

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kennesaw State University Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against Cannabinoid receptor 2 in Guinea pig ileum (GPI) using [3H]CP-55940 ligand | J Med Chem 41: 5177-87 (1999) Article DOI: 10.1021/jm9801197 BindingDB Entry DOI: 10.7270/Q2ST7QJJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50217828 (CHEMBL413707) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity for human cloned 5-hydroxytryptamine 7 receptor in HEK 293 using [3H]5-CT as a radioligand | Bioorg Med Chem Lett 12: 3341-4 (2002) BindingDB Entry DOI: 10.7270/Q2FT8P7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50217831 (CHEMBL430706) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.676 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity for human cloned 5-hydroxytryptamine 7 receptor in HEK 293 using [3H]5-CT as a radioligand | Bioorg Med Chem Lett 12: 3341-4 (2002) BindingDB Entry DOI: 10.7270/Q2FT8P7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50217829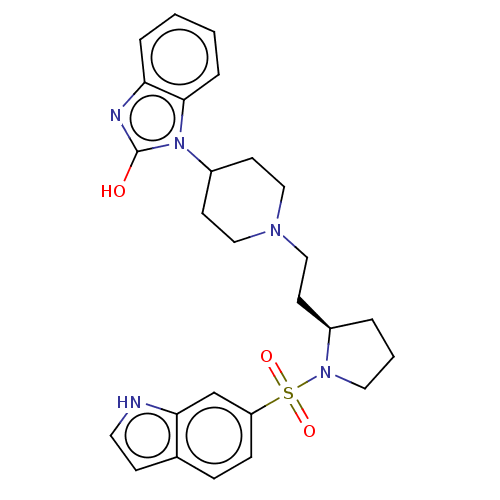 (CHEMBL115262) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.708 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity for human cloned 5-hydroxytryptamine 7 receptor in HEK 293 using [3H]5-CT as a radioligand | Bioorg Med Chem Lett 12: 3341-4 (2002) BindingDB Entry DOI: 10.7270/Q2FT8P7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50556532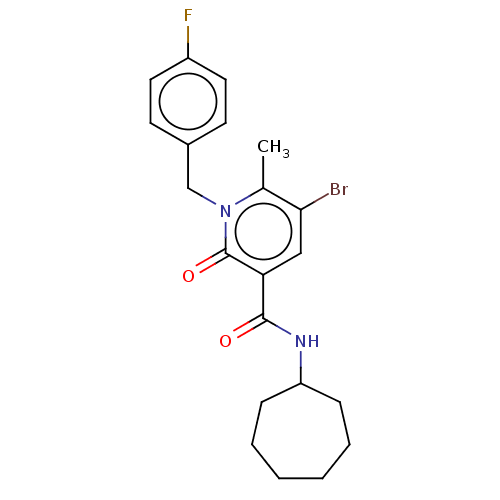 (CHEMBL4753891) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00582 BindingDB Entry DOI: 10.7270/Q2NV9PB9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50217832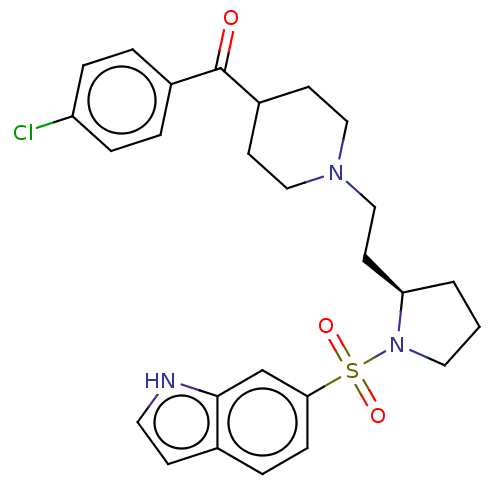 (CHEMBL116292) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity for human cloned 5-hydroxytryptamine 7 receptor in HEK 293 using [3H]5-CT as a radioligand | Bioorg Med Chem Lett 12: 3341-4 (2002) BindingDB Entry DOI: 10.7270/Q2FT8P7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50217835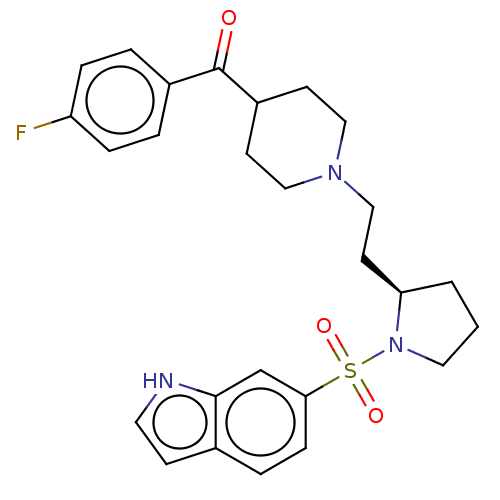 (CHEMBL114345) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.832 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity for human cloned 5-hydroxytryptamine 7 receptor in HEK 293 using [3H]5-CT as a