 Found 123 hits with Last Name = 'tabuchi' and Initial = 's'
Found 123 hits with Last Name = 'tabuchi' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM23954

(5-[(2E,3aS,4R,5R,6aS)-5-hydroxy-4-[(1E,3S)-3-hydro...)Show SMILES [H][C@]12C[C@@H](O)[C@H](\C=C\[C@@H](O)C(C)CC#CC)[C@@]1([H])C\C(C2)=C\CCCC(O)=O Show InChI InChI=1S/C22H32O4/c1-3-4-7-15(2)20(23)11-10-18-19-13-16(8-5-6-9-22(25)26)12-17(19)14-21(18)24/h8,10-11,15,17-21,23-24H,5-7,9,12-14H2,1-2H3,(H,25,26)/b11-10+,16-8+/t15?,17-,18+,19-,20+,21+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
In vitro Prostacyclin (PGI-2) receptor binding assay was determined based on displacement of [3H]-Iloprost radioligand from cloned human IP receptor |
Bioorg Med Chem Lett 13: 4277-9 (2003)
BindingDB Entry DOI: 10.7270/Q22R3R3D |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM23954

(5-[(2E,3aS,4R,5R,6aS)-5-hydroxy-4-[(1E,3S)-3-hydro...)Show SMILES [H][C@]12C[C@@H](O)[C@H](\C=C\[C@@H](O)C(C)CC#CC)[C@@]1([H])C\C(C2)=C\CCCC(O)=O Show InChI InChI=1S/C22H32O4/c1-3-4-7-15(2)20(23)11-10-18-19-13-16(8-5-6-9-22(25)26)12-17(19)14-21(18)24/h8,10-11,15,17-21,23-24H,5-7,9,12-14H2,1-2H3,(H,25,26)/b11-10+,16-8+/t15?,17-,18+,19-,20+,21+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Ability to inhibit binding of [3H]iloprost to cloned human prostaglandin I2 receptor |
Bioorg Med Chem Lett 15: 3279-83 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.042
BindingDB Entry DOI: 10.7270/Q2J103X8 |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50167887

((R)-2-(6-((diphenylcarbamoyloxy)methyl)-6-hydroxy-...)Show SMILES OC(=O)COc1cccc2C[C@@](O)(COC(=O)N(c3ccccc3)c3ccccc3)CCc12 Show InChI InChI=1S/C26H25NO6/c28-24(29)17-32-23-13-7-8-19-16-26(31,15-14-22(19)23)18-33-25(30)27(20-9-3-1-4-10-20)21-11-5-2-6-12-21/h1-13,31H,14-18H2,(H,28,29)/t26-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]iloprost from human Prostanoid IP receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50167890
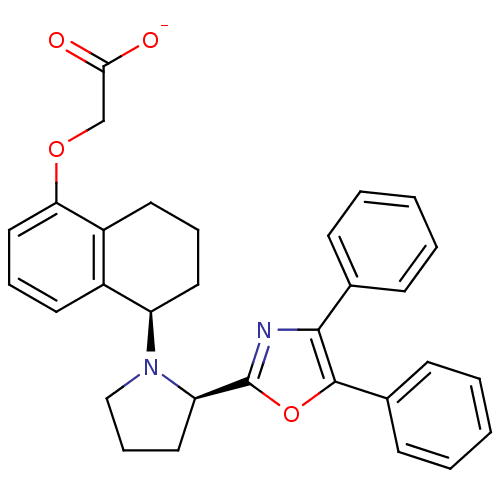
(CHEMBL190497 | FR-193262 | Sodium; {(R)-5-[(R)-2-(...)Show SMILES [O-]C(=O)COc1cccc2[C@@H](CCCc12)N1CCC[C@@H]1c1nc(c(o1)-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C31H30N2O4/c34-28(35)20-36-27-18-8-14-23-24(27)15-7-16-25(23)33-19-9-17-26(33)31-32-29(21-10-3-1-4-11-21)30(37-31)22-12-5-2-6-13-22/h1-6,8,10-14,18,25-26H,7,9,15-17,19-20H2,(H,34,35)/p-1/t25-,26-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]iloprost from human Prostanoid IP receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50167890

(CHEMBL190497 | FR-193262 | Sodium; {(R)-5-[(R)-2-(...)Show SMILES [O-]C(=O)COc1cccc2[C@@H](CCCc12)N1CCC[C@@H]1c1nc(c(o1)-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C31H30N2O4/c34-28(35)20-36-27-18-8-14-23-24(27)15-7-16-25(23)33-19-9-17-26(33)31-32-29(21-10-3-1-4-11-21)30(37-31)22-12-5-2-6-13-22/h1-6,8,10-14,18,25-26H,7,9,15-17,19-20H2,(H,34,35)/p-1/t25-,26-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Ability to inhibit binding of [3H]iloprost to cloned human prostaglandin I2 receptor |
Bioorg Med Chem Lett 15: 3279-83 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.042
BindingDB Entry DOI: 10.7270/Q2J103X8 |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50136234

