

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM335426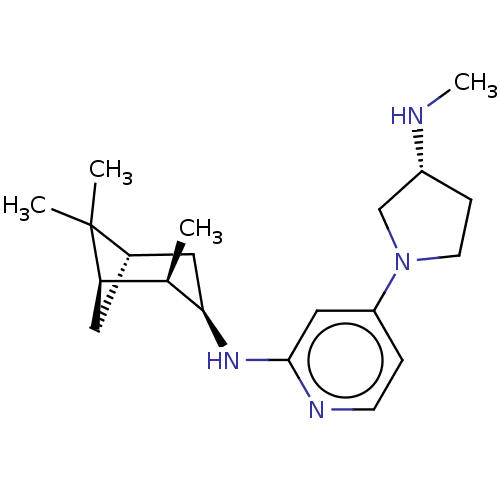 (4-[(3R)-3-(Methylamino)pyrrolidin-1-yl]-N-[(1R,2R,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA N.V. US Patent | Assay Description Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa... | US Patent US9732087 (2017) BindingDB Entry DOI: 10.7270/Q2251M9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50061098 (CHEMBL3393534 | US9732087, 1) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA N.V. US Patent | Assay Description Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa... | US Patent US9732087 (2017) BindingDB Entry DOI: 10.7270/Q2251M9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM335425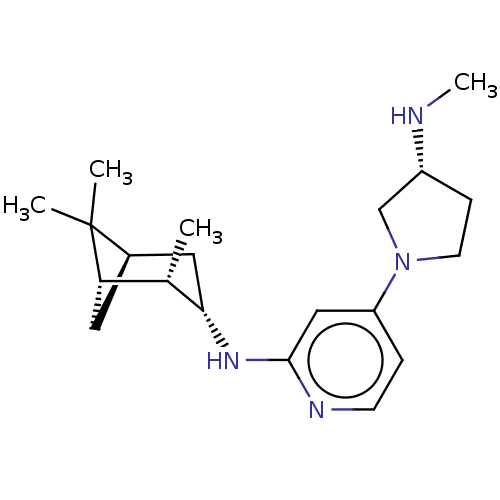 (4-[(3R)-3-(Methylamino)pyrrolidin-1-yl]-N-[(1S,2S,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA N.V. US Patent | Assay Description Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa... | US Patent US9732087 (2017) BindingDB Entry DOI: 10.7270/Q2251M9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50061008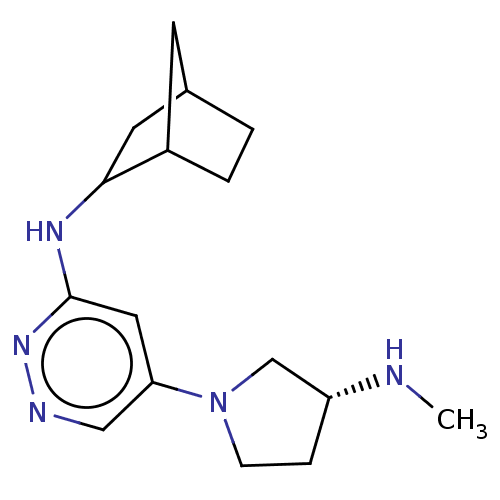 (CHEMBL3393556 | US9732087, 96) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA N.V. US Patent | Assay Description Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa... | US Patent US9732087 (2017) BindingDB Entry DOI: 10.7270/Q2251M9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50061143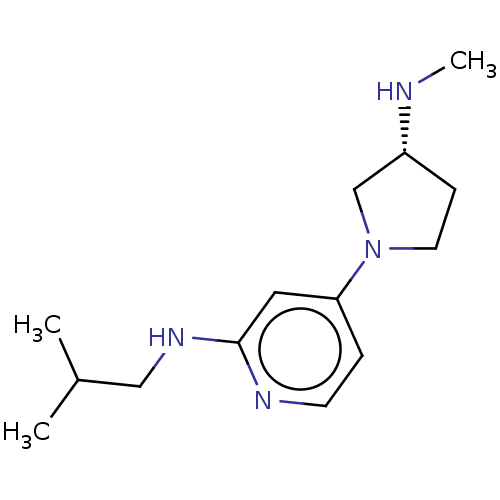 (CHEMBL3393526 | US9732087, 22) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA N.V. US Patent | Assay Description Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa... | US Patent US9732087 (2017) BindingDB Entry DOI: 10.7270/Q2251M9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50061056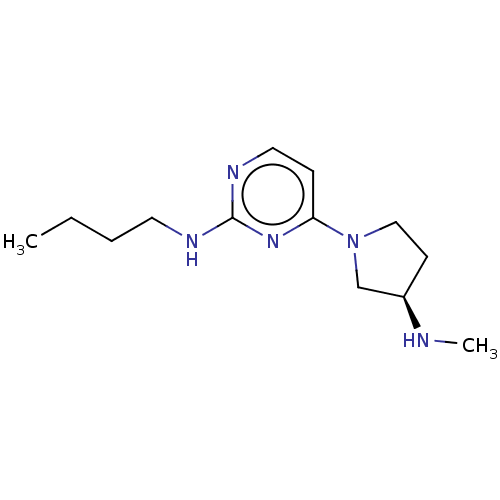 (CHEMBL3393542 | US9732087, 73) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA N.V. US Patent | Assay Description Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa... | US Patent US9732087 (2017) BindingDB Entry DOI: 10.7270/Q2251M9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50061060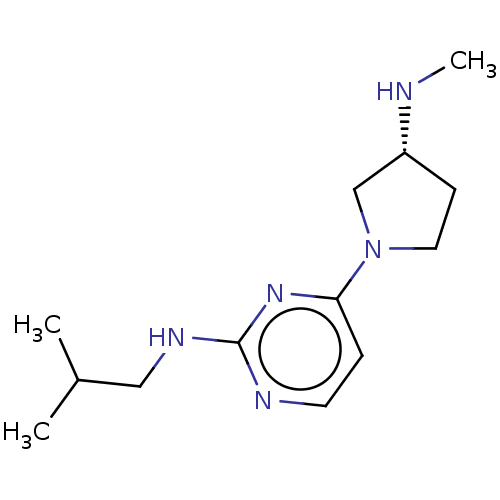 (CHEMBL3393539 | US9732087, 32) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 3.08 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA N.V. US Patent | Assay Description Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa... | US