

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50134709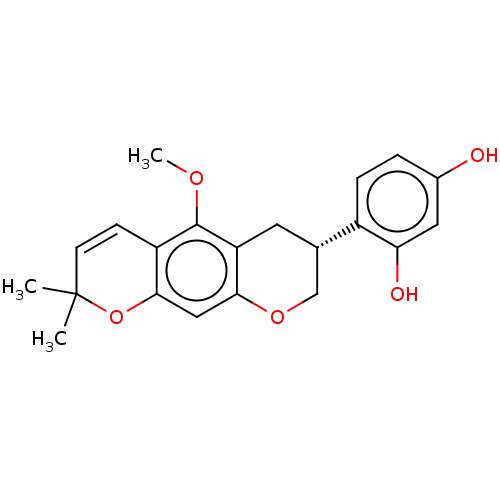 (CHEMBL3747057) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Time dependent inhibition of monophenolase activity of mushroom tyrosinase using L-tyrosine as substrate measured every 30 secs by Morrison and Walsh... | Bioorg Med Chem 24: 153-9 (2016) Article DOI: 10.1016/j.bmc.2015.11.040 BindingDB Entry DOI: 10.7270/Q2RN39PT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50134709 (CHEMBL3747057) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of monophenolase activity of mushroom tyrosinase using L-tyrosine as substrate preincubated for 5 to 60 mins by Lineweaver-Bur... | Bioorg Med Chem 24: 153-9 (2016) Article DOI: 10.1016/j.bmc.2015.11.040 BindingDB Entry DOI: 10.7270/Q2RN39PT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50134709 (CHEMBL3747057) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of diphenolase activity of mushroom tyrosinase using L-DOPA as substrate preincubated for 5 to 60 mins by Lineweaver-Burk and ... | Bioorg Med Chem 24: 153-9 (2016) Article DOI: 10.1016/j.bmc.2015.11.040 BindingDB Entry DOI: 10.7270/Q2RN39PT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50134710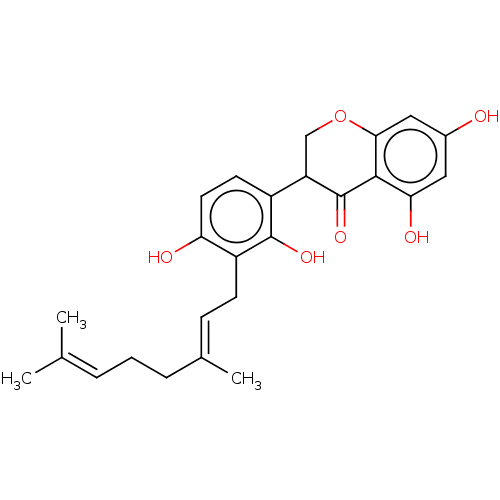 (CHEMBL3745886) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 151 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Time dependent inhibition of monophenolase activity of mushroom tyrosinase using L-tyrosine as substrate measured every 30 secs by Morrison and Walsh... | Bioorg Med Chem 24: 153-9 (2016) Article DOI: 10.1016/j.bmc.2015.11.040 BindingDB Entry DOI: 10.7270/Q2RN39PT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50366237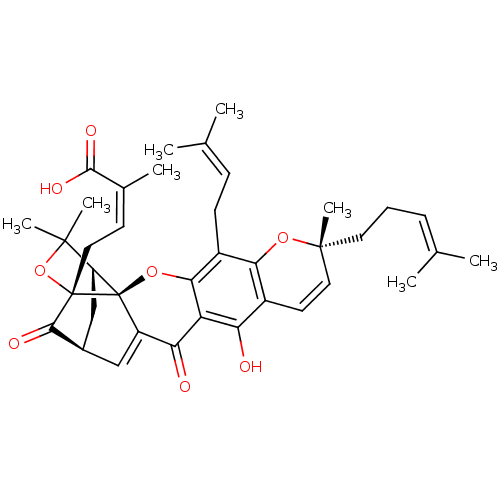 (GAMBOGIC ACID) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Applied Life Science (BK21 Plus), IALS, Gyeongsang National University, Jinju 52828, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate by Dixon plot analysis | Bioorg Med Chem 25: 2498-2506 (2017) Article DOI: 10.1016/j.bmc.2017.03.010 BindingDB Entry DOI: 10.7270/Q2Q81GHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50256793 (CHEMBL4095800) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Applied Life Science (BK21 Plus), IALS, Gyeongsang National University, Jinju 52828, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate by Dixon plot analysis | Bioorg Med Chem 25: 2498-2506 (2017) Article DOI: 