 Found 70 hits with Last Name = 'sim' and Initial = 'at'
Found 70 hits with Last Name = 'sim' and Initial = 'at' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein phosphatase PP1-gamma catalytic subunit
(Homo sapiens (Human)) | BDBM50061067

(15-(3-Guanidino-propyl)-8-isobutyl-18-((1E,3E)-6-m...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](C)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](C)NC(=O)C(=C)N(C)C(=O)CC[C@@H](NC(=O)[C@H]1C)C(O)=O)C(O)=O Show InChI InChI=1S/C49H74N10O12/c1-26(2)23-37-46(66)58-40(48(69)70)30(6)42(62)55-35(17-14-22-52-49(50)51)45(65)54-34(19-18-27(3)24-28(4)38(71-10)25-33-15-12-11-13-16-33)29(5)41(61)56-36(47(67)68)20-21-39(60)59(9)32(8)44(64)53-31(7)43(63)57-37/h11-13,15-16,18-19,24,26,28-31,34-38,40H,8,14,17,20-23,25H2,1-7,9-10H3,(H,53,64)(H,54,65)(H,55,62)(H,56,61)(H,57,63)(H,58,66)(H,67,68)(H,69,70)(H4,50,51,52)/b19-18+,27-24+/t28-,29-,30-,31+,34-,35-,36+,37-,38-,40+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of Serine/threonine protein phosphatase 1 (PP1) mutants by natural toxins in E275R |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase PP1-gamma catalytic subunit
(Homo sapiens (Human)) | BDBM50061067

(15-(3-Guanidino-propyl)-8-isobutyl-18-((1E,3E)-6-m...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](C)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](C)NC(=O)C(=C)N(C)C(=O)CC[C@@H](NC(=O)[C@H]1C)C(O)=O)C(O)=O Show InChI InChI=1S/C49H74N10O12/c1-26(2)23-37-46(66)58-40(48(69)70)30(6)42(62)55-35(17-14-22-52-49(50)51)45(65)54-34(19-18-27(3)24-28(4)38(71-10)25-33-15-12-11-13-16-33)29(5)41(61)56-36(47(67)68)20-21-39(60)59(9)32(8)44(64)53-31(7)43(63)57-37/h11-13,15-16,18-19,24,26,28-31,34-38,40H,8,14,17,20-23,25H2,1-7,9-10H3,(H,53,64)(H,54,65)(H,55,62)(H,56,61)(H,57,63)(H,58,66)(H,67,68)(H,69,70)(H4,50,51,52)/b19-18+,27-24+/t28-,29-,30-,31+,34-,35-,36+,37-,38-,40+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of Serine/threonine protein phosphatase 1 (PP1) mutants by natural toxins in wild type |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase PP1-gamma catalytic subunit
(Homo sapiens (Human)) | BDBM50061067

(15-(3-Guanidino-propyl)-8-isobutyl-18-((1E,3E)-6-m...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](C)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](C)NC(=O)C(=C)N(C)C(=O)CC[C@@H](NC(=O)[C@H]1C)C(O)=O)C(O)=O Show InChI InChI=1S/C49H74N10O12/c1-26(2)23-37-46(66)58-40(48(69)70)30(6)42(62)55-35(17-14-22-52-49(50)51)45(65)54-34(19-18-27(3)24-28(4)38(71-10)25-33-15-12-11-13-16-33)29(5)41(61)56-36(47(67)68)20-21-39(60)59(9)32(8)44(64)53-31(7)43(63)57-37/h11-13,15-16,18-19,24,26,28-31,34-38,40H,8,14,17,20-23,25H2,1-7,9-10H3,(H,53,64)(H,54,65)(H,55,62)(H,56,61)(H,57,63)(H,58,66)(H,67,68)(H,69,70)(H4,50,51,52)/b19-18+,27-24+/t28-,29-,30-,31+,34-,35-,36+,37-,38-,40+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of Serine/threonine protein phosphatase 1 (PP1) mutants by natural toxins in D220V |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B
(Gallus gallus) | BDBM50110686

(14-Ethylidene-2-isopropyl-5-(6-methoxy-3,5-dimethy...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@@H]1NC(=O)[C@@H](NC(=O)[C@@H](C)[C@@H](CC(=O)C(=CC)N(C)C(=O)CC[C@@H](NC(=O)[C@H]1C)C(O)=O)C(O)=O)C(C)C |w:32.33| Show InChI InChI=1S/C41H58N4O10/c1-10-32-33(46)22-29(40(51)52)26(6)37(48)44-36(23(2)3)39(50)42-30(27(7)38(49)43-31(41(53)54)18-19-35(47)45(32)8)17-16-24(4)20-25(5)34(55-9)21-28-14-12-11-13-15-28/h10-17,20,23,25-27,29-31,34,36H,18-19,21-22H2,1-9H3,(H,42,50)(H,43,49)(H,44,48)(H,51,52)(H,53,54)/b17-16+,24-20+,32-10?/t25-,26-,27-,29+,30-,31+,34-,36-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Exogenous inhibition concentration of Serine/threonine protein phosphatase 2A (PP2A) |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B
(Gallus gallus) | BDBM50061067

(15-(3-Guanidino-propyl)-8-isobutyl-18-((1E,3E)-6-m...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](C)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](C)NC(=O)C(=C)N(C)C(=O)CC[C@@H](NC(=O)[C@H]1C)C(O)=O)C(O)=O Show InChI InChI=1S/C49H74N10O12/c1-26(2)23-37-46(66)58-40(48(69)70)30(6)42(62)55-35(17-14-22-52-49(50)51)45(65)54-34(19-18-27(3)24-28(4)38(71-10)25-33-15-12-11-13-16-33)29(5)41(61)56-36(47(67)68)20-21-39(60)59(9)32(8)44(64)53-31(7)43(63)57-37/h11-13,15-16,18-19,24,26,28-31,34-38,40H,8,14,17,20-23,25H2,1-7,9-10H3,(H,53,64)(H,54,65)(H,55,62)(H,56,61)(H,57,63)(H,58,66)(H,67,68)(H,69,70)(H4,50,51,52)/b19-18+,27-24+/t28-,29-,30-,31+,34-,35-,36+,37-,38-,40+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Exogenous inhibition concentration of Serine/threonine protein phosphatase 2A (PP2A) |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase PP1-gamma catalytic subunit
(Homo sapiens (Human)) | BDBM50110681

(CHEMBL430266 | Calyculin-A)Show SMILES COC[C@@H]([C@H](O)[C@H](O)C(=O)NCC[C@H](C)c1nc(\C=C\C[C@@H]2O[C@]3(C[C@@H](O)[C@@H]2C)O[C@H]([C@H](C[C@H](O)[C@H](C)[C@H](O)[C@H](C)\C=C(/C)\C(\C)=C\C=C\C(\C)=C/C#N)OC)[C@H](OP(O)(O)=O)C3(C)C)co1)N(C)C |r| Show InChI InChI=1S/C50H81N4O15P/c1-29(20-22-51)16-14-17-30(2)32(4)24-33(5)42(57)35(7)38(55)25-41(65-13)45-46(69-70(61,62)63)49(8,9)50(68-45)26-39(56)34(6)40(67-50)19-15-18-36-27-66-48(53-36)31(3)21-23-52-47(60)44(59)43(58)37(28-64-12)54(10)11/h14-18,20,24,27,31,33-35,37-46,55-59H,19,21,23,25-26,28H2,1-13H3,(H,52,60)(H2,61,62,63)/b16-14+,18-15+,29-20-,30-17+,32-24+/t31-,33+,34-,35-,37-,38-,39+,40-,41-,42+,43-,44-,45+,46-,50+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of Serine/threonine protein phosphatase 1 (PP1) mutants by natural toxins in D220V |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B
(Gallus gallus) | BDBM50110681