radioligand | Bioorg Med Chem Lett 12: 3341-4 (2002) BindingDB Entry DOI: 10.7270/Q2FT8P7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50114673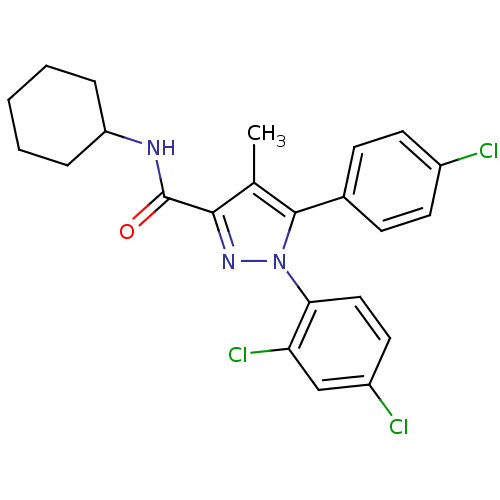 (5-(4-Chloro-phenyl)-1-(2,4-dichloro-phenyl)-4-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.07 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity in a competition assay by displacement of [3H]- SR-141,716 from Cannabinoid receptor 1 in rat whole brain membrane preparation | J Med Chem 45: 2708-19 (2002) BindingDB Entry DOI: 10.7270/Q2TX3DQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity in a competition assay by displacement of [3H]- SR-141,716 from Cannabinoid receptor 1 in rat whole brain membrane preparation | J Med Chem 45: 2708-19 (2002) BindingDB Entry DOI: 10.7270/Q2TX3DQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (Homo sapiens (Human)) | BDBM50217831 (CHEMBL430706) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity towards Alpha-1B adrenergic receptor | Bioorg Med Chem Lett 12: 3341-4 (2002) BindingDB Entry DOI: 10.7270/Q2FT8P7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50098551 ((R)-3-(2-(2-(4-methylpiperidin-1-yl)ethyl)pyrrolid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity for human cloned 5-hydroxytryptamine 7 receptor in HEK 293 using [3H]5-CT as a radioligand | Bioorg Med Chem Lett 12: 3341-4 (2002) BindingDB Entry DOI: 10.7270/Q2FT8P7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50601131 (CHEMBL5193398) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00582 BindingDB Entry DOI: 10.7270/Q2NV9PB9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50217825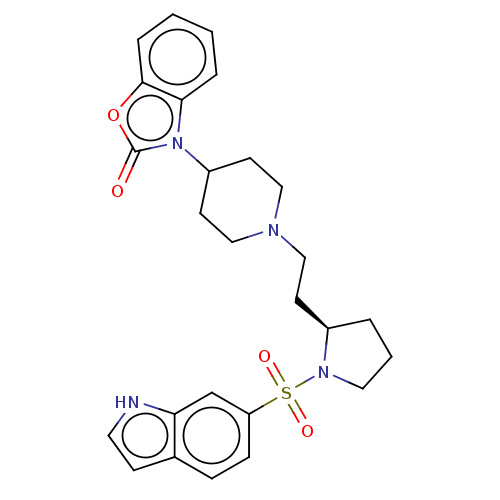 (CHEMBL323778) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity for human cloned 5-hydroxytryptamine 7 receptor in HEK 293 using [3H]5-CT as a radioligand | Bioorg Med Chem Lett 12: 3341-4 (2002) BindingDB Entry DOI: 10.7270/Q2FT8P7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50601132 (CHEMBL5204176) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00582 BindingDB Entry DOI: 10.7270/Q2NV9PB9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50130261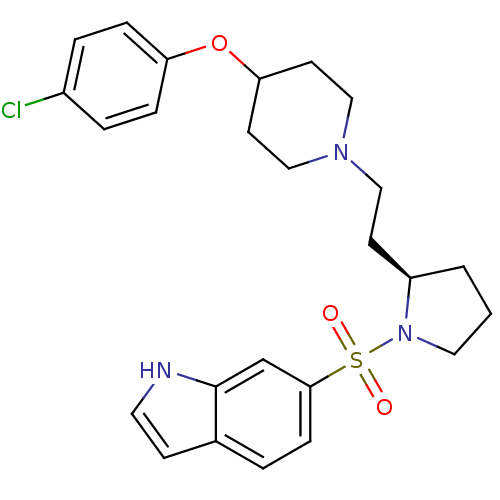 (6-(2-{2-[4-(4-Chloro-phenoxy)-piperidin-1-yl]-ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Compound was tested for its binding affinity in 5-hydroxytryptamine 7 receptor (using human cloned receptors in HEK 293 and [3H]5-CT as a radioligand... | Bioorg Med Chem Lett 12: 3341-4 (2002) BindingDB Entry DOI: 10.7270/Q2FT8P7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50068669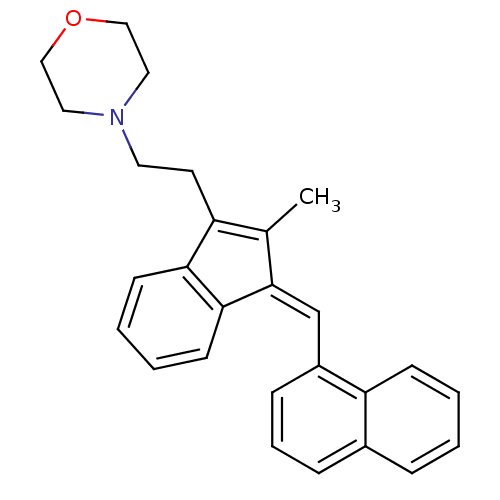 (4-(2-{2-Methyl-3-[1-naphthalen-1-yl-meth-(Z)-ylide...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kennesaw State University Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against Cannabinoid receptor 2 in Guinea pig ileum (GPI) using [3H]CP-55940 ligand | J Med Chem 41: 5177-87 (1999) Article DOI: 10.1021/jm9801197 BindingDB Entry DOI: 10.7270/Q2ST7QJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50130297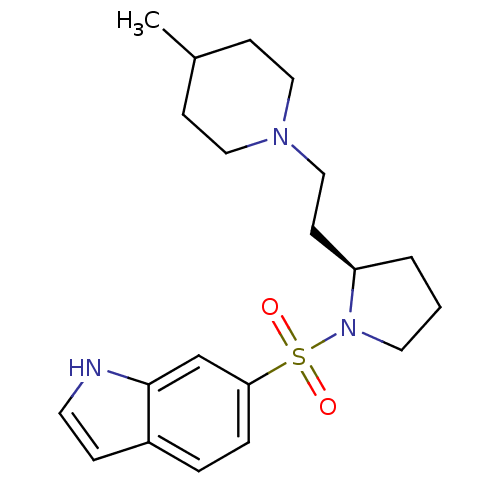 (6-{(R)-2-[2-(4-Methyl-piperidin-1-yl)-ethyl]-pyrro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity for human cloned 5-hydroxytryptamine 7 receptor in HEK 293 using [3H]5-CT as a radioligand | Bioorg Med Chem Lett 12: 3341-4 (2002) BindingDB Entry DOI: 10.7270/Q2FT8P7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50114673 (5-(4-Chloro-phenyl)-1-(2,4-dichloro-phenyl)-4-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity in a competition assay by displacement of [3H]- CP 55 940 from Cannabinoid receptor 1 in rat whole brain membrane preparation | J Med Chem 45: 2708-19 (2002) BindingDB Entry DOI: 10.7270/Q2TX3DQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kennesaw State University Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against Cannabinoid receptor 1 in Guinea pig ileum (GPI) using [3H]CP-55940 ligand | J Med Chem 41: 5177-87 (1999) Article DOI: 10.1021/jm9801197 BindingDB Entry DOI: 10.7270/Q2ST7QJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50068666 (4-(2-{3-[1-Naphthalen-1-yl-meth-(E)-ylidene]-3H-in...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kennesaw State University Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against Cannabinoid receptor 1 in Guinea pig ileum (GPI) using [3H]CP-55940 ligand | J Med Chem 41: 5177-87 (1999) Article DOI: 10.1021/jm9801197 BindingDB Entry DOI: 10.7270/Q2ST7QJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50068666 (4-(2-{3-[1-Naphthalen-1-yl-meth-(E)-ylidene]-3H-in...