10.1016/j.bmc.2017.03.010 BindingDB Entry DOI: 10.7270/Q2Q81GHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50256807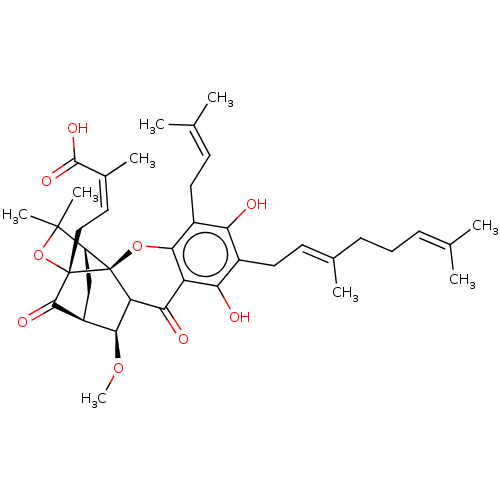 (CHEMBL4069859) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Applied Life Science (BK21 Plus), IALS, Gyeongsang National University, Jinju 52828, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated for 60 mins followed by ... | Bioorg Med Chem 25: 2498-2506 (2017) Article DOI: 10.1016/j.bmc.2017.03.010 BindingDB Entry DOI: 10.7270/Q2Q81GHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50366237 (GAMBOGIC ACID) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 499 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Applied Life Science (BK21 Plus), IALS, Gyeongsang National University, Jinju 52828, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated for 60 mins followed by ... | Bioorg Med Chem 25: 2498-2506 (2017) Article DOI: 10.1016/j.bmc.2017.03.010 BindingDB Entry DOI: 10.7270/Q2Q81GHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50256792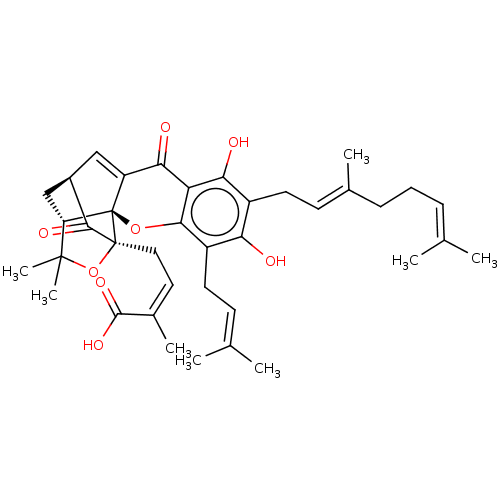 (Gambogenic Acid) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Applied Life Science (BK21 Plus), IALS, Gyeongsang National University, Jinju 52828, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated for 60 mins followed by ... | Bioorg Med Chem 25: 2498-2506 (2017) Article DOI: 10.1016/j.bmc.2017.03.010 BindingDB Entry DOI: 10.7270/Q2Q81GHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable maltase-glucoamylase 2 (Homo sapiens) | BDBM50241453 (1,3,6,7-tetrahydroxy-2,8-bis(3-methylbut-2-enyl)-9...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of alpha-glucosidase (unknown origin) using PNP-G as substrate by Dixon plot analysis | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50256790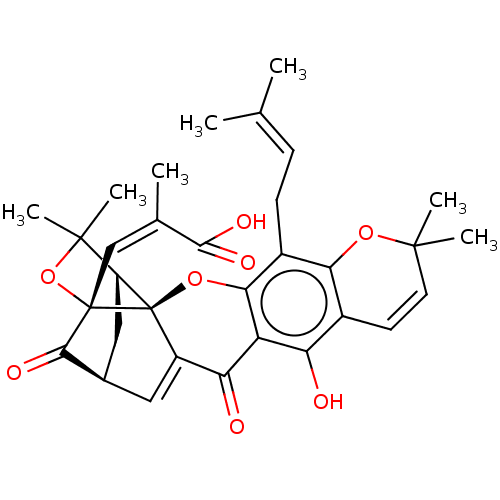 (CHEMBL4068566) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Applied Life Science (BK21 Plus), IALS, Gyeongsang National University, Jinju 52828, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated for 60 mins followed by ... | Bioorg Med Chem 25: 2498-2506 (2017) Article DOI: 10.1016/j.bmc.2017.03.010 BindingDB Entry DOI: 10.7270/Q2Q81GHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50256791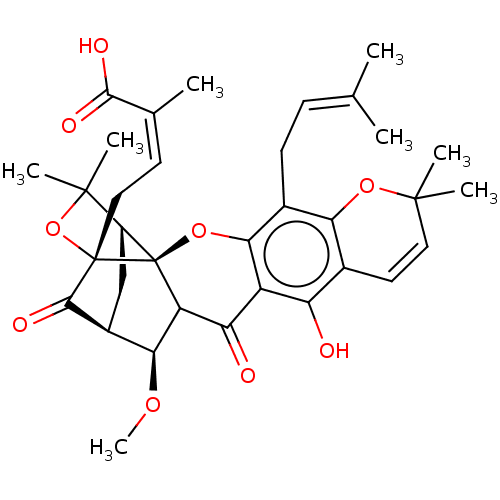 (CHEMBL4090412) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Applied Life Science (BK21 Plus), IALS, Gyeongsang National University, Jinju 