(CHEMBL430266 | Calyculin-A)Show SMILES COC[C@@H]([C@H](O)[C@H](O)C(=O)NCC[C@H](C)c1nc(\C=C\C[C@@H]2O[C@]3(C[C@@H](O)[C@@H]2C)O[C@H]([C@H](C[C@H](O)[C@H](C)[C@H](O)[C@H](C)\C=C(/C)\C(\C)=C\C=C\C(\C)=C/C#N)OC)[C@H](OP(O)(O)=O)C3(C)C)co1)N(C)C |r| Show InChI InChI=1S/C50H81N4O15P/c1-29(20-22-51)16-14-17-30(2)32(4)24-33(5)42(57)35(7)38(55)25-41(65-13)45-46(69-70(61,62)63)49(8,9)50(68-45)26-39(56)34(6)40(67-50)19-15-18-36-27-66-48(53-36)31(3)21-23-52-47(60)44(59)43(58)37(28-64-12)54(10)11/h14-18,20,24,27,31,33-35,37-46,55-59H,19,21,23,25-26,28H2,1-13H3,(H,52,60)(H2,61,62,63)/b16-14+,18-15+,29-20-,30-17+,32-24+/t31-,33+,34-,35-,37-,38-,39+,40-,41-,42+,43-,44-,45+,46-,50+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Exogenous inhibition concentration of Serine/threonine protein phosphatase 2A (PP2A) |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B
(Gallus gallus) | BDBM50110685

(25-Isobutyl-15-(6-methoxy-3,5-dimethyl-7-phenyl-he...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@@H]1NC(=O)[C@H](C)NC(=O)[C@@H](C)[C@@H](CC(=O)[C@H](CC(C)C)NC(=O)[C@@H](C)NC(=O)C(=C)N(C)C(=O)CC[C@@H](NC(=O)[C@H]1C)C(O)=O)C(O)=O Show InChI InChI=1S/C47H68N6O12/c1-25(2)21-37-38(54)24-34(46(61)62)28(5)41(56)48-30(7)43(58)50-35(18-17-26(3)22-27(4)39(65-11)23-33-15-13-12-14-16-33)29(6)42(57)51-36(47(63)64)19-20-40(55)53(10)32(9)45(60)49-31(8)44(59)52-37/h12-18,22,25,27-31,34-37,39H,9,19-21,23-24H2,1-8,10-11H3,(H,48,56)(H,49,60)(H,50,58)(H,51,57)(H,52,59)(H,61,62)(H,63,64)/b18-17+,26-22+/t27-,28-,29-,30-,31+,34+,35-,36+,37-,39-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of microcystin analogues to catalytic subunits of Serine/threonine protein phosphatase 2A (PP2Ac) |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase PP1-gamma catalytic subunit
(Homo sapiens (Human)) | BDBM50110681

(CHEMBL430266 | Calyculin-A)Show SMILES COC[C@@H]([C@H](O)[C@H](O)C(=O)NCC[C@H](C)c1nc(\C=C\C[C@@H]2O[C@]3(C[C@@H](O)[C@@H]2C)O[C@H]([C@H](C[C@H](O)[C@H](C)[C@H](O)[C@H](C)\C=C(/C)\C(\C)=C\C=C\C(\C)=C/C#N)OC)[C@H](OP(O)(O)=O)C3(C)C)co1)N(C)C |r| Show InChI InChI=1S/C50H81N4O15P/c1-29(20-22-51)16-14-17-30(2)32(4)24-33(5)42(57)35(7)38(55)25-41(65-13)45-46(69-70(61,62)63)49(8,9)50(68-45)26-39(56)34(6)40(67-50)19-15-18-36-27-66-48(53-36)31(3)21-23-52-47(60)44(59)43(58)37(28-64-12)54(10)11/h14-18,20,24,27,31,33-35,37-46,55-59H,19,21,23,25-26,28H2,1-13H3,(H,52,60)(H2,61,62,63)/b16-14+,18-15+,29-20-,30-17+,32-24+/t31-,33+,34-,35-,37-,38-,39+,40-,41-,42+,43-,44-,45+,46-,50+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of Serine/threonine protein phosphatase 1 (PP1) mutants by natural toxins in E275R |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine/threonine-protein phosphatase PP1-gamma catalytic subunit
(Homo sapiens (Human)) | BDBM50110681

(CHEMBL430266 | Calyculin-A)Show SMILES COC[C@@H]([C@H](O)[C@H](O)C(=O)NCC[C@H](C)c1nc(\C=C\C[C@@H]2O[C@]3(C[C@@H](O)[C@@H]2C)O[C@H]([C@H](C[C@H](O)[C@H](C)[C@H](O)[C@H](C)\C=C(/C)\C(\C)=C\C=C\C(\C)=C/C#N)OC)[C@H](OP(O)(O)=O)C3(C)C)co1)N(C)C |r| Show InChI InChI=1S/C50H81N4O15P/c1-29(20-22-51)16-14-17-30(2)32(4)24-33(5)42(57)35(7)38(55)25-41(65-13)45-46(69-70(61,62)63)49(8,9)50(68-45)26-39(56)34(6)40(67-50)19-15-18-36-27-66-48(53-36)31(3)21-23-52-47(60)44(59)43(58)37(28-64-12)54(10)11/h14-18,20,24,27,31,33-35,37-46,55-59H,19,21,23,25-26,28H2,1-13H3,(H,52,60)(H2,61,62,63)/b16-14+,18-15+,29-20-,30-17+,32-24+/t31-,33+,34-,35-,37-,38-,39+,40-,41-,42+,43-,44-,45+,46-,50+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of Serine/threonine protein phosphatase 1 (PP1) mutants by natural toxins in wild type |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50110681

(CHEMBL430266 | Calyculin-A)Show SMILES COC[C@@H]([C@H](O)[C@H](O)C(=O)NCC[C@H](C)c1nc(\C=C\C[C@@H]2O[C@]3(C[C@@H](O)[C@@H]2C)O[C@H]([C@H](C[C@H](O)[C@H](C)[C@H](O)[C@H](C)\C=C(/C)\C(\C)=C\C=C\C(\C)=C/C#N)OC)[C@H](OP(O)(O)=O)C3(C)C)co1)N(C)C |r| Show InChI InChI=1S/C50H81N4O15P/c1-29(20-22-51)16-14-17-30(2)32(4)24-33(5)42(57)35(7)38(55)25-41(65-13)45-46(69-70(61,62)63)49(8,9)50(68-45)26-39(56)34(6)40(67-50)19-15-18-36-27-66-48(53-36)31(3)21-23-52-47(60)44(59)43(58)37(28-64-12)54(10)11/h14-18,20,24,27,31,33-35,37-46,55-59H,19,21,23,25-26,28H2,1-13H3,(H,52,60)(H2,61,62,63)/b16-14+,18-15+,29-20-,30-17+,32-24+/t31-,33+,34-,35-,37-,38-,39+,40-,41-,42+,43-,44-,45+,46-,50+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Protein phosphatase 1 was determined; 0.5-1.0 |
Bioorg Med Chem Lett 12: 391-3 (2002)
BindingDB Entry DOI: 10.7270/Q2XS5VX8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase PP1-gamma catalytic subunit
(Homo sapiens (Human)) | BDBM50061067

(15-(3-Guanidino-propyl)-8-isobutyl-18-((1E,3E)-6-m...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](C)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](C)NC(=O)C(=C)N(C)C(=O)CC[C@@H](NC(=O)[C@H]1C)C(O)=O)C(O)=O Show InChI InChI=1S/C49H74N10O12/c1-26(2)23-37-46(66)58-40(48(69)70)30(6)42(62)55-35(17-14-22-52-49(50)51)45(65)54-34(19-18-27(3)24-28(4)38(71-10)25-33-15-12-11-13-16-33)29(5)41(61)56-36(47(67)68)20-21-39(60)59(9)32(8)44(64)53-31(7)43(63)57-37/h11-13,15-16,18-19,24,26,28-31,34-38,40H,8,14,17,20-23,25H2,1-7,9-10H3,(H,53,64)(H,54,65)(H,55,62)(H,56,61)(H,57,63)(H,58,66)(H,67,68)(H,69,70)(H4,50,51,52)/b19-18+,27-24+/t28-,29-,30-,31+,34-,35-,36+,37-,38-,40+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of Serine/threonine protein phosphatase 1 (PP1) mutants by natural toxins in C127S |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B
(Gallus gallus) | BDBM50366883
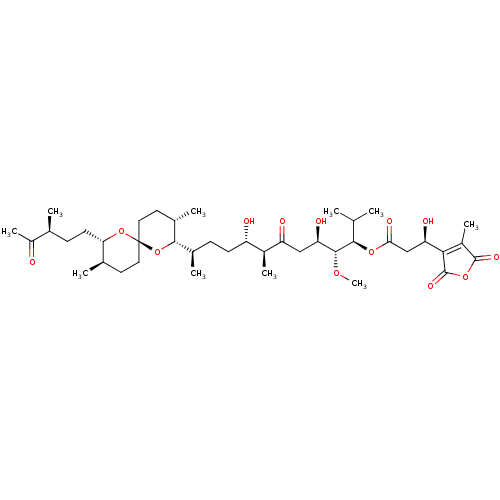
(TAUTOMYCIN)Show SMILES CO[C@H]([C@H](O)CC(=O)[C@@H](C)[C@@H](O)CC[C@@H](C)[C@@H]1O[C@]2(CC[C@@H](C)[C@H](CC[C@H](C)C(C)=O)O2)CC[C@@H]1C)[C@H](OC(=O)C[C@@H](O)C1=C(C)C(=O)OC1=O)C(C)C |r,c:45| Show InChI InChI=1S/C41H66O13/c1-21(2)36(51-34(47)20-31(45)35-27(8)39(48)52-40(35)49)38(50-10)32(46)19-30(44)26(7)29(43)13-11-24(5)37-25(6)16-18-41(54-37)17-15-23(4)33(53-41)14-12-22(3)28(9)42/h21-26,29,31-33,36-38,43,45-46H,11-20H2,1-10H3/t22-,23+,24+,25-,26-,29-,31+,32+,33-,36+,37-,38+,41+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Exogenous inhibition concentration of Serine/threonine protein phosphatase 2A (PP2A) |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase PP1-gamma catalytic subunit
(Homo sapiens (Human)) | BDBM50061067

