

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein-tyrosine kinase 2-beta (Homo sapiens (Human)) | BDBM286349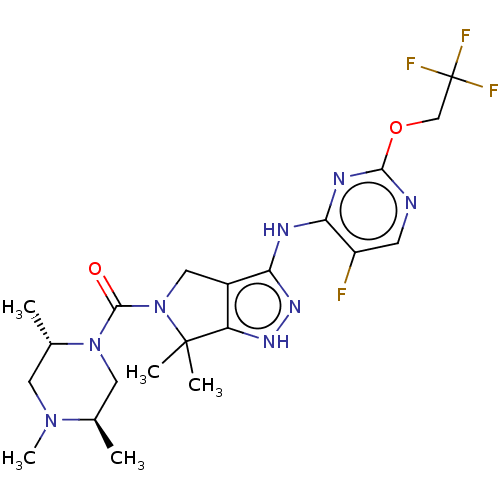 (US11220518, Ex. No. K7 | US11780853, Example K7 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.165 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
PFIZER INC. US Patent | Assay Description Protein Kinase C beta 2 (PKCβII) catalyzes the production of ADP from ATP that accompanies the phosphoryl transfer to the PKC Pseudosubstrate pe... | US Patent US9518060 (2016) BindingDB Entry DOI: 10.7270/Q2ZK5JPN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type Isoform 2 (Homo sapiens (Human)) | BDBM286349 (US11220518, Ex. No. K7 | US11780853, Example K7 | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | 0.165 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Homo sapiens (Human)) | BDBM286349 (US11220518, Ex. No. K7 | US11780853, Example K7 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.165 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A typical assay was carried out on a 96-well, clear microtiter plate in a Molecular Devices spectrophotometer for 20 minutes at 30° C. in 0.1 mL of a... | Citation and Details BindingDB Entry DOI: 10.7270/Q28S4T3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50222915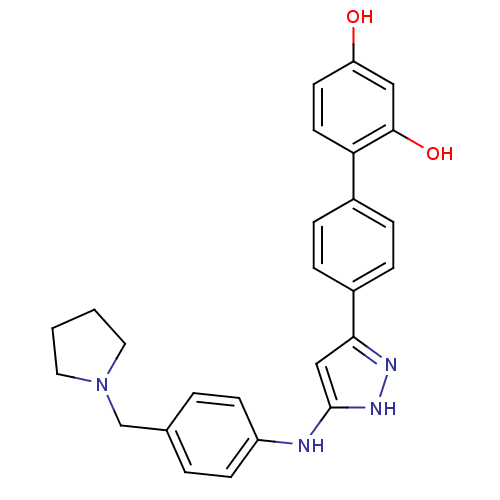 (4'-[5-(4-pyrrolidin-1-ylmethyl-phenylamino)-1H-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human CHK1 expressed in baculovirus/insect cell system | J Med Chem 50: 5253-6 (2007) Article DOI: 10.1021/jm0704604 BindingDB Entry DOI: 10.7270/Q2K0754D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Homo sapiens (Human)) | BDBM286350 (US11220518, Ex. No. K8 | US11780853, Example K8 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.181 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A typical assay was carried out on a 96-well, clear microtiter plate in a Molecular Devices spectrophotometer for 20 minutes at 30° C. in 0.1 mL of a... | Citation and Details BindingDB Entry DOI: 10.7270/Q28S4T3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type Isoform 2 (Homo sapiens (Human)) | BDBM286350 (US11220518, Ex. No. K8 | US11780853, Example K8 | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | 0.181 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 2-beta (Homo sapiens (Human)) | BDBM286350 (US11220518, Ex. No. K8 | US11780853, Example K8 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.181 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