Patent US9732087 (2017) BindingDB Entry DOI: 10.7270/Q2251M9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM335429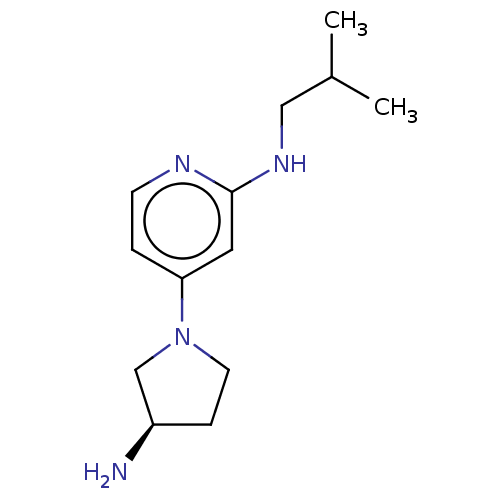 (4-[(3R)-3-Aminopyrrolidin-1-yl]-N-(2-methylpropyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA N.V. US Patent | Assay Description Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa... | US Patent US9732087 (2017) BindingDB Entry DOI: 10.7270/Q2251M9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM335450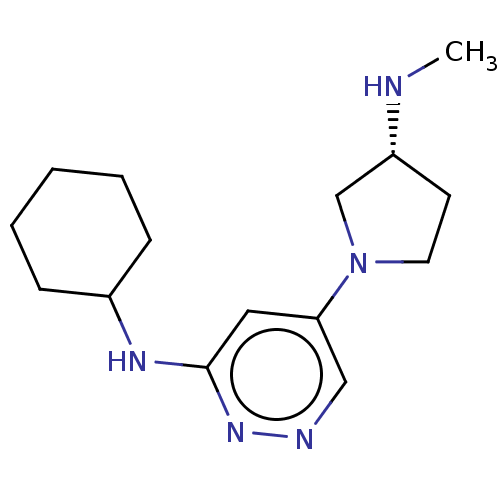 (N-Cyclohexyl-5-[(3R)-3-(methylamino)pyrrolidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA N.V. US Patent | Assay Description Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa... | US Patent US9732087 (2017) BindingDB Entry DOI: 10.7270/Q2251M9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM335449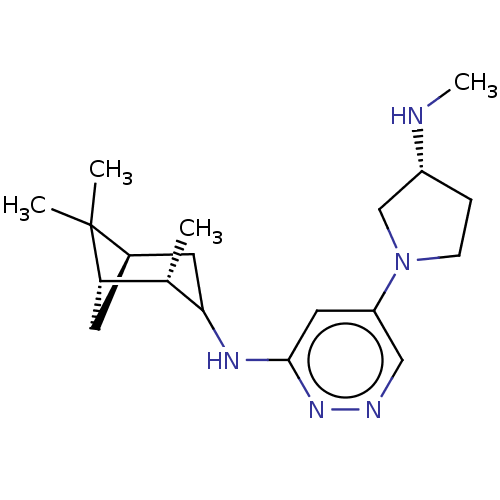 (5-[(3R)-3-(Methylamino)pyrrolidin-1-yl]-N-[(1S,2S,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA N.V. US Patent | Assay Description Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa... | US Patent US9732087 (2017) BindingDB Entry DOI: 10.7270/Q2251M9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM335442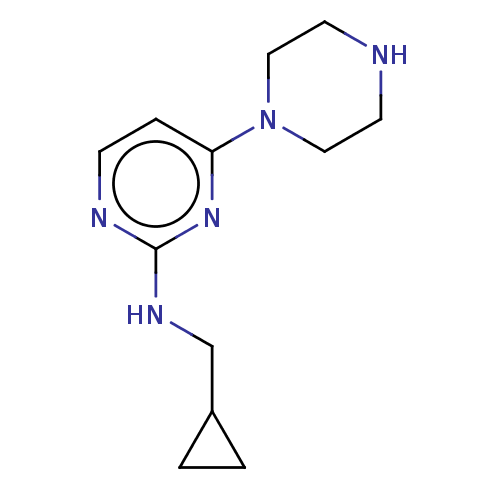 (Cyclopropylmethyl-(4-piperazin-1-yl-pyrimidin-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 12.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA N.V. US Patent | Assay Description Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa... | US Patent US9732087 (2017) BindingDB Entry DOI: 10.7270/Q2251M9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50061014 (CHEMBL3393550 | US9732087, 95) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA N.V. US Patent | Assay Description Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa... | US Patent US9732087 (2017) BindingDB Entry DOI: 10.7270/Q2251M9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM335451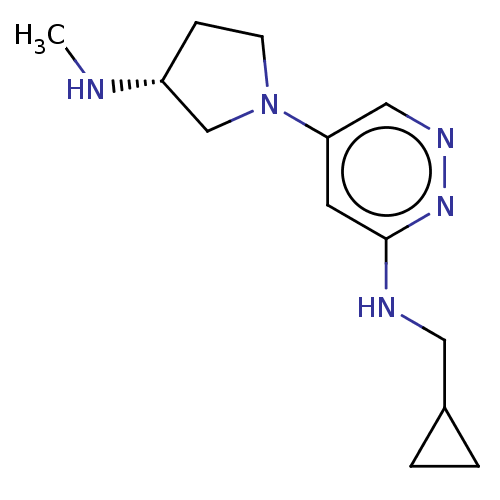 (N-(Cyclopropylmethyl)-5-[(3R)-3-(methylamino)pyrro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 23.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA N.V. US Patent | Assay Description Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa... | US Patent US9732087 (2017) BindingDB Entry DOI: 10.7270/Q2251M9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50061070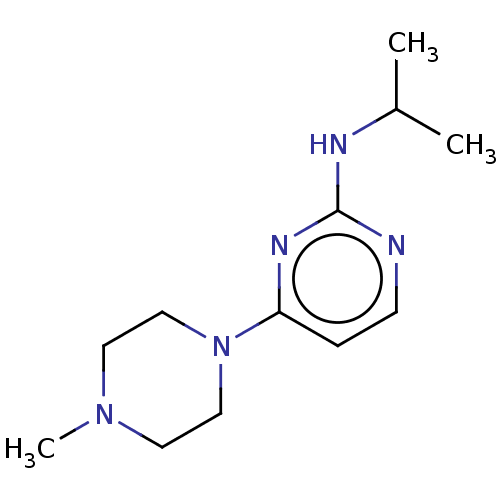 (CHEMBL3393536 | US9732087, 53) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | 24.