(15-(3-Guanidino-propyl)-8-isobutyl-18-((1E,3E)-6-m...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](C)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](C)NC(=O)C(=C)N(C)C(=O)CC[C@@H](NC(=O)[C@H]1C)C(O)=O)C(O)=O Show InChI InChI=1S/C49H74N10O12/c1-26(2)23-37-46(66)58-40(48(69)70)30(6)42(62)55-35(17-14-22-52-49(50)51)45(65)54-34(19-18-27(3)24-28(4)38(71-10)25-33-15-12-11-13-16-33)29(5)41(61)56-36(47(67)68)20-21-39(60)59(9)32(8)44(64)53-31(7)43(63)57-37/h11-13,15-16,18-19,24,26,28-31,34-38,40H,8,14,17,20-23,25H2,1-7,9-10H3,(H,53,64)(H,54,65)(H,55,62)(H,56,61)(H,57,63)(H,58,66)(H,67,68)(H,69,70)(H4,50,51,52)/b19-18+,27-24+/t28-,29-,30-,31+,34-,35-,36+,37-,38-,40+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of Serine/threonine protein phosphatase 1 (PP1) mutants by natural toxins in Y272F |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B
(Gallus gallus) | BDBM50110676

(CHEMBL280487 | okadaic acid)Show SMILES C[C@@H](C[C@H](O)[C@H]1O[C@@H]2CC[C@@]3(CC[C@@H](O3)\C=C\[C@@H](C)[C@@H]3CC(C)=C[C@@]4(O[C@H](C[C@@](C)(O)C(O)=O)CC[C@H]4O)O3)O[C@H]2[C@H](O)C1=C)[C@H]1O[C@@]2(CCCCO2)CC[C@H]1C |r,c:23| Show InChI InChI=1S/C44H68O13/c1-25-21-34(55-44(23-25)35(46)12-11-31(54-44)24-41(6,50)40(48)49)26(2)9-10-30-14-18-43(53-30)19-15-33-39(57-43)36(47)29(5)38(52-33)32(45)22-28(4)37-27(3)13-17-42(56-37)16-7-8-20-51-42/h9-10,23,26-28,30-39,45-47,50H,5,7-8,11-22,24H2,1-4,6H3,(H,48,49)/b10-9+/t26-,27-,28+,30+,31+,32+,33-,34+,35-,36-,37+,38+,39-,41-,42+,43-,44-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Exogenous inhibition concentration of Serine/threonine protein phosphatase 2A (PP2A) |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B
(Gallus gallus) | BDBM50110676

(CHEMBL280487 | okadaic acid)Show SMILES C[C@@H](C[C@H](O)[C@H]1O[C@@H]2CC[C@@]3(CC[C@@H](O3)\C=C\[C@@H](C)[C@@H]3CC(C)=C[C@@]4(O[C@H](C[C@@](C)(O)C(O)=O)CC[C@H]4O)O3)O[C@H]2[C@H](O)C1=C)[C@H]1O[C@@]2(CCCCO2)CC[C@H]1C |r,c:23| Show InChI InChI=1S/C44H68O13/c1-25-21-34(55-44(23-25)35(46)12-11-31(54-44)24-41(6,50)40(48)49)26(2)9-10-30-14-18-43(53-30)19-15-33-39(57-43)36(47)29(5)38(52-33)32(45)22-28(4)37-27(3)13-17-42(56-37)16-7-8-20-51-42/h9-10,23,26-28,30-39,45-47,50H,5,7-8,11-22,24H2,1-4,6H3,(H,48,49)/b10-9+/t26-,27-,28+,30+,31+,32+,33-,34+,35-,36-,37+,38+,39-,41-,42+,43-,44-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the action of protein phosphatase 2A |
Bioorg Med Chem Lett 12: 391-3 (2002)
BindingDB Entry DOI: 10.7270/Q2XS5VX8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B
(Gallus gallus) | BDBM50110682

(25-Isobutyl-15-(6-methoxy-3,5-dimethyl-7-phenyl-he...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@@H]1NC(=O)[C@H](C)NC(=O)[C@@H](C)[C@@H](CC(=O)[C@H](CC(C)C)NC(=O)[C@@H](C)NC(=O)CN(C)C(=O)CC[C@@H](NC(=O)[C@H]1C)C(O)=O)C(O)=O Show InChI InChI=1S/C46H68N6O12/c1-25(2)20-36-37(53)23-33(45(60)61)28(5)41(56)48-31(8)44(59)49-34(17-16-26(3)21-27(4)38(64-10)22-32-14-12-11-13-15-32)29(6)42(57)50-35(46(62)63)18-19-40(55)52(9)24-39(54)47-30(7)43(58)51-36/h11-17,21,25,27-31,33-36,38H,18-20,22-24H2,1-10H3,(H,47,54)(H,48,56)(H,49,59)(H,50,57)(H,51,58)(H,60,61)(H,62,63)/b17-16+,26-21+/t27-,28-,29-,30+,31-,33+,34-,35+,36-,38-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of microcystin analogues to catalytic subunits of Serine/threonine protein phosphatase 2A (PP2Ac) |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B
(Gallus gallus) | BDBM50110700

(7-Cyclohexyl-25-isobutyl-15-(6-methoxy-3,5-dimethy...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@@H]1NC(=O)[C@H](C)NC(=O)[C@@H](C)[C@@H](CC(=O)[C@H](CC(C)C)NC(=O)[C@@H](C)NC(=O)CN(C2CCCCC2)C(=O)CC[C@@H](NC(=O)[C@H]1C)C(O)=O)C(O)=O Show InChI InChI=1S/C51H76N6O12/c1-29(2)24-41-42(58)27-38(50(65)66)32(5)46(61)53-35(8)49(64)54-39(21-20-30(3)25-31(4)43(69-9)26-36-16-12-10-13-17-36)33(6)47(62)55-40(51(67)68)22-23-45(60)57(37-18-14-11-15-19-37)28-44(59)52-34(7)48(63)56-41/h10,12-13,16-17,20-21,25,29,31-35,37-41,43H,11,14-15,18-19,22-24,26-28H2,1-9H3,(H,52,59)(H,53,61)(H,54,64)(H,55,62)(H,56,63)(H,65,66)(H,67,68)/b21-20+,30-25+/t31-,32-,33-,34+,35-,38+,39-,40+,41-,43-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of microcystin analogues to catalytic subunits of Serine/threonine protein phosphatase 2A (PP2Ac) |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase PP1-gamma catalytic subunit
(Homo sapiens (Human)) | BDBM50061067