(CHEMBL132589 | FR-181157 | Sodium; {3-[(R)-2-(4,5-...)Show SMILES [O-]C(=O)COc1cccc(CC2CCCC=C2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 |c:15| Show InChI InChI=1S/C30H27NO4/c32-27(33)20-34-25-16-9-10-21(19-25)18-24-15-7-8-17-26(24)30-31-28(22-11-3-1-4-12-22)29(35-30)23-13-5-2-6-14-23/h1-6,9-14,16-17,19,24H,7-8,15,18,20H2,(H,32,33)/p-1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
In vitro Prostacyclin (PGI-2) receptor binding assay was determined based on displacement of [3H]-Iloprost radioligand from cloned human IP receptor |
Bioorg Med Chem Lett 13: 4277-9 (2003)
BindingDB Entry DOI: 10.7270/Q22R3R3D |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50370452
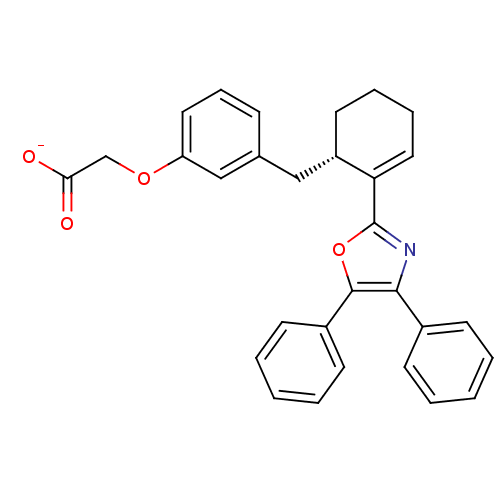
(CHEMBL132589 | FR-181157)Show SMILES [O-]C(=O)COc1cccc(C[C@@H]2CCCC=C2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 |c:15| Show InChI InChI=1S/C30H27NO4/c32-27(33)20-34-25-16-9-10-21(19-25)18-24-15-7-8-17-26(24)30-31-28(22-11-3-1-4-12-22)29(35-30)23-13-5-2-6-14-23/h1-6,9-14,16-17,19,24H,7-8,15,18,20H2,(H,32,33)/p-1/t24-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]iloprost from human Prostanoid IP receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50370452

(CHEMBL132589 | FR-181157)Show SMILES [O-]C(=O)COc1cccc(C[C@@H]2CCCC=C2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 |c:15| Show InChI InChI=1S/C30H27NO4/c32-27(33)20-34-25-16-9-10-21(19-25)18-24-15-7-8-17-26(24)30-31-28(22-11-3-1-4-12-22)29(35-30)23-13-5-2-6-14-23/h1-6,9-14,16-17,19,24H,7-8,15,18,20H2,(H,32,33)/p-1/t24-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Ability to inhibit binding of [3H]iloprost to cloned human prostaglandin I2 receptor |
Bioorg Med Chem Lett 15: 3279-83 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.042
BindingDB Entry DOI: 10.7270/Q2J103X8 |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50136234

(CHEMBL132589 | FR-181157 | Sodium; {3-[(R)-2-(4,5-...)Show SMILES [O-]C(=O)COc1cccc(CC2CCCC=C2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 |c:15| Show InChI InChI=1S/C30H27NO4/c32-27(33)20-34-25-16-9-10-21(19-25)18-24-15-7-8-17-26(24)30-31-28(22-11-3-1-4-12-22)29(35-30)23-13-5-2-6-14-23/h1-6,9-14,16-17,19,24H,7-8,15,18,20H2,(H,32,33)/p-1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]iloprost from cloned human PGI2 receptor |
Bioorg Med Chem Lett 16: 4861-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.076
BindingDB Entry DOI: 10.7270/Q2R49QD9 |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50191133

(CHEMBL439357 | sodium (R)-2-(3-((2-(4,5-diphenylox...)Show SMILES [O-]C(=O)COc1cccc(CN2CCC[C@@H]2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 Show InChI InChI=1S/C28H26N2O4/c31-25(32)19-33-23-14-7-9-20(17-23)18-30-16-8-15-24(30)28-29-26(21-10-3-1-4-11-21)27(34-28)22-12-5-2-6-13-22/h1-7,9-14,17,24H,8,15-16,18-19H2,(H,31,32)/p-1/t24-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]iloprost from cloned human PGI2 receptor |
Bioorg Med Chem Lett 16: 4861-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.076
BindingDB Entry DOI: 10.7270/Q2R49QD9 |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50095202
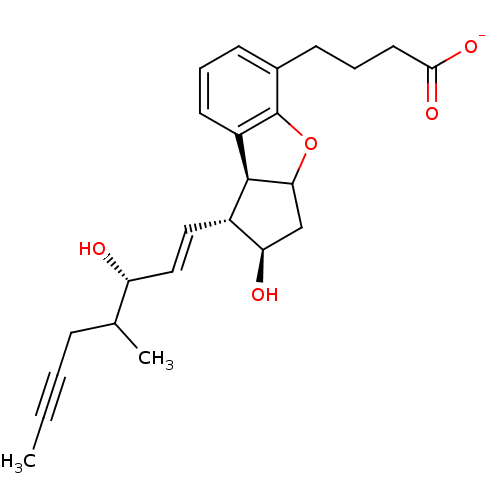
(4-[2-Hydroxy-3-((E)-3-hydroxy-4-methyl-oct-1-en-6-...)Show SMILES CC#CCC(C)[C@H](O)\C=C\[C@H]1[C@H](O)CC2Oc3c(cccc3CCCC([O-])=O)[C@H]12 Show InChI InChI=1S/C24H30O5/c1-3-4-7-15(2)19(25)13-12-17-20(26)14-21-23(17)18-10-5-8-16(24(18)29-21)9-6-11-22(27)28/h5,8,10,12-13,15,17,19-21,23,25-26H,6-7,9,11,14H2,1-2H3,(H,27,28)/p-1/b13-12+/t15?,17-,19+,20+,21?,23-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Tested for inhibition of 3[H]-iloprost binding to human IP receptor |
Bioorg Med Chem Lett 10: 2787-90 (2000)
BindingDB Entry DOI: 10.7270/Q26972TT |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50095203