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA N.V. US Patent | Assay Description Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa... | US Patent US9732087 (2017) BindingDB Entry DOI: 10.7270/Q2251M9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM335435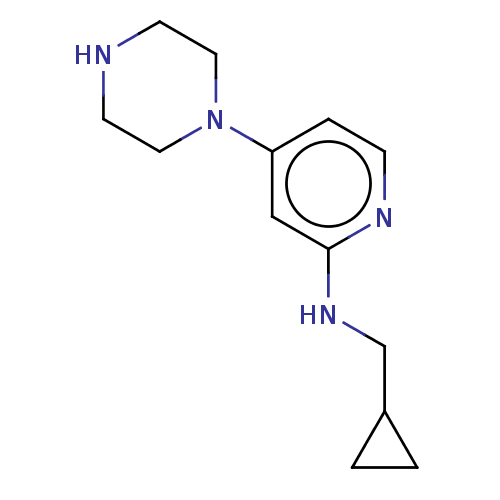 (N-(Cyclopropylmethyl)-4-piperazin-1-ylpyridin-2-am...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 25.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA N.V. US Patent | Assay Description Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa... | US Patent US9732087 (2017) BindingDB Entry DOI: 10.7270/Q2251M9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM335445 (4-(3-Aminoazetidin-1-yl)-N-(2-methylpropyl)pyrimid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 26.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA N.V. US Patent | Assay Description Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa... | US Patent US9732087 (2017) BindingDB Entry DOI: 10.7270/Q2251M9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM335452 (N-Butyl-5-[(3R)-3-(methylamino)pyrrolidin-1-yl]pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA N.V. US Patent | Assay Description Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa... | US Patent US9732087 (2017) BindingDB Entry DOI: 10.7270/Q2251M9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50061018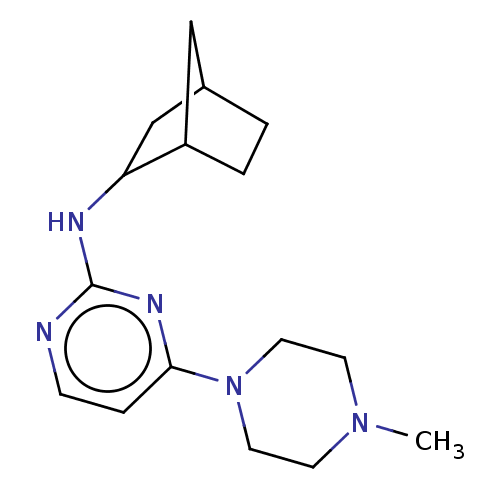 (CHEMBL3393546 | US9732087, 44) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 31.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA N.V. US Patent | Assay Description Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa... | US Patent US9732087 (2017) BindingDB Entry DOI: 10.7270/Q2251M9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM335440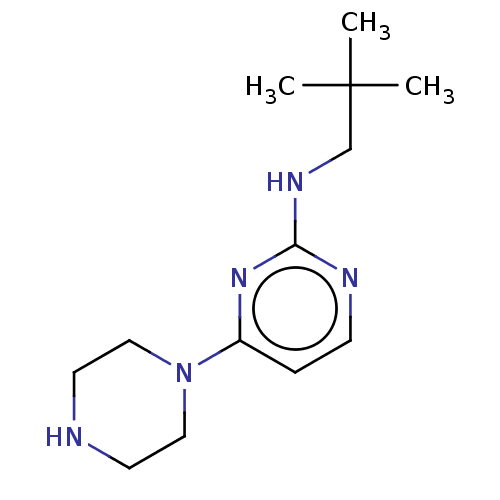 ((2,2-Dimethyl-propyl)-(4-piperazin-1-yl-pyrimidin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA N.V. US Patent | Assay Description Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa... | US Patent US9732087 (2017) BindingDB Entry DOI: 10.7270/Q2251M9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM335432 (4-[(3R)-3-Aminopyrrolidin-1-yl]-N-(tetrahydro-2H-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 42.