 Found 966 hits with Last Name = 'simon' and Initial = 'c'
Found 966 hits with Last Name = 'simon' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
25-hydroxyvitamin D-1 alpha hydroxylase, mitochondrial
(Mus musculus) | BDBM50023359
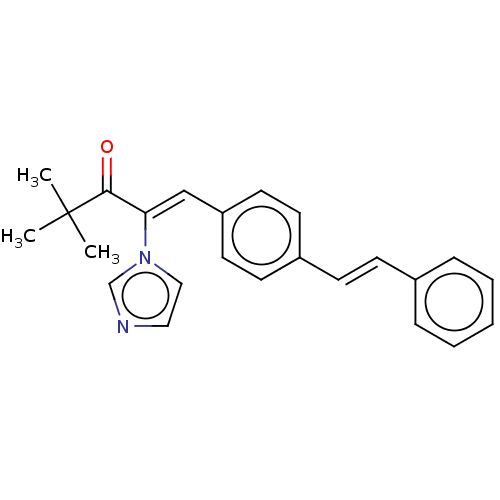
(CHEMBL3329778)Show SMILES CC(C)(C)C(=O)C(=C\c1ccc(\C=C\c2ccccc2)cc1)\n1ccnc1 Show InChI InChI=1S/C24H24N2O/c1-24(2,3)23(27)22(26-16-15-25-18-26)17-21-13-11-20(12-14-21)10-9-19-7-5-4-6-8-19/h4-18H,1-3H3/b10-9+,22-17- | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of mouse CYP27B1 using 1,25(OH)2D3 substrate in presence of bovine adrenodoxin, adrenodoxin reductase and NADPH incubated at 37 degC for 2... |
J Med Chem 57: 7702-15 (2014)
Article DOI: 10.1021/jm5009314
BindingDB Entry DOI: 10.7270/Q2DN46ND |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50023348
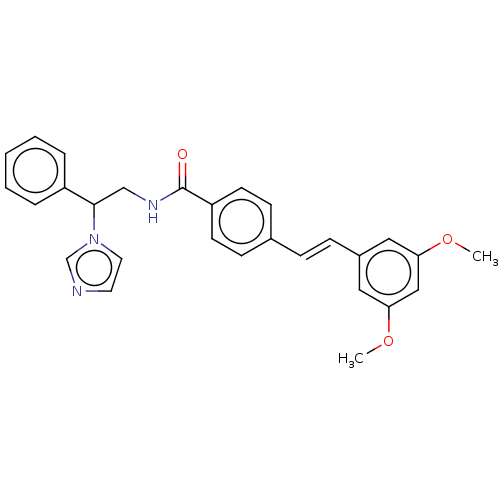
(CHEMBL3329769)Show SMILES COc1cc(OC)cc(\C=C\c2ccc(cc2)C(=O)NCC(c2ccccc2)n2ccnc2)c1 Show InChI InChI=1S/C28H27N3O3/c1-33-25-16-22(17-26(18-25)34-2)9-8-21-10-12-24(13-11-21)28(32)30-19-27(31-15-14-29-20-31)23-6-4-3-5-7-23/h3-18,20,27H,19H2,1-2H3,(H,30,32)/b9-8+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of human MBP-tagged CYP24A1 expressed in Escherichia coli using 1,25(OH)2D3 substrate in presence of bovine adrenodoxin, adrenodoxin reduc... |
J Med Chem 57: 7702-15 (2014)
Article DOI: 10.1021/jm5009314
BindingDB Entry DOI: 10.7270/Q2DN46ND |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50023349
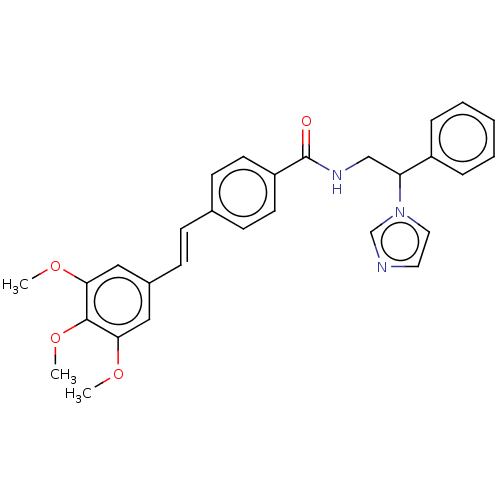
(CHEMBL3329770)Show SMILES COc1cc(\C=C\c2ccc(cc2)C(=O)NCC(c2ccccc2)n2ccnc2)cc(OC)c1OC Show InChI InChI=1S/C29H29N3O4/c1-34-26-17-22(18-27(35-2)28(26)36-3)10-9-21-11-13-24(14-12-21)29(33)31-19-25(32-16-15-30-20-32)23-7-5-4-6-8-23/h4-18,20,25H,19H2,1-3H3,(H,31,33)/b10-9+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of human MBP-tagged CYP24A1 expressed in Escherichia coli using 1,25(OH)2D3 substrate in presence of bovine adrenodoxin, adrenodoxin reduc... |
J Med Chem 57: 7702-15 (2014)
Article DOI: 10.1021/jm5009314
BindingDB Entry DOI: 10.7270/Q2DN46ND |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50023355
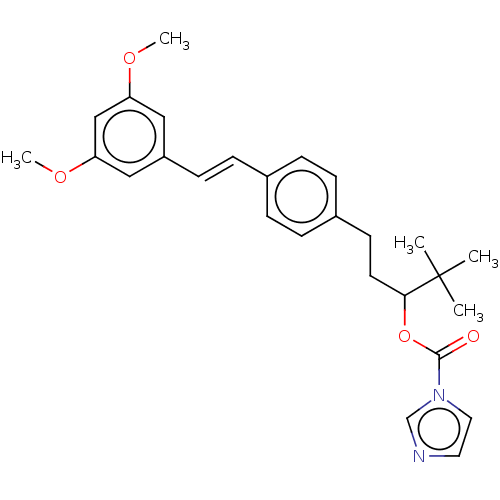
(CHEMBL3329774)Show SMILES COc1cc(OC)cc(\C=C\c2ccc(CCC(OC(=O)n3ccnc3)C(C)(C)C)cc2)c1 Show InChI InChI=1S/C27H32N2O4/c1-27(2,3)25(33-26(30)29-15-14-28-19-29)13-12-21-8-6-20(7-9-21)10-11-22-16-23(31-4)18-24(17-22)32-5/h6-11,14-19,25H,12-13H2,1-5H3/b11-10+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of human MBP-tagged CYP24A1 expressed in Escherichia coli using 1,25(OH)2D3 substrate in presence of bovine adrenodoxin, adrenodoxin reduc... |
J Med Chem 57: 7702-15 (2014)
Article DOI: 10.1021/jm5009314
BindingDB Entry DOI: 10.7270/Q2DN46ND |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50440293
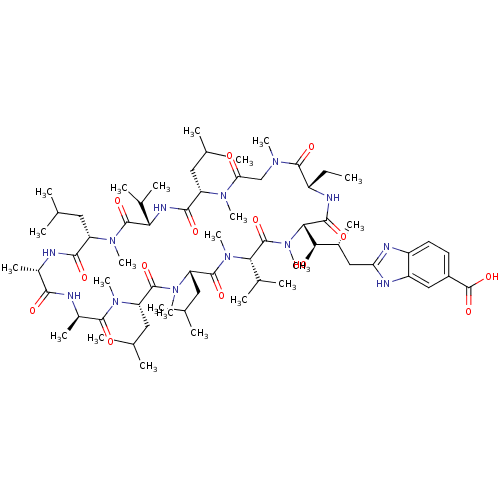
(CHEMBL2424822)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)Cc2nc3ccc(cc3[nH]2)C(O)=O)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C |r| Show InChI InChI=1S/C67H111N13O14/c1-24-44-62(88)74(17)33-52(81)75(18)47(27-34(2)3)59(85)73-53(38(10)11)65(91)76(19)48(28-35(4)5)58(84)68-41(15)57(83)69-42(16)61(87)77(20)49(29-36(6)7)63(89)78(21)50(30-37(8)9)64(90)79(22)54(39(12)13)66(92)80(23)55(60(86)72-44)56(82)40(14)31-51-70-45-26-25-43(67(93)94)32-46(45)71-51/h25-26,32,34-42,44,47-50,53-56,82H,24,27-31,33H2,1-23H3,(H,68,84)(H,69,83)(H,70,71)(H,72,86)(H,73,85)(H,93,94)/t40-,41+,42-,44+,47+,48+,49+,50+,53+,54+,55+,56-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Research Unit for Enzymology of Protein Folding
Curated by ChEMBL
| Assay Description
Inhibition of CypA PPIase activity (unknown origin) using Glt-(Ala)n-Pro-Phe-4-nitroanilides as substrate |
J Med Chem 56: 7302-11 (2013)
Article DOI: 10.1021/jm4007577
BindingDB Entry DOI: 10.7270/Q2NK3GGK |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50022815

