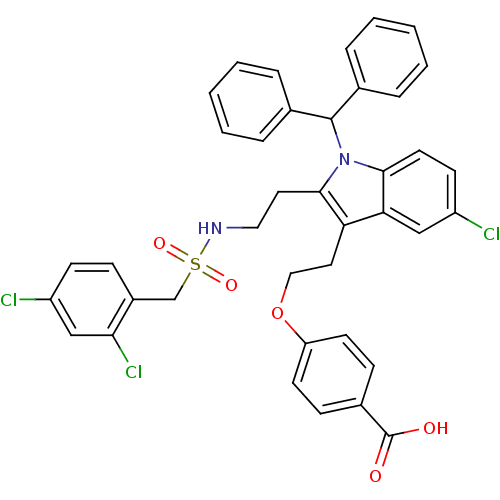
(4-{2-[5-chloro-2-(2-{[(2,4-dichlorobenzyl)sulfonyl...)Show SMILES OC(=O)c1ccc(OCCc2c(CCNS(=O)(=O)Cc3ccc(Cl)cc3Cl)n(C(c3ccccc3)c3ccccc3)c3ccc(Cl)cc23)cc1 Show InChI InChI=1S/C39H33Cl3N2O5S/c40-30-15-18-36-34(23-30)33(20-22-49-32-16-12-28(13-17-32)39(45)46)37(19-21-43-50(47,48)25-29-11-14-31(41)24-35(29)42)44(36)38(26-7-3-1-4-8-26)27-9-5-2-6-10-27/h1-18,23-24,38,43H,19-22,25H2,(H,45,46) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human cPLA2 alpha by GLU micelle assay |
J Med Chem 50: 1380-400 (2007)
Article DOI: 10.1021/jm061131z
BindingDB Entry DOI: 10.7270/Q23F4PB9 |
More data for this
Ligand-Target Pair | |
Cytosolic phospholipase A2
(Homo sapiens (Human)) | BDBM50205537

(4-(2-(1-benzhydryl-5-chloro-2-(2-(phenylmethylsulf...)Show SMILES OC(=O)c1ccc(OCCc2c(CCNS(=O)(=O)Cc3ccccc3)n(C(c3ccccc3)c3ccccc3)c3ccc(Cl)cc23)cc1 Show InChI InChI=1S/C39H35ClN2O5S/c40-32-18-21-36-35(26-32)34(23-25-47-33-19-16-31(17-20-33)39(43)44)37(22-24-41-48(45,46)27-28-10-4-1-5-11-28)42(36)38(29-12-6-2-7-13-29)30-14-8-3-9-15-30/h1-21,26,38,41H,22-25,27H2,(H,43,44) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human cytosolic PLA2alpha by GLU micelle assay |
J Med Chem 52: 1156-71 (2009)
Article DOI: 10.1021/jm8009876
BindingDB Entry DOI: 10.7270/Q20C4VSK |
More data for this
Ligand-Target Pair | |
Cytosolic phospholipase A2
(Homo sapiens (Human)) | BDBM50205537

(4-(2-(1-benzhydryl-5-chloro-2-(2-(phenylmethylsulf...)Show SMILES OC(=O)c1ccc(OCCc2c(CCNS(=O)(=O)Cc3ccccc3)n(C(c3ccccc3)c3ccccc3)c3ccc(Cl)cc23)cc1 Show InChI InChI=1S/C39H35ClN2O5S/c40-32-18-21-36-35(26-32)34(23-25-47-33-19-16-31(17-20-33)39(43)44)37(22-24-41-48(45,46)27-28-10-4-1-5-11-28)42(36)38(29-12-6-2-7-13-29)30-14-8-3-9-15-30/h1-21,26,38,41H,22-25,27H2,(H,43,44) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human cPLA2 alpha by GLU micelle assay |
J Med Chem 50: 1380-400 (2007)
Article DOI: 10.1021/jm061131z
BindingDB Entry DOI: 10.7270/Q23F4PB9 |
More data for this
Ligand-Target Pair | |
Cytosolic phospholipase A2
(Homo sapiens (Human)) | BDBM50205513

(4-{2-[5-chloro-2-(2-{[(2,3-dichlorobenzyl)sulfonyl...)Show SMILES OC(=O)c1ccc(OCCc2c(CCNS(=O)(=O)Cc3cccc(Cl)c3Cl)n(C(c3ccccc3)c3ccccc3)c3ccc(Cl)cc23)cc1 Show InChI InChI=1S/C39H33Cl3N2O5S/c40-30-16-19-35-33(24-30)32(21-23-49-31-17-14-28(15-18-31)39(45)46)36(20-22-43-50(47,48)25-29-12-7-13-34(41)37(29)42)44(35)38(26-8-3-1-4-9-26)27-10-5-2-6-11-27/h1-19,24,38,43H,20-23,25H2,(H,45,46) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human cPLA2 alpha by GLU micelle assay |
J Med Chem 50: 1380-400 (2007)
Article DOI: 10.1021/jm061131z
BindingDB Entry DOI: 10.7270/Q23F4PB9 |
More data for this
Ligand-Target Pair | |
Cytosolic phospholipase A2
(Homo sapiens (Human)) | BDBM50205527

(CHEMBL221504 | N-(((2S,4R)-1-(2-(2,4-difluorobenzo...)Show SMILES Fc1ccc(C(=O)c2ccccc2C(=O)N2C[C@@H](C[C@H]2CNC(=O)c2ccc(\C=C3/SC(=O)NC3=O)cc2)SC(c2ccccc2)(c2ccccc2)c2ccccc2)c(F)c1 Show InChI InChI=1S/C49H37F2N3O5S2/c50-36-24-25-41(42(51)27-36)44(55)39-18-10-11-19-40(39)47(58)54-30-38(28-37(54)29-52-45(56)32-22-20-31(21-23-32)26-43-46(57)53-48(59)60-43)61-49(33-12-4-1-5-13-33,34-14-6-2-7-15-34)35-16-8-3-9-17-35/h1-27,37-38H,28-30H2,(H,52,56)(H,53,57,59)/b43-26-/t37-,38+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human cPLA2 alpha by GLU micelle assay |
J Med Chem 50: 1380-400 (2007)
Article DOI: 10.1021/jm061131z
BindingDB Entry DOI: 10.7270/Q23F4PB9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data