(CHEMBL94751 | [(S)-6-(3-Benzhydryl-6-oxo-6H-pyrida...)Show SMILES OC(=O)COc1cccc2C[C@@H](Cn3nc(ccc3=O)C(c3ccccc3)c3ccccc3)CCc12 Show InChI InChI=1S/C30H28N2O4/c33-28-17-16-26(30(22-8-3-1-4-9-22)23-10-5-2-6-11-23)31-32(28)19-21-14-15-25-24(18-21)12-7-13-27(25)36-20-29(34)35/h1-13,16-17,21,30H,14-15,18-20H2,(H,34,35)/t21-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Tested for inhibition of 3[H]-iloprost binding to human IP receptor |
Bioorg Med Chem Lett 10: 2787-90 (2000)
BindingDB Entry DOI: 10.7270/Q26972TT |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50167892

(CHEMBL195456 | {(S)-6-[(Diphenylcarbamoyloxy)-meth...)Show SMILES O[C@@H]1c2cccc(OCC(O)=O)c2CC[C@]1(O)COC(=O)N(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C26H25NO7/c28-23(29)16-33-22-13-7-12-21-20(22)14-15-26(32,24(21)30)17-34-25(31)27(18-8-3-1-4-9-18)19-10-5-2-6-11-19/h1-13,24,30,32H,14-17H2,(H,28,29)/t24-,26+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]iloprost from human Prostanoid IP receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50167891
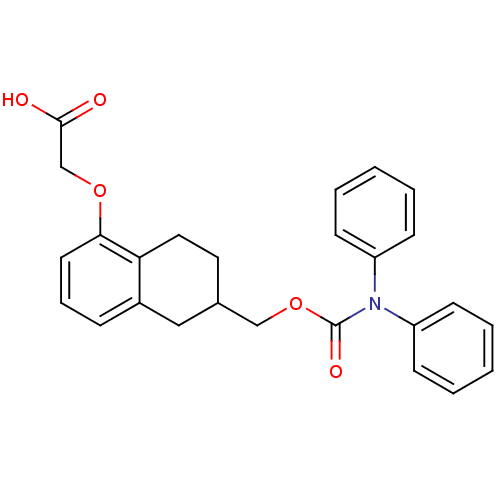
(CHEMBL197319 | {6-[(Diphenylcarbamoyloxy)-methyl]-...)Show SMILES OC(=O)COc1cccc2CC(COC(=O)N(c3ccccc3)c3ccccc3)CCc12 Show InChI InChI=1S/C26H25NO5/c28-25(29)18-31-24-13-7-8-20-16-19(14-15-23(20)24)17-32-26(30)27(21-9-3-1-4-10-21)22-11-5-2-6-12-22/h1-13,19H,14-18H2,(H,28,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]iloprost from human Prostanoid IP receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50167890

(CHEMBL190497 | FR-193262 | Sodium; {(R)-5-[(R)-2-(...)Show SMILES [O-]C(=O)COc1cccc2[C@@H](CCCc12)N1CCC[C@@H]1c1nc(c(o1)-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C31H30N2O4/c34-28(35)20-36-27-18-8-14-23-24(27)15-7-16-25(23)33-19-9-17-26(33)31-32-29(21-10-3-1-4-11-21)30(37-31)22-12-5-2-6-13-22/h1-6,8,10-14,18,25-26H,7,9,15-17,19-20H2,(H,34,35)/p-1/t25-,26-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGD-2 from human Prostanoid DP receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50167890

(CHEMBL190497 | FR-193262 | Sodium; {(R)-5-[(R)-2-(...)Show SMILES [O-]C(=O)COc1cccc2[C@@H](CCCc12)N1CCC[C@@H]1c1nc(c(o1)-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C31H30N2O4/c34-28(35)20-36-27-18-8-14-23-24(27)15-7-16-25(23)33-19-9-17-26(33)31-32-29(21-10-3-1-4-11-21)30(37-31)22-12-5-2-6-13-22/h1-6,8,10-14,18,25-26H,7,9,15-17,19-20H2,(H,34,35)/p-1/t25-,26-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE-2 from human Prostanoid EP1 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Thromboxane A2 receptor
(Homo sapiens (Human)) | BDBM50167890

(CHEMBL190497 | FR-193262 | Sodium; {(R)-5-[(R)-2-(...)Show SMILES [O-]C(=O)COc1cccc2[C@@H](CCCc12)N1CCC[C@@H]1c1nc(c(o1)-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C31H30N2O4/c34-28(35)20-36-27-18-8-14-23-24(27)15-7-16-25(23)33-19-9-17-26(33)31-32-29(21-10-3-1-4-11-21)30(37-31)22-12-5-2-6-13-22/h1-6,8,10-14,18,25-26H,7,9,15-17,19-20H2,(H,34,35)/p-1/t25-,26-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]SQ-29,548 from human Prostanoid TP receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin F2-alpha receptor
(Homo sapiens (Human)) | BDBM50370452

(CHEMBL132589 | FR-181157)Show SMILES [O-]C(=O)COc1cccc(C[C@@H]2CCCC=C2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 |c:15| Show InChI InChI=1S/C30H27NO4/c32-27(33)20-34-25-16-9-10-21(19-25)18-24-15-7-8-17-26(24)30-31-28(22-11-3-1-4-12-22)29(35-30)23-13-5-2-6-14-23/h1-6,9-14,16-17,19,24H,7-8,15,18,20H2,(H,32,33)/p-1/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGF-2 from human Prostanoid FP receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50370452

(CHEMBL132589 | FR-181157)Show SMILES [O-]C(=O)COc1cccc(C[C@@H]2CCCC=C2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 |c:15| Show InChI InChI=1S/C30H27NO4/c32-27(33)20-34-25-16-9-10-21(19-25)18-24-15-7-8-17-26(24)30-31-28(22-11-3-1-4-12-22)29(35-30)23-13-5-2-6-14-23/h1-6,9-14,16-17,19,24H,7-8,15,18,20H2,(H,32,33)/p-1/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE-2 from human Prostanoid EP2 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50167890