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kennesaw State University Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against Cannabinoid receptor 2 in Guinea pig ileum (GPI) using [3H]CP-55940 ligand | J Med Chem 41: 5177-87 (1999) Article DOI: 10.1021/jm9801197 BindingDB Entry DOI: 10.7270/Q2ST7QJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50068669 (4-(2-{2-Methyl-3-[1-naphthalen-1-yl-meth-(Z)-ylide...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kennesaw State University Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against Cannabinoid receptor 1 in Guinea pig ileum (GPI) using [3H]CP-55940 ligand | J Med Chem 41: 5177-87 (1999) Article DOI: 10.1021/jm9801197 BindingDB Entry DOI: 10.7270/Q2ST7QJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 6.18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity in a competition assay by displacement of [3H]- CP 55 940 from Cannabinoid receptor 1 in rat whole brain membrane preparation | J Med Chem 45: 2708-19 (2002) BindingDB Entry DOI: 10.7270/Q2TX3DQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50217833 (CHEMBL331803) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity for human cloned 5-hydroxytryptamine 7 receptor in HEK 293 using [3H]5-CT as a radioligand | Bioorg Med Chem Lett 12: 3341-4 (2002) BindingDB Entry DOI: 10.7270/Q2FT8P7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50217827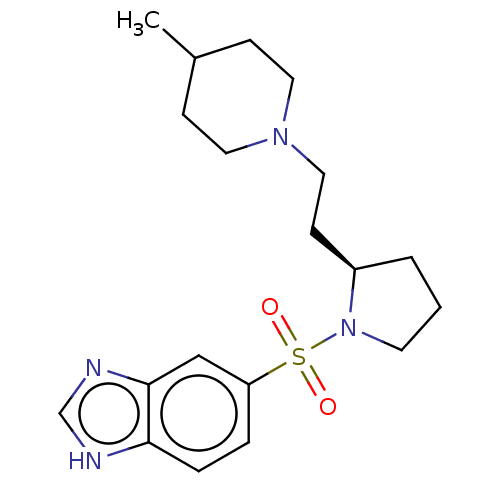 (CHEMBL112876) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity for human cloned 5-hydroxytryptamine 7 receptor in HEK 293 using [3H]5-CT as a radioligand | Bioorg Med Chem Lett 12: 3341-4 (2002) BindingDB Entry DOI: 10.7270/Q2FT8P7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50217837 (CHEMBL111922) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity for human cloned 5-hydroxytryptamine 7 receptor in HEK 293 using [3H]5-CT as a radioligand | Bioorg Med Chem Lett 12: 3341-4 (2002) BindingDB Entry DOI: 10.7270/Q2FT8P7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50072775 (2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00582 BindingDB Entry DOI: 