PFIZER INC. US Patent | Assay Description Protein Kinase C beta 2 (PKCβII) catalyzes the production of ADP from ATP that accompanies the phosphoryl transfer to the PKC Pseudosubstrate pe... | US Patent US9518060 (2016) BindingDB Entry DOI: 10.7270/Q2ZK5JPN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50222916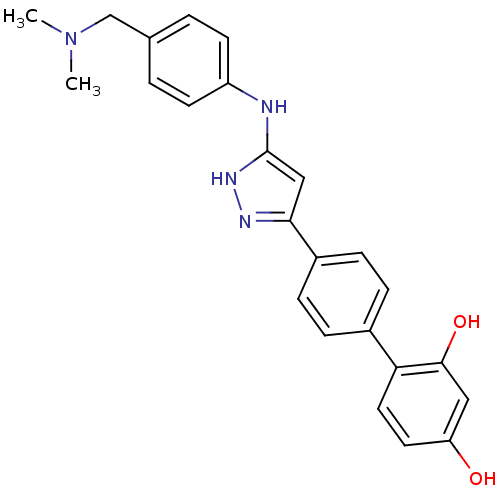 (4'-{5-[4-(dimethylamino-methyl)-phenylamino]-2H-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human CHK1 expressed in baculovirus/insect cell system | J Med Chem 50: 5253-6 (2007) Article DOI: 10.1021/jm0704604 BindingDB Entry DOI: 10.7270/Q2K0754D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50222920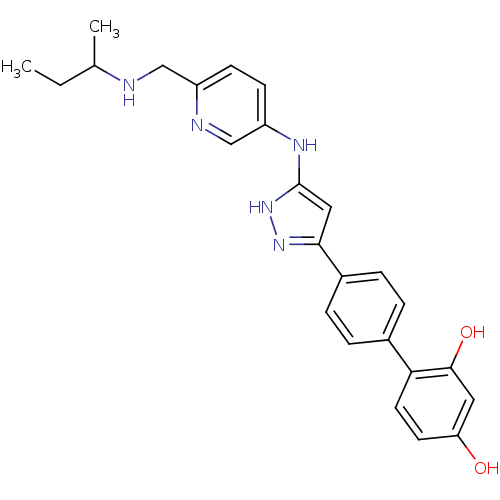 (4'-{5-[6-(sec-butylamino-methyl)-pyridin-3-ylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human CHK1 expressed in baculovirus/insect cell system | J Med Chem 50: 5253-6 (2007) Article DOI: 10.1021/jm0704604 BindingDB Entry DOI: 10.7270/Q2K0754D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50222917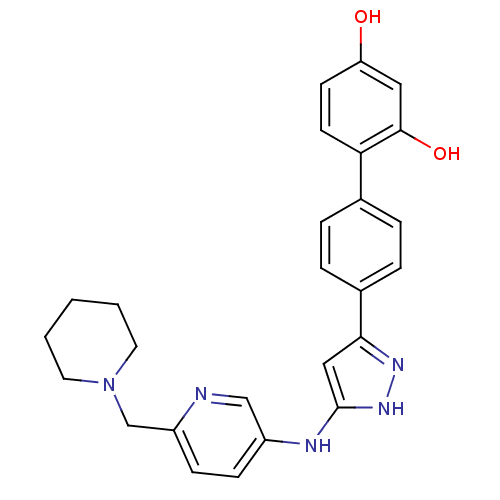 (4'-[5-(6-piperidin-1-ylmethyl-pyridin-3-ylamino)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human CHK1 expressed in baculovirus/insect cell system | J Med Chem 50: 5253-6 (2007) Article DOI: 10.1021/jm0704604 BindingDB Entry DOI: 10.7270/Q2K0754D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50222913 (4'-{5-[4-(isopropylamino-methyl)-phenylamino]-2H-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human CHK1 expressed in baculovirus/insect cell system | J Med Chem 50: 5253-6 (2007) Article DOI: 10.1021/jm0704604 BindingDB Entry DOI: 10.7270/Q2K0754D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type Isoform 2 (Homo sapiens (Human)) | BDBM286348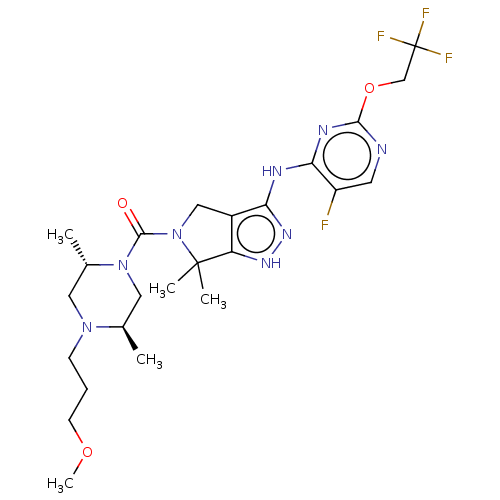 (US11220518, Ex. No. K6 | US11780853, Example K6 | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | 0.376 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Homo sapiens (Human)) | BDBM286348 (US11220518, Ex. No. K6 | US11780853, Example K6 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.376 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A typical assay was carried out on a 96-well, clear microtiter plate in a Molecular Devices spectrophotometer for 20 minutes at 30° C. in 0.1 mL of a... | Citation and Details BindingDB Entry DOI: 10.7270/Q28S4T3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 2-beta (Homo sapiens (Human)) | BDBM286348 (US11220518, Ex. No. K6 | US11780853, Example K6 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.376 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