(CHEMBL190497 | FR-193262 | Sodium; {(R)-5-[(R)-2-(...)Show SMILES [O-]C(=O)COc1cccc2[C@@H](CCCc12)N1CCC[C@@H]1c1nc(c(o1)-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C31H30N2O4/c34-28(35)20-36-27-18-8-14-23-24(27)15-7-16-25(23)33-19-9-17-26(33)31-32-29(21-10-3-1-4-11-21)30(37-31)22-12-5-2-6-13-22/h1-6,8,10-14,18,25-26H,7,9,15-17,19-20H2,(H,34,35)/p-1/t25-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE-2 from human Prostanoid EP2 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50370452

(CHEMBL132589 | FR-181157)Show SMILES [O-]C(=O)COc1cccc(C[C@@H]2CCCC=C2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 |c:15| Show InChI InChI=1S/C30H27NO4/c32-27(33)20-34-25-16-9-10-21(19-25)18-24-15-7-8-17-26(24)30-31-28(22-11-3-1-4-12-22)29(35-30)23-13-5-2-6-14-23/h1-6,9-14,16-17,19,24H,7-8,15,18,20H2,(H,32,33)/p-1/t24-/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE-2 from human Prostanoid EP1 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50167892

(CHEMBL195456 | {(S)-6-[(Diphenylcarbamoyloxy)-meth...)Show SMILES O[C@@H]1c2cccc(OCC(O)=O)c2CC[C@]1(O)COC(=O)N(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C26H25NO7/c28-23(29)16-33-22-13-7-12-21-20(22)14-15-26(32,24(21)30)17-34-25(31)27(18-8-3-1-4-9-18)19-10-5-2-6-11-19/h1-13,24,30,32H,14-17H2,(H,28,29)/t24-,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE-2 from human Prostanoid EP2 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50167892

(CHEMBL195456 | {(S)-6-[(Diphenylcarbamoyloxy)-meth...)Show SMILES O[C@@H]1c2cccc(OCC(O)=O)c2CC[C@]1(O)COC(=O)N(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C26H25NO7/c28-23(29)16-33-22-13-7-12-21-20(22)14-15-26(32,24(21)30)17-34-25(31)27(18-8-3-1-4-9-18)19-10-5-2-6-11-19/h1-13,24,30,32H,14-17H2,(H,28,29)/t24-,26+/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE-2 from human Prostanoid EP1 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin F2-alpha receptor
(Homo sapiens (Human)) | BDBM50167890

(CHEMBL190497 | FR-193262 | Sodium; {(R)-5-[(R)-2-(...)Show SMILES [O-]C(=O)COc1cccc2[C@@H](CCCc12)N1CCC[C@@H]1c1nc(c(o1)-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C31H30N2O4/c34-28(35)20-36-27-18-8-14-23-24(27)15-7-16-25(23)33-19-9-17-26(33)31-32-29(21-10-3-1-4-11-21)30(37-31)22-12-5-2-6-13-22/h1-6,8,10-14,18,25-26H,7,9,15-17,19-20H2,(H,34,35)/p-1/t25-,26-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGF-2 from human Prostanoid FP receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50167891

(CHEMBL197319 | {6-[(Diphenylcarbamoyloxy)-methyl]-...)Show SMILES OC(=O)COc1cccc2CC(COC(=O)N(c3ccccc3)c3ccccc3)CCc12 Show InChI InChI=1S/C26H25NO5/c28-25(29)18-31-24-13-7-8-20-16-19(14-15-23(20)24)17-32-26(30)27(21-9-3-1-4-10-21)22-11-5-2-6-12-22/h1-13,19H,14-18H2,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE-2 from human Prostanoid EP2 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50370452

(CHEMBL132589 | FR-181157)Show SMILES [O-]C(=O)COc1cccc(C[C@@H]2CCCC=C2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 |c:15| Show InChI InChI=1S/C30H27NO4/c32-27(33)20-34-25-16-9-10-21(19-25)18-24-15-7-8-17-26(24)30-31-28(22-11-3-1-4-12-22)29(35-30)23-13-5-2-6-14-23/h1-6,9-14,16-17,19,24H,7-8,15,18,20H2,(H,32,33)/p-1/t24-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGD-2 from human Prostanoid DP receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50167891

(CHEMBL197319 | {6-[(Diphenylcarbamoyloxy)-methyl]-...)Show SMILES OC(=O)COc1cccc2CC(COC(=O)N(c3ccccc3)c3ccccc3)CCc12 Show InChI InChI=1S/C26H25NO5/c28-25(29)18-31-24-13-7-8-20-16-19(14-15-23(20)24)17-32-26(30)27(21-9-3-1-4-10-21)22-11-5-2-6-12-22/h1-13,19H,14-18H2,(H,28,29) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE-2 from human Prostanoid EP1 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Thromboxane A2 receptor
(Homo sapiens (Human)) | BDBM50370452

(CHEMBL132589 | FR-181157)Show SMILES [O-]C(=O)COc1cccc(C[C@@H]2CCCC=C2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 |c:15| Show InChI InChI=1S/C30H27NO4/c32-27(33)20-34-25-16-9-10-21(19-25)18-24-15-7-8-17-26(24)30-31-28(22-11-3-1-4-12-22)29(35-30)23-13-5-2-6-14-23/h1-6,9-14,16-17,19,24H,7-8,15,18,20H2,(H,32,33)/p-1/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]SQ-29,548 from human Prostanoid TP receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50370452