10.7270/Q2NV9PB9 | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50110942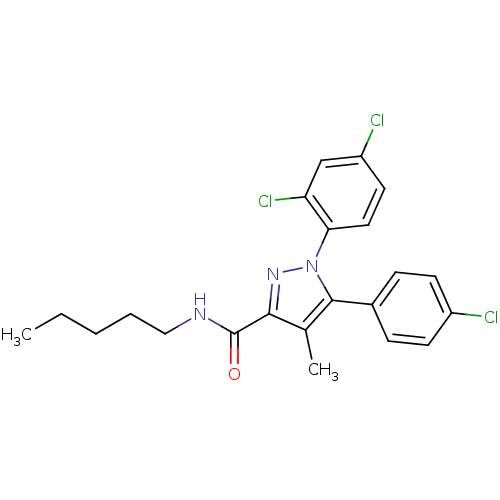 (5-(4-Chloro-phenyl)-1-(2,4-dichloro-phenyl)-4-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity in a competition assay by displacement of [3H]- SR-141,716 from Cannabinoid receptor 1 in rat whole brain membrane preparation | J Med Chem 45: 2708-19 (2002) BindingDB Entry DOI: 10.7270/Q2TX3DQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50556532 (CHEMBL4753891) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00582 BindingDB Entry DOI: 10.7270/Q2NV9PB9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50055824 (2-{[(2R,3S)-3-(4-Chloro-phenyl)-8-methyl-8-aza-bic...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description In vitro binding affinity to Dopamine transporter in rat striatal homogenates using [125I]-IPT20 as the ligand | J Med Chem 40: 9-17 (1997) Article DOI: 10.1021/jm960532j BindingDB Entry DOI: 10.7270/Q2WM1CHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50062680 (2-[[2-[[[3-(4-chlorophenyl)-8-methyl-8-azabicyclo[...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC sid UniChem Similars | Article PubMed | 8.42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description In vitro bindingaffinity towards dopamine transporter in rat striatal homogenatewith [125I]-IPT as the radioligand | J Med Chem 41: 428-36 (1998) Article DOI: 10.1021/jm970742b BindingDB Entry DOI: 10.7270/Q2DN4459 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50114674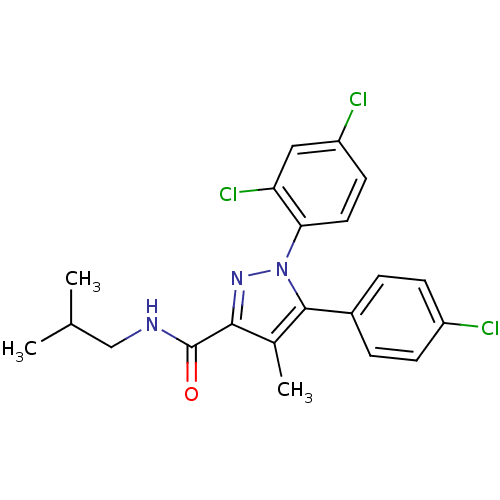 (5-(4-Chloro-phenyl)-1-(2,4-dichloro-phenyl)-4-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity in a competition assay by displacement of [3H]- SR-141,716 from Cannabinoid receptor 1 in rat whole brain membrane preparation | J Med Chem 45: 2708-19 (2002) BindingDB Entry DOI: 10.7270/Q2TX3DQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50068669 (4-(2-{2-Methyl-3-[1-naphthalen-1-yl-meth-(Z)-ylide...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kennesaw State University Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against Cannabinoid receptor 1 in Guinea pig ileum (GPI) using [3H]SR-141,716A ligand | J Med Chem 41: 5177-87 (1999) Article DOI: 10.1021/jm9801197 BindingDB Entry DOI: 10.7270/Q2ST7QJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50068669 (4-(2-{2-Methyl-3-[1-naphthalen-1-yl-meth-(Z)-ylide...