PFIZER INC. US Patent | Assay Description Protein Kinase C beta 2 (PKCβII) catalyzes the production of ADP from ATP that accompanies the phosphoryl transfer to the PKC Pseudosubstrate pe... | US Patent US9518060 (2016) BindingDB Entry DOI: 10.7270/Q2ZK5JPN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50222914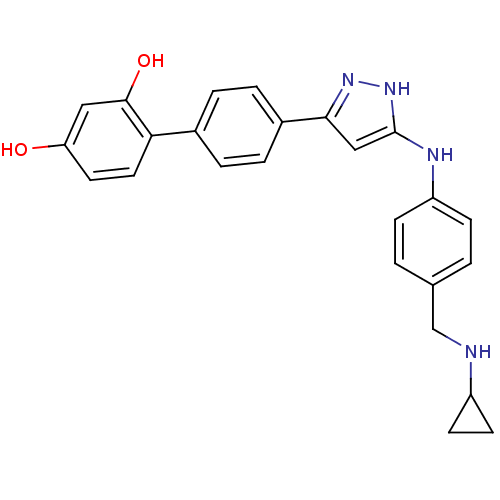 (4'-[5-(4-cyclopropylaminomethyl-phenylamino)-2H-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human CHK1 expressed in baculovirus/insect cell system | J Med Chem 50: 5253-6 (2007) Article DOI: 10.1021/jm0704604 BindingDB Entry DOI: 10.7270/Q2K0754D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50222921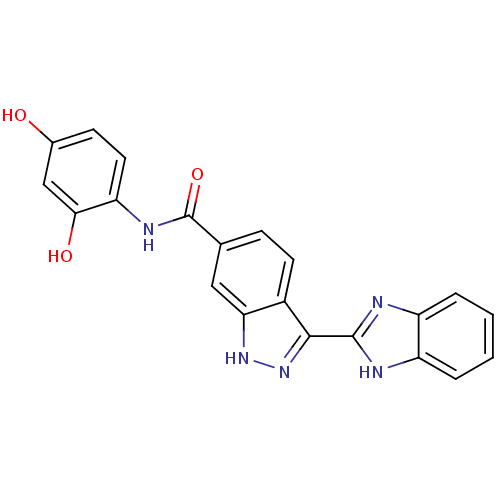 (3-(1H-benzo[d]imidazol-2-yl)-N-(2,4-dihydroxypheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human CHK1 expressed in baculovirus/insect cell system | J Med Chem 50: 5253-6 (2007) Article DOI: 10.1021/jm0704604 BindingDB Entry DOI: 10.7270/Q2K0754D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 2-beta (Homo sapiens (Human)) | BDBM286347 (US11220518, Ex. No. K5 | US11780853, Example K5 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.683 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
PFIZER INC. US Patent | Assay Description Protein Kinase C beta 2 (PKCβII) catalyzes the production of ADP from ATP that accompanies the phosphoryl transfer to the PKC Pseudosubstrate pe... | US Patent US9518060 (2016) BindingDB Entry DOI: 10.7270/Q2ZK5JPN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Homo sapiens (Human)) | BDBM286347 (US11220518, Ex. No. K5 | US11780853, Example K5 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.683 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A typical assay was carried out on a 96-well, clear microtiter plate in a Molecular Devices spectrophotometer for 20 minutes at 30° C. in 0.1 mL of a... | Citation and Details BindingDB Entry DOI: 10.7270/Q28S4T3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type Isoform 2 (Homo sapiens (Human)) | BDBM286347 (US11220518, Ex. No. K5 | US11780853, Example K5 | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | 0.683 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Threonine--tRNA ligase (Yersinia