(15-(3-Guanidino-propyl)-8-isobutyl-18-((1E,3E)-6-m...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](C)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](C)NC(=O)C(=C)N(C)C(=O)CC[C@@H](NC(=O)[C@H]1C)C(O)=O)C(O)=O Show InChI InChI=1S/C49H74N10O12/c1-26(2)23-37-46(66)58-40(48(69)70)30(6)42(62)55-35(17-14-22-52-49(50)51)45(65)54-34(19-18-27(3)24-28(4)38(71-10)25-33-15-12-11-13-16-33)29(5)41(61)56-36(47(67)68)20-21-39(60)59(9)32(8)44(64)53-31(7)43(63)57-37/h11-13,15-16,18-19,24,26,28-31,34-38,40H,8,14,17,20-23,25H2,1-7,9-10H3,(H,53,64)(H,54,65)(H,55,62)(H,56,61)(H,57,63)(H,58,66)(H,67,68)(H,69,70)(H4,50,51,52)/b19-18+,27-24+/t28-,29-,30-,31+,34-,35-,36+,37-,38-,40+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of Serine/threonine protein phosphatase 1 (PP1) mutants by natural toxins in D208A |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B
(Gallus gallus) | BDBM50110681

(CHEMBL430266 | Calyculin-A)Show SMILES COC[C@@H]([C@H](O)[C@H](O)C(=O)NCC[C@H](C)c1nc(\C=C\C[C@@H]2O[C@]3(C[C@@H](O)[C@@H]2C)O[C@H]([C@H](C[C@H](O)[C@H](C)[C@H](O)[C@H](C)\C=C(/C)\C(\C)=C\C=C\C(\C)=C/C#N)OC)[C@H](OP(O)(O)=O)C3(C)C)co1)N(C)C |r| Show InChI InChI=1S/C50H81N4O15P/c1-29(20-22-51)16-14-17-30(2)32(4)24-33(5)42(57)35(7)38(55)25-41(65-13)45-46(69-70(61,62)63)49(8,9)50(68-45)26-39(56)34(6)40(67-50)19-15-18-36-27-66-48(53-36)31(3)21-23-52-47(60)44(59)43(58)37(28-64-12)54(10)11/h14-18,20,24,27,31,33-35,37-46,55-59H,19,21,23,25-26,28H2,1-13H3,(H,52,60)(H2,61,62,63)/b16-14+,18-15+,29-20-,30-17+,32-24+/t31-,33+,34-,35-,37-,38-,39+,40-,41-,42+,43-,44-,45+,46-,50+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the action of protein phosphatase 2A |
Bioorg Med Chem Lett 12: 391-3 (2002)
BindingDB Entry DOI: 10.7270/Q2XS5VX8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase PP1-gamma catalytic subunit
(Homo sapiens (Human)) | BDBM50110681

(CHEMBL430266 | Calyculin-A)Show SMILES COC[C@@H]([C@H](O)[C@H](O)C(=O)NCC[C@H](C)c1nc(\C=C\C[C@@H]2O[C@]3(C[C@@H](O)[C@@H]2C)O[C@H]([C@H](C[C@H](O)[C@H](C)[C@H](O)[C@H](C)\C=C(/C)\C(\C)=C\C=C\C(\C)=C/C#N)OC)[C@H](OP(O)(O)=O)C3(C)C)co1)N(C)C |r| Show InChI InChI=1S/C50H81N4O15P/c1-29(20-22-51)16-14-17-30(2)32(4)24-33(5)42(57)35(7)38(55)25-41(65-13)45-46(69-70(61,62)63)49(8,9)50(68-45)26-39(56)34(6)40(67-50)19-15-18-36-27-66-48(53-36)31(3)21-23-52-47(60)44(59)43(58)37(28-64-12)54(10)11/h14-18,20,24,27,31,33-35,37-46,55-59H,19,21,23,25-26,28H2,1-13H3,(H,52,60)(H2,61,62,63)/b16-14+,18-15+,29-20-,30-17+,32-24+/t31-,33+,34-,35-,37-,38-,39+,40-,41-,42+,43-,44-,45+,46-,50+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of Serine/threonine protein phosphatase 1 (PP1) mutants by natural toxins in C127S |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B
(Gallus gallus) | BDBM50110693
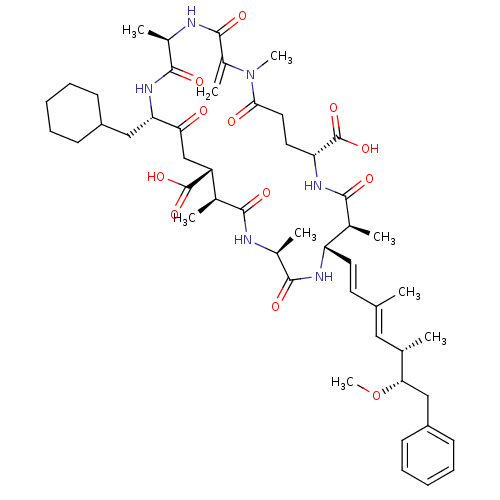
(25-Cyclohexylmethyl-15-(6-methoxy-3,5-dimethyl-7-p...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@@H]1NC(=O)[C@H](C)NC(=O)[C@@H](C)[C@@H](CC(=O)[C@H](CC2CCCCC2)NC(=O)[C@@H](C)NC(=O)C(=C)N(C)C(=O)CC[C@@H](NC(=O)[C@H]1C)C(O)=O)C(O)=O Show InChI InChI=1S/C50H72N6O12/c1-28(24-29(2)42(68-9)26-36-18-14-11-15-19-36)20-21-38-31(4)45(60)54-39(50(66)67)22-23-43(58)56(8)34(7)48(63)52-33(6)47(62)55-40(25-35-16-12-10-13-17-35)41(57)27-37(49(64)65)30(3)44(59)51-32(5)46(61)53-38/h11,14-15,18-21,24,29-33,35,37-40,42H,7,10,12-13,16-17,22-23,25-27H2,1-6,8-9H3,(H,51,59)(H,52,63)(H,53,61)(H,54,60)(H,55,62)(H,64,65)(H,66,67)/b21-20+,28-24+/t29-,30-,31-,32-,33+,37+,38-,39+,40-,42-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of microcystin analogues to catalytic subunits of Serine/threonine protein phosphatase 2A (PP2Ac) |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B
(Gallus gallus) | BDBM50110690
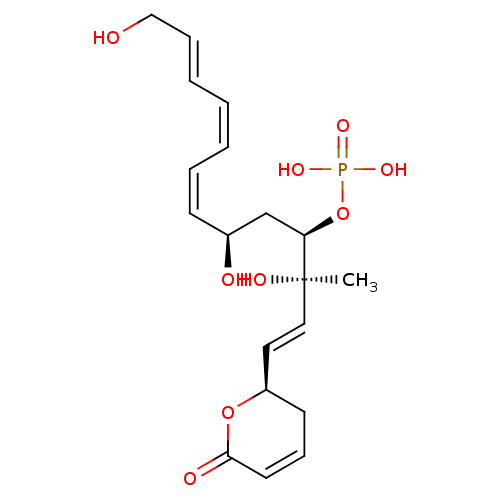
(CHEMBL17377 | FOSTRIECIN | Phosphoric acid mono-{3...)Show SMILES C[C@@](O)(\C=C\[C@H]1CC=CC(=O)O1)[C@@H](C[C@@H](O)\C=C/C=C\C=C\CO)OP(O)(O)=O |c:7| Show InChI InChI=1S/C19H27O9P/c1-19(23,12-11-16-9-7-10-18(22)27-16)17(28-29(24,25)26)14-15(21)8-5-3-2-4-6-13-20/h2-8,10-12,15-17,20-21,23H,9,13-14H2,1H3,(H2,24,25,26)/b3-2-,6-4+,8-5-,12-11+/t15-,16+,17+,19+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Exogenous inhibition concentration of Serine/threonine protein phosphatase 2A (PP2A) |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B
(Gallus gallus) | BDBM50110694

(11,22-Dicarboxy-25-isobutyl-15-(6-methoxy-3,5-dime...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@@H]1NC(=O)[C@H](C)NC(=O)[C@@H](C)[C@@H](CC(=O)[C@H](CC(C)C)NC(=O)[C@@H](C[NH3+])NC(=O)C(=C)N(C)C(=O)CC[C@@H](NC(=O)[C@H]1C)C(O)=O)C(O)=O Show InChI InChI=1S/C47H69N7O12/c1-25(2)20-36-38(55)23-33(46(62)63)28(5)41(57)49-30(7)43(59)50-34(17-16-26(3)21-27(4)39(66-10)22-32-14-12-11-13-15-32)29(6)42(58)51-35(47(64)65)18-19-40(56)54(9)31(8)44(60)53-37(24-48)45(61)52-36/h11-17,21,25,27-30,33-37,39H,8,18-20,22-24,48H2,1-7,9-10H3,(H,49,57)(H,50,59)(H,51,58)(H,52,61)(H,53,60)(H,62,63)(H,64,65)/p+1/b17-16+,26-21+/t27-,28-,29-,30-,33+,34-,35+,36-,37+,39-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of microcystin analogues to catalytic subunits of Serine/threonine protein phosphatase 2A (PP2Ac) |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase PP1-gamma catalytic subunit
(Homo sapiens (Human)) | BDBM50110676