((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33...)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C |r| Show InChI InChI=1S/C62H111N11O12/c1-25-27-28-40(15)52(75)51-56(79)65-43(26-2)58(81)67(18)33-48(74)68(19)44(29-34(3)4)55(78)66-49(38(11)12)61(84)69(20)45(30-35(5)6)54(77)63-41(16)53(76)64-42(17)57(80)70(21)46(31-36(7)8)59(82)71(22)47(32-37(9)10)60(83)72(23)50(39(13)14)62(85)73(51)24/h25,27,34-47,49-52,75H,26,28-33H2,1-24H3,(H,63,77)(H,64,76)(H,65,79)(H,66,78)/b27-25+/t40-,41+,42-,43+,44+,45+,46+,47+,49+,50+,51+,52-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Research Unit for Enzymology of Protein Folding
Curated by ChEMBL
| Assay Description
Inhibition of CypA PPIase activity (unknown origin) using Glt-(Ala)n-Pro-Phe-4-nitroanilides as substrate |
J Med Chem 56: 7302-11 (2013)
Article DOI: 10.1021/jm4007577
BindingDB Entry DOI: 10.7270/Q2NK3GGK |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50440297
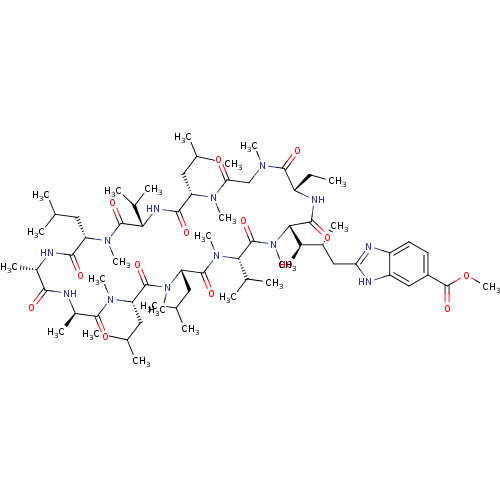
(CHEMBL2424817)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)Cc2nc3ccc(cc3[nH]2)C(=O)OC)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C |r| Show InChI InChI=1S/C68H113N13O14/c1-25-45-63(89)75(17)34-53(82)76(18)48(28-35(2)3)60(86)74-54(39(10)11)66(92)77(19)49(29-36(4)5)59(85)69-42(15)58(84)70-43(16)62(88)78(20)50(30-37(6)7)64(90)79(21)51(31-38(8)9)65(91)80(22)55(40(12)13)67(93)81(23)56(61(87)73-45)57(83)41(14)32-52-71-46-27-26-44(68(94)95-24)33-47(46)72-52/h26-27,33,35-43,45,48-51,54-57,83H,25,28-32,34H2,1-24H3,(H,69,85)(H,70,84)(H,71,72)(H,73,87)(H,74,86)/t41-,42+,43-,45+,48+,49+,50+,51+,54+,55+,56+,57-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Research Unit for Enzymology of Protein Folding
Curated by ChEMBL
| Assay Description
Inhibition of CypA PPIase activity (unknown origin) using Glt-(Ala)n-Pro-Phe-4-nitroanilides as substrate |
J Med Chem 56: 7302-11 (2013)
Article DOI: 10.1021/jm4007577
BindingDB Entry DOI: 10.7270/Q2NK3GGK |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50440299
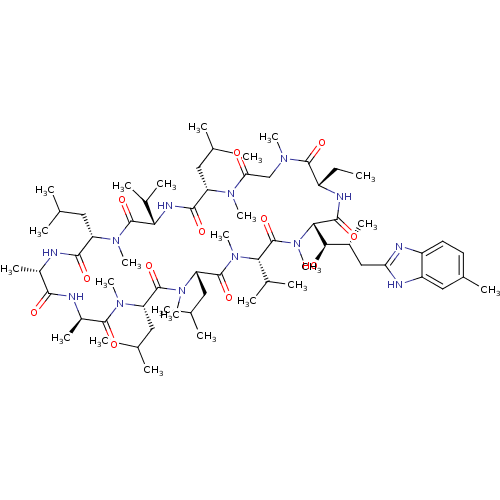
(CHEMBL2424823)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)Cc2nc3ccc(C)cc3[nH]2)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C |r| Show InChI InChI=1S/C67H113N13O12/c1-25-45-63(88)74(18)34-53(81)75(19)48(28-35(2)3)60(85)73-54(39(10)11)66(91)76(20)49(29-36(4)5)59(84)68-43(16)58(83)69-44(17)62(87)77(21)50(30-37(6)7)64(89)78(22)51(31-38(8)9)65(90)79(23)55(40(12)13)67(92)80(24)56(61(86)72-45)57(82)42(15)33-52-70-46-27-26-41(14)32-47(46)71-52/h26-27,32,35-40,42-45,48-51,54-57,82H,25,28-31,33-34H2,1-24H3,(H,68,84)(H,69,83)(H,70,71)(H,72,86)(H,73,85)/t42-,43+,44-,45+,48+,49+,50+,51+,54+,55+,56+,57-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Research Unit for Enzymology of Protein Folding
Curated by ChEMBL
| Assay Description
Inhibition of CypA PPIase activity (unknown origin) using Glt-(Ala)n-Pro-Phe-4-nitroanilides as substrate |
J Med Chem 56: 7302-11 (2013)
Article DOI: 10.1021/jm4007577
BindingDB Entry DOI: 10.7270/Q2NK3GGK |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50023359

(CHEMBL3329778)Show SMILES CC(C)(C)C(=O)C(=C\c1ccc(\C=C\c2ccccc2)cc1)\n1ccnc1 Show InChI InChI=1S/C24H24N2O/c1-24(2,3)23(27)22(26-16-15-25-18-26)17-21-13-11-20(12-14-21)10-9-19-7-5-4-6-8-19/h4-18H,1-3H3/b10-9+,22-17- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of human MBP-tagged CYP24A1 expressed in Escherichia coli using 1,25(OH)2D3 substrate in presence of bovine adrenodoxin, adrenodoxin reduc... |
J Med Chem 57: 7702-15 (2014)
Article DOI: 10.1021/jm5009314
BindingDB Entry DOI: 10.7270/Q2DN46ND |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50027700