pestis) | BDBM50426188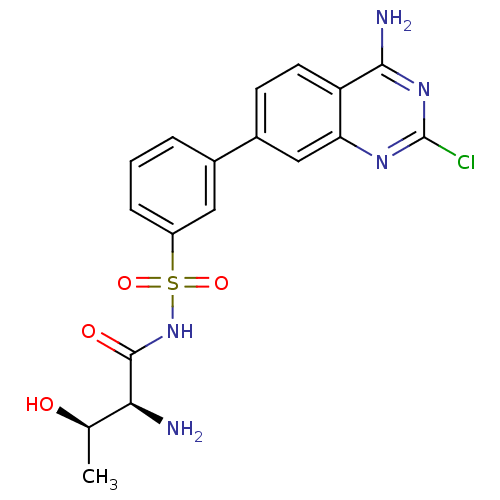 (CHEMBL2311920) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. Curated by ChEMBL | Assay Description Binding affinity to Yersinia pestis ThrRS by coupled spectrophotometry | J Med Chem 56: 1748-60 (2013) Article DOI: 10.1021/jm301756m BindingDB Entry DOI: 10.7270/Q2GQ7036 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50222919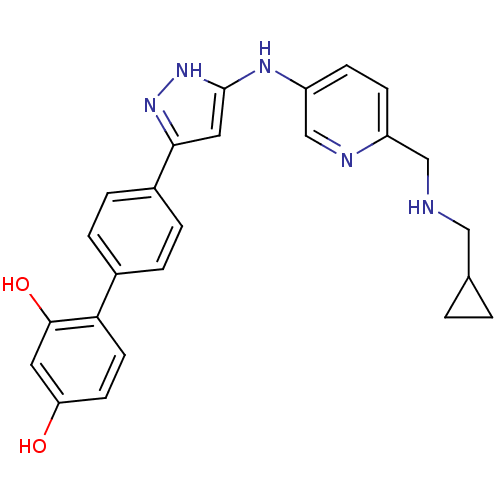 (4'-(5-{6-[(cyclopropylmethyl-amino)-methyl]-pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human CHK1 expressed in baculovirus/insect cell system | J Med Chem 50: 5253-6 (2007) Article DOI: 10.1021/jm0704604 BindingDB Entry DOI: 10.7270/Q2K0754D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Homo sapiens (Human)) | BDBM286345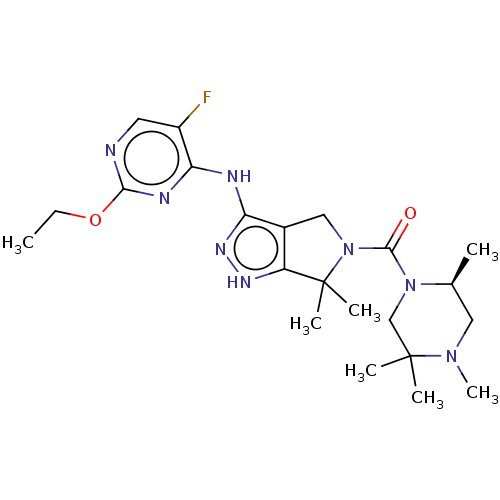 (US11220518, Ex. No. K3 | US11780853, Example K3 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.912 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A typical assay was carried out on a 96-well, clear microtiter plate in a Molecular Devices spectrophotometer for 20 minutes at 30° C. in 0.1 mL of a... | Citation and Details BindingDB Entry DOI: 10.7270/Q28S4T3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type Isoform 2 (Homo sapiens (Human)) | BDBM286345 (US11220518, Ex. No. K3 | US11780853, Example K3 | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | 0.912 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 2-beta (Homo sapiens (Human)) | BDBM286345 (US11220518, Ex. No. K3 | US11780853, Example K3 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.912 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
PFIZER INC. US Patent | Assay Description Protein Kinase C beta 2 (PKCβII) catalyzes the production of ADP from ATP that accompanies the phosphoryl transfer to the PKC Pseudosubstrate pe... | US Patent US9518060 (2016) BindingDB Entry DOI: 10.7270/Q2ZK5JPN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Homo sapiens (Human)) | BDBM286246 (5-{[(8S)-6,8-dimethyl-6,9-diazaspiro[4.5]dec-9-yl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A typical assay was carried out on a 96-well, clear microtiter plate in a Molecular Devices spectrophotometer for 20 minutes at 30° C. in 0.1 mL of a... | Citation and Details BindingDB Entry DOI: 10.7270/Q28S4T3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 2-beta (Homo sapiens (Human)) | BDBM286246 (5-{[(8S)-6,8-dimethyl-6,9-diazaspiro[4.5]dec-9-yl]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.01 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