(CHEMBL132589 | FR-181157)Show SMILES [O-]C(=O)COc1cccc(C[C@@H]2CCCC=C2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 |c:15| Show InChI InChI=1S/C30H27NO4/c32-27(33)20-34-25-16-9-10-21(19-25)18-24-15-7-8-17-26(24)30-31-28(22-11-3-1-4-12-22)29(35-30)23-13-5-2-6-14-23/h1-6,9-14,16-17,19,24H,7-8,15,18,20H2,(H,32,33)/p-1/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE-2 from human Prostanoid EP4 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50167891

(CHEMBL197319 | {6-[(Diphenylcarbamoyloxy)-methyl]-...)Show SMILES OC(=O)COc1cccc2CC(COC(=O)N(c3ccccc3)c3ccccc3)CCc12 Show InChI InChI=1S/C26H25NO5/c28-25(29)18-31-24-13-7-8-20-16-19(14-15-23(20)24)17-32-26(30)27(21-9-3-1-4-10-21)22-11-5-2-6-12-22/h1-13,19H,14-18H2,(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE-2 from human Prostanoid EP3 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50167887

((R)-2-(6-((diphenylcarbamoyloxy)methyl)-6-hydroxy-...)Show SMILES OC(=O)COc1cccc2C[C@@](O)(COC(=O)N(c3ccccc3)c3ccccc3)CCc12 Show InChI InChI=1S/C26H25NO6/c28-24(29)17-32-23-13-7-8-19-16-26(31,15-14-22(19)23)18-33-25(30)27(20-9-3-1-4-10-20)21-11-5-2-6-12-21/h1-13,31H,14-18H2,(H,28,29)/t26-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGD-2 from human Prostanoid DP receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50370452

(CHEMBL132589 | FR-181157)Show SMILES [O-]C(=O)COc1cccc(C[C@@H]2CCCC=C2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 |c:15| Show InChI InChI=1S/C30H27NO4/c32-27(33)20-34-25-16-9-10-21(19-25)18-24-15-7-8-17-26(24)30-31-28(22-11-3-1-4-12-22)29(35-30)23-13-5-2-6-14-23/h1-6,9-14,16-17,19,24H,7-8,15,18,20H2,(H,32,33)/p-1/t24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE-2 from human Prostanoid EP3 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50167887

((R)-2-(6-((diphenylcarbamoyloxy)methyl)-6-hydroxy-...)Show SMILES OC(=O)COc1cccc2C[C@@](O)(COC(=O)N(c3ccccc3)c3ccccc3)CCc12 Show InChI InChI=1S/C26H25NO6/c28-24(29)17-32-23-13-7-8-19-16-26(31,15-14-22(19)23)18-33-25(30)27(20-9-3-1-4-10-20)21-11-5-2-6-12-21/h1-13,31H,14-18H2,(H,28,29)/t26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE-2 from human Prostanoid EP3 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50167887

((R)-2-(6-((diphenylcarbamoyloxy)methyl)-6-hydroxy-...)Show SMILES OC(=O)COc1cccc2C[C@@](O)(COC(=O)N(c3ccccc3)c3ccccc3)CCc12 Show InChI InChI=1S/C26H25NO6/c28-24(29)17-32-23-13-7-8-19-16-26(31,15-14-22(19)23)18-33-25(30)27(20-9-3-1-4-10-20)21-11-5-2-6-12-21/h1-13,31H,14-18H2,(H,28,29)/t26-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE-2 from human Prostanoid EP1 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50167890

(CHEMBL190497 | FR-193262 | Sodium; {(R)-5-[(R)-2-(...)Show SMILES [O-]C(=O)COc1cccc2[C@@H](CCCc12)N1CCC[C@@H]1c1nc(c(o1)-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C31H30N2O4/c34-28(35)20-36-27-18-8-14-23-24(27)15-7-16-25(23)33-19-9-17-26(33)31-32-29(21-10-3-1-4-11-21)30(37-31)22-12-5-2-6-13-22/h1-6,8,10-14,18,25-26H,7,9,15-17,19-20H2,(H,34,35)/p-1/t25-,26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE-2 from human Prostanoid EP3 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin F2-alpha receptor
(Homo sapiens (Human)) | BDBM50167887

((R)-2-(6-((diphenylcarbamoyloxy)methyl)-6-hydroxy-...)Show SMILES OC(=O)COc1cccc2C[C@@](O)(COC(=O)N(c3ccccc3)c3ccccc3)CCc12 Show InChI InChI=1S/C26H25NO6/c28-24(29)17-32-23-13-7-8-19-16-26(31,15-14-22(19)23)18-33-25(30)27(20-9-3-1-4-10-20)21-11-5-2-6-12-21/h1-13,31H,14-18H2,(H,28,29)/t26-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGF-2 from human Prostanoid FP receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50167887

((R)-2-(6-((diphenylcarbamoyloxy)methyl)-6-hydroxy-...)Show SMILES OC(=O)COc1cccc2C[C@@](O)(COC(=O)N(c3ccccc3)c3ccccc3)CCc12 Show InChI InChI=1S/C26H25NO6/c28-24(29)17-32-23-13-7-8-19-16-26(31,15-14-22(19)23)18-33-25(30)27(20-9-3-1-4-10-20)21-11-5-2-6-12-21/h1-13,31H,14-18H2,(H,28,29)/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE-2 from human Prostanoid EP2 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Thromboxane A2 receptor
(Homo sapiens (Human)) | BDBM50167887