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kennesaw State University Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against Cannabinoid receptor 1 in Guinea pig ileum (GPI) using [3H]SR-141,716A ligand | J Med Chem 41: 5177-87 (1999) Article DOI: 10.1021/jm9801197 BindingDB Entry DOI: 10.7270/Q2ST7QJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50114682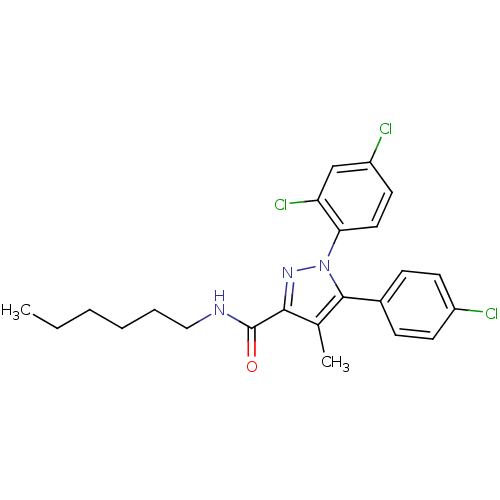 (5-(4-Chloro-phenyl)-1-(2,4-dichloro-phenyl)-4-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity in a competition assay by displacement of [3H]- SR-141,716 from Cannabinoid receptor 1 in rat whole brain membrane preparation | J Med Chem 45: 2708-19 (2002) BindingDB Entry DOI: 10.7270/Q2TX3DQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50068666 (4-(2-{3-[1-Naphthalen-1-yl-meth-(E)-ylidene]-3H-in...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kennesaw State University Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against Cannabinoid receptor 1 in Guinea pig ileum (GPI) using [3H]SR-141,716A ligand at site 1 | J Med Chem 41: 5177-87 (1999) Article DOI: 10.1021/jm9801197 BindingDB Entry DOI: 10.7270/Q2ST7QJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50110942 (5-(4-Chloro-phenyl)-1-(2,4-dichloro-phenyl)-4-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity in a competition assay by displacement of [3H]- CP 55 940 from Cannabinoid receptor 1 in rat whole brain membrane preparation | J Med Chem 45: 2708-19 (2002) BindingDB Entry DOI: 10.7270/Q2TX3DQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50114674 (5-(4-Chloro-phenyl)-1-(2,4-dichloro-phenyl)-4-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity in a competition assay by displacement of [3H]- CP 55 940 from Cannabinoid receptor 1 in rat whole brain membrane preparation | J Med Chem 45: 2708-19 (2002) BindingDB Entry DOI: 10.7270/Q2TX3DQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50114685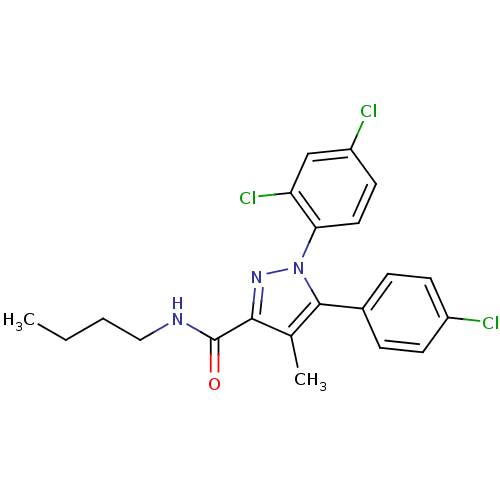 (5-(4-Chloro-phenyl)-1-(2,4-dichloro-phenyl)-4-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity in a competition assay by displacement of [3H]- SR-141,716 from Cannabinoid receptor 1 in rat whole brain membrane preparation | J Med Chem 45: 2708-19 (2002) BindingDB Entry DOI: 10.7270/Q2TX3DQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50114676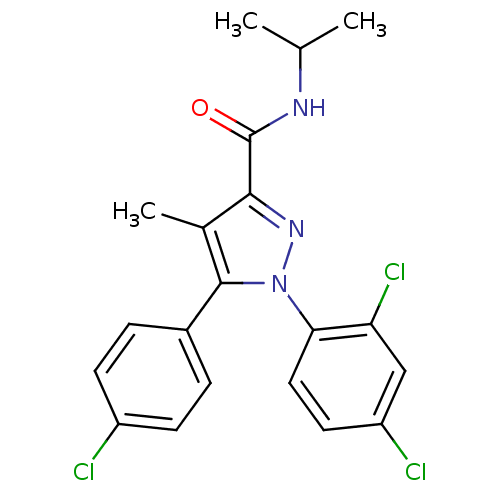 (5-(4-Chloro-phenyl)-1-(2,4-dichloro-phenyl)-4-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity in a competition assay by displacement of [3H]- SR-141,716 from Cannabinoid receptor 1 in rat whole brain membrane preparation | J Med Chem 45: 2708-19 (2002) BindingDB Entry DOI: 10.7270/Q2TX3DQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50114685 (5-(4-Chloro-phenyl)-1-(2,4-dichloro-phenyl)-4-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity in a competition assay by displacement of [3H]- CP 55 940 from Cannabinoid receptor 1 in rat whole brain membrane preparation | J Med Chem 45: 2708-19 (2002) BindingDB Entry DOI: 10.7270/Q2TX3DQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50062679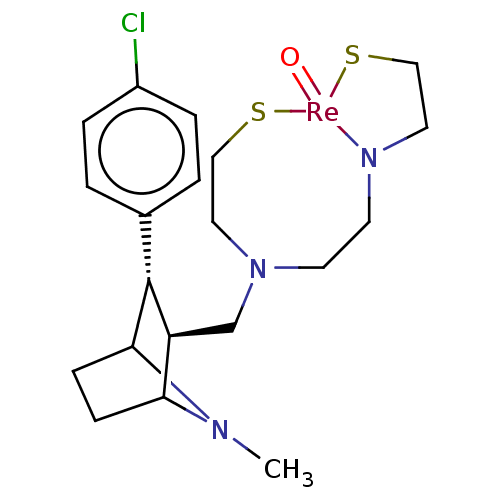 (2-[[2-[[[3-(4-chlorophenyl)-8-methyl-8-azabicyclo[...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC sid UniChem Similars | Article PubMed | 13.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description In vitro bindingaffinity towards dopamine transporter in rat striatal homogenatewith [125I]-IPT as the radioligand | J Med Chem 41: 428-36 (1998) Article DOI: 10.1021/jm970742b BindingDB Entry DOI: 10.7270/Q2DN4459 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50055825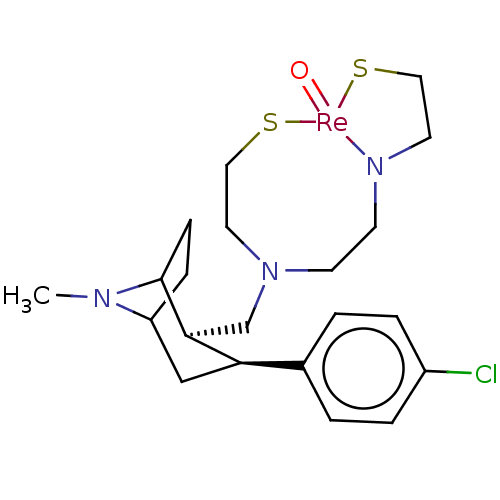 (CHEMBL295441 | Rhenium Compound) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description In vitro binding affinity of the compound towards dopamine transporter using [125I]-IPT20 as radioligand in rat striatal homogenates | J Med Chem 40: 9-17 (1997) Article DOI: 10.1021/jm960532j BindingDB Entry DOI: 10.7270/Q2WM1CHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50475919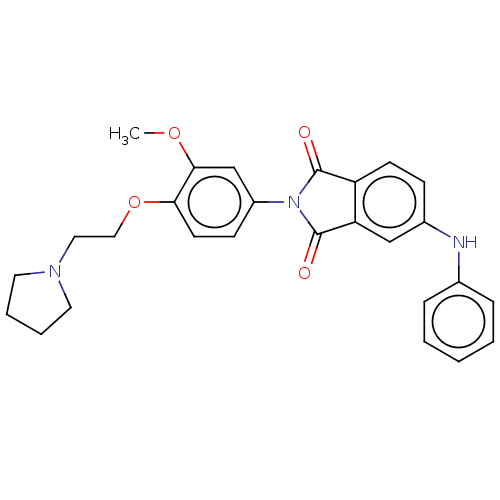 (CHEMBL386050) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of radiolabeled iodo-MCH from human MCHR1 expressed in HEK293 cells | Bioorg Med Chem Lett 16: 4872-8 (2006) Article DOI: 