PFIZER INC. US Patent | Assay Description Protein Kinase C beta 2 (PKCβII) catalyzes the production of ADP from ATP that accompanies the phosphoryl transfer to the PKC Pseudosubstrate pe... | US Patent US9518060 (2016) BindingDB Entry DOI: 10.7270/Q2ZK5JPN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type Isoform 2 (Homo sapiens (Human)) | BDBM286246 (5-{[(8S)-6,8-dimethyl-6,9-diazaspiro[4.5]dec-9-yl]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | 1.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Homo sapiens (Human)) | BDBM533472 (US11220518, Ex. No. K1 | US11780853, Example K1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A typical assay was carried out on a 96-well, clear microtiter plate in a Molecular Devices spectrophotometer for 20 minutes at 30° C. in 0.1 mL of a... | Citation and Details BindingDB Entry DOI: 10.7270/Q28S4T3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 2-beta (Homo sapiens (Human)) | BDBM286343 (N-(2-ethoxy-5-fluoropyrimidin-4-yl)-6,6-dimethyl-5...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.12 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
PFIZER INC. US Patent | Assay Description Protein Kinase C beta 2 (PKCβII) catalyzes the production of ADP from ATP that accompanies the phosphoryl transfer to the PKC Pseudosubstrate pe... | US Patent US9518060 (2016) BindingDB Entry DOI: 10.7270/Q2ZK5JPN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type Isoform 2 (Homo sapiens (Human)) | BDBM533472 (US11220518, Ex. No. K1 | US11780853, Example K1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | 1.12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 2-beta (Homo sapiens (Human)) | BDBM286320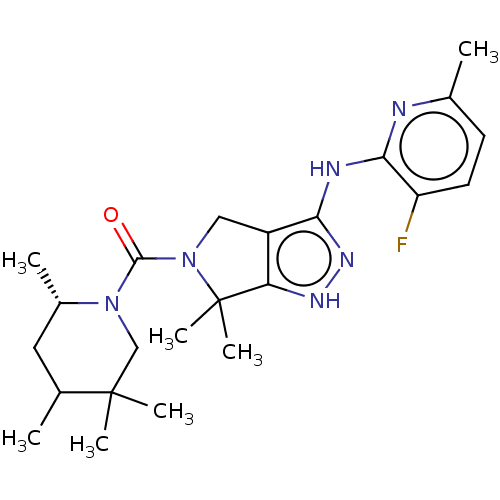 (US9518060, Example J6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.17 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
PFIZER INC. US Patent | Assay Description Protein Kinase C beta 2 (PKCβII) catalyzes the production of ADP from ATP that accompanies the phosphoryl transfer to the PKC Pseudosubstrate pe... | US Patent US9518060 (2016) BindingDB Entry DOI: 10.7270/Q2ZK5JPN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type Isoform 2 (Homo sapiens (Human)) | BDBM533459 (US11220518, Ex. No. J6 | US11780853, Example J6) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | 1.17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Homo