(CHEMBL3344395)Show SMILES COc1cc(OC)cc(\C=C\c2cccc3n(CCCCn4ccnc4)ccc23)c1 Show InChI InChI=1S/C25H27N3O2/c1-29-22-16-20(17-23(18-22)30-2)8-9-21-6-5-7-25-24(21)10-14-28(25)13-4-3-12-27-15-11-26-19-27/h5-11,14-19H,3-4,12-13H2,1-2H3/b9-8+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminally MBP-fused human CYP24A1 by cell-free assay |
Eur J Med Chem 87: 39-51 (2014)
Article DOI: 10.1016/j.ejmech.2014.09.035
BindingDB Entry DOI: 10.7270/Q2SF2XR1 |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50027702
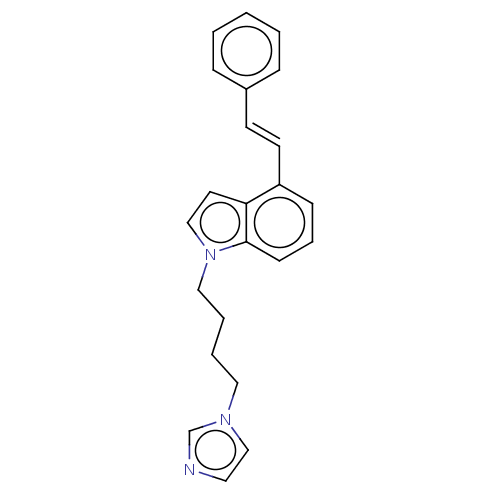
(CHEMBL3344394)Show InChI InChI=1S/C23H23N3/c1-2-7-20(8-3-1)11-12-21-9-6-10-23-22(21)13-17-26(23)16-5-4-15-25-18-14-24-19-25/h1-3,6-14,17-19H,4-5,15-16H2/b12-11+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminally MBP-fused human CYP24A1 by cell-free assay |
Eur J Med Chem 87: 39-51 (2014)
Article DOI: 10.1016/j.ejmech.2014.09.035
BindingDB Entry DOI: 10.7270/Q2SF2XR1 |
More data for this
Ligand-Target Pair | |
25-hydroxyvitamin D-1 alpha hydroxylase, mitochondrial
(Mus musculus) | BDBM50027700

(CHEMBL3344395)Show SMILES COc1cc(OC)cc(\C=C\c2cccc3n(CCCCn4ccnc4)ccc23)c1 Show InChI InChI=1S/C25H27N3O2/c1-29-22-16-20(17-23(18-22)30-2)8-9-21-6-5-7-25-24(21)10-14-28(25)13-4-3-12-27-15-11-26-19-27/h5-11,14-19H,3-4,12-13H2,1-2H3/b9-8+ | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of mouse CYP27B1 by cell-free assay |
Eur J Med Chem 87: 39-51 (2014)
Article DOI: 10.1016/j.ejmech.2014.09.035
BindingDB Entry DOI: 10.7270/Q2SF2XR1 |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50023346
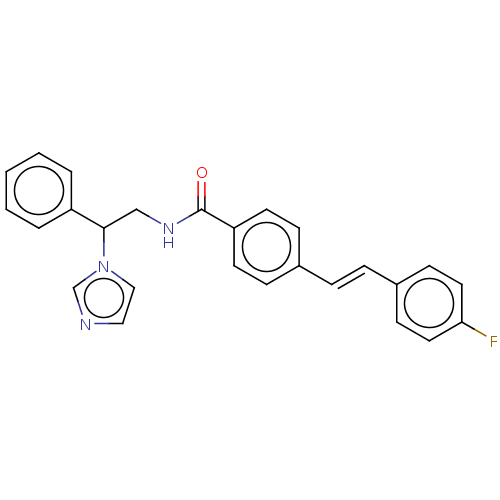
(CHEMBL3329767)Show SMILES Fc1ccc(\C=C\c2ccc(cc2)C(=O)NCC(c2ccccc2)n2ccnc2)cc1 Show InChI InChI=1S/C26H22FN3O/c27-24-14-10-21(11-15-24)7-6-20-8-12-23(13-9-20)26(31)29-18-25(30-17-16-28-19-30)22-4-2-1-3-5-22/h1-17,19,25H,18H2,(H,29,31)/b7-6+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of human MBP-tagged CYP24A1 expressed in Escherichia coli using 1,25(OH)2D3 substrate in presence of bovine adrenodoxin, adrenodoxin reduc... |
J Med Chem 57: 7702-15 (2014)
Article DOI: 10.1021/jm5009314
BindingDB Entry DOI: 10.7270/Q2DN46ND |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50023363
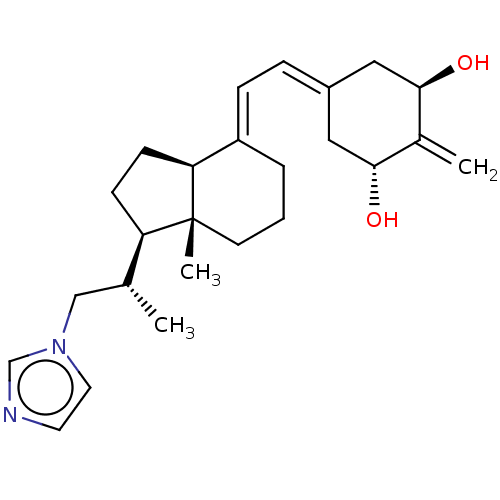
(CHEMBL3360784)Show SMILES [H][C@@]1([#6]-[#6][C@@]2([H])\[#6](-[#6]-[#6]-[#6][C@]12[#6])=[#6]\[#6]=[#6]-1\[#6]-[#6@@H](-[#8])-[#6](=[#6])-[#6@H](-[#8])-[#6]-1)[#6@H](-[#6])-[#6]-n1ccnc1 |r| Show InChI InChI=1S/C25H36N2O2/c1-17(15-27-12-11-26-16-27)21-8-9-22-20(5-4-10-25(21,22)3)7-6-19-13-23(28)18(2)24(29)14-19/h6-7,11-12,16-17,21-24,28-29H,2,4-5,8-10,13-15H2,1,3H3/b20-7+/t17-,21-,22+,23-,24-,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of human MBP-tagged CYP24A1 expressed in Escherichia coli using 1,25(OH)2D3 substrate in presence of bovine adrenodoxin, adrenodoxin reduc... |
J Med Chem 57: 7702-15 (2014)
Article DOI: 10.1021/jm5009314
BindingDB Entry DOI: 10.7270/Q2DN46ND |
More data for this
Ligand-Target Pair | |
25-hydroxyvitamin D-1 alpha hydroxylase, mitochondrial
(Mus musculus) | BDBM50027682
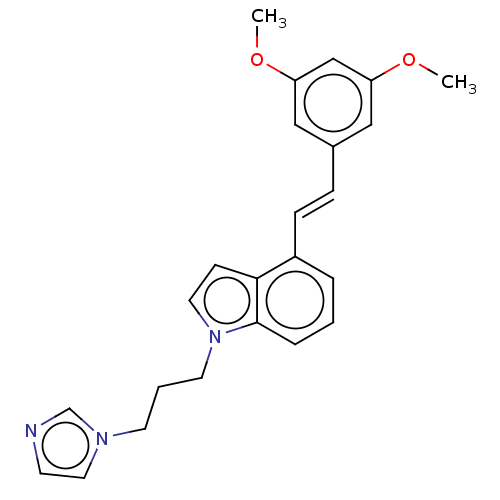
(CHEMBL3344393)Show SMILES COc1cc(OC)cc(\C=C\c2cccc3n(CCCn4ccnc4)ccc23)c1 Show InChI InChI=1S/C24H25N3O2/c1-28-21-15-19(16-22(17-21)29-2)7-8-20-5-3-6-24-23(20)9-13-27(24)12-4-11-26-14-10-25-18-26/h3,5-10,13-18H,4,11-12H2,1-2H3/b8-7+ | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of mouse CYP27B1 by cell-free assay |
Eur J Med Chem 87: 39-51 (2014)
Article DOI: 10.1016/j.ejmech.2014.09.035
BindingDB Entry DOI: 10.7270/Q2SF2XR1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50440300