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA N.V. US Patent | Assay Description Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa... | US Patent US9732087 (2017) BindingDB Entry DOI: 10.7270/Q2251M9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM335428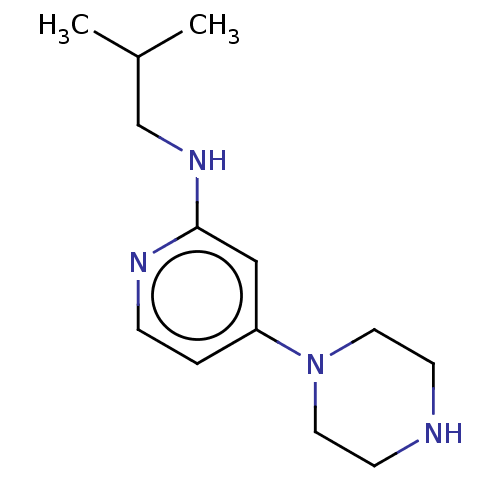 (N-(2-Methylpropyl)-4-piperazin-1-ylpyridin-2-amine...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 45.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA N.V. US Patent | Assay Description Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa... | US Patent US9732087 (2017) BindingDB Entry DOI: 10.7270/Q2251M9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM335441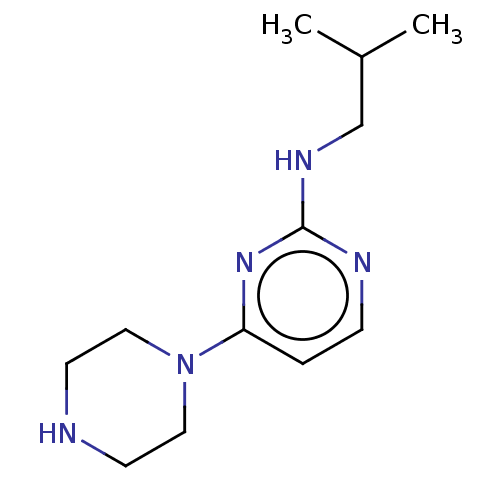 (Isobutyl-(4-piperazin-1-yl-pyrimidin-2-yl)-amine |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | 46.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA N.V. US Patent | Assay Description Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa... | US Patent US9732087 (2017) BindingDB Entry DOI: 10.7270/Q2251M9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50061144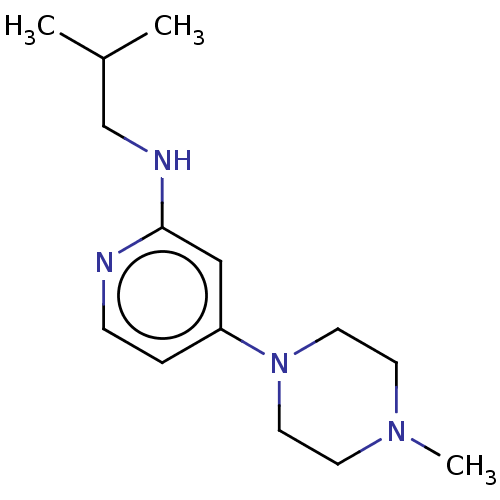 (CHEMBL3393525 | US9732087, 21) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 51.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA N.V. US Patent | Assay Description Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa... | US Patent US9732087 (2017) BindingDB Entry DOI: 10.7270/Q2251M9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50061142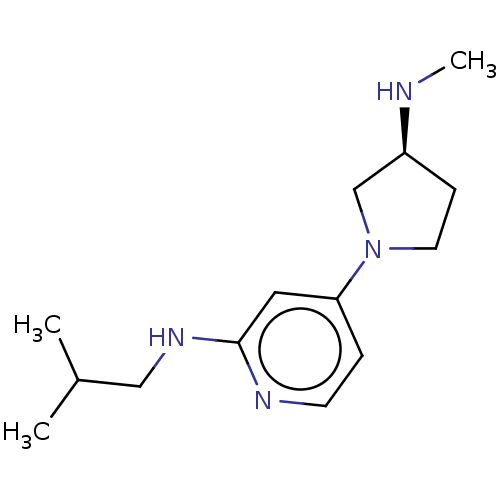 (CHEMBL3393527 | US9732087, 15) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 54.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA N.V. US Patent | Assay Description Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa... | US Patent US9732087 (2017) BindingDB Entry DOI: 10.7270/Q2251M9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM335446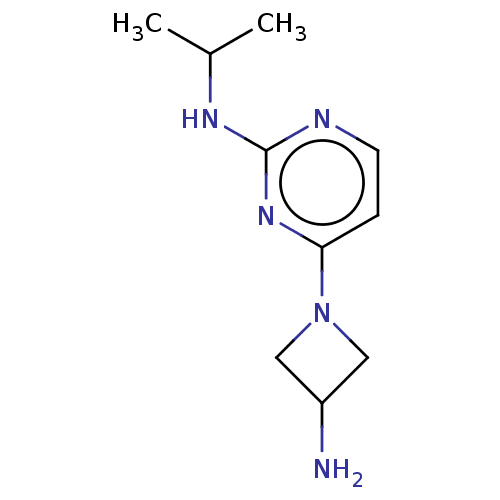 (4-(3-Aminoazetidin-1-yl)-N-(1-methylethyl)pyrimidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 68.