sapiens (Human)) | BDBM533459 (US11220518, Ex. No. J6 | US11780853, Example J6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A typical assay was carried out on a 96-well, clear microtiter plate in a Molecular Devices spectrophotometer for 20 minutes at 30° C. in 0.1 mL of a... | Citation and Details BindingDB Entry DOI: 10.7270/Q28S4T3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Threonine--tRNA ligase 1, cytoplasmic (Homo sapiens (Human)) | BDBM50426188 (CHEMBL2311920) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. Curated by ChEMBL | Assay Description Binding affinity to human cytoplasmic ThrRS by coupled spectrophotometry | J Med Chem 56: 1748-60 (2013) Article DOI: 10.1021/jm301756m BindingDB Entry DOI: 10.7270/Q2GQ7036 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50222918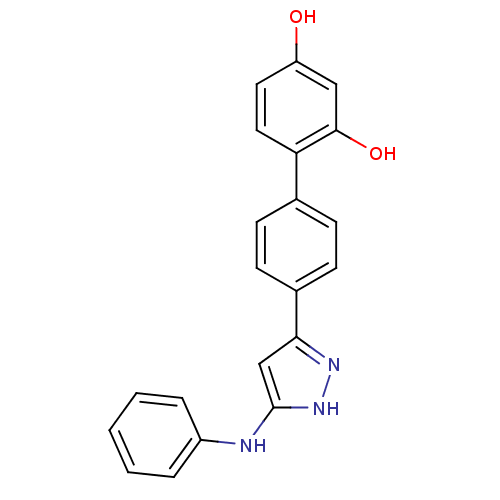 (4'-(5-phenylamino-2H-pyrazol-3-yl)-biphenyl-2,4-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human CHK1 expressed in baculovirus/insect cell system | J Med Chem 50: 5253-6 (2007) Article DOI: 10.1021/jm0704604 BindingDB Entry DOI: 10.7270/Q2K0754D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Homo sapiens (Human)) | BDBM286270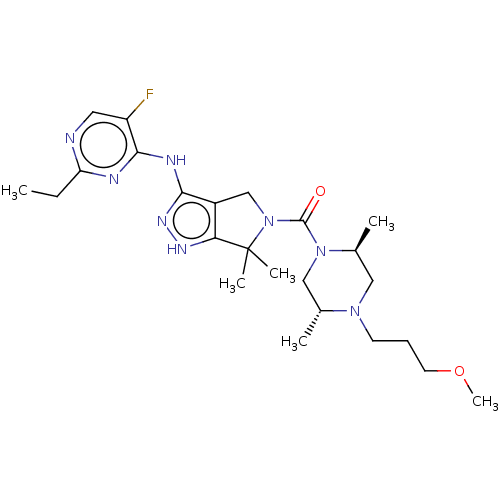 (US11220518, Ex. No. E2 | US11780853, Example E2 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A typical assay was carried out on a 96-well, clear microtiter plate in a Molecular Devices spectrophotometer for 20 minutes at 30° C. in 0.1 mL of a... | Citation and Details BindingDB Entry DOI: 10.7270/Q28S4T3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type Isoform 2 (Homo sapiens (Human)) | BDBM286270 (US11220518, Ex. No. E2 | US11780853, Example E2 | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | 1.64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 2-beta (Homo sapiens (Human)) | BDBM286270 (US11220518, Ex. No. E2 | US11780853, Example E2 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.64 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
PFIZER INC. US Patent | Assay Description Protein Kinase C beta 2 (PKCβII) catalyzes the production of ADP from ATP that accompanies the phosphoryl transfer to the PKC Pseudosubstrate pe... | US Patent US9518060 (2016) BindingDB Entry DOI: 10.7270/Q2ZK5JPN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Homo sapiens (Human)) | BDBM153901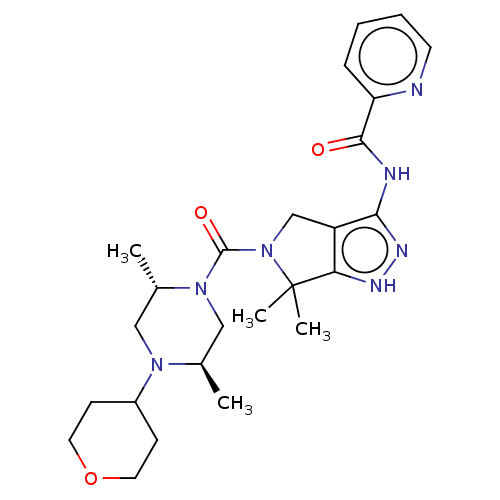 (US8999981, A146) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.70 | -50.