(CHEMBL2424821)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)Cc2nc3ccccc3[nH]2)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C |r| Show InChI InChI=1S/C66H111N13O12/c1-24-44-62(87)73(17)34-52(80)74(18)47(29-35(2)3)59(84)72-53(39(10)11)65(90)75(19)48(30-36(4)5)58(83)67-42(15)57(82)68-43(16)61(86)76(20)49(31-37(6)7)63(88)77(21)50(32-38(8)9)64(89)78(22)54(40(12)13)66(91)79(23)55(60(85)71-44)56(81)41(14)33-51-69-45-27-25-26-28-46(45)70-51/h25-28,35-44,47-50,53-56,81H,24,29-34H2,1-23H3,(H,67,83)(H,68,82)(H,69,70)(H,71,85)(H,72,84)/t41-,42+,43-,44+,47+,48+,49+,50+,53+,54+,55+,56-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Research Unit for Enzymology of Protein Folding
Curated by ChEMBL
| Assay Description
Inhibition of CypA PPIase activity (unknown origin) using Glt-(Ala)n-Pro-Phe-4-nitroanilides as substrate |
J Med Chem 56: 7302-11 (2013)
Article DOI: 10.1021/jm4007577
BindingDB Entry DOI: 10.7270/Q2NK3GGK |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50023347
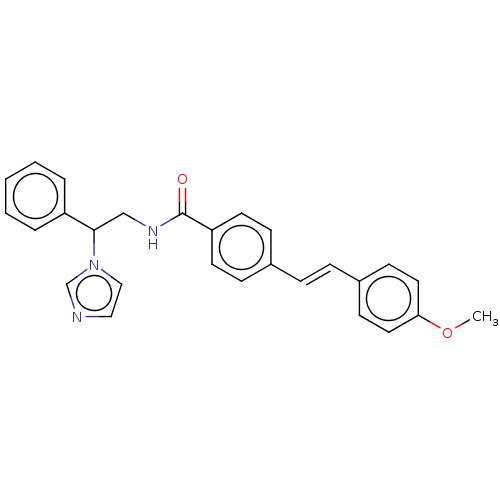
(CHEMBL3329768)Show SMILES COc1ccc(\C=C\c2ccc(cc2)C(=O)NCC(c2ccccc2)n2ccnc2)cc1 Show InChI InChI=1S/C27H25N3O2/c1-32-25-15-11-22(12-16-25)8-7-21-9-13-24(14-10-21)27(31)29-19-26(30-18-17-28-20-30)23-5-3-2-4-6-23/h2-18,20,26H,19H2,1H3,(H,29,31)/b8-7+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of human MBP-tagged CYP24A1 expressed in Escherichia coli using 1,25(OH)2D3 substrate in presence of bovine adrenodoxin, adrenodoxin reduc... |
J Med Chem 57: 7702-15 (2014)
Article DOI: 10.1021/jm5009314
BindingDB Entry DOI: 10.7270/Q2DN46ND |
More data for this
Ligand-Target Pair | |
25-hydroxyvitamin D-1 alpha hydroxylase, mitochondrial
(Mus musculus) | BDBM50027694
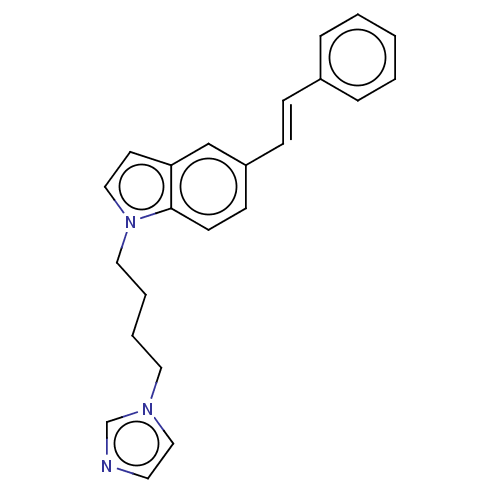
(CHEMBL3344397)Show InChI InChI=1S/C23H23N3/c1-2-6-20(7-3-1)8-9-21-10-11-23-22(18-21)12-16-26(23)15-5-4-14-25-17-13-24-19-25/h1-3,6-13,16-19H,4-5,14-15H2/b9-8+ | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of mouse CYP27B1 by cell-free assay |
Eur J Med Chem 87: 39-51 (2014)
Article DOI: 10.1016/j.ejmech.2014.09.035
BindingDB Entry DOI: 10.7270/Q2SF2XR1 |
More data for this
Ligand-Target Pair | |
25-hydroxyvitamin D-1 alpha hydroxylase, mitochondrial
(Mus musculus) | BDBM50027702

(CHEMBL3344394)Show InChI InChI=1S/C23H23N3/c1-2-7-20(8-3-1)11-12-21-9-6-10-23-22(21)13-17-26(23)16-5-4-15-25-18-14-24-19-25/h1-3,6-14,17-19H,4-5,15-16H2/b12-11+ | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of mouse CYP27B1 by cell-free assay |
Eur J Med Chem 87: 39-51 (2014)
Article DOI: 10.1016/j.ejmech.2014.09.035
BindingDB Entry DOI: 10.7270/Q2SF2XR1 |
More data for this
Ligand-Target Pair | |
25-hydroxyvitamin D-1 alpha hydroxylase, mitochondrial
(Mus musculus) | BDBM50023348

(CHEMBL3329769)Show SMILES COc1cc(OC)cc(\C=C\c2ccc(cc2)C(=O)NCC(c2ccccc2)n2ccnc2)c1 Show InChI InChI=1S/C28H27N3O3/c1-33-25-16-22(17-26(18-25)34-2)9-8-21-10-12-24(13-11-21)28(32)30-19-27(31-15-14-29-20-31)23-6-4-3-5-7-23/h3-18,20,27H,19H2,1-2H3,(H,30,32)/b9-8+ | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of mouse CYP27B1 using 1,25(OH)2D3 substrate in presence of bovine adrenodoxin, adrenodoxin reductase and NADPH incubated at 37 degC for 2... |
J Med Chem 57: 7702-15 (2014)
Article DOI: 10.1021/jm5009314
BindingDB Entry DOI: 10.7270/Q2DN46ND |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50027683

(CHEMBL3344392)Show InChI InChI=1S/C22H21N3/c1-2-6-19(7-3-1)10-11-20-8-4-9-22-21(20)12-16-25(22)15-5-14-24-17-13-23-18-24/h1-4,6-13,16-18H,5,14-15H2/b11-10+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminally MBP-fused human CYP24A1 by cell-free assay |
Eur J Med Chem 87: 39-51 (2014)
Article DOI: 10.1016/j.ejmech.2014.09.035
BindingDB Entry DOI: 10.7270/Q2SF2XR1 |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50322357
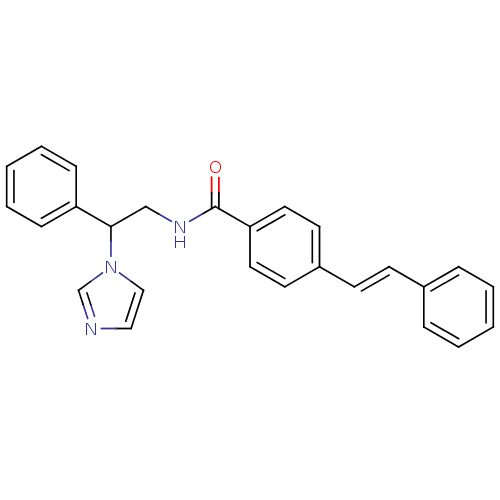
((E)-4-N-(2-(1H-imidazol-1-yl)-2-phenylethyl)-4-sty...)Show SMILES O=C(NCC(c1ccccc1)n1ccnc1)c1ccc(\C=C\c2ccccc2)cc1 Show InChI InChI=1S/C26H23N3O/c30-26(24-15-13-22(14-16-24)12-11-21-7-3-1-4-8-21)28-19-25(29-18-17-27-20-29)23-9-5-2-6-10-23/h1-18,20,25H,19H2,(H,28,30)/b12-11+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of human MBP-tagged CYP24A1 expressed in Escherichia coli using 1,25(OH)2D3 substrate in presence of bovine adrenodoxin, adrenodoxin reduc... |
J Med Chem 57: 7702-15 (2014)
Article DOI: 10.1021/jm5009314
BindingDB Entry DOI: 10.7270/Q2DN46ND |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50027682