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA N.V. US Patent | Assay Description Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa... | US Patent US9732087 (2017) BindingDB Entry DOI: 10.7270/Q2251M9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50061065 (CHEMBL3393538 | US9732087, 31) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA N.V. US Patent | Assay Description Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa... | US Patent US9732087 (2017) BindingDB Entry DOI: 10.7270/Q2251M9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM335436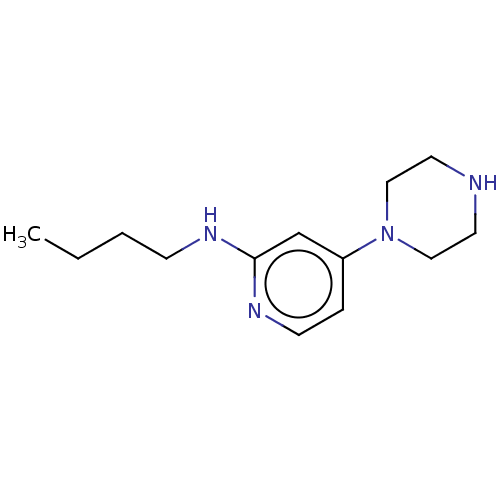 (N-Butyl-4-piperazin-1-ylpyridin-2-amine | US973208...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 91.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA N.V. US Patent | Assay Description Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa... | US Patent US9732087 (2017) BindingDB Entry DOI: 10.7270/Q2251M9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM335439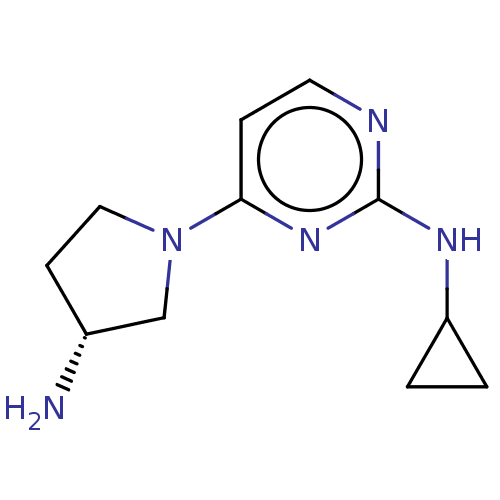 (4-[(3R)-3-Aminopyrrolidin-1-yl]-N-cyclopropylpyrim...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA N.V. US Patent | Assay Description Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa... | US Patent US9732087 (2017) BindingDB Entry DOI: 10.7270/Q2251M9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM335443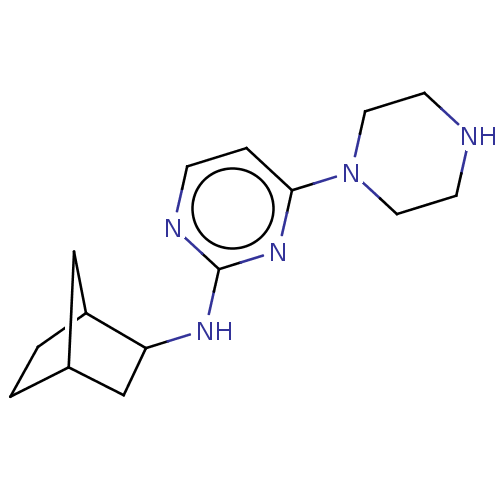 (Bicyclo[2.2.1]hept-2-yl-(4-piperazin-1-yl-pyrimidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA N.V. US Patent | Assay Description Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa... | US Patent US9732087 (2017) BindingDB Entry DOI: 10.7270/Q2251M9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50061015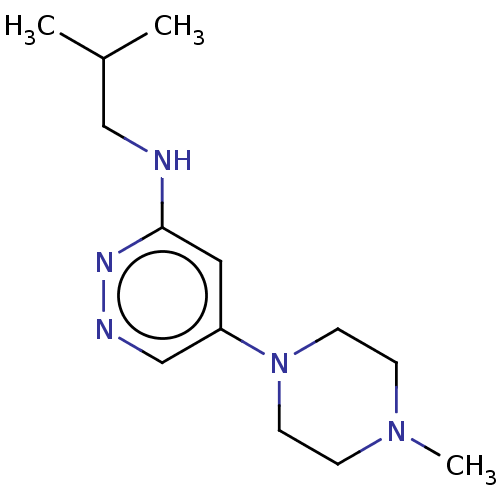 (CHEMBL3393549 | US9732087, 101) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA N.V. US Patent | Assay Description Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa... | US Patent US9732087 (2017) BindingDB Entry DOI: 10.7270/Q2251M9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Rattus norvegicus) | BDBM17659 ((R,S)-2-phosphonomethylpentanedioic acid | 2-(phos...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration against rat brain NAALADase (Folate hydrolase) | J Med Chem 44: 4170-5 (2001) BindingDB Entry DOI: 10.7270/Q2D21WX8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate carboxypeptidase 2 (Rattus norvegicus) | BDBM50085699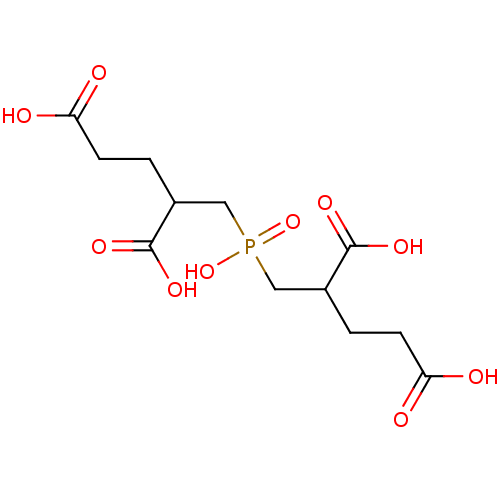 ((S,S / R,R)4-Carboxy-5-[(2,4-dicarboxy-butyl)-hydr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration against rat brain NAALADase (Folate hydrolase) | J Med Chem 44: 4170-5 (2001) BindingDB Entry DOI: 