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer Inc.; Pfizer Products Inc. US Patent | Assay Description Protein Kinase C beta 2 (PKC beta II) catalyzes the production of ADP from ATP that accompanies the phosphoryl transfer to the PKC Pseudosubstrate pe... | US Patent US8999981 (2015) BindingDB Entry DOI: 10.7270/Q2RR1X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type Isoform 2 (Homo sapiens (Human)) | BDBM286377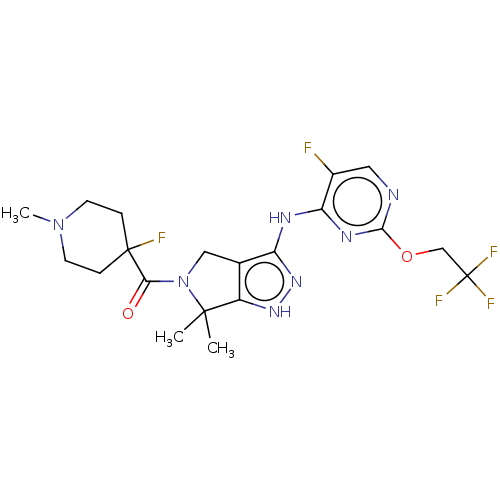 (US11220518, Ex. No. L4 | US11780853, Example L4 | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | 1.71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 2-beta (Homo sapiens (Human)) | BDBM286377 (US11220518, Ex. No. L4 | US11780853, Example L4 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.71 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
PFIZER INC. US Patent | Assay Description Protein Kinase C beta 2 (PKCβII) catalyzes the production of ADP from ATP that accompanies the phosphoryl transfer to the PKC Pseudosubstrate pe... | US Patent US9518060 (2016) BindingDB Entry DOI: 10.7270/Q2ZK5JPN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Homo sapiens (Human)) | BDBM286377 (US11220518, Ex. No. L4 | US11780853, Example L4 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A typical assay was carried out on a 96-well, clear microtiter plate in a Molecular Devices spectrophotometer for 20 minutes at 30° C. in 0.1 mL of a... | Citation and Details BindingDB Entry DOI: 10.7270/Q28S4T3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 2-beta (Homo sapiens (Human)) | BDBM286310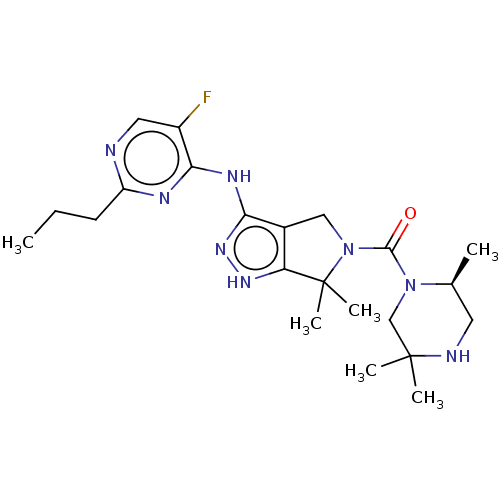 (N-(5-fluoro-2-propylpyrimidin-4-yl)-6,6-dimethyl-5...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.73 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
PFIZER INC. US Patent | Assay Description Protein Kinase C beta 2 (PKCβII) catalyzes the production of ADP from ATP that accompanies the phosphoryl transfer to the PKC Pseudosubstrate pe... | US Patent US9518060 (2016) BindingDB Entry DOI: 10.7270/Q2ZK5JPN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Homo sapiens (Human)) | BDBM533452 (US11220518, Ex. No. I1 | US11780853, Example I1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A typical assay was carried out on a 96-well, clear microtiter plate in a Molecular Devices spectrophotometer for 20 minutes at 30° C. in 0.1 mL of a... | Citation and Details BindingDB Entry DOI: 