52828, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate by Dixon plot analysis | Bioorg Med Chem 25: 2498-2506 (2017) Article DOI: 10.1016/j.bmc.2017.03.010 BindingDB Entry DOI: 10.7270/Q2Q81GHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50134710 (CHEMBL3745886) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of monophenolase activity of mushroom tyrosinase using L-tyrosine as substrate preincubated for 5 to 60 mins by Lineweaver-Bur... | Bioorg Med Chem 24: 153-9 (2016) Article DOI: 10.1016/j.bmc.2015.11.040 BindingDB Entry DOI: 10.7270/Q2RN39PT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50454316 (CHEMBL4211682) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using P-NPP as substrate after 10 mins by Dixon plot analysis | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50454316 (CHEMBL4211682) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Time dependent inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using P-NPP as substrate preincubated with enz... | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50442401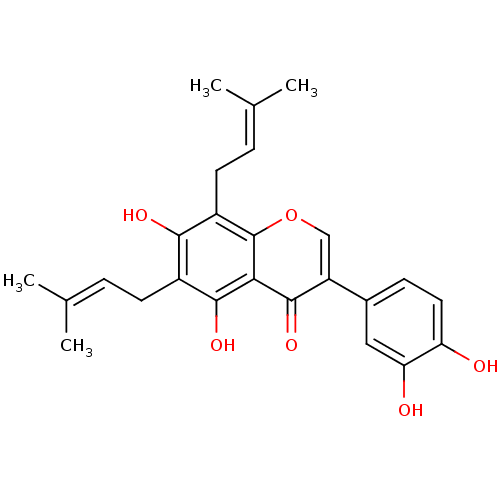 (CHEMBL2442947) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Qiqihar University Curated by ChEMBL | Assay Description Non-competitive inhibition of human recombinant PTP1B using p-nitrophenyl phosphate as substrate by spectrophotometry based Lineweaver-Burk plot | Bioorg Med Chem Lett 26: 318-21 (2016) Article DOI: 10.1016/j.bmcl.2015.12.021 BindingDB Entry DOI: 10.7270/Q2C82C4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50241453 (1,3,6,7-tetrahydroxy-2,8-bis(3-methylbut-2-enyl)-9...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using P-NPP as substrate after 10 mins by Dixon plot analysis | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50346335 (CHEMBL1782241 | Cochinchinone A) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using P-NPP as substrate after 10 mins by Dixon plot analysis | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50214969 (1,3,6-Trihydroxy-7-methoxy-2,8-bis-(3-methyl-but-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using P-NPP as substrate after 10 mins by Dixon plot analysis | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50136569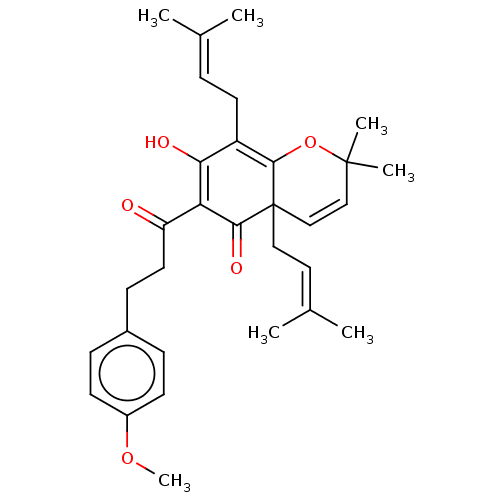 (CHEMBL3754629) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Qiqihar University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant PTP1B using p-nitrophenyl phosphate as substrate by spectrophotometry based