(CHEMBL3344393)Show SMILES COc1cc(OC)cc(\C=C\c2cccc3n(CCCn4ccnc4)ccc23)c1 Show InChI InChI=1S/C24H25N3O2/c1-28-21-15-19(16-22(17-21)29-2)7-8-20-5-3-6-24-23(20)9-13-27(24)12-4-11-26-14-10-25-18-26/h3,5-10,13-18H,4,11-12H2,1-2H3/b8-7+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminally MBP-fused human CYP24A1 by cell-free assay |
Eur J Med Chem 87: 39-51 (2014)
Article DOI: 10.1016/j.ejmech.2014.09.035
BindingDB Entry DOI: 10.7270/Q2SF2XR1 |
More data for this
Ligand-Target Pair | |
25-hydroxyvitamin D-1 alpha hydroxylase, mitochondrial
(Mus musculus) | BDBM50023355

(CHEMBL3329774)Show SMILES COc1cc(OC)cc(\C=C\c2ccc(CCC(OC(=O)n3ccnc3)C(C)(C)C)cc2)c1 Show InChI InChI=1S/C27H32N2O4/c1-27(2,3)25(33-26(30)29-15-14-28-19-29)13-12-21-8-6-20(7-9-21)10-11-22-16-23(31-4)18-24(17-22)32-5/h6-11,14-19,25H,12-13H2,1-5H3/b11-10+ | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of mouse CYP27B1 using 1,25(OH)2D3 substrate in presence of bovine adrenodoxin, adrenodoxin reductase and NADPH incubated at 37 degC for 2... |
J Med Chem 57: 7702-15 (2014)
Article DOI: 10.1021/jm5009314
BindingDB Entry DOI: 10.7270/Q2DN46ND |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM31768

(CHEMBL295698 | Ketoconazole | Nizoral | Panfungol)Show SMILES CC(=O)N1CCN(CC1)c1ccc(OC[C@@H]2CO[C@](Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of human MBP-tagged CYP24A1 expressed in Escherichia coli using 1,25(OH)2D3 substrate in presence of bovine adrenodoxin, adrenodoxin reduc... |
J Med Chem 57: 7702-15 (2014)
Article DOI: 10.1021/jm5009314
BindingDB Entry DOI: 10.7270/Q2DN46ND |
More data for this
Ligand-Target Pair | |
25-hydroxyvitamin D-1 alpha hydroxylase, mitochondrial
(Mus musculus) | BDBM50027683

(CHEMBL3344392)Show InChI InChI=1S/C22H21N3/c1-2-6-19(7-3-1)10-11-20-8-4-9-22-21(20)12-16-25(22)15-5-14-24-17-13-23-18-24/h1-4,6-13,16-18H,5,14-15H2/b11-10+ | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of mouse CYP27B1 by cell-free assay |
Eur J Med Chem 87: 39-51 (2014)
Article DOI: 10.1016/j.ejmech.2014.09.035
BindingDB Entry DOI: 10.7270/Q2SF2XR1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase B
(Homo sapiens (Human)) | BDBM50440293

(CHEMBL2424822)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)Cc2nc3ccc(cc3[nH]2)C(O)=O)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C |r| Show InChI InChI=1S/C67H111N13O14/c1-24-44-62(88)74(17)33-52(81)75(18)47(27-34(2)3)59(85)73-53(38(10)11)65(91)76(19)48(28-35(4)5)58(84)68-41(15)57(83)69-42(16)61(87)77(20)49(29-36(6)7)63(89)78(21)50(30-37(8)9)64(90)79(22)54(39(12)13)66(92)80(23)55(60(86)72-44)56(82)40(14)31-51-70-45-26-25-43(67(93)94)32-46(45)71-51/h25-26,32,34-42,44,47-50,53-56,82H,24,27-31,33H2,1-23H3,(H,68,84)(H,69,83)(H,70,71)(H,72,86)(H,73,85)(H,93,94)/t40-,41+,42-,44+,47+,48+,49+,50+,53+,54+,55+,56-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Research Unit for Enzymology of Protein Folding
Curated by ChEMBL
| Assay Description
Inhibition of CypB PPIase activity (unknown origin) |
J Med Chem 56: 7302-11 (2013)
Article DOI: 10.1021/jm4007577
BindingDB Entry DOI: 10.7270/Q2NK3GGK |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM31768

(CHEMBL295698 | Ketoconazole | Nizoral | Panfungol)Show SMILES CC(=O)N1CCN(CC1)c1ccc(OC[C@@H]2CO[C@](Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminally MBP-fused human CYP24A1 by cell-free assay |
Eur J Med Chem 87: 39-51 (2014)
Article DOI: 10.1016/j.ejmech.2014.09.035
BindingDB Entry DOI: 10.7270/Q2SF2XR1 |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50023350
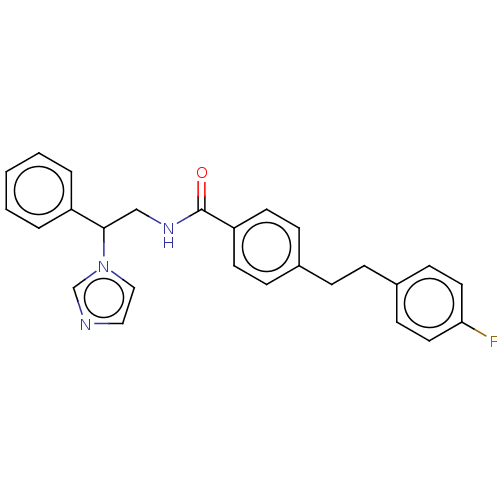
(CHEMBL3329771)Show SMILES Fc1ccc(CCc2ccc(cc2)C(=O)NCC(c2ccccc2)n2ccnc2)cc1 Show InChI InChI=1S/C26H24FN3O/c27-24-14-10-21(11-15-24)7-6-20-8-12-23(13-9-20)26(31)29-18-25(30-17-16-28-19-30)22-4-2-1-3-5-22/h1-5,8-17,19,25H,6-7,18H2,(H,29,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of human MBP-tagged CYP24A1 expressed in Escherichia coli using 1,25(OH)2D3 substrate in presence of bovine adrenodoxin, adrenodoxin reduc... |
J Med Chem 57: 7702-15 (2014)
Article DOI: 10.1021/jm5009314
BindingDB Entry DOI: 10.7270/Q2DN46ND |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50027694