(CHEMBL280487 | okadaic acid)Show SMILES C[C@@H](C[C@H](O)[C@H]1O[C@@H]2CC[C@@]3(CC[C@@H](O3)\C=C\[C@@H](C)[C@@H]3CC(C)=C[C@@]4(O[C@H](C[C@@](C)(O)C(O)=O)CC[C@H]4O)O3)O[C@H]2[C@H](O)C1=C)[C@H]1O[C@@]2(CCCCO2)CC[C@H]1C |r,c:23| Show InChI InChI=1S/C44H68O13/c1-25-21-34(55-44(23-25)35(46)12-11-31(54-44)24-41(6,50)40(48)49)26(2)9-10-30-14-18-43(53-30)19-15-33-39(57-43)36(47)29(5)38(52-33)32(45)22-28(4)37-27(3)13-17-42(56-37)16-7-8-20-51-42/h9-10,23,26-28,30-39,45-47,50H,5,7-8,11-22,24H2,1-4,6H3,(H,48,49)/b10-9+/t26-,27-,28+,30+,31+,32+,33-,34+,35-,36-,37+,38+,39-,41-,42+,43-,44-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of Serine/threonine protein phosphatase 1 (PP1) mutant type D220V by compound was evalutade |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein phosphatase PP1-gamma catalytic subunit
(Homo sapiens (Human)) | BDBM50061067

(15-(3-Guanidino-propyl)-8-isobutyl-18-((1E,3E)-6-m...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](C)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](C)NC(=O)C(=C)N(C)C(=O)CC[C@@H](NC(=O)[C@H]1C)C(O)=O)C(O)=O Show InChI InChI=1S/C49H74N10O12/c1-26(2)23-37-46(66)58-40(48(69)70)30(6)42(62)55-35(17-14-22-52-49(50)51)45(65)54-34(19-18-27(3)24-28(4)38(71-10)25-33-15-12-11-13-16-33)29(5)41(61)56-36(47(67)68)20-21-39(60)59(9)32(8)44(64)53-31(7)43(63)57-37/h11-13,15-16,18-19,24,26,28-31,34-38,40H,8,14,17,20-23,25H2,1-7,9-10H3,(H,53,64)(H,54,65)(H,55,62)(H,56,61)(H,57,63)(H,58,66)(H,67,68)(H,69,70)(H4,50,51,52)/b19-18+,27-24+/t28-,29-,30-,31+,34-,35-,36+,37-,38-,40+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of Serine/threonine protein phosphatase 1 9PP1) mutants by natural toxins in R221S |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase PP1-gamma catalytic subunit
(Homo sapiens (Human)) | BDBM50061067

(15-(3-Guanidino-propyl)-8-isobutyl-18-((1E,3E)-6-m...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](C)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](C)NC(=O)C(=C)N(C)C(=O)CC[C@@H](NC(=O)[C@H]1C)C(O)=O)C(O)=O Show InChI InChI=1S/C49H74N10O12/c1-26(2)23-37-46(66)58-40(48(69)70)30(6)42(62)55-35(17-14-22-52-49(50)51)45(65)54-34(19-18-27(3)24-28(4)38(71-10)25-33-15-12-11-13-16-33)29(5)41(61)56-36(47(67)68)20-21-39(60)59(9)32(8)44(64)53-31(7)43(63)57-37/h11-13,15-16,18-19,24,26,28-31,34-38,40H,8,14,17,20-23,25H2,1-7,9-10H3,(H,53,64)(H,54,65)(H,55,62)(H,56,61)(H,57,63)(H,58,66)(H,67,68)(H,69,70)(H4,50,51,52)/b19-18+,27-24+/t28-,29-,30-,31+,34-,35-,36+,37-,38-,40+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of Serine/threonine protein phosphatase 1 (PP1) mutants by natural toxins in H248N |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase PP1-gamma catalytic subunit
(Homo sapiens (Human)) | BDBM50061067

(15-(3-Guanidino-propyl)-8-isobutyl-18-((1E,3E)-6-m...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](C)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](C)NC(=O)C(=C)N(C)C(=O)CC[C@@H](NC(=O)[C@H]1C)C(O)=O)C(O)=O Show InChI InChI=1S/C49H74N10O12/c1-26(2)23-37-46(66)58-40(48(69)70)30(6)42(62)55-35(17-14-22-52-49(50)51)45(65)54-34(19-18-27(3)24-28(4)38(71-10)25-33-15-12-11-13-16-33)29(5)41(61)56-36(47(67)68)20-21-39(60)59(9)32(8)44(64)53-31(7)43(63)57-37/h11-13,15-16,18-19,24,26,28-31,34-38,40H,8,14,17,20-23,25H2,1-7,9-10H3,(H,53,64)(H,54,65)(H,55,62)(H,56,61)(H,57,63)(H,58,66)(H,67,68)(H,69,70)(H4,50,51,52)/b19-18+,27-24+/t28-,29-,30-,31+,34-,35-,36+,37-,38-,40+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of Serine/threonine protein phosphatase 1 (PP1) mutants by natural toxins in R96A |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase PP1-gamma catalytic subunit
(Homo sapiens (Human)) | BDBM50110676

(CHEMBL280487 | okadaic acid)Show SMILES C[C@@H](C[C@H](O)[C@H]1O[C@@H]2CC[C@@]3(CC[C@@H](O3)\C=C\[C@@H](C)[C@@H]3CC(C)=C[C@@]4(O[C@H](C[C@@](C)(O)C(O)=O)CC[C@H]4O)O3)O[C@H]2[C@H](O)C1=C)[C@H]1O[C@@]2(CCCCO2)CC[C@H]1C |r,c:23| Show InChI InChI=1S/C44H68O13/c1-25-21-34(55-44(23-25)35(46)12-11-31(54-44)24-41(6,50)40(48)49)26(2)9-10-30-14-18-43(53-30)19-15-33-39(57-43)36(47)29(5)38(52-33)32(45)22-28(4)37-27(3)13-17-42(56-37)16-7-8-20-51-42/h9-10,23,26-28,30-39,45-47,50H,5,7-8,11-22,24H2,1-4,6H3,(H,48,49)/b10-9+/t26-,27-,28+,30+,31+,32+,33-,34+,35-,36-,37+,38+,39-,41-,42+,43-,44-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of Serine/threonine protein phosphatase 1 (PP1) mutants by natural toxins in wild type |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein phosphatase PP1-gamma catalytic subunit
(Homo sapiens (Human)) | BDBM50110676

(CHEMBL280487 | okadaic acid)Show SMILES C[C@@H](C[C@H](O)[C@H]1O[C@@H]2CC[C@@]3(CC[C@@H](O3)\C=C\[C@@H](C)[C@@H]3CC(C)=C[C@@]4(O[C@H](C[C@@](C)(O)C(O)=O)CC[C@H]4O)O3)O[C@H]2[C@H](O)C1=C)[C@H]1O[C@@]2(CCCCO2)CC[C@H]1C |r,c:23| Show InChI InChI=1S/C44H68O13/c1-25-21-34(55-44(23-25)35(46)12-11-31(54-44)24-41(6,50)40(48)49)26(2)9-10-30-14-18-43(53-30)19-15-33-39(57-43)36(47)29(5)38(52-33)32(45)22-28(4)37-27(3)13-17-42(56-37)16-7-8-20-51-42/h9-10,23,26-28,30-39,45-47,50H,5,7-8,11-22,24H2,1-4,6H3,(H,48,49)/b10-9+/t26-,27-,28+,30+,31+,32+,33-,34+,35-,36-,37+,38+,39-,41-,42+,43-,44-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of Serine/threonine protein phosphatase 1 (PP1) mutant type E275R by compound was evalutade |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein phosphatase PP1-gamma catalytic subunit
(Homo sapiens (Human)) | BDBM50110681