((R)-2-(6-((diphenylcarbamoyloxy)methyl)-6-hydroxy-...)Show SMILES OC(=O)COc1cccc2C[C@@](O)(COC(=O)N(c3ccccc3)c3ccccc3)CCc12 Show InChI InChI=1S/C26H25NO6/c28-24(29)17-32-23-13-7-8-19-16-26(31,15-14-22(19)23)18-33-25(30)27(20-9-3-1-4-10-20)21-11-5-2-6-12-21/h1-13,31H,14-18H2,(H,28,29)/t26-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]SQ-29,548 from human Prostanoid TP receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50167891

(CHEMBL197319 | {6-[(Diphenylcarbamoyloxy)-methyl]-...)Show SMILES OC(=O)COc1cccc2CC(COC(=O)N(c3ccccc3)c3ccccc3)CCc12 Show InChI InChI=1S/C26H25NO5/c28-25(29)18-31-24-13-7-8-20-16-19(14-15-23(20)24)17-32-26(30)27(21-9-3-1-4-10-21)22-11-5-2-6-12-22/h1-13,19H,14-18H2,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE-2 from human Prostanoid EP4 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50167887

((R)-2-(6-((diphenylcarbamoyloxy)methyl)-6-hydroxy-...)Show SMILES OC(=O)COc1cccc2C[C@@](O)(COC(=O)N(c3ccccc3)c3ccccc3)CCc12 Show InChI InChI=1S/C26H25NO6/c28-24(29)17-32-23-13-7-8-19-16-26(31,15-14-22(19)23)18-33-25(30)27(20-9-3-1-4-10-20)21-11-5-2-6-12-21/h1-13,31H,14-18H2,(H,28,29)/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE-2 from human Prostanoid EP4 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50167892

(CHEMBL195456 | {(S)-6-[(Diphenylcarbamoyloxy)-meth...)Show SMILES O[C@@H]1c2cccc(OCC(O)=O)c2CC[C@]1(O)COC(=O)N(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C26H25NO7/c28-23(29)16-33-22-13-7-12-21-20(22)14-15-26(32,24(21)30)17-34-25(31)27(18-8-3-1-4-9-18)19-10-5-2-6-11-19/h1-13,24,30,32H,14-17H2,(H,28,29)/t24-,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE-2 from human Prostanoid EP4 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50167890

(CHEMBL190497 | FR-193262 | Sodium; {(R)-5-[(R)-2-(...)Show SMILES [O-]C(=O)COc1cccc2[C@@H](CCCc12)N1CCC[C@@H]1c1nc(c(o1)-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C31H30N2O4/c34-28(35)20-36-27-18-8-14-23-24(27)15-7-16-25(23)33-19-9-17-26(33)31-32-29(21-10-3-1-4-11-21)30(37-31)22-12-5-2-6-13-22/h1-6,8,10-14,18,25-26H,7,9,15-17,19-20H2,(H,34,35)/p-1/t25-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE-2 from human Prostanoid EP4 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50167892

(CHEMBL195456 | {(S)-6-[(Diphenylcarbamoyloxy)-meth...)Show SMILES O[C@@H]1c2cccc(OCC(O)=O)c2CC[C@]1(O)COC(=O)N(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C26H25NO7/c28-23(29)16-33-22-13-7-12-21-20(22)14-15-26(32,24(21)30)17-34-25(31)27(18-8-3-1-4-9-18)19-10-5-2-6-11-19/h1-13,24,30,32H,14-17H2,(H,28,29)/t24-,26+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE-2 from human Prostanoid EP3 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50005463

((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...)Show SMILES CN1c2ccccc2C(=N[C@H](NC(=O)c2cc3ccccc3[nH]2)C1=O)c1ccccc1 |r,c:9| Show InChI InChI=1S/C25H20N4O2/c1-29-21-14-8-6-12-18(21)22(16-9-3-2-4-10-16)27-23(25(29)31)28-24(30)20-15-17-11-5-7-13-19(17)26-20/h2-15,23,26H,1H3,(H,28,30)/t23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-Cholecystokinin-8 (125I-CCK-8) binding to Cholecystokinin type A receptor of rat pancreatic membranes |
Bioorg Med Chem Lett 7: 169-174 (1997)
Article DOI: 10.1016/S0960-894X(96)00609-9
BindingDB Entry DOI: 10.7270/Q27M07ZF |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50070467
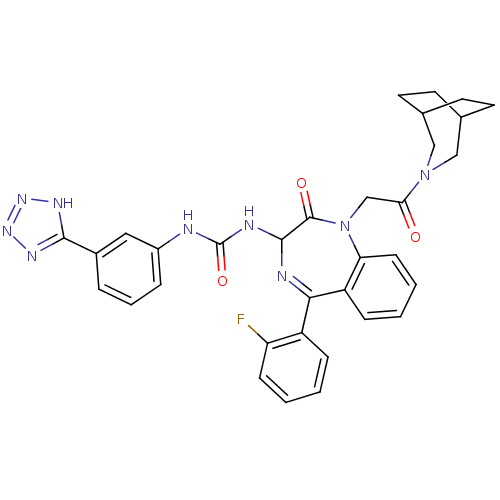
(1-[1-[2-(3-Aza-bicyclo[3.2.2]non-3-yl)-2-oxo-ethyl...)Show SMILES Fc1ccccc1C1=NC(NC(=O)Nc2cccc(c2)-c2nnn[nH]2)C(=O)N(CC(=O)N2CC3CCC(CC3)C2)c2ccccc12 |t:8,(1.42,-6,;-.06,-6.42,;-.53,-7.87,;-2.05,-8.21,;-3.07,-7.07,;-2.61,-5.6,;-1.1,-5.28,;-.62,-3.81,;.87,-3.58,;1.59,-2.15,;2.92,-2.92,;4.25,-2.15,;4.27,-.61,;5.58,-2.92,;6.93,-2.17,;8.26,-2.95,;9.59,-2.18,;9.6,-.64,;8.24,.13,;6.93,-.64,;8.24,1.67,;7,2.57,;7.47,4.04,;9.01,4.04,;9.5,2.58,;.92,-.7,;1.87,.51,;-.58,-.4,;-1,1.07,;.05,2.19,;-.37,3.66,;1.55,1.81,;1.24,3.1,;2.22,4.25,;3.8,4.12,;4.65,2.79,;4.13,1.39,;3.69,3.12,;2.2,2.71,;2.88,1.05,;-1.56,-1.34,;-2.89,-.57,;-4.22,-1.35,;-4.22,-2.89,;-2.89,-3.66,;-1.56,-2.9,)| Show InChI InChI=1S/C33H32FN9O3/c34-26-10-3-1-8-24(26)29-25-9-2-4-11-27(25)43(19-28(44)42-17-20-12-13-21(18-42)15-14-20)32(45)31(36-29)37-33(46)35-23-7-5-6-22(16-23)30-38-40-41-39-30/h1-11,16,20-21,31H,12-15,17-19H2,(H2,35,37,46)(H,38,39,40,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.0870 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of 124 I-CCK-8 binding at Cholecystokinin type B receptor on guinea pig cerebral cortical membranes |
Bioorg Med Chem Lett 8: 1449-54 (1999)
BindingDB Entry DOI: 10.7270/Q28P5ZMJ |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50290398