Lineweaver-Burk plot | Bioorg Med Chem Lett 26: 318-21 (2016) Article DOI: 10.1016/j.bmcl.2015.12.021 BindingDB Entry DOI: 10.7270/Q2C82C4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50454321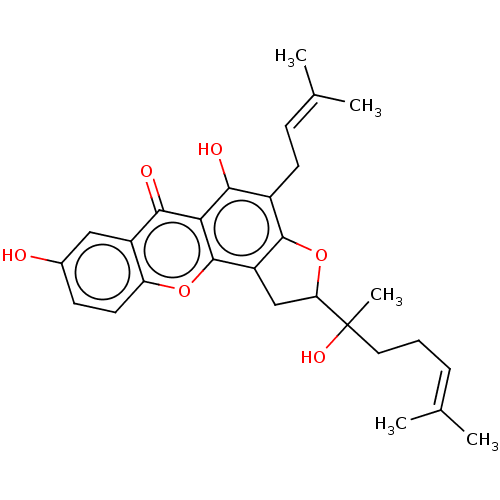 (CHEMBL4208135) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using P-NPP as substrate after 10 mins by Dixon plot analysis | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable maltase-glucoamylase 2 (Homo sapiens) | BDBM50454316 (CHEMBL4211682) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of alpha-glucosidase (unknown origin) using PNP-G as substrate by Dixon plot analysis | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50256794 (CHEMBL4059800) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 4.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Applied Life Science (BK21 Plus), IALS, Gyeongsang National University, Jinju 52828, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate by Dixon plot analysis | Bioorg Med Chem 25: 2498-2506 (2017) Article DOI: 10.1016/j.bmc.2017.03.010 BindingDB Entry DOI: 10.7270/Q2Q81GHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50442400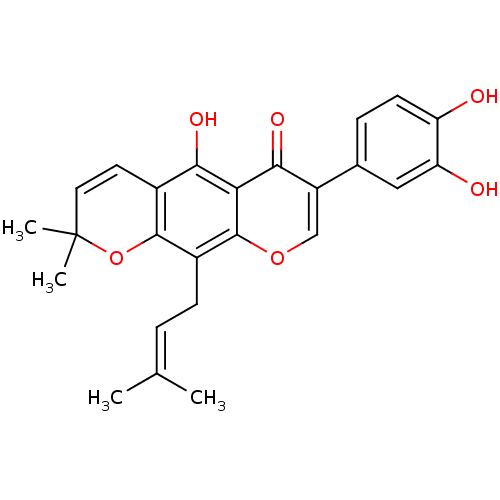 (AURICULASIN) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Qiqihar University Curated by ChEMBL | Assay Description Non-competitive inhibition of human recombinant PTP1B using p-nitrophenyl phosphate as substrate by spectrophotometry based Lineweaver-Burk plot | Bioorg Med Chem Lett 26: 318-21 (2016) Article DOI: 10.1016/j.bmcl.2015.12.021 BindingDB Entry DOI: 10.7270/Q2C82C4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50454319 (CHEMBL4214309) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using P-NPP as substrate after 10 mins by Dixon plot analysis | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable maltase-glucoamylase 2 (Homo sapiens) | BDBM50454316 (CHEMBL4211682) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Mixed type inhibition of alpha-glucosidase (unknown origin) assessed as enzyme-substrate-inhibitor complex formation using PNP-G as substrate by Line... | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable maltase-glucoamylase 2 (Homo sapiens) | BDBM50214969 (1,3,6-Trihydroxy-7-methoxy-2,8-bis-(3-methyl-but-2...