(CHEMBL3344397)Show InChI InChI=1S/C23H23N3/c1-2-6-20(7-3-1)8-9-21-10-11-23-22(18-21)12-16-26(23)15-5-4-14-25-17-13-24-19-25/h1-3,6-13,16-19H,4-5,14-15H2/b9-8+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminally MBP-fused human CYP24A1 by cell-free assay |
Eur J Med Chem 87: 39-51 (2014)
Article DOI: 10.1016/j.ejmech.2014.09.035
BindingDB Entry DOI: 10.7270/Q2SF2XR1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50440296
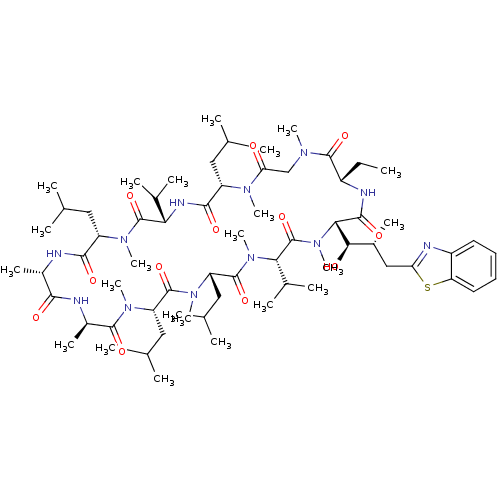
(CHEMBL2424818)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)Cc2nc3ccccc3s2)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C |r| Show InChI InChI=1S/C66H110N12O12S/c1-24-44-62(86)72(17)34-52(79)73(18)46(29-35(2)3)59(83)71-53(39(10)11)65(89)74(19)47(30-36(4)5)58(82)67-42(15)57(81)68-43(16)61(85)75(20)48(31-37(6)7)63(87)76(21)49(32-38(8)9)64(88)77(22)54(40(12)13)66(90)78(23)55(60(84)70-44)56(80)41(14)33-51-69-45-27-25-26-28-50(45)91-51/h25-28,35-44,46-49,53-56,80H,24,29-34H2,1-23H3,(H,67,82)(H,68,81)(H,70,84)(H,71,83)/t41-,42+,43-,44+,46+,47+,48+,49+,53+,54+,55+,56-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Research Unit for Enzymology of Protein Folding
Curated by ChEMBL
| Assay Description
Inhibition of CypA PPIase activity (unknown origin) using Glt-(Ala)n-Pro-Phe-4-nitroanilides as substrate |
J Med Chem 56: 7302-11 (2013)
Article DOI: 10.1021/jm4007577
BindingDB Entry DOI: 10.7270/Q2NK3GGK |
More data for this
Ligand-Target Pair | |
25-hydroxyvitamin D-1 alpha hydroxylase, mitochondrial
(Mus musculus) | BDBM50023360
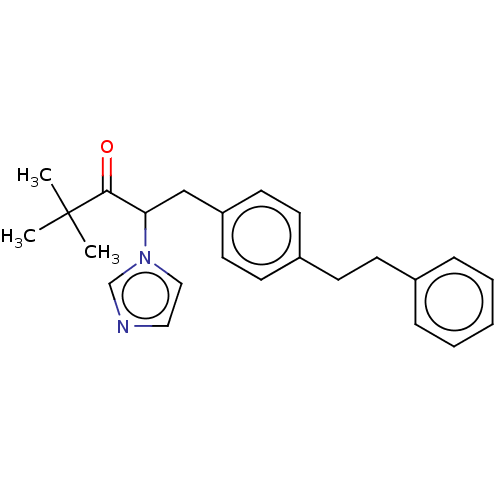
(CHEMBL3329779)Show SMILES CC(C)(C)C(=O)C(Cc1ccc(CCc2ccccc2)cc1)n1ccnc1 Show InChI InChI=1S/C24H28N2O/c1-24(2,3)23(27)22(26-16-15-25-18-26)17-21-13-11-20(12-14-21)10-9-19-7-5-4-6-8-19/h4-8,11-16,18,22H,9-10,17H2,1-3H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of mouse CYP27B1 using 1,25(OH)2D3 substrate in presence of bovine adrenodoxin, adrenodoxin reductase and NADPH incubated at 37 degC for 2... |
J Med Chem 57: 7702-15 (2014)
Article DOI: 10.1021/jm5009314
BindingDB Entry DOI: 10.7270/Q2DN46ND |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
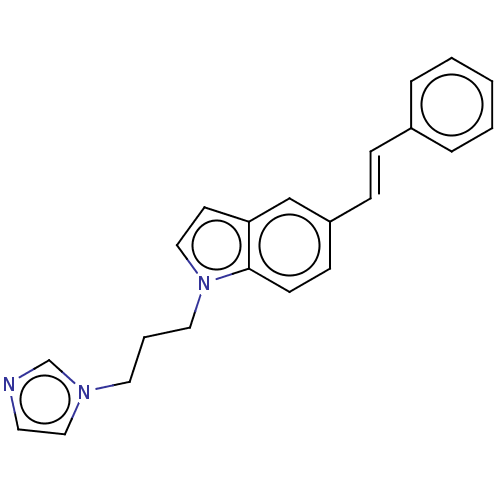
(Homo sapiens (Human)) | BDBM50027699
(CHEMBL3344396)Show InChI InChI=1S/C22H21N3/c1-2-5-19(6-3-1)7-8-20-9-10-22-21(17-20)11-15-25(22)14-4-13-24-16-12-23-18-24/h1-3,5-12,15-18H,4,13-14H2/b8-7+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminally MBP-fused human CYP24A1 by cell-free assay |
Eur J Med Chem 87: 39-51 (2014)
Article DOI: 10.1016/j.ejmech.2014.09.035
BindingDB Entry DOI: 10.7270/Q2SF2XR1 |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50023362

(CHEMBL3360783)Show SMILES [H][C@@]1(CC[C@@]2([H])\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)CCC1=C)[C@H](C)CCCOS(=O)(=O)c1ccc(C)cc1 |r| Show InChI InChI=1S/C31H44O4S/c1-22-9-15-28(16-10-22)36(33,34)35-20-6-7-24(3)29-17-18-30-25(8-5-19-31(29,30)4)12-13-26-21-27(32)14-11-23(26)2/h9-10,12-13,15-16,24,27,29-30,32H,2,5-8,11,14,17-21H2,1,3-4H3/b25-12+,26-13-/t24-,27+,29-,30+,31-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of human MBP-tagged CYP24A1 expressed in Escherichia coli using 1,25(OH)2D3 substrate in presence of bovine adrenodoxin, adrenodoxin reduc... |
J Med Chem 57: 7702-15 (2014)
Article DOI: 10.1021/jm5009314
BindingDB Entry DOI: 10.7270/Q2DN46ND |
More data for this
Ligand-Target Pair | |
25-hydroxyvitamin D-1 alpha hydroxylase, mitochondrial
(Mus musculus) | BDBM50023347

(CHEMBL3329768)Show SMILES COc1ccc(\C=C\c2ccc(cc2)C(=O)NCC(c2ccccc2)n2ccnc2)cc1 Show InChI InChI=1S/C27H25N3O2/c1-32-25-15-11-22(12-16-25)8-7-21-9-13-24(14-10-21)27(31)29-19-26(30-18-17-28-20-30)23-5-3-2-4-6-23/h2-18,20,26H,19H2,1H3,(H,29,31)/b8-7+ | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of mouse CYP27B1 using 1,25(OH)2D3 substrate in presence of bovine adrenodoxin, adrenodoxin reductase and NADPH incubated at 37 degC for 2... |
J Med Chem 57: 7702-15 (2014)
Article DOI: 10.1021/jm5009314
BindingDB Entry DOI: 10.7270/Q2DN46ND |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50023364
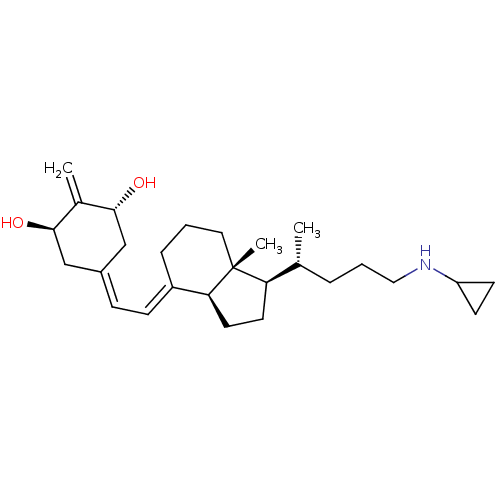
(CHEMBL3360785)Show SMILES [H][C@@]1([#6]-[#6][C@@]2([H])\[#6](-[#6]-[#6]-[#6][C@]12[#6])=[#6]\[#6]=[#6]-1\[#6]-[#6@@H](-[#8])-[#6](=[#6])-[#6@H](-[#8])-[#6]-1)[#6@H](-[#6])-[#6]-[#6]-[#6]-[#7]-[#6]-1-[#6]-[#6]-1 |r| Show InChI InChI=1S/C27H43NO2/c1-18(6-5-15-28-22-10-11-22)23-12-13-24-21(7-4-14-27(23,24)3)9-8-20-16-25(29)19(2)26(30)17-20/h8-9,18,22-26,28-30H,2,4-7,10-17H2,1,3H3/b21-9+/t18-,23-,24+,25-,26-,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of human MBP-tagged CYP24A1 expressed in Escherichia coli using 1,25(OH)2D3 substrate in presence of bovine adrenodoxin, adrenodoxin reduc... |
J Med Chem 57: 7702-15 (2014)
Article DOI: 10.1021/jm5009314
BindingDB Entry DOI: 10.7270/Q2DN46ND |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50023360