(1-[1-[2-(3-Aza-bicyclo[3.2.2]non-3-yl)-2-oxo-ethyl...)Show SMILES Cc1cccc(NC(=O)NC2N=C(c3ccccc3F)c3ccccc3N(CC(=O)N3CC4CCC(CC4)C3)C2=O)c1 |t:11,(11,-6.73,;10.23,-8.06,;11,-9.39,;10.23,-10.71,;8.7,-10.71,;7.96,-9.39,;6.61,-8.62,;5.28,-9.39,;5.3,-10.93,;3.94,-8.64,;2.6,-9.42,;1.92,-10.82,;.44,-11.15,;.1,-12.65,;-1.37,-13.13,;-1.73,-14.65,;-.58,-15.7,;.89,-15.22,;1.24,-13.72,;2.71,-13.24,;-.77,-10.2,;-2.12,-11,;-3.47,-10.23,;-3.47,-8.66,;-2.12,-7.89,;-.77,-8.66,;.36,-7.63,;-.28,-6.21,;.61,-4.95,;2.15,-5.11,;,-3.55,;.99,-2.36,;.68,-.85,;-.7,-.15,;-2.09,-.79,;-2.47,-2.29,;-1,-2.67,;-.77,-1.17,;-1.54,-3.51,;1.92,-8.03,;2.88,-6.82,;8.7,-8.06,)| Show InChI InChI=1S/C33H34FN5O3/c1-21-7-6-8-24(17-21)35-33(42)37-31-32(41)39(20-29(40)38-18-22-13-14-23(19-38)16-15-22)28-12-5-3-10-26(28)30(36-31)25-9-2-4-11-27(25)34/h2-12,17,22-23,31H,13-16,18-20H2,1H3,(H2,35,37,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.0870 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-Cholecystokinin-8 binding to Cholecystokinin type B receptor of guinea pig cerebral cortical membranes |
Bioorg Med Chem Lett 7: 169-174 (1997)
Article DOI: 10.1016/S0960-894X(96)00609-9
BindingDB Entry DOI: 10.7270/Q27M07ZF |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50111656