10.7270/Q2D21WX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Rattus norvegicus) | BDBM50106576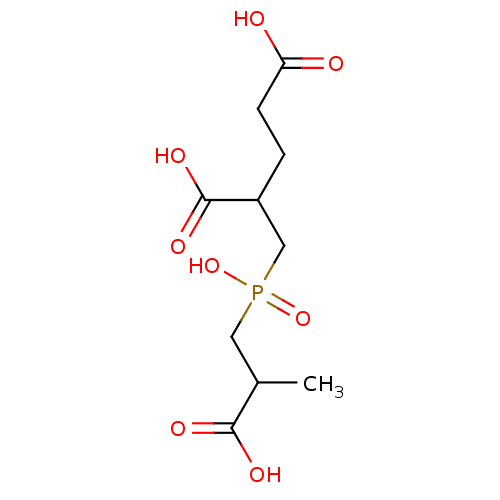 (2-[(2-Carboxy-propyl)-hydroxy-phosphinoylmethyl]-p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration against rat brain NAALADase (Folate hydrolase) | J Med Chem 44: 4170-5 (2001) BindingDB Entry DOI: 10.7270/Q2D21WX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Rattus norvegicus) | BDBM50106577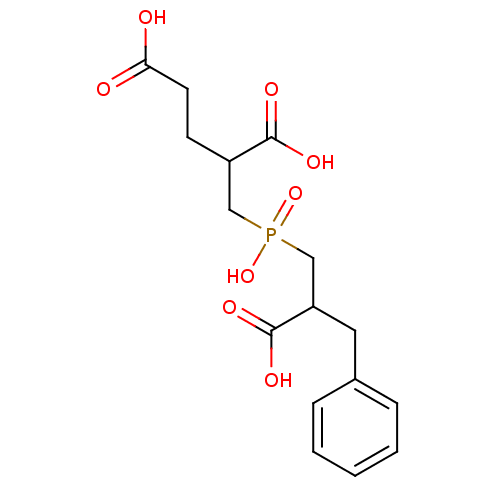 (2-[(2-Carboxy-3-phenyl-propyl)-hydroxy-phosphinoyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration against rat brain NAALADase (Folate hydrolase) | J Med Chem 44: 4170-5 (2001) BindingDB Entry DOI: 10.7270/Q2D21WX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Rattus norvegicus) | BDBM50106570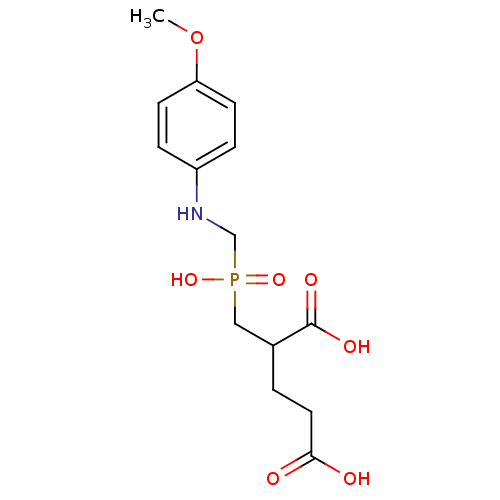 (2-{Hydroxy-[(4-methoxy-phenylamino)-methyl]-phosph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration against rat brain NAALADase (Folate hydrolase) | J Med Chem 44: 4170-5 (2001) BindingDB Entry DOI: 10.7270/Q2D21WX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24206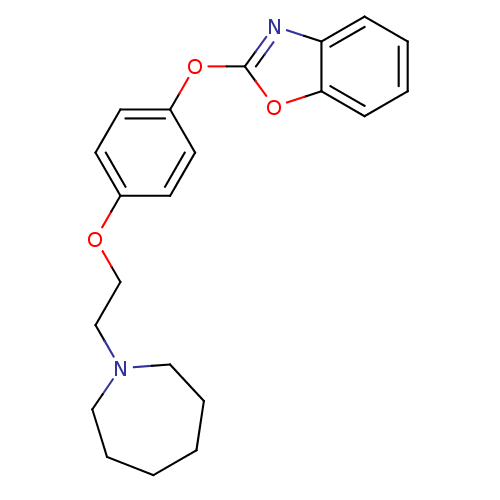 (2-{4-[2-(azepan-1-yl)ethoxy]phenoxy}-1,3-benzoxazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Johnson & Johnson Pharmaceutical | Assay Description Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... | J Med Chem 51: 4150-69 (2008) Article DOI: 10.1021/jm701575k BindingDB Entry DOI: 10.7270/Q2GB22CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Rattus norvegicus) | BDBM50106566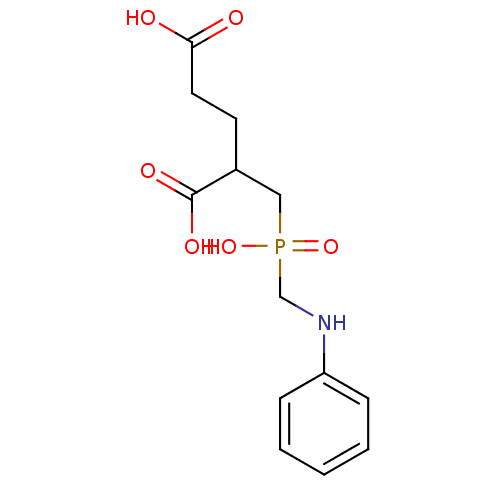 (2-(Hydroxy-phenylaminomethyl-phosphinoylmethyl)-pe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration against rat brain NAALADase (Folate hydrolase) | J Med Chem 44: 4170-5 (2001) BindingDB Entry DOI: 10.7270/Q2D21WX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24216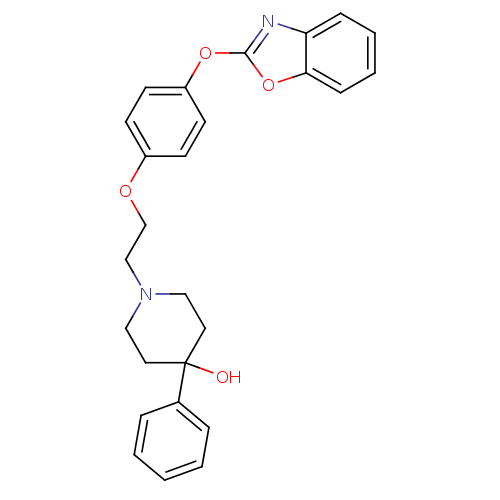 (1-{2-[4-(1,3-benzoxazol-2-yloxy)phenoxy]ethyl}-4-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Johnson & Johnson Pharmaceutical | Assay Description Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... | J Med Chem 51: 4150-69 (2008) Article DOI: 10.1021/jm701575k BindingDB Entry DOI: 10.7270/Q2GB22CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24199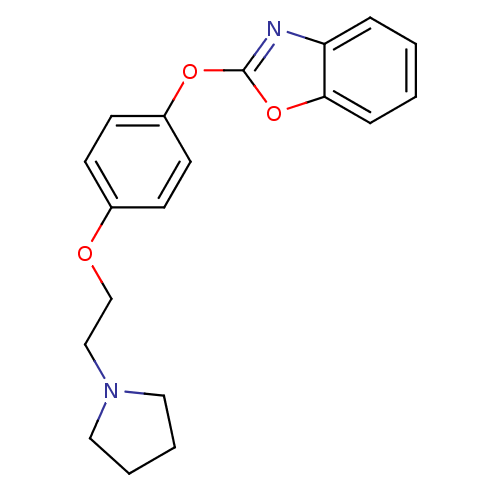 (2-{4-[2-(pyrrolidin-1-yl)ethoxy]phenoxy}-1,3-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Johnson & Johnson Pharmaceutical | Assay Description Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... | J Med Chem 51: 4150-69 (2008) Article DOI: 10.1021/jm701575k BindingDB Entry DOI: 10.7270/Q2GB22CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24238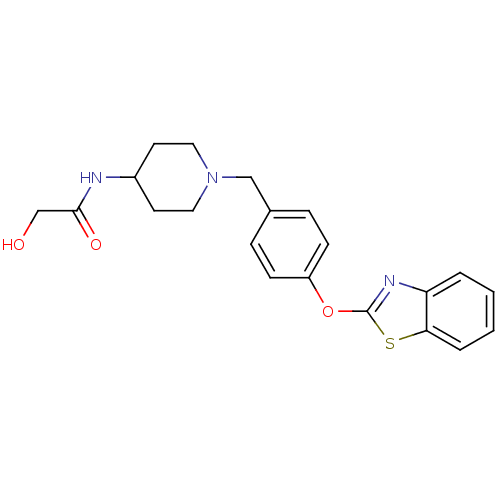 (Benzthiazole compound, 33p | N-(1-{[4-(1,3-benzoth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | 25 |
Johnson & Johnson Pharmaceutical | Assay Description Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... | J Med Chem 51: 4150-69 (2008) Article DOI: 10.1021/jm701575k BindingDB Entry DOI: 10.7270/Q2GB22CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24224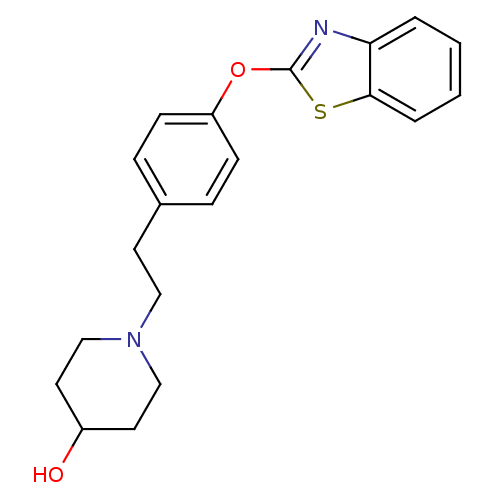 (1-{2-[4-(1,3-benzothiazol-2-yloxy)phenyl]ethyl}pip...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Johnson & Johnson Pharmaceutical | Assay Description Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... | J Med Chem 51: 4150-69 (2008) Article DOI: 10.1021/jm701575k BindingDB Entry DOI: 10.7270/Q2GB22CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24212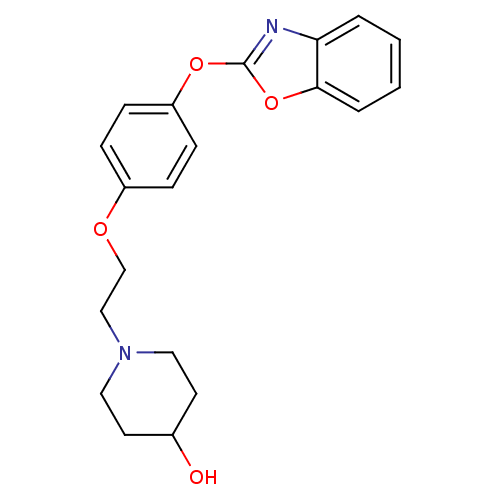 (1-{2-[4-(1,3-benzoxazol-2-yloxy)phenoxy]ethyl}pipe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Johnson & Johnson Pharmaceutical | Assay Description Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... | J Med Chem 51: 4150-69 (2008) Article DOI: 10.1021/jm701575k BindingDB Entry DOI: 10.7270/Q2GB22CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24235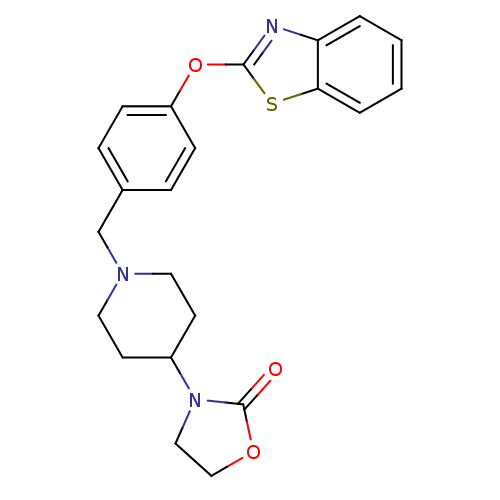 (3-(1-{[4-(1,3-benzothiazol-2-yloxy)phenyl]methyl}p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | 25 |
Johnson & Johnson Pharmaceutical | Assay Description Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... | J Med Chem 51: 4150-69 (2008) Article DOI: 10.1021/jm701575k BindingDB Entry DOI: 10.7270/Q2GB22CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Rattus norvegicus) | BDBM50106575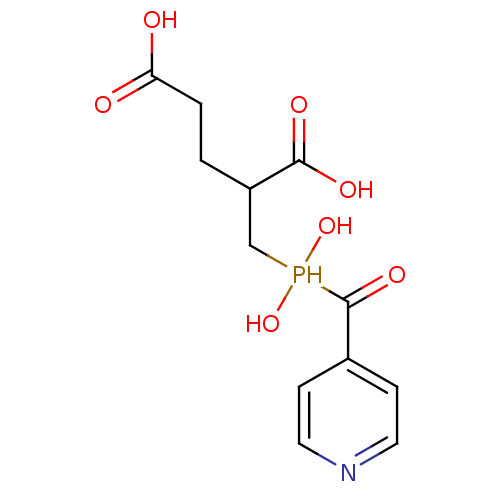 (2-[Hydroxy-(hydroxy-pyridin-4-yl-methyl)-phosphino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration against rat brain NAALADase (Folate hydrolase) | J Med Chem 44: 4170-5 (2001) BindingDB Entry DOI: 10.7270/Q2D21WX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50101114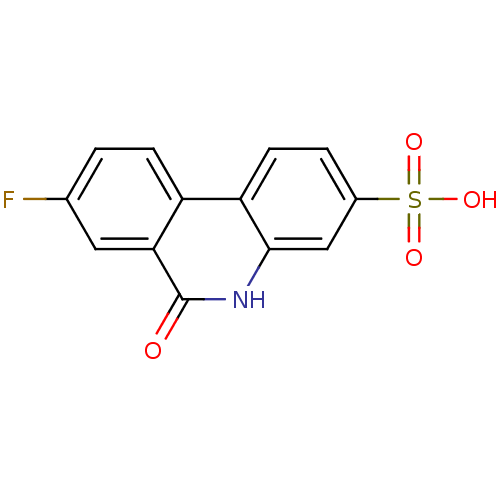 (8-Fluoro-6-oxo-5,6-dihydro-phenanthridine-3-sulfon...