(CHEMBL430266 | Calyculin-A)Show SMILES COC[C@@H]([C@H](O)[C@H](O)C(=O)NCC[C@H](C)c1nc(\C=C\C[C@@H]2O[C@]3(C[C@@H](O)[C@@H]2C)O[C@H]([C@H](C[C@H](O)[C@H](C)[C@H](O)[C@H](C)\C=C(/C)\C(\C)=C\C=C\C(\C)=C/C#N)OC)[C@H](OP(O)(O)=O)C3(C)C)co1)N(C)C |r| Show InChI InChI=1S/C50H81N4O15P/c1-29(20-22-51)16-14-17-30(2)32(4)24-33(5)42(57)35(7)38(55)25-41(65-13)45-46(69-70(61,62)63)49(8,9)50(68-45)26-39(56)34(6)40(67-50)19-15-18-36-27-66-48(53-36)31(3)21-23-52-47(60)44(59)43(58)37(28-64-12)54(10)11/h14-18,20,24,27,31,33-35,37-46,55-59H,19,21,23,25-26,28H2,1-13H3,(H,52,60)(H2,61,62,63)/b16-14+,18-15+,29-20-,30-17+,32-24+/t31-,33+,34-,35-,37-,38-,39+,40-,41-,42+,43-,44-,45+,46-,50+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of Serine/threonine protein phosphatase 1 (PP1) mutants by natural toxins in Y272F |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B
(Gallus gallus) | BDBM50110677
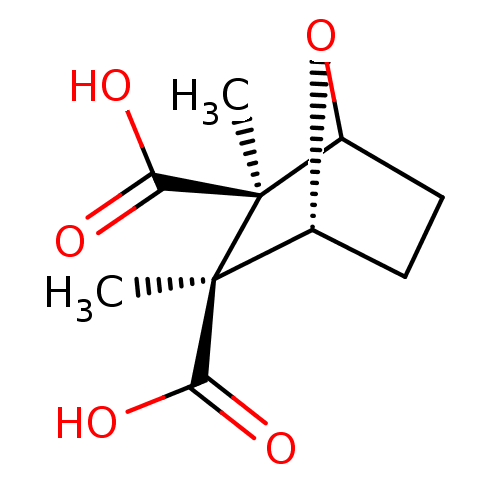
(2,3-Dimethyl-7-oxa-bicyclo[2.2.1]heptane-2,3-dicar...)Show SMILES C[C@@]1(C2CC[C@@H](O2)[C@@]1(C)C(O)=O)C(O)=O Show InChI InChI=1S/C10H14O5/c1-9(7(11)12)5-3-4-6(15-5)10(9,2)8(13)14/h5-6H,3-4H2,1-2H3,(H,11,12)(H,13,14)/t5-,6?,9+,10-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Exogenous inhibition concentration of Serine/threonine protein phosphatase 2A (PP2A) |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase PP1-gamma catalytic subunit
(Homo sapiens (Human)) | BDBM50110681

(CHEMBL430266 | Calyculin-A)Show SMILES COC[C@@H]([C@H](O)[C@H](O)C(=O)NCC[C@H](C)c1nc(\C=C\C[C@@H]2O[C@]3(C[C@@H](O)[C@@H]2C)O[C@H]([C@H](C[C@H](O)[C@H](C)[C@H](O)[C@H](C)\C=C(/C)\C(\C)=C\C=C\C(\C)=C/C#N)OC)[C@H](OP(O)(O)=O)C3(C)C)co1)N(C)C |r| Show InChI InChI=1S/C50H81N4O15P/c1-29(20-22-51)16-14-17-30(2)32(4)24-33(5)42(57)35(7)38(55)25-41(65-13)45-46(69-70(61,62)63)49(8,9)50(68-45)26-39(56)34(6)40(67-50)19-15-18-36-27-66-48(53-36)31(3)21-23-52-47(60)44(59)43(58)37(28-64-12)54(10)11/h14-18,20,24,27,31,33-35,37-46,55-59H,19,21,23,25-26,28H2,1-13H3,(H,52,60)(H2,61,62,63)/b16-14+,18-15+,29-20-,30-17+,32-24+/t31-,33+,34-,35-,37-,38-,39+,40-,41-,42+,43-,44-,45+,46-,50+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of Serine/threonine protein phosphatase 1 (PP1) mutants by natural toxins in R96A |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50110676

(CHEMBL280487 | okadaic acid)Show SMILES C[C@@H](C[C@H](O)[C@H]1O[C@@H]2CC[C@@]3(CC[C@@H](O3)\C=C\[C@@H](C)[C@@H]3CC(C)=C[C@@]4(O[C@H](C[C@@](C)(O)C(O)=O)CC[C@H]4O)O3)O[C@H]2[C@H](O)C1=C)[C@H]1O[C@@]2(CCCCO2)CC[C@H]1C |r,c:23| Show InChI InChI=1S/C44H68O13/c1-25-21-34(55-44(23-25)35(46)12-11-31(54-44)24-41(6,50)40(48)49)26(2)9-10-30-14-18-43(53-30)19-15-33-39(57-43)36(47)29(5)38(52-33)32(45)22-28(4)37-27(3)13-17-42(56-37)16-7-8-20-51-42/h9-10,23,26-28,30-39,45-47,50H,5,7-8,11-22,24H2,1-4,6H3,(H,48,49)/b10-9+/t26-,27-,28+,30+,31+,32+,33-,34+,35-,36-,37+,38+,39-,41-,42+,43-,44-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Protein phosphatase 1 was determined |
Bioorg Med Chem Lett 12: 391-3 (2002)
BindingDB Entry DOI: 10.7270/Q2XS5VX8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase PP1-gamma catalytic subunit
(Homo sapiens (Human)) | BDBM50110676

(CHEMBL280487 | okadaic acid)Show SMILES C[C@@H](C[C@H](O)[C@H]1O[C@@H]2CC[C@@]3(CC[C@@H](O3)\C=C\[C@@H](C)[C@@H]3CC(C)=C[C@@]4(O[C@H](C[C@@](C)(O)C(O)=O)CC[C@H]4O)O3)O[C@H]2[C@H](O)C1=C)[C@H]1O[C@@]2(CCCCO2)CC[C@H]1C |r,c:23| Show InChI InChI=1S/C44H68O13/c1-25-21-34(55-44(23-25)35(46)12-11-31(54-44)24-41(6,50)40(48)49)26(2)9-10-30-14-18-43(53-30)19-15-33-39(57-43)36(47)29(5)38(52-33)32(45)22-28(4)37-27(3)13-17-42(56-37)16-7-8-20-51-42/h9-10,23,26-28,30-39,45-47,50H,5,7-8,11-22,24H2,1-4,6H3,(H,48,49)/b10-9+/t26-,27-,28+,30+,31+,32+,33-,34+,35-,36-,37+,38+,39-,41-,42+,43-,44-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of Serine/threonine protein phosphatase 1 (PP1) mutant type C127S by compound was evalutade |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B
(Gallus gallus) | BDBM50110675
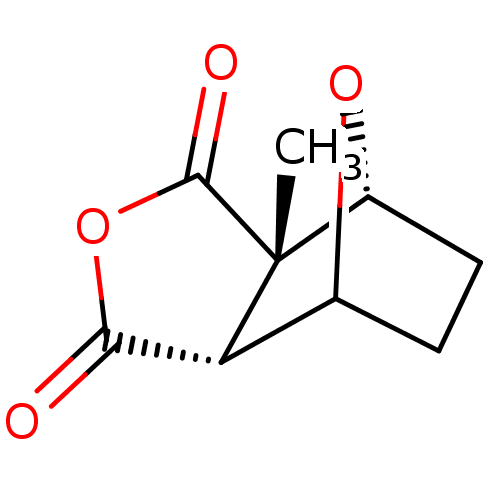
(2-Methyl-4,10-dioxa-tricyclo[5.2.1.0*2,6*]decane-3...)Show InChI InChI=1S/C9H10O4/c1-9-5-3-2-4(12-5)6(9)7(10)13-8(9)11/h4-6H,2-3H2,1H3/t4?,5-,6-,9-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Exogenous inhibition concentration of Serine/threonine protein phosphatase 2A (PP2A) |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B
(Gallus gallus) | BDBM50090505
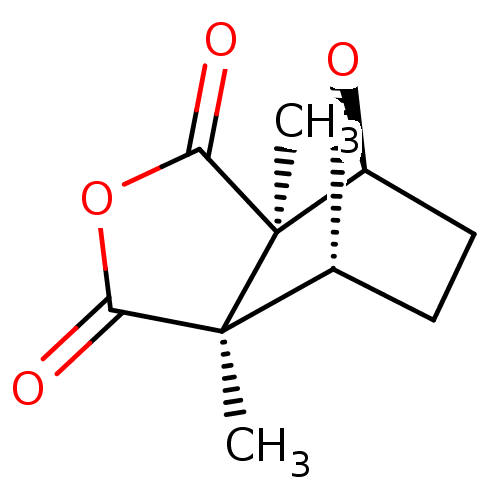
((1R,2S,6R,7S)-2,6-Dimethyl-4,10-dioxa-tricyclo[5.2...)Show SMILES C[C@]12[C@@H]3CC[C@@H](O3)[C@@]1(C)C(=O)OC2=O Show InChI InChI=1S/C10H12O4/c1-9-5-3-4-6(13-5)10(9,2)8(12)14-7(9)11/h5-6H,3-4H2,1-2H3/t5-,6+,9+,10- | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Exogenous inhibition concentration of Serine/threonine protein phosphatase 2A (PP2A) |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase PP1-gamma catalytic subunit
(Homo sapiens (Human)) | BDBM50061067