10.1016/j.bmcl.2006.06.061 BindingDB Entry DOI: 10.7270/Q29889S7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50475894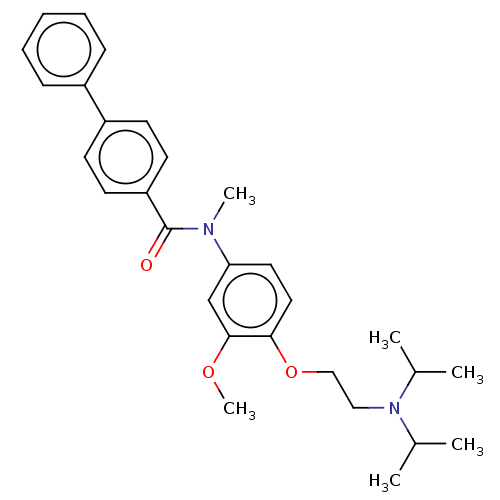 (CHEMBL441855) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of radiolabeled iodo-MCH from human MCHR1 expressed in HEK293 cells | Bioorg Med Chem Lett 16: 4865-71 (2006) Article DOI: 10.1016/j.bmcl.2006.06.056 BindingDB Entry DOI: 10.7270/Q2F192GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50114682 (5-(4-Chloro-phenyl)-1-(2,4-dichloro-phenyl)-4-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 18.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity in a competition assay by displacement of [3H]- CP 55 940 from Cannabinoid receptor 1 in rat whole brain membrane preparation | J Med Chem 45: 2708-19 (2002) BindingDB Entry DOI: 10.7270/Q2TX3DQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (Homo sapiens (Human)) | BDBM50217835 (CHEMBL114345) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity towards Alpha-1B adrenergic receptor | Bioorg Med Chem Lett 12: 3341-4 (2002) BindingDB Entry DOI: 10.7270/Q2FT8P7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50570253 (CHEMBL4467500) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00582 BindingDB Entry DOI: 10.7270/Q2NV9PB9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1/2 (Rattus norvegicus (Rat)-Rattus norvegicus (rat)) | BDBM50054472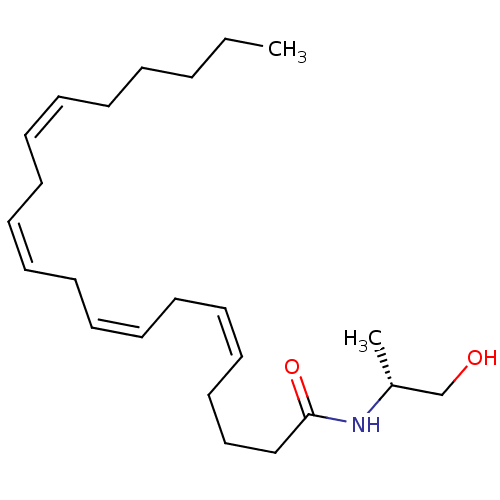 ((5Z,8Z,11Z,14Z)-Icosa-5,8,11,14-tetraenoic acid ((...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Binding affinity for the cannabinoid receptor in the presence of phenylmethanesulfonyl fluoride (PMSF) was determined in rat fore brain membranes | J Med Chem 37: 1889-93 (1994) Article DOI: 10.1021/jm00038a020 BindingDB Entry DOI: 10.7270/Q2JQ13QV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50475902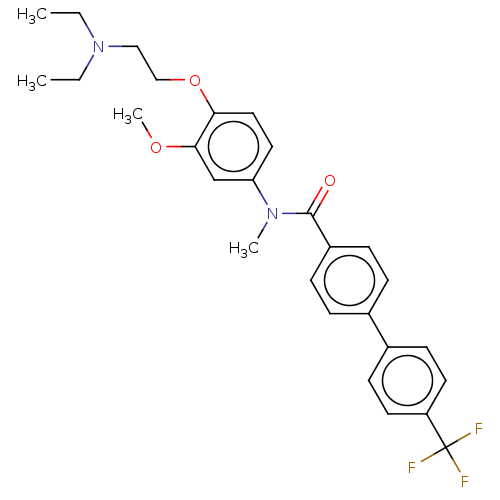 (CHEMBL2110360 | SB-568849) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of radiolabeled iodo-MCH from human MCHR1 expressed in HEK293 cells | Bioorg Med Chem Lett 16: 4865-71 (2006) Article DOI: 10.1016/j.bmcl.2006.06.056 BindingDB Entry DOI: 10.7270/Q2F192GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2085 total ) | Next | Last >> |