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration against purified recombinant human Poly (ADP-ribose) polymerase 1 | Bioorg Med Chem Lett 11: 1687-90 (2001) BindingDB Entry DOI: 10.7270/Q2348KWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24239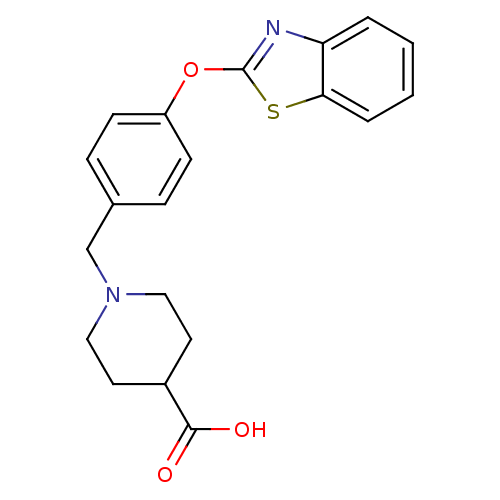 (1-{[4-(1,3-benzothiazol-2-yloxy)phenyl]methyl}pipe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | 25 |
Johnson & Johnson Pharmaceutical | Assay Description Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... | J Med Chem 51: 4150-69 (2008) Article DOI: 10.1021/jm701575k BindingDB Entry DOI: 10.7270/Q2GB22CX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24222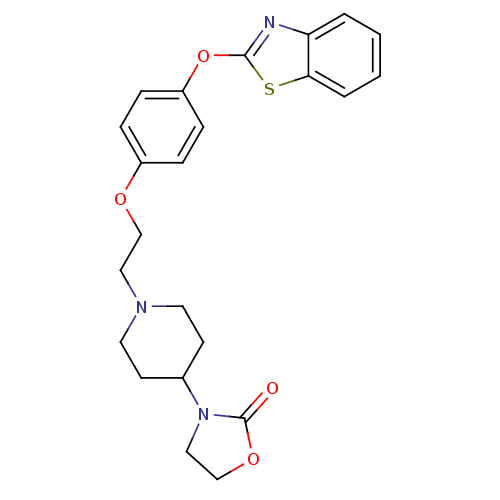 (3-(1-{2-[4-(1,3-benzothiazol-2-yloxy)phenoxy]ethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Johnson & Johnson Pharmaceutical | Assay Description Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... | J Med Chem 51: 4150-69 (2008) Article DOI: 10.1021/jm701575k BindingDB Entry DOI: 10.7270/Q2GB22CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24203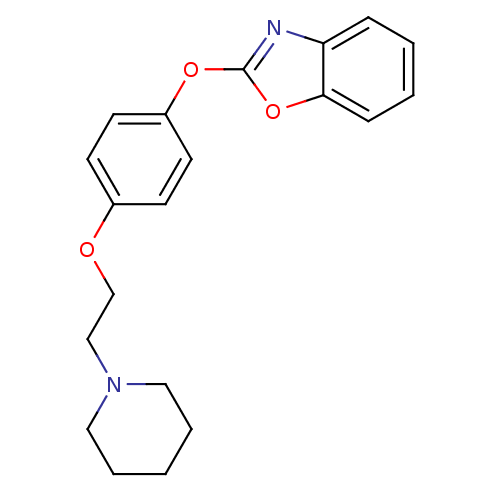 (2-{4-[2-(piperidin-1-yl)ethoxy]phenoxy}-1,3-benzox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Johnson & Johnson Pharmaceutical | Assay Description Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... | J Med Chem 51: 4150-69 (2008) Article DOI: 10.1021/jm701575k BindingDB Entry DOI: 10.7270/Q2GB22CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24234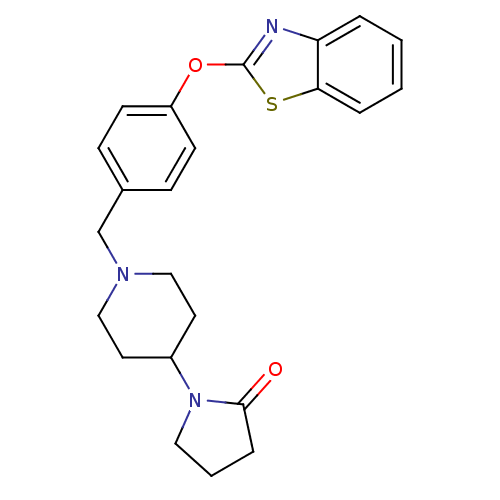 (1-(1-{[4-(1,3-benzothiazol-2-yloxy)phenyl]methyl}p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | 25 |
Johnson & Johnson Pharmaceutical | Assay Description Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... | J Med Chem 51: 4150-69 (2008) Article DOI: 10.1021/jm701575k BindingDB Entry DOI: 10.7270/Q2GB22CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24236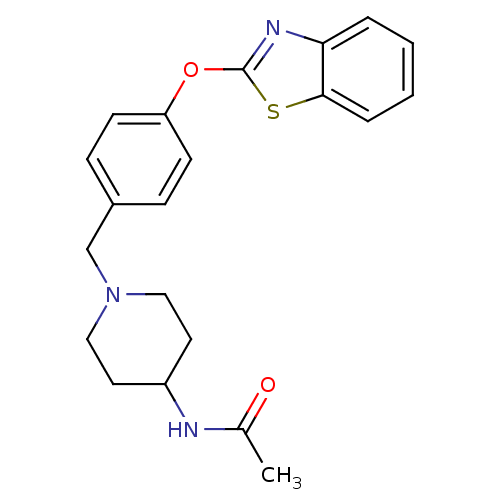 (Benzthiazole compound, 33n | N-(1-{[4-(1,3-benzoth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | 25 |
Johnson & Johnson Pharmaceutical | Assay Description Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... | J Med Chem 51: 4150-69 (2008) Article DOI: 10.1021/jm701575k BindingDB Entry DOI: 10.7270/Q2GB22CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 151 total ) | Next | Last >> |