10.7270/Q28S4T3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type Isoform 2 (Homo sapiens (Human)) | BDBM533452 (US11220518, Ex. No. I1 | US11780853, Example I1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | 1.73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Threonine--tRNA ligase (Yersinia pestis) | BDBM50426183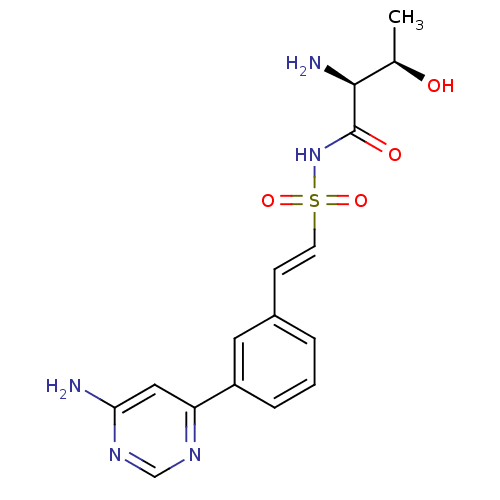 (CHEMBL2311925) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. Curated by ChEMBL | Assay Description Binding affinity to Yersinia pestis ThrRS by coupled spectrophotometry | J Med Chem 56: 1748-60 (2013) Article DOI: 10.1021/jm301756m BindingDB Entry DOI: 10.7270/Q2GQ7036 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Threonine--tRNA ligase (Yersinia pestis) | BDBM50426182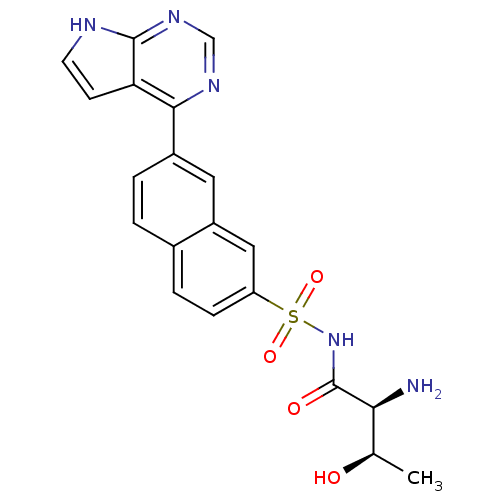 (CHEMBL2311926) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. Curated by ChEMBL | Assay Description Binding affinity to Yersinia pestis ThrRS by coupled spectrophotometry | J Med Chem 56: 1748-60 (2013) Article DOI: 10.1021/jm301756m BindingDB Entry DOI: 10.7270/Q2GQ7036 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Threonine--tRNA ligase 1, cytoplasmic (Homo sapiens (Human)) | BDBM50426182 (CHEMBL2311926) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. Curated by ChEMBL | Assay Description Binding affinity to human cytoplasmic ThrRS by coupled spectrophotometry | J Med Chem 56: 1748-60 (2013) Article DOI: 10.1021/jm301756m BindingDB Entry DOI: 10.7270/Q2GQ7036 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Homo sapiens (Human)) | BDBM153898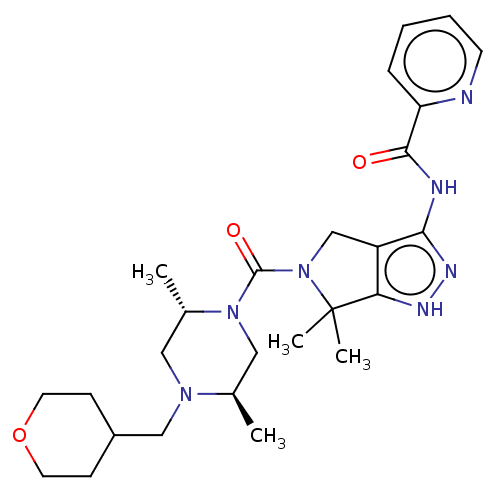 (US8999981, A143) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | 1.90 | -50.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer Inc.; Pfizer Products Inc. US Patent | Assay Description Protein Kinase C beta 2 (PKC beta II) catalyzes the production of ADP from ATP that accompanies the phosphoryl transfer to the PKC Pseudosubstrate pe... | US Patent US8999981 (2015) BindingDB Entry DOI: 10.7270/Q2RR1X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Threonine--tRNA ligase (Yersinia pestis) | BDBM50426184 (CHEMBL2311924) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. Curated by ChEMBL | Assay Description Binding affinity to Yersinia pestis ThrRS by coupled spectrophotometry | J Med Chem 56: 1748-60 (2013) Article DOI: 10.1021/jm301756m BindingDB Entry DOI: 10.7270/Q2GQ7036 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1770 total ) | Next | Last >> |