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of alpha-glucosidase (unknown origin) using PNP-G as substrate by Dixon plot analysis | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50134712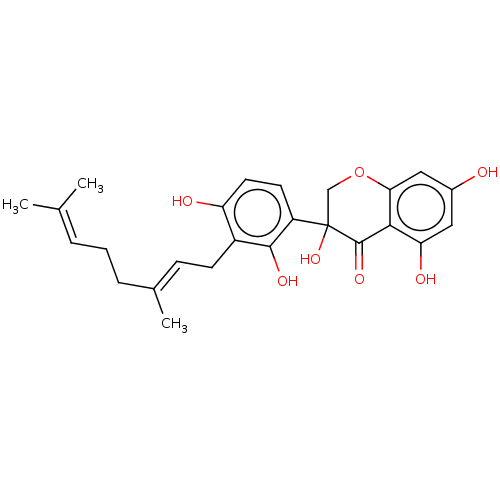 (CHEMBL3746218) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of monophenolase activity of mushroom tyrosinase using L-tyrosine as substrate preincubated for 5 to 60 mins by Lineweaver-Bur... | Bioorg Med Chem 24: 153-9 (2016) Article DOI: 10.1016/j.bmc.2015.11.040 BindingDB Entry DOI: 10.7270/Q2RN39PT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable maltase-glucoamylase 2 (Homo sapiens) | BDBM50454317 (CHEMBL4203265) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of alpha-glucosidase (unknown origin) using PNP-G as substrate by Dixon plot analysis | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50454317 (CHEMBL4203265) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using P-NPP as substrate after 10 mins by Dixon plot analysis | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50442403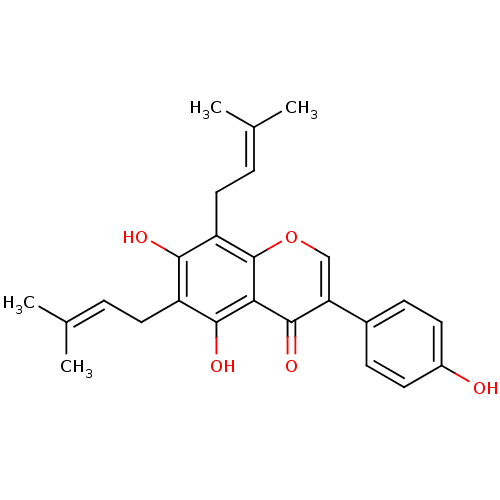 (CHEMBL494252) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Qiqihar University Curated by ChEMBL | Assay Description Non-competitive inhibition of human recombinant PTP1B using p-nitrophenyl phosphate as substrate by spectrophotometry based Lineweaver-Burk plot | Bioorg Med Chem Lett 26: 318-21 (2016) Article DOI: 10.1016/j.bmcl.2015.12.021 BindingDB Entry DOI: 10.7270/Q2C82C4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable maltase-glucoamylase 2 (Homo sapiens) | BDBM50454321 (CHEMBL4208135) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of alpha-glucosidase (unknown origin) using PNP-G as substrate by Dixon plot analysis | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50346334 (1,3,7-trihydroxy-2,4-diisoprenylxanthone | CHEMBL1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using P-NPP as substrate after 10 mins by Dixon plot analysis | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50136568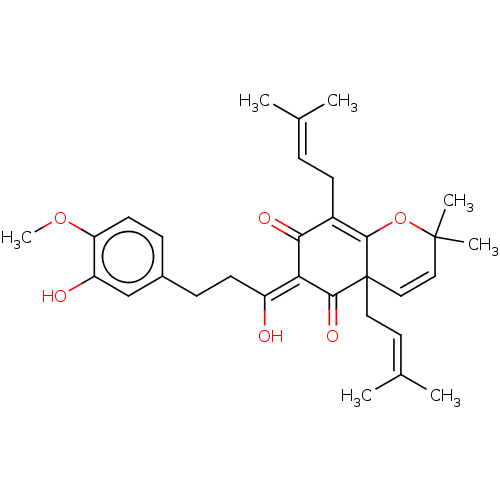 (CHEMBL3754570) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Qiqihar University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant PTP1B using p-nitrophenyl phosphate as substrate by spectrophotometry based Lineweaver-Burk plot | Bioorg Med Chem Lett 26: 318-21 (2016) Article DOI: 10.1016/j.bmcl.2015.12.021 BindingDB Entry DOI: 10.7270/Q2C82C4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable maltase-glucoamylase 2 (Homo sapiens) | BDBM50454318 (CHEMBL4202640) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of alpha-glucosidase (unknown