(CHEMBL3329779)Show SMILES CC(C)(C)C(=O)C(Cc1ccc(CCc2ccccc2)cc1)n1ccnc1 Show InChI InChI=1S/C24H28N2O/c1-24(2,3)23(27)22(26-16-15-25-18-26)17-21-13-11-20(12-14-21)10-9-19-7-5-4-6-8-19/h4-8,11-16,18,22H,9-10,17H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of human MBP-tagged CYP24A1 expressed in Escherichia coli using 1,25(OH)2D3 substrate in presence of bovine adrenodoxin, adrenodoxin reduc... |
J Med Chem 57: 7702-15 (2014)
Article DOI: 10.1021/jm5009314
BindingDB Entry DOI: 10.7270/Q2DN46ND |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50440298

(CHEMBL2424824)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)Cc2nc3ccc(cc3[nH]2)C(C)C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C |r| Show InChI InChI=1S/C69H117N13O12/c1-26-47-65(90)76(19)35-55(83)77(20)50(29-36(2)3)62(87)75-56(41(12)13)68(93)78(21)51(30-37(4)5)61(86)70-44(17)60(85)71-45(18)64(89)79(22)52(31-38(6)7)66(91)80(23)53(32-39(8)9)67(92)81(24)57(42(14)15)69(94)82(25)58(63(88)74-47)59(84)43(16)33-54-72-48-28-27-46(40(10)11)34-49(48)73-54/h27-28,34,36-45,47,50-53,56-59,84H,26,29-33,35H2,1-25H3,(H,70,86)(H,71,85)(H,72,73)(H,74,88)(H,75,87)/t43-,44+,45-,47+,50+,51+,52+,53+,56+,57+,58+,59-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Research Unit for Enzymology of Protein Folding
Curated by ChEMBL
| Assay Description
Inhibition of CypA PPIase activity (unknown origin) using Glt-(Ala)n-Pro-Phe-4-nitroanilides as substrate |
J Med Chem 56: 7302-11 (2013)
Article DOI: 10.1021/jm4007577
BindingDB Entry DOI: 10.7270/Q2NK3GGK |
More data for this
Ligand-Target Pair | |
25-hydroxyvitamin D-1 alpha hydroxylase, mitochondrial
(Mus musculus) | BDBM50023350

(CHEMBL3329771)Show SMILES Fc1ccc(CCc2ccc(cc2)C(=O)NCC(c2ccccc2)n2ccnc2)cc1 Show InChI InChI=1S/C26H24FN3O/c27-24-14-10-21(11-15-24)7-6-20-8-12-23(13-9-20)26(31)29-18-25(30-17-16-28-19-30)22-4-2-1-3-5-22/h1-5,8-17,19,25H,6-7,18H2,(H,29,31) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of mouse CYP27B1 using 1,25(OH)2D3 substrate in presence of bovine adrenodoxin, adrenodoxin reductase and NADPH incubated at 37 degC for 2... |
J Med Chem 57: 7702-15 (2014)
Article DOI: 10.1021/jm5009314
BindingDB Entry DOI: 10.7270/Q2DN46ND |
More data for this
Ligand-Target Pair | |
25-hydroxyvitamin D-1 alpha hydroxylase, mitochondrial
(Mus musculus) | BDBM50027699

(CHEMBL3344396)Show InChI InChI=1S/C22H21N3/c1-2-5-19(6-3-1)7-8-20-9-10-22-21(17-20)11-15-25(22)14-4-13-24-16-12-23-18-24/h1-3,5-12,15-18H,4,13-14H2/b8-7+ | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of mouse CYP27B1 by cell-free assay |
Eur J Med Chem 87: 39-51 (2014)
Article DOI: 10.1016/j.ejmech.2014.09.035
BindingDB Entry DOI: 10.7270/Q2SF2XR1 |
More data for this
Ligand-Target Pair | |
25-hydroxyvitamin D-1 alpha hydroxylase, mitochondrial
(Mus musculus) | BDBM50023349

(CHEMBL3329770)Show SMILES COc1cc(\C=C\c2ccc(cc2)C(=O)NCC(c2ccccc2)n2ccnc2)cc(OC)c1OC Show InChI InChI=1S/C29H29N3O4/c1-34-26-17-22(18-27(35-2)28(26)36-3)10-9-21-11-13-24(14-12-21)29(33)31-19-25(32-16-15-30-20-32)23-7-5-4-6-8-23/h4-18,20,25H,19H2,1-3H3,(H,31,33)/b10-9+ | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of mouse CYP27B1 using 1,25(OH)2D3 substrate in presence of bovine adrenodoxin, adrenodoxin reductase and NADPH incubated at 37 degC for 2... |
J Med Chem 57: 7702-15 (2014)
Article DOI: 10.1021/jm5009314
BindingDB Entry DOI: 10.7270/Q2DN46ND |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50440294

(CHEMBL2424820)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)CC2=Nc3ccccc3CN2)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C |r,t:12| Show InChI InChI=1S/C67H113N13O12/c1-24-46-63(88)74(17)35-53(81)75(18)48(29-36(2)3)60(85)73-54(40(10)11)66(91)76(19)49(30-37(4)5)59(84)69-43(15)58(83)70-44(16)62(87)77(20)50(31-38(6)7)64(89)78(21)51(32-39(8)9)65(90)79(22)55(41(12)13)67(92)80(23)56(61(86)72-46)57(82)42(14)33-52-68-34-45-27-25-26-28-47(45)71-52/h25-28,36-44,46,48-51,54-57,82H,24,29-35H2,1-23H3,(H,68,71)(H,69,84)(H,70,83)(H,72,86)(H,73,85)/t42-,43+,44-,46+,48+,49+,50+,51+,54+,55+,56+,57-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Research Unit for Enzymology of Protein Folding
Curated by ChEMBL
| Assay Description
Inhibition of CypA PPIase activity (unknown origin) using Glt-(Ala)n-Pro-Phe-4-nitroanilides as substrate |
J Med Chem 56: 7302-11 (2013)
Article DOI: 10.1021/jm4007577
BindingDB Entry DOI: 10.7270/Q2NK3GGK |
More data for this
Ligand-Target Pair | |
25-hydroxyvitamin D-1 alpha hydroxylase, mitochondrial
(Mus musculus) | BDBM31768

(CHEMBL295698 | Ketoconazole | Nizoral | Panfungol)Show SMILES CC(=O)N1CCN(CC1)c1ccc(OC[C@@H]2CO[C@](Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of mouse CYP27B1 by cell-free assay |
Eur J Med Chem 87: 39-51 (2014)
Article DOI: 10.1016/j.ejmech.2014.09.035
BindingDB Entry DOI: 10.7270/Q2SF2XR1 |
More data for this
Ligand-Target Pair | |
25-hydroxyvitamin D-1 alpha hydroxylase, mitochondrial
(Mus musculus) | BDBM31768