(15-(3-Guanidino-propyl)-8-isobutyl-18-((1E,3E)-6-m...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](C)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](C)NC(=O)C(=C)N(C)C(=O)CC[C@@H](NC(=O)[C@H]1C)C(O)=O)C(O)=O Show InChI InChI=1S/C49H74N10O12/c1-26(2)23-37-46(66)58-40(48(69)70)30(6)42(62)55-35(17-14-22-52-49(50)51)45(65)54-34(19-18-27(3)24-28(4)38(71-10)25-33-15-12-11-13-16-33)29(5)41(61)56-36(47(67)68)20-21-39(60)59(9)32(8)44(64)53-31(7)43(63)57-37/h11-13,15-16,18-19,24,26,28-31,34-38,40H,8,14,17,20-23,25H2,1-7,9-10H3,(H,53,64)(H,54,65)(H,55,62)(H,56,61)(H,57,63)(H,58,66)(H,67,68)(H,69,70)(H4,50,51,52)/b19-18+,27-24+/t28-,29-,30-,31+,34-,35-,36+,37-,38-,40+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of Serine/threonine protein phosphatase 1 (PP1) mutants by natural toxins in N124D |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase PP1-gamma catalytic subunit
(Homo sapiens (Human)) | BDBM50110676

(CHEMBL280487 | okadaic acid)Show SMILES C[C@@H](C[C@H](O)[C@H]1O[C@@H]2CC[C@@]3(CC[C@@H](O3)\C=C\[C@@H](C)[C@@H]3CC(C)=C[C@@]4(O[C@H](C[C@@](C)(O)C(O)=O)CC[C@H]4O)O3)O[C@H]2[C@H](O)C1=C)[C@H]1O[C@@]2(CCCCO2)CC[C@H]1C |r,c:23| Show InChI InChI=1S/C44H68O13/c1-25-21-34(55-44(23-25)35(46)12-11-31(54-44)24-41(6,50)40(48)49)26(2)9-10-30-14-18-43(53-30)19-15-33-39(57-43)36(47)29(5)38(52-33)32(45)22-28(4)37-27(3)13-17-42(56-37)16-7-8-20-51-42/h9-10,23,26-28,30-39,45-47,50H,5,7-8,11-22,24H2,1-4,6H3,(H,48,49)/b10-9+/t26-,27-,28+,30+,31+,32+,33-,34+,35-,36-,37+,38+,39-,41-,42+,43-,44-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of Serine/threonine protein phosphatase 1 (PP1) mutant type R96A by compound was evalutade |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B
(Gallus gallus) | BDBM50090505

((1R,2S,6R,7S)-2,6-Dimethyl-4,10-dioxa-tricyclo[5.2...)Show SMILES C[C@]12[C@@H]3CC[C@@H](O3)[C@@]1(C)C(=O)OC2=O Show InChI InChI=1S/C10H12O4/c1-9-5-3-4-6(13-5)10(9,2)8(12)14-7(9)11/h5-6H,3-4H2,1-2H3/t5-,6+,9+,10- | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of Serine/threonine protein phosphatase 2A by anhydride modified Cantharidin analogues |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B
(Gallus gallus) | BDBM50110696
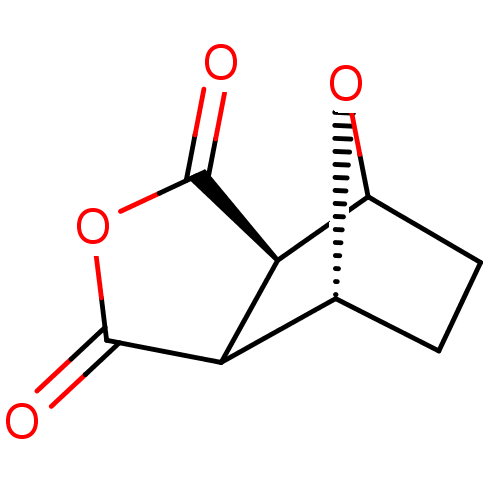
(4,10-Dioxa-tricyclo[5.2.1.0*2,6*]decane-3,5-dione ...)Show InChI InChI=1S/C8H8O4/c9-7-5-3-1-2-4(11-3)6(5)8(10)12-7/h3-6H,1-2H2/t3-,4?,5?,6+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of Serine/threonine protein phosphatase 2A by anhydride modified Cantharidin analogues |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B
(Gallus gallus) | BDBM50110695
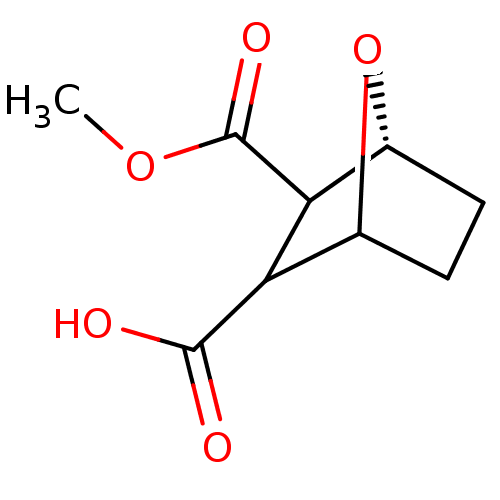
(7-Oxa-bicyclo[2.2.1]heptane-2,3-dicarboxylic acid ...)Show InChI InChI=1S/C9H12O5/c1-13-9(12)7-5-3-2-4(14-5)6(7)8(10)11/h4-7H,2-3H2,1H3,(H,10,11)/t4?,5-,6?,7?/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of Serine/threonine protein phosphatase 2A by anhydride modified Cantharidin analogues |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B
(Gallus gallus) | BDBM50110692
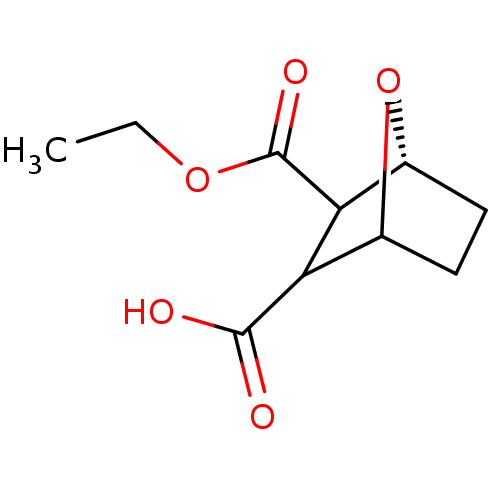
(7-Oxa-bicyclo[2.2.1]heptane-2,3-dicarboxylic acid ...)Show InChI InChI=1S/C10H14O5/c1-2-14-10(13)8-6-4-3-5(15-6)7(8)9(11)12/h5-8H,2-4H2,1H3,(H,11,12)/t5?,6-,7?,8?/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of Serine/threonine protein phosphatase 2A by anhydride modified Cantharidin analogues |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B
(Gallus gallus) | BDBM50110698