origin) using PNP-G as substrate by Dixon plot analysis | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable maltase-glucoamylase 2 (Homo sapiens) | BDBM50346335 (CHEMBL1782241 | Cochinchinone A) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of alpha-glucosidase (unknown origin) using PNP-G as substrate by Dixon plot analysis | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50136567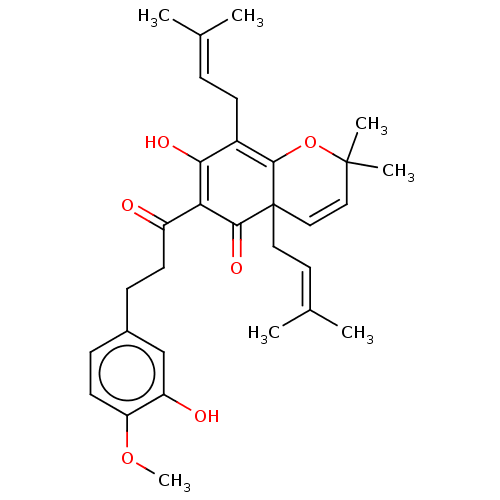 (CHEMBL3753821) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Qiqihar University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant PTP1B using p-nitrophenyl phosphate as substrate by spectrophotometry based Lineweaver-Burk plot | Bioorg Med Chem Lett 26: 318-21 (2016) Article DOI: 10.1016/j.bmcl.2015.12.021 BindingDB Entry DOI: 10.7270/Q2C82C4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable maltase-glucoamylase 2 (Homo sapiens) | BDBM50454323 (CHEMBL4209191) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of alpha-glucosidase (unknown origin) using PNP-G as substrate by Dixon plot analysis | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50442402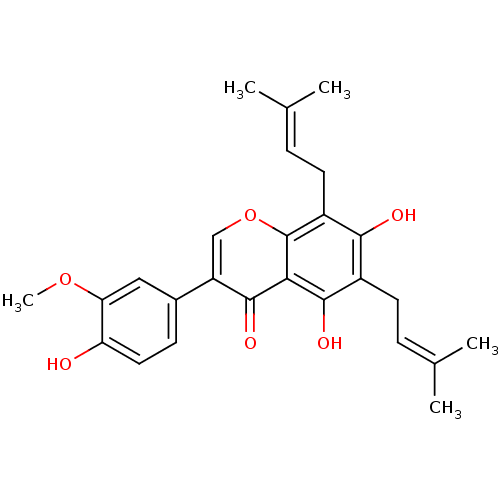 (FLEMINGSIN) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Qiqihar University Curated by ChEMBL | Assay Description Non-competitive inhibition of human recombinant PTP1B using p-nitrophenyl phosphate as substrate by spectrophotometry based Lineweaver-Burk plot | Bioorg Med Chem Lett 26: 318-21 (2016) Article DOI: 10.1016/j.bmcl.2015.12.021 BindingDB Entry DOI: 10.7270/Q2C82C4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable maltase-glucoamylase 2 (Homo sapiens) | BDBM50346334 (1,3,7-trihydroxy-2,4-diisoprenylxanthone | CHEMBL1...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of alpha-glucosidase (unknown origin) using PNP-G as substrate by Dixon plot analysis | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50454323 (CHEMBL4209191) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using P-NPP as substrate after 10 mins by Dixon plot analysis | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50454320 (CHEMBL4217516) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 2.