(1-[2-(5-Chloro-2-oxo-benzothiazol-3-yl)-acetyl]-pi...)Show SMILES Clc1ccc2sc(=O)n(CC(=O)N3CCC(CC3)C(=O)Nc3ccc(Cl)c4ccccc34)c2c1 Show InChI InChI=1S/C25H21Cl2N3O3S/c26-16-5-8-22-21(13-16)30(25(33)34-22)14-23(31)29-11-9-15(10-12-29)24(32)28-20-7-6-19(27)17-3-1-2-4-18(17)20/h1-8,13,15H,9-12,14H2,(H,28,32) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Tested for the inhibition of [125I]-PYY binding to HEK 293 cells stably expressed with human neuropeptide NPY Y5 receptor |
Bioorg Med Chem Lett 12: 1171-5 (2002)
BindingDB Entry DOI: 10.7270/Q26M365J |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50290397
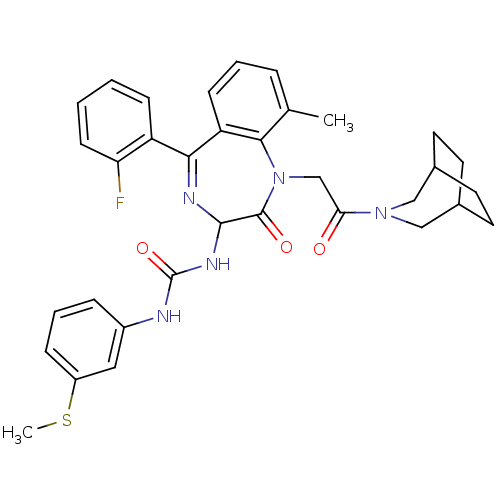
(1-[1-[2-(3-Aza-bicyclo[3.2.2]non-3-yl)-2-oxo-ethyl...)Show SMILES CSc1cccc(NC(=O)NC2N=C(c3ccccc3F)c3cccc(C)c3N(CC(=O)N3CC4CCC(CC4)C3)C2=O)c1 |t:12,(8.7,-.09,;7.17,-.12,;6.41,-1.43,;7.17,-2.77,;6.41,-4.09,;4.89,-4.09,;4.13,-2.78,;2.59,-2.78,;1.28,-3.54,;1.29,-5.08,;-.06,-2.8,;-1.4,-3.58,;-2.07,-4.98,;-3.55,-5.3,;-3.9,-6.8,;-5.37,-7.29,;-5.72,-8.79,;-4.58,-9.84,;-3.09,-9.36,;-2.76,-7.86,;-1.3,-7.38,;-4.77,-4.36,;-6.11,-5.15,;-7.45,-4.38,;-7.45,-2.82,;-6.11,-2.05,;-6.13,-.5,;-4.77,-2.82,;-3.65,-1.79,;-4.28,-.37,;-3.39,.88,;-1.85,.72,;-4.03,2.27,;-3.02,3.46,;-3.32,4.97,;-4.69,5.67,;-6.09,5.04,;-6.46,3.54,;-4.98,3.14,;-4.77,4.65,;-5.54,2.32,;-2.07,-2.19,;-1.13,-.99,;4.87,-1.45,)| Show InChI InChI=1S/C34H36FN5O3S/c1-21-7-5-11-27-30(26-10-3-4-12-28(26)35)37-32(38-34(43)36-24-8-6-9-25(17-24)44-2)33(42)40(31(21)27)20-29(41)39-18-22-13-14-23(19-39)16-15-22/h3-12,17,22-23,32H,13-16,18-20H2,1-2H3,(H2,36,38,43) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-Cholecystokinin-8 binding to Cholecystokinin type B receptor of guinea pig cerebral cortical membranes |
Bioorg Med Chem Lett 7: 169-174 (1997)
Article DOI: 10.1016/S0960-894X(96)00609-9
BindingDB Entry DOI: 10.7270/Q27M07ZF |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50290396
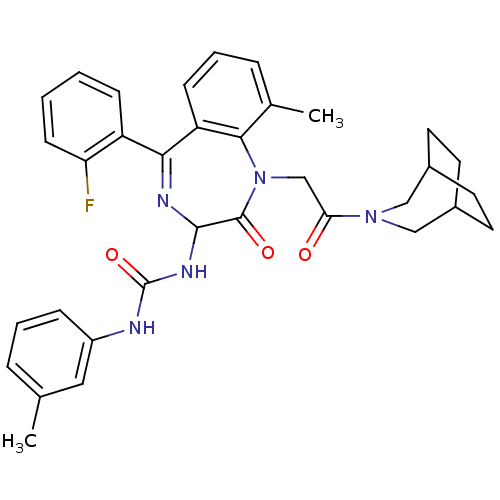
(1-[1-[2-(3-Aza-bicyclo[3.2.2]non-3-yl)-2-oxo-ethyl...)Show SMILES Cc1cccc(NC(=O)NC2N=C(c3ccccc3F)c3cccc(C)c3N(CC(=O)N3CC4CCC(CC4)C3)C2=O)c1 |t:11,(9.11,-4.37,;8.34,-5.7,;9.11,-7.02,;8.37,-8.34,;6.84,-8.37,;6.07,-7.02,;4.53,-7.02,;3.22,-7.79,;3.22,-9.35,;1.87,-7.06,;.52,-7.82,;-.13,-9.24,;-1.62,-9.58,;-1.97,-11.08,;-3.45,-11.55,;-3.79,-13.05,;-2.65,-14.11,;-1.18,-13.62,;-.83,-12.13,;.64,-11.66,;-2.84,-8.63,;-4.19,-9.4,;-5.54,-8.63,;-5.54,-7.09,;-4.19,-6.29,;-4.2,-4.75,;-2.84,-7.06,;-1.72,-6.04,;-2.35,-4.62,;-1.45,-3.36,;.08,-3.53,;-2.07,-1.97,;-1.09,-.8,;-1.4,.71,;-2.84,.39,;-3.05,-1.1,;-4.54,-.71,;-4.16,.77,;-2.77,1.41,;-3.61,-1.93,;-.16,-6.44,;.82,-5.23,;6.83,-5.72,)| Show InChI InChI=1S/C34H36FN5O3/c1-21-7-5-9-25(17-21)36-34(43)38-32-33(42)40(20-29(41)39-18-23-13-14-24(19-39)16-15-23)31-22(2)8-6-11-27(31)30(37-32)26-10-3-4-12-28(26)35/h3-12,17,23-24,32H,13-16,18-20H2,1-2H3,(H2,36,38,43) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-Cholecystokinin-8 binding to Cholecystokinin type B receptor of guinea pig cerebral cortical membranes |
Bioorg Med Chem Lett 7: 169-174 (1997)
Article DOI: 10.1016/S0960-894X(96)00609-9
BindingDB Entry DOI: 10.7270/Q27M07ZF |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50111645

(1-[2-(5-Chloro-2-oxo-benzothiazol-3-yl)-acetyl]-pi...)Show SMILES Fc1ccc(NC(=O)C2CCN(CC2)C(=O)Cn2c3cc(Cl)ccc3sc2=O)cc1C(F)(F)F Show InChI InChI=1S/C22H18ClF4N3O3S/c23-13-1-4-18-17(9-13)30(21(33)34-18)11-19(31)29-7-5-12(6-8-29)20(32)28-14-2-3-16(24)15(10-14)22(25,26)27/h1-4,9-10,12H,5-8,11H2,(H,28,32) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory concentration of total specific binding of [125I]- -PYY to HEK 293 cells transfected with Human NPY-Y5 cDNA (compound prepared by manual s... |
Bioorg Med Chem Lett 12: 1171-5 (2002)
BindingDB Entry DOI: 10.7270/Q26M365J |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data