(7-Oxa-bicyclo[2.2.1]heptane-2,3-dicarboxylic acid ...)Show InChI InChI=1S/C11H16O5/c1-2-5-15-11(14)9-7-4-3-6(16-7)8(9)10(12)13/h6-9H,2-5H2,1H3,(H,12,13)/t6?,7-,8?,9?/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of Serine/threonine protein phosphatase 2A by anhydride modified Cantharidin analogues |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase PP1-gamma catalytic subunit
(Homo sapiens (Human)) | BDBM50110681

(CHEMBL430266 | Calyculin-A)Show SMILES COC[C@@H]([C@H](O)[C@H](O)C(=O)NCC[C@H](C)c1nc(\C=C\C[C@@H]2O[C@]3(C[C@@H](O)[C@@H]2C)O[C@H]([C@H](C[C@H](O)[C@H](C)[C@H](O)[C@H](C)\C=C(/C)\C(\C)=C\C=C\C(\C)=C/C#N)OC)[C@H](OP(O)(O)=O)C3(C)C)co1)N(C)C |r| Show InChI InChI=1S/C50H81N4O15P/c1-29(20-22-51)16-14-17-30(2)32(4)24-33(5)42(57)35(7)38(55)25-41(65-13)45-46(69-70(61,62)63)49(8,9)50(68-45)26-39(56)34(6)40(67-50)19-15-18-36-27-66-48(53-36)31(3)21-23-52-47(60)44(59)43(58)37(28-64-12)54(10)11/h14-18,20,24,27,31,33-35,37-46,55-59H,19,21,23,25-26,28H2,1-13H3,(H,52,60)(H2,61,62,63)/b16-14+,18-15+,29-20-,30-17+,32-24+/t31-,33+,34-,35-,37-,38-,39+,40-,41-,42+,43-,44-,45+,46-,50+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >500 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of Serine/threonine protein phosphatase 1 (PP1) mutants by natural toxins in N124D |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine/threonine-protein phosphatase PP1-gamma catalytic subunit
(Homo sapiens (Human)) | BDBM50110681

(CHEMBL430266 | Calyculin-A)Show SMILES COC[C@@H]([C@H](O)[C@H](O)C(=O)NCC[C@H](C)c1nc(\C=C\C[C@@H]2O[C@]3(C[C@@H](O)[C@@H]2C)O[C@H]([C@H](C[C@H](O)[C@H](C)[C@H](O)[C@H](C)\C=C(/C)\C(\C)=C\C=C\C(\C)=C/C#N)OC)[C@H](OP(O)(O)=O)C3(C)C)co1)N(C)C |r| Show InChI InChI=1S/C50H81N4O15P/c1-29(20-22-51)16-14-17-30(2)32(4)24-33(5)42(57)35(7)38(55)25-41(65-13)45-46(69-70(61,62)63)49(8,9)50(68-45)26-39(56)34(6)40(67-50)19-15-18-36-27-66-48(53-36)31(3)21-23-52-47(60)44(59)43(58)37(28-64-12)54(10)11/h14-18,20,24,27,31,33-35,37-46,55-59H,19,21,23,25-26,28H2,1-13H3,(H,52,60)(H2,61,62,63)/b16-14+,18-15+,29-20-,30-17+,32-24+/t31-,33+,34-,35-,37-,38-,39+,40-,41-,42+,43-,44-,45+,46-,50+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >500 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of Serine/threonine protein phosphatase 1 (PP1) mutants by natural toxins in R221S |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine/threonine-protein phosphatase PP1-gamma catalytic subunit
(Homo sapiens (Human)) | BDBM50110681

(CHEMBL430266 | Calyculin-A)Show SMILES COC[C@@H]([C@H](O)[C@H](O)C(=O)NCC[C@H](C)c1nc(\C=C\C[C@@H]2O[C@]3(C[C@@H](O)[C@@H]2C)O[C@H]([C@H](C[C@H](O)[C@H](C)[C@H](O)[C@H](C)\C=C(/C)\C(\C)=C\C=C\C(\C)=C/C#N)OC)[C@H](OP(O)(O)=O)C3(C)C)co1)N(C)C |r| Show InChI InChI=1S/C50H81N4O15P/c1-29(20-22-51)16-14-17-30(2)32(4)24-33(5)42(57)35(7)38(55)25-41(65-13)45-46(69-70(61,62)63)49(8,9)50(68-45)26-39(56)34(6)40(67-50)19-15-18-36-27-66-48(53-36)31(3)21-23-52-47(60)44(59)43(58)37(28-64-12)54(10)11/h14-18,20,24,27,31,33-35,37-46,55-59H,19,21,23,25-26,28H2,1-13H3,(H,52,60)(H2,61,62,63)/b16-14+,18-15+,29-20-,30-17+,32-24+/t31-,33+,34-,35-,37-,38-,39+,40-,41-,42+,43-,44-,45+,46-,50+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >500 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of Serine/threonine protein phosphatase 1 (PP1) mutants by natural toxins in D208A |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine/threonine-protein phosphatase PP1-gamma catalytic subunit
(Homo sapiens (Human)) | BDBM50110681

(CHEMBL430266 | Calyculin-A)Show SMILES COC[C@@H]([C@H](O)[C@H](O)C(=O)NCC[C@H](C)c1nc(\C=C\C[C@@H]2O[C@]3(C[C@@H](O)[C@@H]2C)O[C@H]([C@H](C[C@H](O)[C@H](C)[C@H](O)[C@H](C)\C=C(/C)\C(\C)=C\C=C\C(\C)=C/C#N)OC)[C@H](OP(O)(O)=O)C3(C)C)co1)N(C)C |r| Show InChI InChI=1S/C50H81N4O15P/c1-29(20-22-51)16-14-17-30(2)32(4)24-33(5)42(57)35(7)38(55)25-41(65-13)45-46(69-70(61,62)63)49(8,9)50(68-45)26-39(56)34(6)40(67-50)19-15-18-36-27-66-48(53-36)31(3)21-23-52-47(60)44(59)43(58)37(28-64-12)54(10)11/h14-18,20,24,27,31,33-35,37-46,55-59H,19,21,23,25-26,28H2,1-13H3,(H,52,60)(H2,61,62,63)/b16-14+,18-15+,29-20-,30-17+,32-24+/t31-,33+,34-,35-,37-,38-,39+,40-,41-,42+,43-,44-,45+,46-,50+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >500 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of Serine/threonine protein phosphatase 1 (PP1) mutants by natural toxins in H248N |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine/threonine-protein phosphatase PP1-gamma catalytic subunit
(Homo sapiens (Human)) | BDBM50110676

(CHEMBL280487 | okadaic acid)Show SMILES C[C@@H](C[C@H](O)[C@H]1O[C@@H]2CC[C@@]3(CC[C@@H](O3)\C=C\[C@@H](C)[C@@H]3CC(C)=C[C@@]4(O[C@H](C[C@@](C)(O)C(O)=O)CC[C@H]4O)O3)O[C@H]2[C@H](O)C1=C)[C@H]1O[C@@]2(CCCCO2)CC[C@H]1C |r,c:23| Show InChI InChI=1S/C44H68O13/c1-25-21-34(55-44(23-25)35(46)12-11-31(54-44)24-41(6,50)40(48)49)26(2)9-10-30-14-18-43(53-30)19-15-33-39(57-43)36(47)29(5)38(52-33)32(45)22-28(4)37-27(3)13-17-42(56-37)16-7-8-20-51-42/h9-10,23,26-28,30-39,45-47,50H,5,7-8,11-22,24H2,1-4,6H3,(H,48,49)/b10-9+/t26-,27-,28+,30+,31+,32+,33-,34+,35-,36-,37+,38+,39-,41-,42+,43-,44-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
PubMed
| n/a | n/a | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of Serine/threonine protein phosphatase 1 (PP1) mutant type Y272F by compound was evalutade |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B
(Gallus gallus) | BDBM50090505

((1R,2S,6R,7S)-2,6-Dimethyl-4,10-dioxa-tricyclo[5.2...)Show SMILES C[C@]12[C@@H]3CC[C@@H](O3)[C@@]1(C)C(=O)OC2=O Show InChI InChI=1S/C10H12O4/c1-9-5-3-4-6(13-5)10(9,2)8(12)14-7(9)11/h5-6H,3-4H2,1-2H3/t5-,6+,9+,10- | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the action of protein phosphatase 2A |
Bioorg Med Chem Lett 12: 391-3 (2002)
BindingDB Entry DOI: 10.7270/Q2XS5VX8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data