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using P-NPP as substrate after 10 mins by Dixon plot analysis | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable maltase-glucoamylase 2 (Homo sapiens) | BDBM50454320 (CHEMBL4217516) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of alpha-glucosidase (unknown origin) using PNP-G as substrate by Dixon plot analysis | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable maltase-glucoamylase 2 (Homo sapiens) | BDBM50454319 (CHEMBL4214309) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of alpha-glucosidase (unknown origin) using PNP-G as substrate by Dixon plot analysis | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50256806 (CHEMBL4068458) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Applied Life Science (BK21 Plus), IALS, Gyeongsang National University, Jinju 52828, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated for 60 mins followed by ... | Bioorg Med Chem 25: 2498-2506 (2017) Article DOI: 10.1016/j.bmc.2017.03.010 BindingDB Entry DOI: 10.7270/Q2Q81GHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50454318 (CHEMBL4202640) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using P-NPP as substrate after 10 mins by Dixon plot analysis | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50442397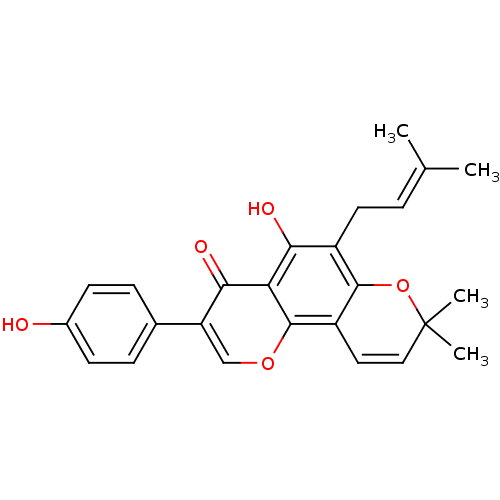 (OSAJIN) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Qiqihar University Curated by ChEMBL | Assay Description Non-competitive inhibition of human recombinant PTP1B using p-nitrophenyl phosphate as substrate by spectrophotometry based Lineweaver-Burk plot | Bioorg Med Chem Lett 26: 318-21 (2016) Article DOI: 10.1016/j.bmcl.2015.12.021 BindingDB Entry DOI: 10.7270/Q2C82C4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50454322 (CHEMBL4218731) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using P-NPP as substrate after 10 mins by Dixon plot analysis | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable maltase-glucoamylase 2 (Homo sapiens) | BDBM50454322 (CHEMBL4218731) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of alpha-glucosidase (unknown origin) using PNP-G as substrate by Dixon plot analysis | Bioorg Med Chem 26: 737-746 (2018) Article DOI: 10.1016/j.bmc.2017.12.043 BindingDB Entry DOI: 10.7270/Q2CJ8H34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50134713 (CHEMBL3746757) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of monophenolase activity of mushroom tyrosinase using L-tyrosine as substrate preincubated for 5 to 60 mins by Lineweaver-Bur... | Bioorg Med Chem 24: 153-9 (2016) Article DOI: 10.1016/j.bmc.2015.11.040 BindingDB Entry DOI: 10.7270/Q2RN39PT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 128 total ) | Next | Last >> |