(CHEMBL295698 | Ketoconazole | Nizoral | Panfungol)Show SMILES CC(=O)N1CCN(CC1)c1ccc(OC[C@@H]2CO[C@](Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of mouse CYP27B1 using 1,25(OH)2D3 substrate in presence of bovine adrenodoxin, adrenodoxin reductase and NADPH incubated at 37 degC for 2... |
J Med Chem 57: 7702-15 (2014)
Article DOI: 10.1021/jm5009314
BindingDB Entry DOI: 10.7270/Q2DN46ND |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50440295

(CHEMBL2424819)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)Cc2nc3ccccc3c(=O)[nH]2)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C |r| Show InChI InChI=1S/C67H111N13O13/c1-24-45-63(89)74(17)34-52(81)75(18)47(29-35(2)3)60(86)73-53(39(10)11)66(92)76(19)48(30-36(4)5)59(85)68-42(15)57(83)69-43(16)62(88)77(20)49(31-37(6)7)64(90)78(21)50(32-38(8)9)65(91)79(22)54(40(12)13)67(93)80(23)55(61(87)71-45)56(82)41(14)33-51-70-46-28-26-25-27-44(46)58(84)72-51/h25-28,35-43,45,47-50,53-56,82H,24,29-34H2,1-23H3,(H,68,85)(H,69,83)(H,71,87)(H,73,86)(H,70,72,84)/t41-,42+,43-,45+,47+,48+,49+,50+,53+,54+,55+,56-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Research Unit for Enzymology of Protein Folding
Curated by ChEMBL
| Assay Description
Inhibition of CypA PPIase activity (unknown origin) using Glt-(Ala)n-Pro-Phe-4-nitroanilides as substrate |
J Med Chem 56: 7302-11 (2013)
Article DOI: 10.1021/jm4007577
BindingDB Entry DOI: 10.7270/Q2NK3GGK |
More data for this
Ligand-Target Pair | |
25-hydroxyvitamin D-1 alpha hydroxylase, mitochondrial
(Mus musculus) | BDBM50322357

((E)-4-N-(2-(1H-imidazol-1-yl)-2-phenylethyl)-4-sty...)Show SMILES O=C(NCC(c1ccccc1)n1ccnc1)c1ccc(\C=C\c2ccccc2)cc1 Show InChI InChI=1S/C26H23N3O/c30-26(24-15-13-22(14-16-24)12-11-21-7-3-1-4-8-21)28-19-25(29-18-17-27-20-29)23-9-5-2-6-10-23/h1-18,20,25H,19H2,(H,28,30)/b12-11+ | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of mouse CYP27B1 using 1,25(OH)2D3 substrate in presence of bovine adrenodoxin, adrenodoxin reductase and NADPH incubated at 37 degC for 2... |
J Med Chem 57: 7702-15 (2014)
Article DOI: 10.1021/jm5009314
BindingDB Entry DOI: 10.7270/Q2DN46ND |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50023351

(CHEMBL3329772)Show SMILES O=C(NCC(c1ccccc1)n1ccnc1)c1ccc(cc1)S(=O)(=O)Nc1ccccc1 Show InChI InChI=1S/C24H22N4O3S/c29-24(26-17-23(28-16-15-25-18-28)19-7-3-1-4-8-19)20-11-13-22(14-12-20)32(30,31)27-21-9-5-2-6-10-21/h1-16,18,23,27H,17H2,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of human MBP-tagged CYP24A1 expressed in Escherichia coli using 1,25(OH)2D3 substrate in presence of bovine adrenodoxin, adrenodoxin reduc... |
J Med Chem 57: 7702-15 (2014)
Article DOI: 10.1021/jm5009314
BindingDB Entry DOI: 10.7270/Q2DN46ND |
More data for this
Ligand-Target Pair | |
25-hydroxyvitamin D-1 alpha hydroxylase, mitochondrial
(Mus musculus) | BDBM50023346

(CHEMBL3329767)Show SMILES Fc1ccc(\C=C\c2ccc(cc2)C(=O)NCC(c2ccccc2)n2ccnc2)cc1 Show InChI InChI=1S/C26H22FN3O/c27-24-14-10-21(11-15-24)7-6-20-8-12-23(13-9-20)26(31)29-18-25(30-17-16-28-19-30)22-4-2-1-3-5-22/h1-17,19,25H,18H2,(H,29,31)/b7-6+ | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of mouse CYP27B1 using 1,25(OH)2D3 substrate in presence of bovine adrenodoxin, adrenodoxin reductase and NADPH incubated at 37 degC for 2... |
J Med Chem 57: 7702-15 (2014)
Article DOI: 10.1021/jm5009314
BindingDB Entry DOI: 10.7270/Q2DN46ND |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase C
(Homo sapiens (Human)) | BDBM50440293

(CHEMBL2424822)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)Cc2nc3ccc(cc3[nH]2)C(O)=O)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C |r| Show InChI InChI=1S/C67H111N13O14/c1-24-44-62(88)74(17)33-52(81)75(18)47(27-34(2)3)59(85)73-53(38(10)11)65(91)76(19)48(28-35(4)5)58(84)68-41(15)57(83)69-42(16)61(87)77(20)49(29-36(6)7)63(89)78(21)50(30-37(8)9)64(90)79(22)54(39(12)13)66(92)80(23)55(60(86)72-44)56(82)40(14)31-51-70-45-26-25-43(67(93)94)32-46(45)71-51/h25-26,32,34-42,44,47-50,53-56,82H,24,27-31,33H2,1-23H3,(H,68,84)(H,69,83)(H,70,71)(H,72,86)(H,73,85)(H,93,94)/t40-,41+,42-,44+,47+,48+,49+,50+,53+,54+,55+,56-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 118 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Research Unit for Enzymology of Protein Folding
Curated by ChEMBL
| Assay Description
Inhibition of CypC PPIase activity (unknown origin) |
J Med Chem 56: 7302-11 (2013)
Article DOI: 10.1021/jm4007577
BindingDB Entry DOI: 10.7270/Q2NK3GGK |
More data for this
Ligand-Target Pair | |
25-hydroxyvitamin D-1 alpha hydroxylase, mitochondrial
(Mus musculus) | BDBM50023357
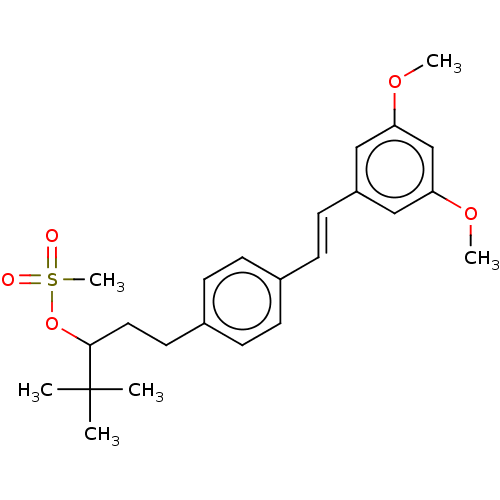
(CHEMBL3329776)Show SMILES COc1cc(OC)cc(\C=C\c2ccc(CCC(OS(C)(=O)=O)C(C)(C)C)cc2)c1 Show InChI InChI=1S/C24H32O5S/c1-24(2,3)23(29-30(6,25)26)14-13-19-9-7-18(8-10-19)11-12-20-15-21(27-4)17-22(16-20)28-5/h7-12,15-17,23H,13-14H2,1-6H3/b12-11+ | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of mouse CYP27B1 using 1,25(OH)2D3 substrate in presence of bovine adrenodoxin, adrenodoxin reductase and NADPH incubated at 37 degC for 2... |
J Med Chem 57: 7702-15 (2014)
Article DOI: 10.1021/jm5009314
BindingDB Entry DOI: 10.7270/Q2DN46ND |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data